Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


168
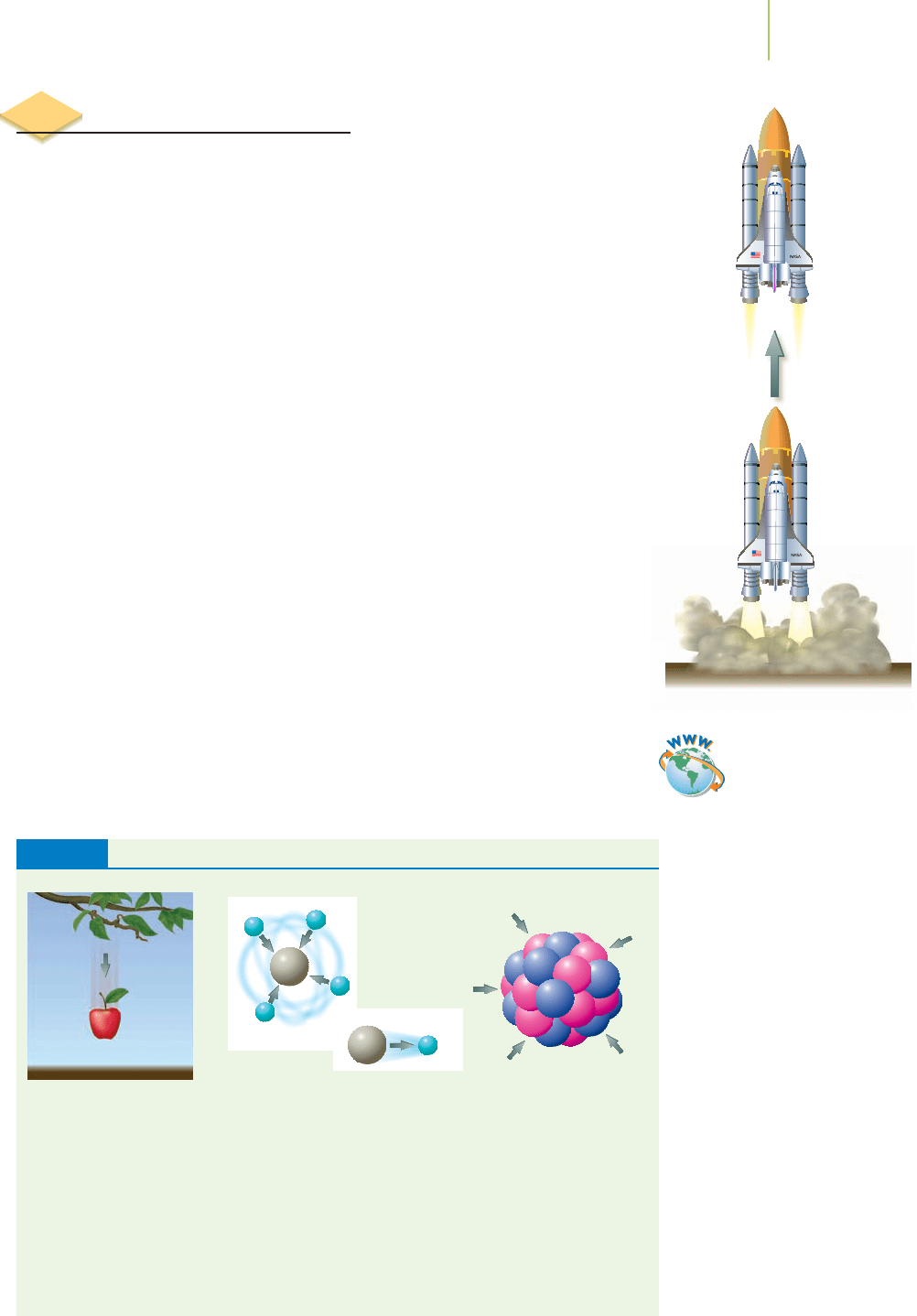
A space shuttle launch is an awe-inspiring
demonstration of the ability of energy to trans-
port humans and material away from Earth’s surface
with an eye toward planetary exploration. The energy to
launch the craft and its crew comes from the explosive vio-
lence of chemical reactions. When these reactions are used in a
carefully controlled way, they can lift the massive shuttle (which
weighs in at a robust 2,000,000 kg), the booster rockets, and the fuel tank
and propel the ship into orbit hundreds of miles above Earth in a very brief
10-minute ride.
Two chemical reactions, indicated in Figure 5.1, power the launch of the space
shuttle. The combustion of hydrogen gas (the combination of hydrogen and oxygen
to form water) takes place in the main engines. At the same time, the solid rocket
boosters attached to the sides of the shuttle release a host of products resulting from
the oxidation of aluminum by ammonium perchlorate, though we show only the
primary equation here.
Main engines: 2H
2
(g) + O
2
(g) →2H
2
O(g)
Boosters: 10Al(s) + 6NH
4
ClO
4
(s) → 5Al
2
O
3
(s) + 6HCl(g) + 3N
2
(g) + 9H
2
O(g)
As the shuttle sits on the launch pad, it is hard to believe that the en-
ergy that will launch it with such a spectacular display of chemical mus-
cle is quietly present within the main fuel tank and the solid fuel of the
booster rockets. Chemicals can store huge amounts of energy in this
way—and then release it, with very dramatic effects, as soon as a chemi-
cal reaction begins.
These thoughts raise many questions about energy:
What is energy?
The rocket fuels used to lift the space shuttle store energy and release it
during takeoff. How is energy stored within molecules and compounds?
Can we calculate how much energy will be released from a molecule or
compound during a shuttle launch so that enough will be harnessed to
allow the shuttle to climb to exactly the intended orbit? These questions
can be answered by focusing our attention on a branch of science known
as
thermodynamics. Thermodynamics is concerned with the interconver-
sion of different forms of energy. The specific area of thermodynamics
most relevant to chemical reactions is known as
thermochemistry, the
study of energy changes and exchanges in chemical processes.
