Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

2. Determine the limiting reagent for the reaction.
3. Calculate the number of grams of product formed.
Solution
The balanced molecular equation is
AlBr
3
(aq) + 3NaOH(aq) →Al(OH)
3
(s) + 3NaBr(aq)
Examination of the solubility rules indicates that the aluminum hydroxide is insol-
uble in water. How many grams of Al(OH)
3
(s) are produced if AlBr
3
is the limiting
reagent?
1.30 L AlBr
3
solution ×
0.0200 mol AlBr
3
1LAlBr
3
solution
×
1 mol Al(OH)
3
1 mol AlBr
3
×
78.00 g Al(OH)
3
1 mol Al(OH)
3
= 2.03 g Al(OH)
3
How many grams of Al(OH)
3
(s) are produced if NaOH is the limiting reagent?
3.00 L NaOH solution ×
0.0350 mol NaOH
1 L NaOH solution
×
1 mol Al(OH)
3
3 mol NaOH
×
78.00 g Al(OH)
3
1 mol Al(OH)
3
= 2.73 g Al(OH)
3
Our calculations indicate that the AlBr
3
is the limiting reagent because it, rather
than the NaOH, limits the mass of Al(OH)
3
formed. Mixing the two reagents to-
gether will produce 2.03 grams of aluminum hydroxide as a precipitate.
PRACTICE 4.9
What mass of precipitate is produced when 0.35 L of 0.25 M sodium carbonate
(Na
2
CO
3
) reacts with 0.55 L of 0.35 M barium chloride?
See Problems 67, 68, and 91.
4.6 Acid–Base Reactions
Drainage from a gold mine is often very acidic (see Figure 4.17). In fact, the water
from a gold mine can be acidic enough to seriously burn anyone who touches it.
To make this water suitable for the environment, cleanup crews often add a com-
pound that decreases the amount of acid in the waste. What is an
acid? A good
working definition is that an acid is a substance that releases hydrogen ions (H
+
)
in a solution. The pain of “acid indigestion,” familiar to most of us, is caused by
too many hydrogen ions in the gastric fluid that fills the stomach. The chemical
opposite of an acid is a base. A
base is a substance that releases hydroxide ions
(OH
−
) in a solution. A base that is soluble in water is also called an alkali. Acids,
bases, and their solutions are explored in detail in Chapters 17 and 18. In this
chapter we introduce some principles that govern the reactions between them
and examine the stoichiometry of their reactions.
An
acid–base reaction is the reaction between an acid and a base. The result of
this reaction is neutralization of the acid by the base, and vice versa, resulting in
a solution that is neither acidic nor basic. For this reason, acid–base reactions are
often referred to as neutralization reactions.
Strong Acids and Bases
One very common acid is hydrochloric acid (HCl). In the industrial world, the
startling quantities of HCl produced (5.0 billion kg prepared in 2004, easily
148 Chapter 4 Solution Stoichiometry and Types of Reactions
Application
Tutorial: Neutralization
Reactions
Video Lesson: Acid–Base
Reactions
Video Lesson: Strong
Acid–Strong Base and
Weak Acid–Strong Base
Reactions
Video Lesson: Strong
Acid–Weak Base and
Weak Acid–Weak Base
Reactions
Visualization: Neutralization of
a Strong Acid by a Strong Base
Visualization: Proton Transfer
within the top 20 amounts of industrial chemicals produced) are used in the
isolation and cleaning of metals and in the production of solvents and chlori-
nated organic compounds, as well as in acid–base reactions. As we have noted,
HCl is a strong electrolyte. When HCl molecules dissolve in water, they com-
pletely “ionize” (dissociate completely into ions) to form hydrogen ions and chlo-
ride ions:
HCl(g) → HCl(aq) → H
+
(aq) + Cl
−
(aq)
Because HCl is acidic and ionizes completely in aqueous solution (i.e., because it is
a strong electrolyte), we call it a
strong acid. Examples of other strong acids are
given in Table 4.4.
A
strong base is one that ionizes completely to produce OH
−
in water.Sodium
hydroxide (NaOH), which we first introduced in Exercise 4.1, is one of the more
common strong bases. It is often used in bathroom cleaners, in drain uncloggers,
and in chemical reactions that are used to prepare soaps. Because it is a strong
electrolyte, it dissociates completely when it is added to water:
NaOH(s) → NaOH(aq) →Na
+
(aq) + OH
−
(aq)
Examples of other strong bases are given in Table 4.4.
