Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

about 160 km south-southwest of Dublin (Figure 4.11), receives periodic ship-
ments of a concentrated aqueous HFSA solution from a chemical manufacturer
in Spain. The concentration of this HFSA solution, shipped in 4000-gallon con-
tainers, is about 22 M HFSA. The solution is then diluted with water to 7.8 M and
shipped to cities and towns all over Ireland for use in the nation’s many water
treatment plants. At these plants, the HFSA solution is further diluted in the
water supply to a final fluoride ion concentration of 1 ppm.
How do the workers know how much to dilute? There are really two questions
here. One is a mathematics question, and one is a chemical process question
(“How do I prepare this solution?”). In terms of the chemical process, it has long
been known that unless you are adding two of the exact same liquids to each
other, the volumes are not additive. That is, adding 100 mL of ethanol to 100 mL
of water does not give 200 mL of solution. Rather, it gives a volume that is a little
bit less than 200 mL. The practical outcome of this is that for laboratory-scale
preparation of a solution of known molarity, we must use volumetric glassware
(shown in Figure 4.12), in which the etched mark on the flask indicates the listed
volume to a very high accuracy, typically within +/−0.05% of the stated volume.
We add the reagent to the flask and then dilute with solvent (water, in this case)
to the mark.
We can now deal with the mathematics question. Let’s assume we wish to pre-
pare 500.0 mL of 7.8 M HFSA solution from a sample of 22 M HFSA. To do this,
we need to calculate the volume of 22 M HFSA that can be added to the volu-
metric flask and diluted with water. How many moles of HFSA will be in the final
solution? We are given the molarity (7.8 M) and volume (500.0 mL) of the final
solution, so we can do the following calculation:
7.8 mol HFSA
L HFSA solution
× 0.5000 L solution = 3.9 mol HFSA
What volume of the concentrated (22 M) HFSA solution must we dispense into
the volumetric flask and dilute with water in order to give us 3.9 moles of HFSA?
3.9 mol HFSA ×
1 L HFSA
22 mol HFSA
= 0.1773 L HFSA solution
= 177.3 mL HFSA solution = 180 mL HFSA solution (to 2 significant figures)
This means that we can measure 180 mL of HFSA solution and dilute to 500.0 mL