In order to properly describe energy exchanges, we must clearly state
where they occur. We define the
system as that object
in which we are interested. In our discussion, the
main shuttle craft is the system. Everything else is
defined as the
surroundings. Energy that is lost by the
shuttle’s main engines in our system is gained by
the atmosphere, the surroundings. The system and
the surroundings combine to make up the
universe.
These terms are fundamental to our discussion of
energy in chemistry.
Application
C
HEMICAL ENCOUNTERS:
Setting the Stage with
the Space Shuttle
2H
2
(g) + O
2
(g)
10Al(s) + 6NH
4
ClO
4
(s)
5 Al
2
O
3
(s) + 6HCl(g) +
3N
2
(g) + 9H
2
O(g)
2H
2
O(g)
FIGURE 5.1
The reactions that provide the energy to
lift the space shuttle into orbit.
System
(shuttle,
payload, people)
Surroundings
Universe = System + Surroundings
The system and the
surroundings make up
the universe.
5.1 The Concept of Energy
The simplest commonly used definition of energy is that it is “the capacity to do
work.”Often it is defined as “the capacity to do work or produce heat.”In order to
fully understand the definition of energy, we need to define work.
Work is done whenever any force is used to move an object some distance. For
example, suppose we use a chemical reaction to propel a heavy weight, such as the
space shuttle, upward. We are using the reaction to provide the force that makes
the shuttle move. The chemicals that are combined in the reaction are doing work
on the shuttle, so they are releasing energy. Why does it take so much energy to lift
the shuttle? You might say, “Because the shuttle is very heavy,” but that is only the
beginning of the answer. The shuttle is heavy because of the force of gravitational
attraction between the particles in this system and the rest of planet Earth. To
raise the shuttle, we must make it move against the force of gravity, one of the
three known fundamental forces of nature listed in Table 5.1. Since the force of
gravitational attraction is so large between the shuttle and Earth, we must supply
a lot of energy to raise the shuttle into orbit. The result is that a tremendous
amount of work is done on the shuttle.
We can therefore also describe energy as the capacity to move something
against a fundamental force. This idea can help us develop a good understanding
of what energy is all about. In terms of the system and the surroundings, we can
make the following statement:
Energy is absorbed by the system from the surroundings in order to oppose a
natural attraction.
This means, for example, that energy must be added to two magnets that are stuck
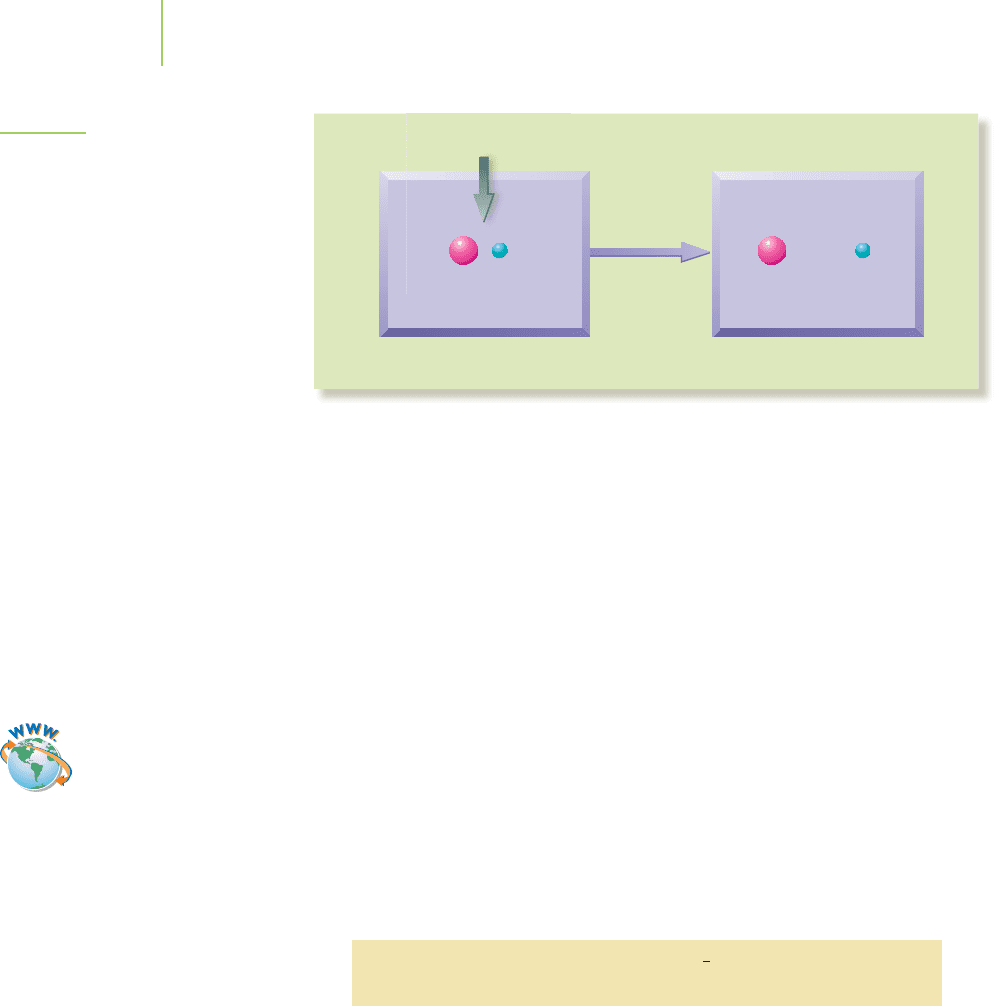
together (a system) in order to pull them apart, because we are opposing their nat-
ural magnetic attraction.Similarly,whenwe pull an electronaway from a nucleus in
an atom (another system),energy is required (must be added to the system) because
opposite charges attract and we are opposing that natural attraction. Figure 5.2
illustrates the energy exchange between the system and surroundings in this case.
5.1 The Concept of Energy 169
Earth
Work moves
the space
shuttle away
from Earth.
The Three Known Fundamental Forces of Nature
TABLE 5.1
e
–
e
–
P
+
N
Gravitational force
causes all objects with
mass to be attracted to
one another.