4.6 Acid–Base Reactions 149
2Fe
2
(SO
4
)
3
+ 2H
2
SO
4
4Fe
3+
(aq) + 6SO
4
2–
(aq)
Fe
3+
(aq) + 3OH
–
(aq)
From exposure
to air
As wastewater is
diluted in the stream,
the iron(III) reacts
with OH
–
in the water.
Strong acid
Yellow-brown
Dissolved in
mine wastewater
4FeS
2
+ 15O
2
+ 2H
2
O
Fe(OH)
3
(s)
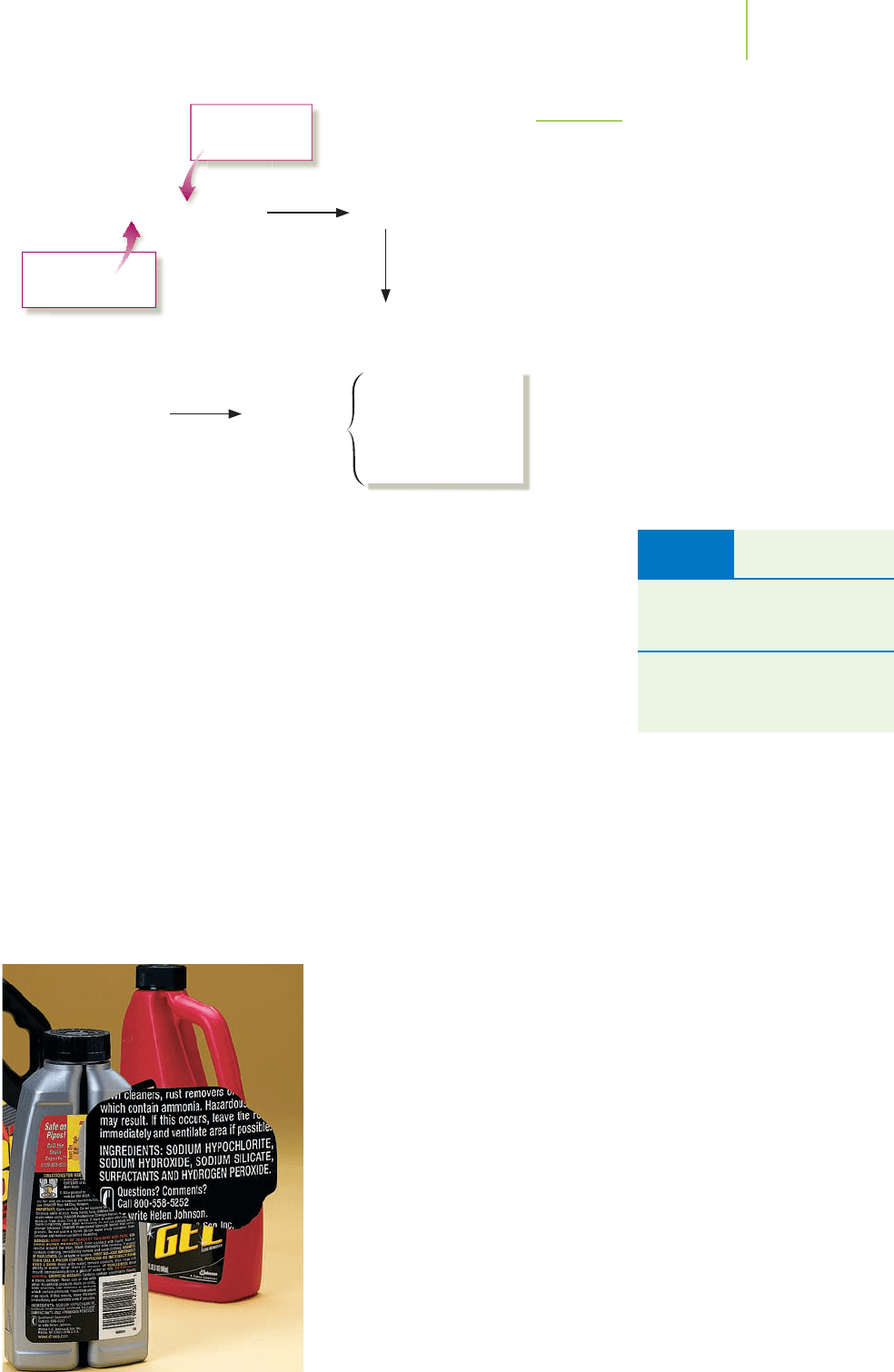
FIGURE 4.17
Water that fills a gold mine dissolves small amounts of
FeS
2
(iron pyrite, also known as fool’s gold) that is often
found in gold deposits. This compound undergoes oxida-
tion when it reaches the air outside the mine. The result
is a mixture of insoluble Fe(OH)
3
and sulfuric acid. Gold
mine runoff typically has a rust color and extremely high
acidity.