in our volumetric flask and, after mixing, have a 7.8 M HFSA solution.
138 Chapter 4 Solution Stoichiometry and Types of Reactions
Dublin
New Ross
A
tlantic
Ocean
Irish
Sea
IRELAND
50 Miles0
Scale
FIGURE 4.11
New Ross, Ireland, is about 100 miles
(160 km) from Dublin.
FIGURE 4.12
Using a volumetric flask. A solute is added to the volumetric flask and dissolved in water. The resulting solution
is then diluted to the mark on the neck of the flask. The result is a precise and accurate volume of solution.
Visualization: Dilution
Video Lesson: CIA
Demonstration: Dilutions
Tutorial: Dilution
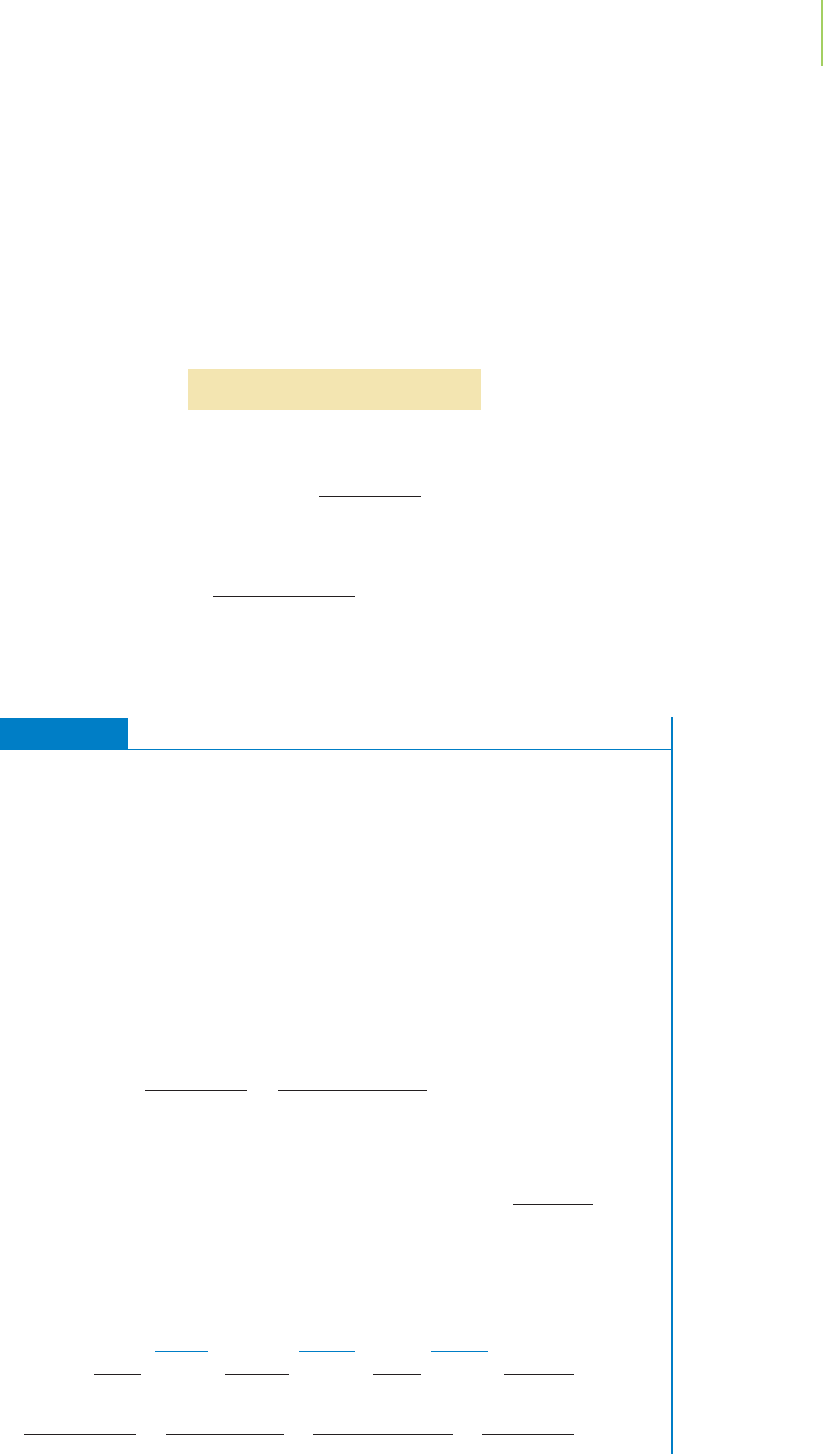
Note that in our calculations, we have compared the number of moles of solute
in the concentrated solution that we are measuring to the number of moles of the
diluted solution that we want to prepare. Mathematically, this can be written:
number of moles measured
=
number of moles needed
from the concentrated solution in the dilute solution
Because we calculated the number of moles needed in the dilute (“final”) solution
by multiplying its concentration (C
final
) by its total solution volume (V
final
), and
because this is equal to the number of moles of concentrated (“initial”) solution
measured, we can say that
C
initial
× V
initial
= C
final
× V
final
Our goal is to find the volume of the initial solution of HFSA to dilute, so we can
solve for V
initial
.
V
initial
=
C
final
× V
final
C
initial
Using our data for HFSA, we find that
V
initial
=
7.8 M × 0.5000 L
22 M
= 0.177 L = 180 mL
The total volume of 22 M HFSA needed to prepare large, intercity shipments of
7.8 M HFSA is far larger, but the problem-solving strategy is the same.
EXERCISE 4.5 Practice with Dilution of Solutions
Hydrochloric acid is typically shipped as a 12 M aqueous solution. If we are to do a
laboratory analysis that requires 250.0 mL of a 1.0 M solution, how many milliliters
of the concentrated solution should be diluted?
First Thoughts
What range of volumes would make sense as an answer? We want to dilute our solu-
tion by a factor of 12, to go from 12 M to 1.0 M. If the total volume of the solution is
to be 250.0 mL, and we are diluting a solution with water by a factor of 12,this would
suggest that we need about 20 mL of the concentrated solution (roughly 1/12 of
250.0 mL). The remainder of the solution is water, added to dilute the concentrated
acid.