Electroweak force is responsi-
ble for the attraction between
objects with opposite electric
charges and the repulsion
between objects with the same
electric charge (when it is
known as the electric force), the
phenomena of magnetism and
light, and some transformations
within subatomic particles.
Strong nuclear force
binds protons and
neutrons together
within atomic nuclei.
Tutorial: Work, Heat, and
Energy Flow
Because energy is needed to oppose a natural attraction, the reverse process allows
us to draw the following opposite, perhaps counterintuitive, conclusion:
Energy is released from the system to the surroundings when a natural attrac-
tion occurs.
This happens when, for example, magnets are made to stick together, an electron
is attracted to an atom, or a ball falls to Earth toward the planet’s center of gravity.
Kinetic Energy Versus Potential Energy
Energy can be relegated to two basic forms: kinetic energy and potential energy.
In addition to these two forms, energy can also be propagated through space in
the form of
electromagnetic radiation, such as light, which we will discuss in detail
in Chapter 6.
Kinetic energy is the energy of movement. Everything that is moving has an
amount of kinetic energy that depends on its mass and velocity, as described by
the equation
Kinetic energy =
1
2
mv
2
where m = mass in kilograms, v = velocity in meters per second.
For example, a 36,000-kg 18-wheeled truck moving at the same speed as an 800-kg
subcompact automobile has more kinetic energy than the car because its mass is
greater.A truck movingfaster thananother,identicaltruck has more kinetic energy
than the slower truck because its velocity is greater. The relationship of kinetic en-
ergy to mass and velocity is true for a car, a truck, a space shuttle—or a molecule.
If movement is associated with energy, then everything that is moving must,
in appropriate circumstances, be able to do work. You can convince yourself of
this by thinking about the steel ball shown in Figure 5.3. If the ball is moving
toward the slope, its energy of movement (kinetic energy) allows it to rise a
certain distance up the slope. The height to which it rises depends on how fast it
was originally moving. Kinetic energy is associated with the work of raising the
ball, and the amount of kinetic energy that the rolling ball possesses will deter-
mine how high it can rise in defiance of the gravitational force.
The second fundamental form of energy is
potential energy, which is the en-
ergy that objects possess because of their positions. We often think of potential
energy as stored energy, which, if released, can be transformed into kinetic energy.
For example, any object raised up against the force of gravity gains an amount of
170 Chapter 5 Energy
Energy
Initial state
System:
E
Surroundings: E
Final state
System:
E
Surroundings: E
System System
Surroundings Surroundings
FIGURE 5.2
Energy exchange between the system
and the surroundings during the ioniza-
tion of an atom.
Video Lesson: The Nature
of Energy
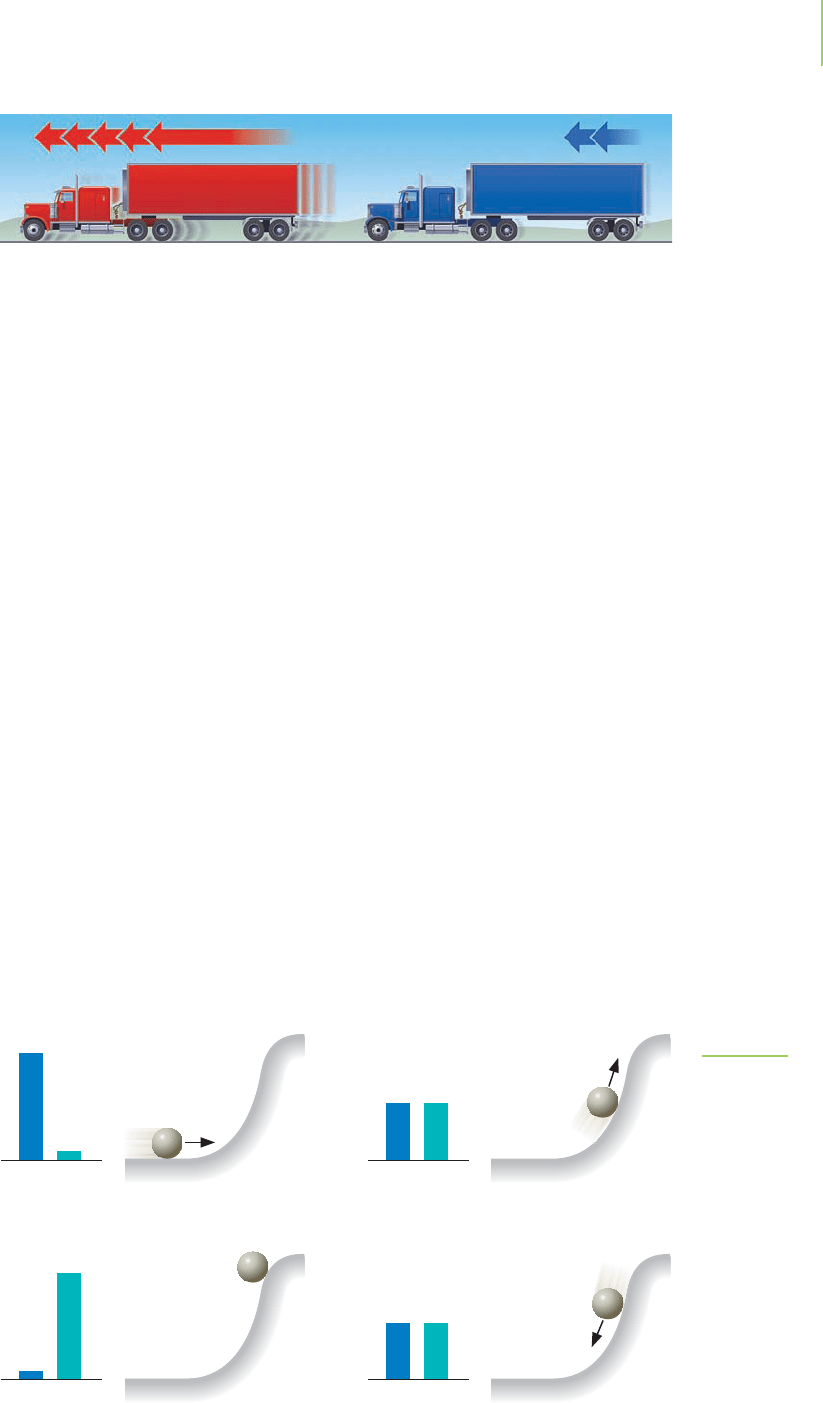
potential energy because of its position relative to the gravitational attraction to
Earth’s center. Recall our key idea that energy is absorbed by the system from the
surroundings in order to oppose a natural attraction. The farther we pull our object
away from Earth’s center of gravity, the more energy is required, and the greater
will be the object’s potential energy.