Drain decloggers contain strong bases
such as sodium hydroxide.
TABLE 4.4 Common Strong
Acids and Bases
Strong Acids
HCl, HBr, HI, HNO
3
,HClO
4
,
H
2
SO
4
Strong Bases
Hydroxides of Group IA metals,
such as LiOH, NaOH, and KOH
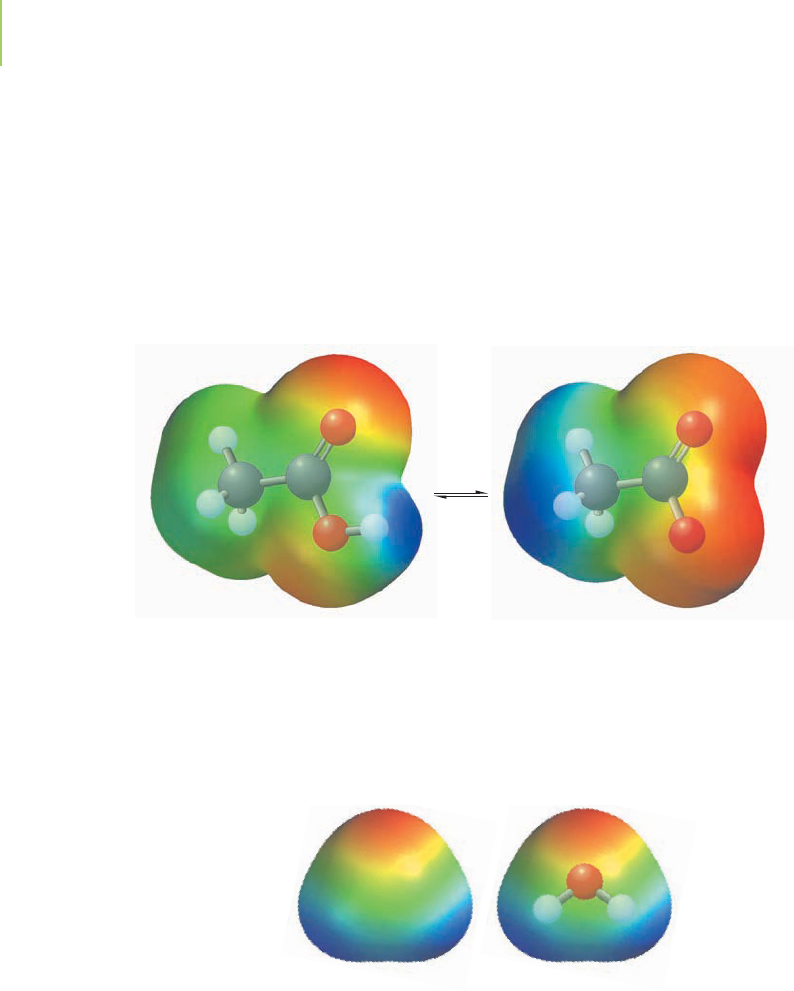
150 Chapter 4 Solution Stoichiometry and Types of Reactions
Weak Acids and Bases
A weak acid differs from a strong acid in that it does not dissociate completely in
aqueous solution. Most acids (except those in Table 4.4) are considered weak
acids. For example, acetic acid is added to water to make vinegar. The reaction
illustrating its acidity is shown below. Note that the reaction doesn’t proceed
completely to products, and because of this, we refer to weak acids as weak
electrolytes.
CH
3
COOH(l) → CH
3
COOH(aq)
CH
3
COO
−
(aq) + H
+
(aq)
Weak bases don’t react extensively to produce hydroxide ions. When ammo-
nia is added to water, only a small proportion of the ammonia reacts to produce
the ammonium ion (NH
4
+
) and hydroxide ion (OH
−
). Most bases are weak. In a
fashion similar to the weak acids, weak bases are known as weak electrolytes.