Solution
V
initial
=
C
final
× V
final
C
initial
=
1.0 M × 250.0mL
12 M
= 20.8mL= 21 mL
Further Insights
It is quite common for industrially prepared concentrated acids to be labeled for
shipment on the basis of grams of acid per grams of solution,
g acid
g solution
. This is
known as weight-to-weight percent, or (w/w). Hydrochloric acid, for example, is
shipped as a 37% (w/w) solution and has a density of 1.18 grams of acid per milli-
liter of solution. How do we relate w/w to molarity? Here are the flowchart and the
calculation.
g soln
mL soln
1000 mL
1L
mol HCl
gHCl
gHCl
g soln
−−−−−→
gHCl
mL soln
−−−−−→
gHCl
L soln
−−−−−→
mol HCl
L soln
37 g HCl
100 g solution
×
1.18 g solution
1 mL solution
×
1000 mL solution
1 L solution
×
1 mole HCl
36.5gHCl
= 12 M
4.2 The Concentration of Solutions 139
PRACTICE 4.5
Industrially prepared nitric acid (HNO
3
) has a concentration of 16 molar. How
many milliliters of nitric acid are required to prepare 2.00 L of 0.15 M HNO
3
?
See Problems 29 and 30.
4.3 Stoichiometric Analysis of Solutions
The concepts of concentration and solution stoichiometry are put to important
use every day to examine all manner of solutions. In medicine, they are used to an-
alyze bodily fluids for evidence of illness and to prepare pharmaceuticals in appro-
priate amounts. In industry, they are used to ensure that the correct quantities of
dissolved reactants are mixed together. In forensic chemistry they can determine
the levels of drugs and poisons in a victim or suspect of crime. And in the food
industry they make it possible to measure and modify the nutritional content of
foods.
For example, the amount of vitamin C in a sample of fruit juice can be deter-
mined by adding iodine to the fruit juice via the following reaction:
C
6
H
8
O
6
+ I
2
→ C
6
H
6
O
6
+ 2HI
vitamin C brownish-red colorless colorless
colorless
140 Chapter 4 Solution Stoichiometry and Types of Reactions
The controlled addition of a solution of known concentration to react with the
substance of interest to determine its concentration is called a
titration. In the
titration of fruit juice with iodine shown in Figure 4.13, we slowly add iodine so-
lution from a
buret (a laboratory device used to precisely and accurately add
small known quantities of solution) to a sample of fruit juice. When all the vita-
min C in the sample has reacted, excess iodine will begin to accumulate. The
point at which the reactants have been completely consumed is known as the
equivalence point (very close to the visual end point) of the titration. We can de-
tect when the reaction has reached this stage by including a compound, in this
case starch, that reacts with the excess iodine to produce a color change.Although
the iodine itself is colored, it is often difficult to see this color, especially in fruit
juice. However, the addition of starch results in a very obvious color change.
starch + I
2
→ I
2
−starch
colorless brownish-red deep blue
Here is how we can use this reaction to determine the vitamin C content of a
fruit juice in moles per liter and percent by mass. We begin by preparing a solu-
tion of a known concentration of iodine that turns out to be, for example,
0.01052 M I
2
. This is known as a standard solution, because we use it as a standard
of known concentration against which other solutions can be tested. We then
measure out 50.00 mL (0.05000 L) of fruit juice and add some starch to react with
excess iodine. By adding our standard solution of iodine from a buret, we titrate
the fruit juice until a color change is observed. Typically, this process is repeated
several times to minimize error. In our hypothetical titrations, let’s assume that
Vitamin C
O
O
OH
OH
HO
HO
Starch–iodine end point.
Application

we averaged 16.97 mL of iodine solution before the color change was observed.
How much vitamin C is in the fruit juice? We can quantify the vitamin C content
of the fruit juice using the following strategy:
1. Write the balanced chemical equation relating the reaction of iodine with
vitamin C, C
6
H
8
O
6
.
2. Use dimensional analysis to convert the units of 16.97 mL of iodine solution
into grams of vitamin C.
C
6
H
8
O
6
+ I
2
→ C
6
H
6
O
6
+ 2HI
1 mole + 1 mole 1 mole + 2 moles
16.97 mL I
2
solution ×
1LI
2
solution
1000 mL I
2
solution
×
0.01052 mol I
2
1LI
2
solution
×
1 mol vitamin C
1 mol I
2
×
176.1 g vitamin C
1 mol vitamin C
= 0.03144 g vitamin C
That is the number of grams of vitamin C in our 50.00 mL (0.05000 L) sample
of fruit juice. We can determine the amount of vitamin C in 1 L by doing the
following calculation:
0.03144 g vitamin C
50.00 mL fruit juice solution
×
1000 mL fruit juice solution
1 L fruit juice solution
=
0.6288 g vitamin C
L fruit juice solution
Our calculations reveal that there are 0.03144 g of vitamin C in our 50.00 mL
sample of fruit juice. Alternatively, this is equal to 0.6288 g of vitamin C per liter
of fruit juice. If we assume that the density of the fruit juice is
1.0g
mL
, what is the
concentration of vitamin C in ppm?