As our steel ball in Figure 5.3 rises upward, it begins to slow down. In fact, if
we ignore the loss of energy due to friction, the steel ball steadily gains potential
energy in an amount that is equal to the kinetic energy it loses because it is slow-
ing down. By the time the ball becomes stationary at the top of its climb, kinetic
energy due to the rolling of the ball has been converted into potential energy.
What happens next? The potential energy stored in the steel ball due to its rela-
tively high altitude is released as the ball begins to run downhill. The amount of
potential energy is reduced as the kinetic energy, and therefore the velocity of the
ball, increases. At the bottom of the hill, this potential energy has been com-
pletely converted into kinetic energy, and the ball is traveling at its maximum
velocity.
This example of the interconversion between kinetic and potential energy
illustrates one of the most significant fundamental laws of nature. It is known as
the
law of conservation of energy:
Energy is neither created nor destroyed. It is only transferred from place to place
and transformed from one form into another.
The energy contained in molecules and compounds, such as those that power
the space shuttle, is a constantly interconverting mixture of kinetic and potential
energy. The particles in the system are moving, bouncing off one another, vibrat-
ing, and rotating as the bonds between atoms stretch in and out, atoms and larger
groups rotate around bonds, and entire molecules cartwheel through space. All
of this motion is associated with a corresponding amount of kinetic energy. The
available potential energy in chemical compounds is stored in the positions and
arrangements of the electrons and nuclei that make up those compounds. For
5.1 The Concept of Energy 171
(a) KE > PE
KE PE
(b) KE = PE
KE PE
(c) KE < PE
KE PE
(d) KE = PE
KE PE
FIGURE 5.3
A steel ball moving toward a hill demon-
strates that energy can do work and that
kinetic energy and potential energy can
be interconverted. As the ball moves up
the slope, its kinetic energy is converted
into potential energy. When it reaches its
maximum height and begins to fall back,
that potential energy is converted back
into kinetic energy. Work is done when
the mass of the ball rises up the slope.
Assuming they have equal mass, the
faster truck has greater kinetic energy.

instance, as we discussed previously, it takes energy to move an electron away
from an atom’s nucleus, against the pull of the electric force, in just the same way
as it takes energy to lift a weight up from the ground against the pull of the grav-
itational force illustrated in Figure 5.4.
It also requires an influx of energy to a system of atoms in order to force two or
more (like-charged) electrons closer together against the repulsion due to the
electric force, or to force two nuclei together against that force. The electrons and
nuclei of chemicals have potential energy as a consequence of their positions in the
electric force field, as shown in Figure 5.5, just as objects we lift and throw around
have potential energy due to their positions in the gravitational force field.
Any set of compounds, at a given temperature and pressure, contains chemical
energy stored as a result of the motions and positions of their atomic nuclei
and electrons.
When a chemical reaction occurs in a system, new chemicals are formed with
different internal motions, different electron arrangements, and a different total
energy content. Any excess energy must be released to the surroundings, usually
as heat and possibly as light and/or sound. Any energy acquired by the new chem-
icals must come from the surroundings. For example, when hydrogen and oxygen
react to form water in the main engines of the space shuttle, a tremendous
amount of chemical energy is released. This release of energy occurs because the
structure of water molecules embodies much less energy than that of the oxygen
and hydrogen molecules used to form the water. A small fraction of the released
energy is converted into light and sound. The majority of the released energy dra-
matically increases the speed of motion of the particles involved, causing them to
undergo a massive and explosive expansion as all of the very fast-moving parti-
cles bounce off one another. This chemical cyclone of particles within the ex-
panding gas pounds against the inner upper surface of the shuttle engines and
literally pushes the shuttle upward while water vapor escapes from the opening at
the bottom of the engines, as shown in Figure 5.6.
EXERCISE 5.1 Energy in Various Forms
In our day-to-day chats and political or environmental discussions, we speak of
many different kinds of energy, such as that from wind, waves, or fossil fuels. Can
you explain how these forms of energy (wind, waves, and fossil fuels) are related to
the fundamental forms of energy discussed above?
Solution
Wind energy is the energy associated with the movement of air, so it is a form of
kinetic energy. Similarly, wave energy is the energy associated with the movement of
172 Chapter 5 Energy
Force of expanding gases
Gravity
Net force
FIGURE 5.6
Forces acting on the shuttle’s main
engine.
FIGURE 5.4
The gravitational force. Energy is re-
quired to lift an apple from the ground.
The opposing force is gravity.
FIGURE 5.5
Chemical energy. The positions of the nuclei and electrons, as
well as the motions of the particles, are part of the energy
stored in a molecule.
Application
5.1 The Concept of Energy 173
The trapping of the energy of sunlight by green plants is
one of the most significant chemical processes on Earth.
What makes it even more interesting is that the process
is achieved by the smallest chemical change. That tiny
change, powered by the absorption of energy from the
Sun, is the transfer of an electron from one molecule to
another. This change begins the process that provides
energy to the plant and allows it to grow.