NH
3
(aq) + H
2
O(l)
NH
4
+
(aq) + OH
−
(aq)
When Acids and Bases Combine
In a reaction between a strong acid and a strong base, hydrogen ions (H
+
) and
hydroxide ions (OH
−
) come together to form water. This is illustrated by writing
the chemical equation for the reaction of HCl with NaOH. In the molecular
equation, we can see that the products are an ionic compound (in this case, NaCl)
and water.
HCl(aq) + NaOH(aq) →NaCl(aq) + H
2
O(l)
The complete ionic equation shows exactly which ions are involved in the
reaction.
H
+
(aq) + Cl
−
(aq) + Na
+
(aq) + OH
−
(aq) → Na
+
(aq) + Cl
−
(aq) + H
2
O(l)
The sodium and chloride ions are spectator ions, so we can simplify to the net
ionic equation:
H
+
(aq) + OH
−
(aq) → H
2
O(l)
This is the formation of water from its ions. This is the typical outcome for strong-
acid–strong-base reactions; the formation of water as the net ionic equation for
Acetic acid Acetate ion
+ H
+
Water

the reaction indicates a neutralization reaction. In other words, the acid (H
+
) and
the base (OH
−
) react to make a compound that is neither acidic nor basic but,
rather, the neutral liquid water (H
2
O).
Hydrochloric acid is also known as a
monoprotic acid, because it produces just
one mole of hydrogen ions (H
+
, which is a proton) from each mole of HCl when
it dissociates. This results in one mole of water forming when the acid is com-
pletely neutralized in an acid–base reaction. Other common acids can be
diprotic
or triprotic and so generate two or three moles of water, respectively, for each
mole of acid neutralized. Compare the molecular and net ionic equations of
hydrochloric acid (HCl), sulfuric acid (H
2
SO
4
), and phosphoric acid (H
3
PO
4
),
each reacting with sodium hydroxide:
HCl(aq) + NaOH(aq) → NaCl(aq) + H
2
O(l)
hydrochloric 1 mole NaOH 1 mole water
acid (monoprotic) required formed per mole
acid
H
2
SO
4
(aq) + 2NaOH(aq) → Na
2
SO
4
(aq) + 2H
2
O(l)
sulfuric acid 2 mole NaOH 2 mole water
(diprotic) required formed per mole
acid
H
3
PO
4
(aq) + 3NaOH(aq) → Na
3
PO
4
(aq) + 3H
2
O(l)
phosphoric acid 3 mole NaOH 3 mole water
(triprotic) required formed per mole
acid
EXERCISE 4.10 Stoichiometry of a Neutralization Reaction
What volume of a 0.200 M H
2
SO
4
solution is needed to neutralize 25.0 mL of a
0.330 M NaOH solution?
First Thoughts
As usual, our first step in answering a question about a reaction is to write the bal-
anced equation for the reaction. Then we can use dimensional analysis to determine
the volume of sulfuric acid solution that is needed. Remember, it’s a good idea to
map out the process before you begin the calculation.
Solution
The balanced molecular equation is
H
2
SO
4
(aq) + 2NaOH(aq) → Na
2
SO
4
(aq) + 2H
2
O(l)
sulfuric acid 2 mole NaOH 2 mole water
(diprotic) required formed per mole
acid
Using unit conversion, we develop the entire flowchart to calculate the number of
liters of sulfuric acid solution that will react with the base:
L
mL
mol NaOH
L NaOH solution
mol H
2
SO
4
mol NaOH
mL NaOH −−−−→L NaOH −−−−−−−−−−−→mol NaOH −−−−−−−→mol H
2
SO
4
LH
2
SO
4
solution
mol H
2
SO
4
−−−−−−−−−−→LH
2
SO
4
solution
25.0 mL NaOH ×
1L
1000 mL
×
0.330 mol NaOH
L NaOH solution
×
1 mol H
2
SO
4
2 mol NaOH
×
1LH
2
SO
4
solution
0.200 mol H
2
SO
4
= 0.0206 L H
2
SO
4
solution
4.6 Acid–Base Reactions 151
A total of 0.0206 L (or 20.6 mL) of 0.200 M H
2
SO
4
is required. Note that the mole
ratio of 2 moles of NaOH to 1 mole of H
2
SO
4
is used because sulfuric acid is dipro-
tic, having two protons that can react with sodium hydroxide.