Recall from Table 4.2 that parts per million (ppm) of vitamin C can be
expressed as
mg vitamin C
L fruit juice solution
. We know the vitamin C concentration in
g
L
, so
all we need to do is convert from grams to milligrams of vitamin C.
0.6288 g vitamin C
L fruit juice solution
×
1000 mg vitamin C
1 g vitamin C
=
628.8 mg vitamin C
L fruit juice solution
= 628.8 ppm vitamin C
4.3 Stoichiometric Analysis of Solutions 141
FIGURE 4.13
The process of titration.
Video Lesson:
Acid–Base
Titrations
Video Lesson: CIA
Demonstration:
Titrations

142 Chapter 4 Solution Stoichiometry and Types of Reactions
Water is such a precious resource that
countries have been known to threaten
one another with war over water rights. In the United
States, individual states quarrel and even sue each other
over access to fresh water. For example, Nebraska and
Kansas have fought for decades over who owns the water
that flows from Colorado through Nebraska and into
Kansas via the Republican River.
We are so used to thinking of water as essential and
beneficial that it is easy to overlook the many chemical
situations in which too much water can be very undesir-
able. What would be the effect of too much water in the
Republican River? On a smaller scale, what would be the
effect of too much water in a chocolate bar or potato
chips? Canned cooking fats and oils certainly do not
benefit from the presence of water; and there are limits
to the amount of water that can be in pharmaceutical
products that are ingested as tablets, gelcaps, and caplets.
Therefore, it is important to be able to determine how
much water is present in samples of foods, medicines,
and other industrial products, even if the quantities of
water involved are very small.
One chemical method
used to quantify the water
content of a wide range of
samples, such as chewing
gum, jelly beans, and peanuts,
is the Karl Fischer titration,
named after the scientist who
devised the basic method in
1935. The Karl Fischer titra-
tion makes use of the reaction
of iodine (I
2
) with the water in
the sample being analyzed.
The reaction is performed in
the presence of organic
solvents such as pyridine
(C
5
H
5
N) and methanol
(CH
3
OH). The precise details
of the chemical reaction are
quite complex, to the extent that even now, more than 70
years after the method was first developed, the exact
processes involved in the reaction are still the subject of
research. We can summarize it, however, as follows:
(Reactants) +I
2
+ H
2
O → (products)
The chemical details vary with the actual method used.
In all cases, however, the crucial quantitative fact that al-