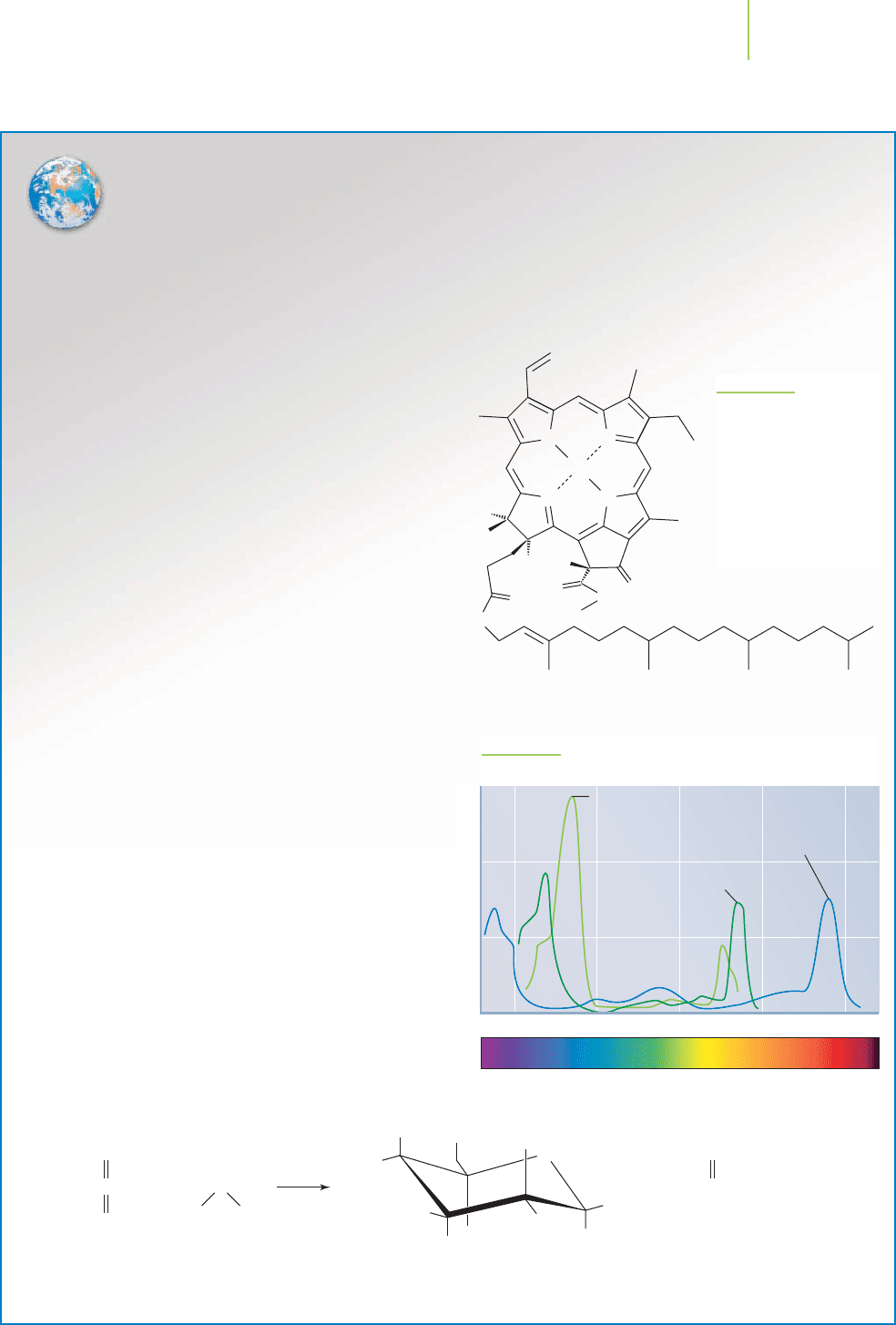
Why are plants green? They contain molecules of
chlorophyll,which absorb light in the red and blue parts
of the visible spectrum (the range of visible colors)
more than in the green parts, leaving the green light to
reach our eyes. There are actually several different types
of molecules known as chlorophyll. Chlorophyll a is
shown in Figure 5.7.
The absorption spectra of some chlorophylls, mean-
ing the extent to which they absorb light of different
wavelengths, is shown in Figure 5.8. Because of differ-
ences in structure, every atom and molecule has its own
absorption fingerprint, which we will discuss more fully
in Chapter 6.
When a molecule absorbs light energy, it usually
raises one or more of the electrons into a higher energy
configuration. When this happens to the chlorophyll in
a plant, an excited higher-energy electron can be trans-
ferred to an adjacent receptor molecule. This electron
transfer requires an input of energy as the negatively
charged electron moves away from a positively charged
atomic nucleus within chlorophyll, against the pull of the
electric force. The transferred electron carries much of
this energy to the receptor molecule.
This is the tiny nanoscale event that powers photo-
synthesis. The transferred electron then moves among a
series of other molecules involved in photosynthesis.
The complex series of steps that each of the molecules
goes through allows some of the energy originally ab-
sorbed from the Sun to be stored within other com-
pounds. Ultimately, the energy from the Sun converts
carbon dioxide and water into carbohydrate and
oxygen—the overall energy-requiring process of
photosynthesis.
The path the energy takes in completing this task is
long. The chemical processes are very complex, and the
involvement of huge numbers of electrons is necessary
to generate significant amounts of carbohydrate and
oxygen. But the crucial event begins with a single electron.
NanoWorld / MacroWorld
Big effects of the very small:
Electrons capturing energy in photosynthesis
Chlorophyll a
N
N
CH
3
H
H
H
O
O
O
O
O
N
N
Mg
FIGURE 5.7
Chlorophyll a is a complex
naturally occurring mole-
cule. The transfer of an
electron from chlorophyll,
powered by the energy of
sunlight, is the key event
of photosynthesis.
FIGURE 5.8
The absorption spectra of a series of chlorophylls.
OH
O
H
H
H
OH
OH
HO
HO
O
C
O
O
O
H
O+66 +
HH
H
6
Carbon dioxide and water combine to make carbohydrates and oxygen.This reaction is driven by the trans-
fer of an electron from chlorophyll.
400 500 600
Wavelength (nm)
Absorption
700 800
Chlorophyll b
Chlorophyll a
Bacteriochlorophyll a

water, so it is a form of kinetic energy. Fossil fuel energy is the energy that we release
from the fossil fuels—coal, oil and natural gas—when we burn them. The energy is
stored within the chemicals involved as a mixture of kinetic and potential energy,
associated with the movement and positions of the particles of the chemicals.