Further Insights
We didn’t need to determine the net ionic equation to answer how many liters of an
acid will react with a particular amount of base. However, we did need to determine
the balanced molecular equation. In general, stoichiometry calculations require
only that we know one form of the balanced chemical equation.
PRACTICE 4.10
What volume of a 0.550 M H
3
PO
4
solution is needed to neutralize 50.0 mL of a
0.250 M NaOH solution?
See Problems 69–72 and 87.
4.7 Oxidation–Reduction Reactions
Living in an atmosphere rich in oxygen makes life possible, but it also gives us
the continual challenge of preventing unwanted reactions between oxygen and
chemicals on which we rely. One kind of very fast reaction with oxygen, called
combustion, yields fire, and we go to great lengths to protect ourselves against
unwanted fires. A slow but almost equally troublesome reaction of metals with
oxygen causes corrosion, such as that which occurs when iron rusts. Rust forms
when iron reacts with oxygen (in the presence of water) to form various forms of
iron oxide, such as Fe
2
O
3
. The presence of water is crucial for this reaction to
occur at a significant rate, because the oxygen must be in solution. This explains
why rusting of iron and steel can be prevented by protecting the metal from water
and oxygen with paint, plastic coatings, and so on.
When chemists examine what happens in such
oxidation reactions, they find
that electrons are lost from the reactant that is being
oxidized. Many oxidations
do involve oxygen, but what is really happening is the transfer of electrons in the
reaction. In forming rust, for example, elemental iron atoms lose electrons to
become iron ions:
Fe → Fe
3+
+ 3e
−
All processes in which chemicals lose electrons, regardless of whether or not oxygen
is involved, are known as oxidations. An oxidation cannot happen on its own,
because the electrons must have somewhere to go after they are lost from the
reactants that are oxidized. That is, they must be gained by some other chemical
species. When rust forms, for example, the electrons from the iron atoms are
transferred to molecular oxygen to form oxide ions:
O
2
+ 4e
−
→ 2O
2−
We say the chemical that accepts the electrons has been reduced or has undergone
reduction. A reduction is any process in which electrons are gained by a chemical.
One useful mnemonic to help you remember oxidation and reduction is
OIL RIG:
Oxidation Involves Loss of electrons, Reduction Involves Gain of electrons
Some prefer the mnemonic LEO says GER:
Loss of Electrons is Oxidation, Gain of Electrons is Reduction
152 Chapter 4 Solution Stoichiometry and Types of Reactions
Metal
panel
on a car
Paint
Water
Oxygen
Metal is protected from the
reactants that cause rusting.
Painted surfaces of a car are protected
from oxidation.
Video Lesson: Oxidation–
Reduction Reactions
Visualization: Sugar and
Potassium Chlorate
Visualization: Oxidation of Zinc
with Iodine
Video Lesson: CIA
Demonstration: The Reaction
between Al and Br
2

No matter which mnemonic you prefer, the chemical point remains the same:
Oxidation and reduction processes must occur together in
oxidation–reduction
reactions
(also called redox reactions, for reduction–oxidation). The chemicals
concerned must react in the proportions that allow the number of electrons lost
via oxidation to equal the number of electrons gained via reduction. The
processes that we discussed above do not exist alone. Instead, each is one-half of
the complete electron-exchange process that produces iron(III) oxide (Fe
2
O
3
)
from iron and oxygen. Still, we find it convenient to separate the overall reaction
into individual oxidation and reduction
half-reactions. Let’s examine these half-
reactions more closely.
The oxidation half-reaction is: Fe → Fe
3+
+ 3e
−
(3 electrons are lost)
The reduction half-reaction is: O
2
+ 4e
−
→ 2O
2−
(4 electrons are gained)
To obtain the complete oxidation–reduction reaction that results from the
iron and oxygen half-reactions, we need to make sure that the number of
electrons involved is the same on both sides of the equation. The number of elec-
trons lost by the iron must be equal to the number gained by the oxygen. Charge, like
mass, is conserved.
Multiplying the entire oxidation half-reaction by 4 and the entire reduction
half-reaction by 3 will allow 12 electrons to be lost from iron as the same 12 elec-
trons are gained by oxygen.
4Fe → 4Fe
3+
+ 12e
−
(oxidation)
3O
2
+ 12e
−
→ 6O
2−
(reduction)
The overall oxidation–reduction equation is the sum of the two half-reactions:
4Fe → 4Fe
3+
+ 12e
−
3O
2
+ 12e
−
→ 6O
2−
4Fe + 3O
2
→4Fe
3+
+ 6O
2−
When the electrons are transferred in this reaction, the resulting iron ions and
oxide ions combine to form Fe
2
O
3
.