lows the water content to be measured is that the iodine
and water always react in a known mole ratio, which is 1:1
in the case shown above. The end point of the titration,
when we can tell that all the water has been consumed, is
How do we know?
How to test for small amounts of water
The Republican River supplies Harlan County Reservoir with water
used for irrigation and recreation.
Hot oil spatters violently
when water, such as that
on the surface of this
turkey, is present.
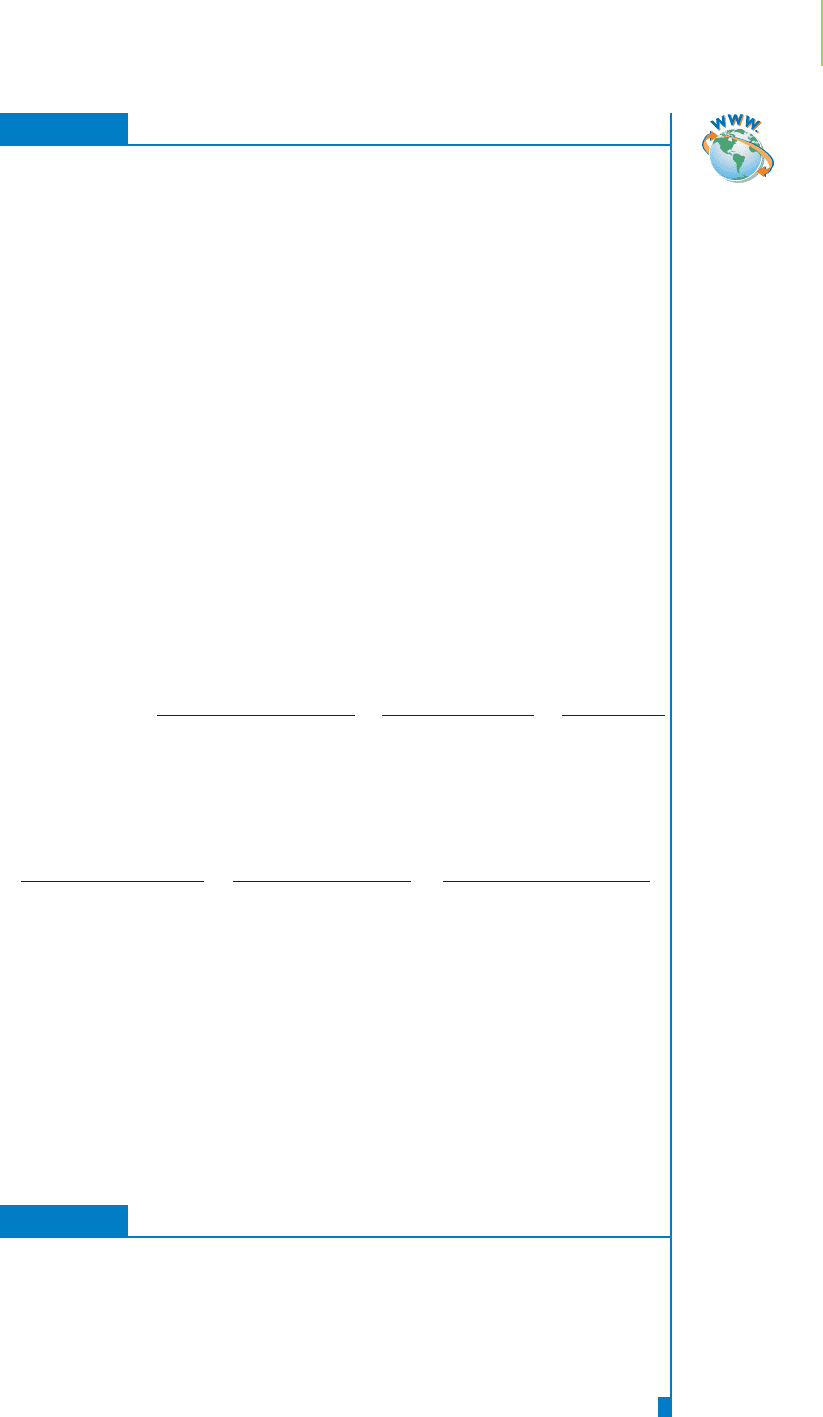
FIGURE 4.14
Automated Fischer titration
apparatus.
marked by a distinctive color change, which enables us
to determine the quantity of iodine required to achieve
that color change. The amount of water that must
have reacted with that quantity of iodine can then be
calculated.
Modern laboratory instrumentation has allowed
the titration process to be automated, as shown in
Figure 4.14. Instead of direct observation of a color
change, the automated instruments may rely on mea-
surement of a flow of electrons to excess iodine as soon
as all the water has reacted. This flow of electrons is due
to the process I
2
+ 2e
−
→ 2I
−
. Another option is to use
the reversal of the process shown above—namely
2I
−
→ I
2
+ 2e
−
—to generate the iodine needed to react
with the water.
The details vary depending on the machines used,
but automated Karl Fischer titrations allow accurate de-
termination of the water content in tiny samples con-
taining hardly any water at all. What do we mean by
“hardly any” and “tiny”? The method is useful down to
the level of 50 ppm water in a sample that can have a
volume as small as 10 microliters.
EXERCISE 4.6 Practice with Titration
A common type of titration is the reaction of a solution of sodium hydroxide (with
a known concentration) with a solution of an acid (with an unknown concentra-
tion). The result can be used to determine the molarity of the acid solution. What is
the molarity of a solution of 50.00 mL of HCl if 31.98 mL of 0.1253 M NaOH is re-
quired to react with it? The products of this reaction are sodium chloride and water.
First Thoughts
We should begin this problem as we begin all stoichiometry problems in chemistry,
with a balanced chemical equation. From the problem we determine the equation
for the titration of hydrochloric acid with sodium hydroxide:
HCl(aq) + NaOH(aq) →H
2
O(l) + NaCl(aq)
Our flowchart for the dimensional analysis can be expressed as follows:
mL NaOH → mol NaOH →mol HCl → M HCl
Remember to change the milliliters of NaOH and HCl to liters in order to work with
molarity in the proper units.