PRACTICE 5.1
Explain how the “activities” of plants are related to the fundamental forms of en-
ergy. Include in your discussion the interaction of plants with the environment and
their ultimate fate as food.
See Problems 1, 2, 5–8, 17, and 18.
Heat and Reaction Profile Diagrams
During a chemical change, energy is exchanged between the system and the sur-
roundings. Much of this energy exchange is as heat.
Heat (q) is the energy that is
exchanged between a system and its surroundings because of a difference in tem-
perature between the two. The temperature of a system is proportional to the
kinetic energy of its particles, and the energy flow as heat between a system and
its surroundings is essentially a transfer of kinetic energy due to the collisions
between particles.
We can portray the change in the internal energy content of chemicals during
a reaction using “reaction profile diagrams” like those shown in Figure 5.9. These
diagrams illustrate the changes in the energy of the components of a reaction as
it progresses. The horizontal axis in the diagram is known as the reaction coordi-
nate, an arbitrary scale that denotes the progress of the reaction from start (left-
hand side) to finish (right-hand side). The vertical axis represents the relative
energy of the individual components of the system. By comparing the energy of
the starting materials with the products, we can determine the direction of the
flow of energy in the reaction (either from the system to the surroundings or
from the surroundings to the system.)
From the diagram, we note that the curve of the reaction rises to a peak as the
reaction proceeds. This indicates an input of energy needed to drive the reaction
toward products. Virtually all reactions begin with an input of energy, known as
the
activation energy. This energy may be supplied when the overall kinetic
energy of the moving particles is converted into internal potential and kinetic en-
ergy. The chemicals, after colliding, lose energy as they settle into the new chem-
ical arrangement of the products.
A chemical reaction that releases energy as heat to the surroundings, like that
shown in Figure 5.10, is known as an
exothermic reaction. Energy, as heat, flows
out of the system and into the surroundings. A forest fire includes all manner of
174 Chapter 5 Energy
Progress of reaction
Energy
Reactants
Products
Activation
energy: E
A
Heat evolved
during reaction
Progress of reaction
Energy
Reactants
Products
Activation
energy: E
A
Heat absorbed
during reaction
FIGURE 5.9
Reaction profile dia-
grams. The exothermic
reaction on the left in-
volves a loss of heat.
The endothermic reac-
tion on the right re-
quires the absorption
of heat.
A forest fire results from many different
exothermic reactions.
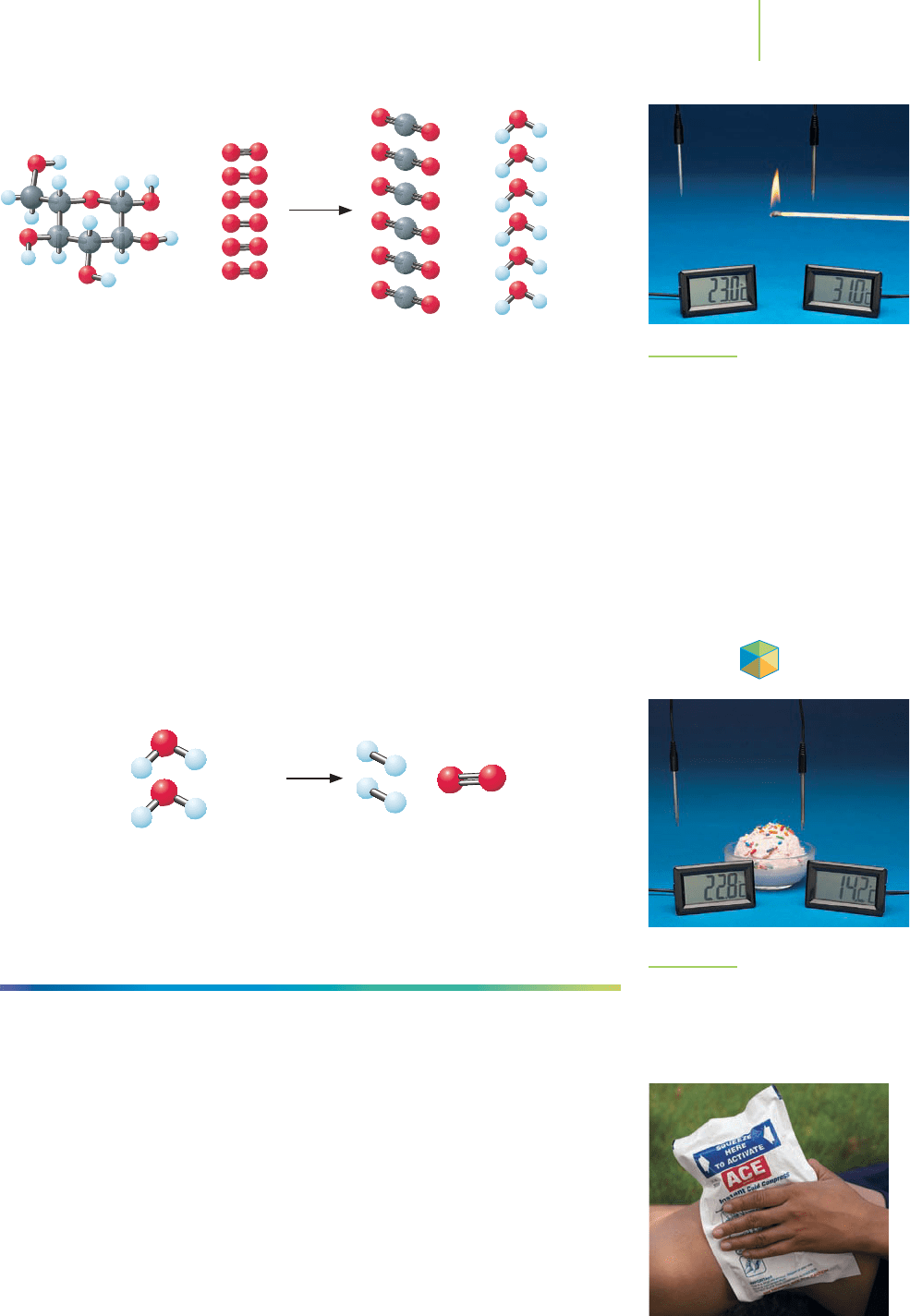
exothermic reactions, releasing torrents of energy as heat to the surroundings.