Oxidation Numbers
In business, bookkeepers are hired to keep track of the money coming in to and
that paid out by the company. They keep the financial books. In chemistry, our
4.7 Oxidation–Reduction Reactions 153
Combustion and corrosion are redox processes.

154 Chapter 4 Solution Stoichiometry and Types of Reactions
electronic bookkeeping tool is called an oxidation number, which we assign to in-
dividual atoms on the basis of where electrons in a bond are likely to be found.
For example, in the ionic compound NaCl, in which sodium has given an electron
to chlorine, we say that the sodium ion has an oxidation number of +1, because
it has one less electron than the sodium atom. The chloride ion has an oxidation
number of −1, because it has one more electron than the chlorine atom. We use
oxidation numbers to keep track of electrons as they move among atoms, mole-
cules, and ions in redox reactions and, in fact, in all types of reactions. The term
oxidation number is often used interchangeably with the term
oxidation state.
In the iron and oxygen reaction that produces iron(III) oxide,
4Fe → 4Fe
3+
+ 12e
−
(oxidation)
3O
2
+ 12e
−
→ 6O
2−
(reduction)
each iron started as a neutral atom (sometimes noted with a superscript “
0
”, F e
0
)
and was oxidized, losing three electrons, to form Fe
3+
. The oxidation number (or
oxidation state) of the iron is now +3. The oxygen molecule includes two oxygen
atoms that share electrons equally—that is, neither atom exerts a strong prefer-
ence for the bonding electrons—so each oxygen atom is assigned (remember,
we are bookkeepers here!) an oxidation state of 0. When oxygen reacts with
iron, the electrons gained produce oxide ions (O
2−
) that have an oxidation num-
ber of −2.
When atoms are combined to make a molecule, they neither lose nor gain
electrons to form ions. Instead, the electrons in molecules are shared, to one de-
gree or another, between the atoms. Overall, the molecule is electrically neutral,
with a net charge of zero and, therefore, a net oxidation number of 0. However, in
many molecules, there is a tendency for electrons to be closer to the nuclei of
some atoms than of others. For example, in water (H
2
O), there is a marked ten-
dency for the electrons in each of the two hydrogen-to-oxygen bonds to be found
closer to the oxygen nucleus than to the hydrogen nucleus (Figure 4.3). This
behavior is indicated in our electron bookkeeping system by giving oxygen an ox-
idation state of −2. The electron from each hydrogen atom is drawn away from
its nucleus more of the time, and we denote this by giving each hydrogen atom an
oxidation number of +1. Our chemical understanding has led to a host of book-
keeping conventions to assign oxidation numbers to the individual atoms that
make up a molecule. These rules are listed in order of importance in Table 4.5. In
cases where the rules appear to contradict each other, follow the rule that comes
first in the table.
What oxidation numbers would we assign to the carbon and hydrogen atoms
of methane (CH
4
)? By following the rules in Table 4.5, we determine that each hy-
drogen atom has a +1 oxidation number. Because the molecule is neutral overall,
we assign the oxidation number −4 to this carbon atom. The carbon atom in car-
bon monoxide (CO), on the other hand, is assigned a different oxidation number.
According to the rules, the oxygen atom has an oxidation number of −2. The car-
bon atom must, then, have an oxidation number of +2.Carbon monoxide is more
oxidized than methane. The oxidation number of the carbon atom in each com-
pound bears this out.
It is important that we understand the difference between the oxidation
numbers of atoms in molecules and the oxidation numbers of ions in ionic com-
pounds. In molecules, assigning oxidation states is simply an accounting proce-
dure used to keep track of electrons; it does not imply that electrons have really
been lost or gained by any atoms. Remember that we have just assigned the
oxidation numbers on the basis of some simple rules that hypothetically assume
the electrons are transferred when the molecule is made. In ionic compounds, on
the other hand, the oxidation number of an ion is the real charge on the ion.
Video Lesson: Oxidation
Numbers

EXERCISE 4.11 Assigning Oxidation States
Assign oxidation states to all the atoms in the following elements, compounds and
ions:
a. He c. Al
2
O
3
e. NO
3
−
g. F
2
b. C
6
H
6
d. Zn f. SF
6
h. CO
3
2−
Solution
a. This is an element, so the oxidation number is 0.
b. This is a neutral compound. Each hydrogen atom has an oxidation number +1
per atom, and because there are the same number of carbon atoms as of hydro-
gen atoms, each carbon atom must have an oxidation number of −1.
c. This is a neutral compound. Oxygen has an oxidation number of −2, so in this
case the aluminum must have an oxidation number of +3, because there are
three oxygen atoms (total =−6), and the oxidation numbers must sum to 0
overall.
d. This is an element, so the oxidation number =0.
4.7 Oxidation–Reduction Reactions 155
Rules for Assigning Oxidation Numbers
1. The oxidation number of an atom in an element
is 0.
2. The oxidation number of a monatomic ion is the
same as its charge.
3. The oxidation number of a Group IA metal in a
compound is always +1; the oxidation number of
a Group IIA metal in a compound is always +2.
4. The oxidation number of fluorine in its
compounds is always −1.
5. The oxidation number of hydrogen is +1 in its
nonmetal compounds. It is −1 when combining with
many metals in compounds known as hydrides.
6. The oxidation number of oxygen in its covalent
compounds is usually −2. Exceptions include
peroxides such as hydrogen peroxide (H
2
O
2
), in
which oxygen is in the −1 oxidation state.
7. In binary compounds containing metals, Group
VIIA elements typically have an oxidation number
of −1; Group VIA elements have an oxidation
number of −2; and Group VA elements have an
oxidation number of −3.
8. The sum of the oxidation numbers of all atoms
in the molecule or ion must equal the total charge
of the molecule (zero) or ion.
TABLE 4.5
Na, H
2
,N
2
,O
2
,O
3
, and He, all have atoms with oxidation
number = 0
The oxidation number of Li
+
is +1, of Ca
2+
is +2, of Cl
−
is −1,
and of O
2−
is −2.
The oxidation number of sodium in NaCl is +1. The oxidation
number of calcium in CaBr
2
is +2.
In HF, the oxidation number of F is −1 and of H is +1.
In HCl, the oxidation number of H is +1 and of Cl is −1. In
H
2
O, the oxidation number of H is +1 and of O is −2. However,
in NaH, the oxidation number of H is −1 and of Na is +1.
In CO
2
, the oxidation number of oxygen is −2 and of carbon is
+4. In CO, the oxidation number of oxygen is also −2, but that
of carbon is +2.
The oxidation number of sulfur in FeS is −2. The oxidation
number of nitrogen in Mg
3
N
2
is −3.
In the water molecule (H
2
O), each H has an oxidation number
of +1 and the O has an oxidation number of −2, so the sum
of all the oxidation numbers =(+1 ×2) +(−2) =0. In the ionic
compound calcium fluoride (CaF
2
), each Ca
2+
ion has an
oxidation number of +2 and each F
−
ion has an oxidation
number of −1, so the sum of all the oxidation numbers =
(+2) + (2 ×−1) = 0. In the NH
4
+
ion, each H has an oxidation
number of +1 and the N has an oxidation number of −3, so the
sum of all the oxidation states = (−3) + (+1 × 4) =+1, which
is the charge on the polyatomic ion.
e. This is an ion with a −1 charge overall, so the oxidation number of the atoms
present must sum to −1. Oxygen has an oxidation number of −2, so the three
oxygen atoms contribute a total of −6. Nitrogen must therefore have the oxida-
tion number of +5.
f. This is a neutral compound. Fluorine has an oxidation number of −1, and
there are six fluorine atoms (total =−6), so the sulfur must have an oxidation
number of +6.
g. This is an element, so each atom has an oxidation number of 0.
h. This is an ion with a −2 charge overall, so the oxidation number of the atoms
present must sum to −2. Oxygen has an oxidation number of −2, so the three
oxygen atoms contribute a total of −6. The carbon, therefore, must have an
oxidation number of +4.
PRACTICE 4.11
Use Table 4.5 to assign the oxidation numbers of each of the species in the com-
pounds below.
KCl Fe
2
O
3
P
4
CH
2
Cl
2
Al PBr
3
HCN
See Problems 81 and 82.
Identifying Oxidation–Reduction Reactions
Redox reactions are often disguised and can be difficult to identify without close
examination. Let’s examine a redox reaction to see how it is identified as such.