Cellular respiration, a complex process that we can summarize with the above
equation, is also exothermic. Note that in an exothermic reaction, we can think of
heat as a product of the reaction.
A chemical reaction that absorbs energy as heat is known as an
endothermic
reaction
. In this process, energy as heat flows into the system from the surround-
ings, as illustrated in the melting of ice cream (Figure 5.11). In an endothermic
reaction, such as the formation of oxygen and hydrogen from water, we can think
of heat as a reactant.
Many ammonium salts, such as ammonium nitrate, endothermically dissolve
in water, absorbing energy as heat from the surroundings. Chemical “cold packs”
that are used to cool the injured knee or elbow of an athlete use this concept.
5.1 The Concept of Energy 175
C
6
H
12
O
6
(s) + 6O
2
(g) 6CO
2
(g) + 6H
2
O(l) + heat
++
+
Heat
→
The overall equation describing cellular respiration.
FIGURE 5.10
An exothermic process. During chemical
reactions, energy is transferred between
the chemical system and its surround-
ings. Exothermic reactions release energy
from the system into the surroundings.
FIGURE 5.11
An endothermic process. Some chemical
reactions require the transfer of energy
from the surroundings to the system. This
is an endothermic process.
2H
2
O(l) + heat 2H
2
(g) + O
2
(g)
++
Heat
→
An endothermic reaction requires the addition of heat. In addition to boiling water, the
addition of heat can, under certain conditions, induce a chemical reaction that produces
hydrogen and oxygen.
HERE’S WHAT WE KNOW SO FAR
■
There are three natural forces: the gravitational, electroweak, and strong
nuclear forces.
■
Potential energy is the energy stored within a system.
■
Kinetic energy is the energy associated with motion.
■
The law of conservation of energy states that energy can be neither created nor
destroyed. Instead, it just moves between the system and the surroundings
and can be transformed from one form to another.
■
Work and heat are two ways in which energy can move between the system
and the surroundings.
■
The flow of energy as heat from the system to the surroundings involves an
exothermic process. An endothermic process involves the flow of energy as
heat from the surroundings to the system.
■
Changes in the energy of a reaction can be studied by examining a reaction
profile diagram.
Cold packs use endothermic reactions.
Application
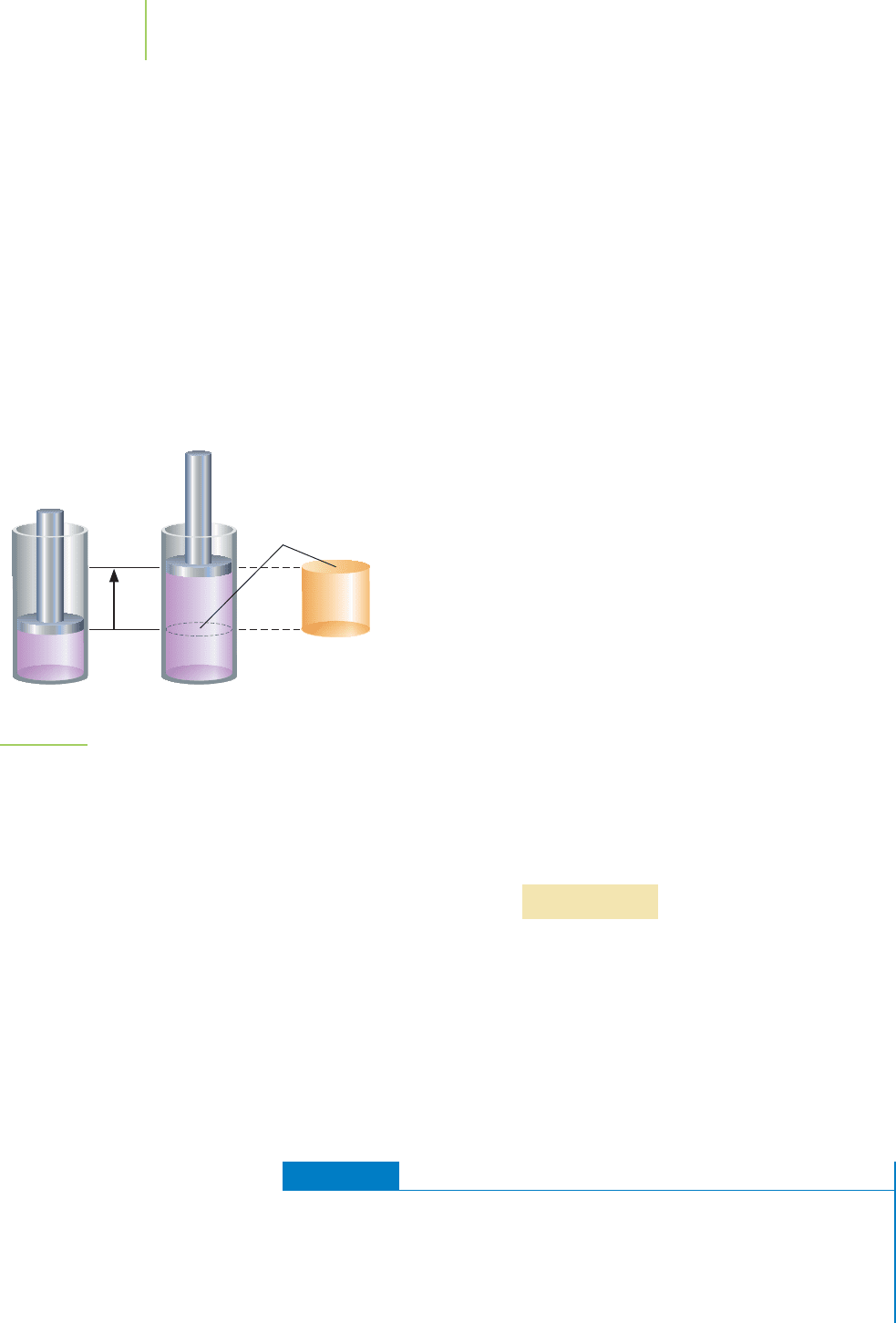
A Closer Look at Work
A system doesn’t contain work. Rather, we can calculate the amount of work that
a system does in moving an object through a distance. Mathematically, we can de-
fine work as the product of the applied force and the distance the object moved.