The reaction of hydrogen and nitrogen to make ammonia, known as the Haber
process, is a redox reaction that is vital to farming. The ammonia made by this
process is used to fertilize fields for the production of most crops, including corn,
wheat, and sorghum (a versatile crop used to make syrup, hay, flour, animal feed,
and even brooms!). The reaction is
3H
2
(g) + N
2
(g) → 2NH
3
(g)
A first look at the reaction does not reveal that any electrons are being trans-
ferred in the process, so at first we might incorrectly assume that this is not a
redox reaction. However, if we examine the oxidation numbers of the individual
atoms involved, we can see that reduction and oxidation are taking place.
H
2
Hydrogen is assigned the oxidation number 0.
N
2
Nitrogen is assigned the oxidation number 0.
NH
3
Hydrogen is assigned the oxidation number +1, nitrogen −3.
Overall, the hydrogen changes its oxidation state from 0 (in H
2
) to +1 (in NH
3
),
losing electrons in this reaction as it is oxidized. The nitrogen changes its oxida-
tion state from 0 (in N
2
) to −3 (in NH
3
), gaining electrons as it is reduced. Be-
cause the Haber process includes reduction and oxidation, it is a redox reaction.
Redox reactions are characterized by the exchange of electrons, which makes
balancing these equations difficult to do by trial and error. We will discuss a
method for balancing these reactions in Chapter 19.
EXERCISE 4.12 Identifying Redox Reactions
Is the combustion of ethane a redox reaction? Prove your answer.
2C
2
H
6
(g) + 7O
2
(g) → 4CO
2
(g) + 6H
2
O(g)
156 Chapter 4 Solution Stoichiometry and Types of Reactions
Application
First Thoughts
What should we look for that is common to all redox reactions? The single common
feature is the exchange of electrons. How do we know whether there is an electron
exchange? The best way is to assign oxidation numbers to each atom and see
whether there are changes going from reactants to products.
Solution
Judging on the basis of the oxidation number rules in Table 4.5, we can make the
following assignments:
2C
2
H
6
(g) + 7O
2
(g) → 4CO
2
(g) + 6H
2
O(g)
↑↑↑↑
−3 +10 +4 −2 +1 −2
Carbon goes from −3 to +4. (oxidation)
Oxygen goes from 0 to −2. (reduction)
Hydrogen does not change oxidation number.
Because electrons have been exchanged, this a redox reaction.
Further Insights
Combustion is the high-temperature reaction of oxygen with another compound.
All combustion reactions are redox reactions, because electron exchange is occur-
ring. However, not all redox reactions are combustion reactions. For example, bat-
teries work because of redox reactions that include metals such as cadmium, lead, or
lithium, along with acids such as sulfuric acid or bases such as potassium hydroxide.
PRACTICE 4.12
Is the reaction of aqueous solution of phosphoric acid and sodium hydroxide a
redox reaction?
See Problems 55, 56, 83, and 84.
4.8 Fresh Water—Issues of Quantitative Chemistry
Nothing is more important to us than our supplies of fresh water, which we
largely draw from rivers, lakes (Figure 4.18), and underground aquifers. Manag-
ing these precious freshwater resources requires our understanding of quantita-
tive chemistry and aqueous solutions. If fresh water is to be of use to us as a
supply of drinking water or for agricultural use, we must ensure that it is clean,
meaning that certain dissolved solutes must occur only in quantities that are
within the acceptable limits for good health. Table 4.6 lists, for selected chemicals,
the EPA’s maximum permitted level, below which there is minimal risk to human
health (this is called the maximum contaminant level goal, or MCLG).
Chemists in water collection and treatment facilities check for compliance
with safe water standards by quantitative analysis of the water. According to those
safe-water standards, they also administer appropriate quantities of disinfect-
ing chemicals. One such disinfectant is chlorine, which is toxic to freshwater life
above about 19 parts per billion. The good news is that chlorination of drinking
water has been instrumental in reducing the risk of microbial disease transmitted
through a water supply.
For instance, cholera (a bacterial infection of the intestines that causes vom-
iting, diarrhea, and dehydration) claims thousands of lives each year and is par-
ticularly invasive in countries in which the population has been uprooted as a
consequence of civil war and grinding poverty. In 2005, cholera outbreaks were
4.8 Fresh Water—Issues of Quantitative Chemistry 157
Application
C
HEMICAL ENCOUNTERS:
Revisiting the
Maximum Levels of
Chemicals in Drinking
Water