Work (w) = force (F) × distance (d)
To relate this equation to a chemical system, as is done by the rocket scientist, the
force exerted by an expanding gas is directly related to the product of the pressure
of a system and the area in which it is expanding. Pressure, as we’ll discover in
Chapter 11, is the force exerted by a gas per unit area on the walls of its container.
Force (F) = pressure (P) × area (A)
Substituting this equation into that for the calculation of the amount of work,
we get
Work (w) = P × A × d
This equation can be simplified by considering the product of
the area (A) multiplied by the height (h), a measure of distance. As
shown in Figure 5.12, the base of the cylinder in the initial figure
has a known area (A). Moreover, the cylinder has a known height
(h), the distance from the bottom to the top of the cylinder. There-
fore, the gas inside the cylinder has a known volume (V), because
V
= A × h. In the final state in Figure 5.12, the piston has been dis-
placed by a known distance (h). We use the Greek letter (delta)
to represent the change in some quantity. Therefore, h means “the
change in height.” The resulting three-dimensional measurement
is the change in the volume of the system (V). Specifically,for this
case, V is the final volume of the system minus the initial volume
of the system: V = V
final
− V
initial
. In other words, A × hfora
reaction is best represented by V.
Work (w) = pressure (P) × change in volume (V)
Our final modification to the equation that defines work is an
adjustment to account for the loss of energy when a system does
work and the gain of energy when a system has work done on it.
We add a negative sign to the equation to illustrate this fact:
w =−PV
This equation indicates that when the volume of a system expands (i.e., V is pos-
itive), work is done by the system, and, as a result, energy is lost from the system
(w
system
= “−”). Conversely, when the volume of a system contracts (i.e., V is
negative), the system gains energy, and work is done on the system (w
system
= “+”).
Because pressure is recorded in atmospheres and volume in liters, the units for
work (energy) are in L·atm. This is mathematically related to the SI unit of energy
known as the joule (J), in honor of the English physicist James Prescott Joule
(1818–1889). We will derive this relationship later, but for now we can assume
that 1 L·atm = 101.3 J.
EXERCISE 5.2 Work, Work, and More Work
Calculate, in L·atm and joules, the work associated with the contraction of a gas
from 75.0 L to 30.0 L at a constant external pressure of 6.20 atm. (1 L·atm = 101.3 J)
First Thoughts
The road signs concerning work can be confusing. When a system expands, work
is done by the system (w
system
= “−”) on the surroundings (w
surroundings
= “+”).
176 Chapter 5 Energy
∆h
(a) Initial state (b) Final state
Area = A
∆V
FIGURE 5.12
Gas expansion does work. The initial volume of the gas in-
creases by an amount ∆V. When the pressure remains
constant, this becomes P∆V, or work.
Conversely, when the system contracts, the surroundings do work on the system
(w
system
= “+”). In this case, the work (from the standpoint of the system) is posi-
tive because the gas is being compressed. In other words, work is being done on the
system.
Solution
V = change in volume = V
final
−V
initial
= 30.0 L − 75.0 L =−45.0 L
w =−PV =−6.20 atm × (−45.0 L) =+279 L·atm
converting this to joules, we get
w =+279 L·atm ×
101.3J
L ·atm
=+2.83 × 10
4
J = 28.3 kJ
Further Insights
The sign on the value of the work term is important to us. Not only does it reflect
how much the internal energy of the system changes, but it also indicates the direc-
tion in which it changes. Mathematically, the sign was determined by the direction
of the change in the volume. In our example, the volume of the system is reduced,
so the work done on the system is positive.
PRACTICE 5.2
Calculate, in L·atm and joules, the work associated with the expansion of a gas from
30 L to 300 L at a constant external pressure of 1.0 atm.
See Problems 9, 13d, and 14d.
Internal Energy
The reaction of hydrogen and oxygen in the shuttle’s main engines causes energy
transfer as heat and as work. The clearest result of the work done by this system
is that the shuttle rises upward through Earth’s atmosphere. The reaction also re-
leases energy that heats the internal parts of the craft, along with many molecules
in the atmosphere that come into contact with the hot exhaust vapors from the
reaction.
The total energy of the space shuttle, just as in any other system, is known as
the
internal energy (U) of the system. The internal energy is made up of the sum of
the system’s kinetic and potential energies. Unfortunately, we cannot calculate the
absolute value for the internal energy of a system, because it is extremely difficult
to account for all the individual energies that give rise to the kinetic and potential
energies. However, because the change in the internal energy (U) in a chemical
process is measurable—if we can calculate the exchange of energy as heat (q)
within, and the work done (w) by the system—we can determine the energy
change within the system. This is the
first law of thermodynamics:
U = q + w
A word of caution: We must take care with the way in which we attribute positive
or negative values to q and w. If we are looking at things from the point of view of
the system, the loss of heat from the system to the surroundings will carry a nega-
tive sign (q
system
= “−”). Similarly, if the system does work on the surroundings,
w
system
will have a negative sign (w
system
= “−”). Conversely, the flow of energy as
heat (q) into the system will be a positive value (q = “+”), and if work (w)isdone
on the system by the surroundings, w
system
will be positive (w
system
= “+”).
Because energy can be neither created nor destroyed in a process, the total
amount of energy transferred as heat and work to or from the system is exactly
equal and opposite to that transferred from or to the surroundings.
U
system
=−U
surroundings
5.1 The Concept of Energy 177
The reaction of hydrogen and oxygen in
the main shuttle engine produces heat
and does work on the surroundings.
Video Lesson: The First Law
of Thermodynamics
Video Lesson: Work
Video Lesson: Heat
Visualization: Work vs. Energy
Flow