Solution
From the equation, 1 mole of HCl reacts with 1 mole of NaOH. Therefore, we can
calculate the molarity of the HCl. (Remember to construct the flowchart first.)
31.98 mL NaOH ×
1 L NaOH solution
1000 mL NaOH solution
×
0.1253 mol NaOH
1 L NaOH solution
×
1 mol HCl
1 mol NaOH
= 0.004007 mol HCl
Now that we know the quantity of HCl in moles, we can determine the molarity by
dividing by the volume of the HCl.
0.004007 mol HCl
50.00 mL HCl solution
×
1000 mL HCl solution
1 L HCl solution
=
0.08014 mol HCl solution
1 L HCl solution
= 0.08014 M HCl
Since the titration used more of the HCl solution than of the NaOH solution, we
would expect the HCl solution to be more dilute. Our answer makes sense.
Further Insights
Titration is just one (albeit one very important) technique for finding out how
much of something you have. We will learn about many other “quantitative analy-
sis” procedures in this textbook, based on physical properties such as color, electri-
cal conductivity, and the formation of a precipitate.
PRACTICE 4.6
The equation for the reaction between a sodium hydroxide solution and dilute sul-
furic acid is
2NaOH(aq) + H
2
SO
4
(aq) → Na
2
SO
4
(aq) + 2H
2
O(l)
What is the molarity of a solution of 25.00 mL of H
2
SO
4
if 22.25 mL of 0.100 M
NaOH is required to reach equivalence?
See Problems 47–54.
4.3 Stoichiometric Analysis of Solutions 143
Video Lesson: Solving Titration
Problems

144 Chapter 4 Solution Stoichiometry and Types of Reactions
4.4 Types of Chemical Reactions
The vast number of aqueous reactions that have been identified since the begin-
ning of chemistry would stun the ancient alchemists. Fortunately, most of these
reactions fall within certain general types or categories because of key similarities
among them. Three of the more important types of chemical reactions are
■
Precipitation reactions
■
Acid–base reactions
■
Oxidation–reduction reactions
In order to more fully understand the three types of processes, we will take this
opportunity to build on our ability to write chemical equations in aqueous solu-
tions. Our first step is determining what happens to the individual components
when they are added to water. We’ll use our knowledge of electrolytes to assist us
in this process.
Molecular and Ionic Equations
The reaction that was the focus of Exercise 4.6 includes three strong electrolytes:
hydrochloric acid (HCl), sodium hydroxide (NaOH), and sodium chloride
(NaCl). The reaction equation, containing the individual components written as
compounds, is known as the
molecular equation. The molecular equation illus-
trates that the individual substances exist as hydrated compounds in the aqueous
solution. The molecular equation representing the reaction of hydrochloric acid
and sodium hydroxide can be written
HCl(aq) + NaOH(aq) →H
2
O(l) + NaCl(aq)
molecular equation
However, as we have already discussed, strong electrolytes dissolve in water, dis-
sociate into their component ions, and become relatively independent within the
solution. For this reason, all the individual ions can be shown separately, in what
is known as a
complete ionic equation.
H
+
(aq) + Cl
−
(aq) + Na
+
(aq) + OH
−
(aq) → Na
+
(aq) + Cl
−
(aq) + H
2
O(l)
complete ionic equation
Note that only the strong electrolytes have been written as individual ions.Unlike
the electrolytes, molecules such as water do not dissociate into ions to any appre-
ciable extent. They are not written as individual ions.
Complete ionic equations list all substances as they exist in the solution,
whether or not they participate in the reaction. For example, in the complete
ionic equation above, the sodium (Na
+
) and chloride (Cl
−
) ions are present at
both the start and the end of the reaction, yet they do not participate at all during
the reaction; they “sit on the sidelines,” like spectators at a sporting event. For this
reason, they are known as
spectator ions. The real action during this reaction is
the combination of hydrogen ions (H
+
) and hydroxide ions (OH
−
) to form water
(H
2
O). If we remove the spectator ions from the equation, we can simplify the
equation to better show the combination of hydrogen ions and hydroxide ions:
H
+
(aq) + Cl
−
(aq) + Na
+
(aq) + OH
−
(aq) → Na
+
(aq) + Cl
−
(aq) + H
2
O(l)
The result represents the overall, or “net,” chemical change that occurs in this reac-
tion and is known as the
net ionic equation:
H
+
(aq) + OH
−
(aq) → H
2
O(l)
net ionic equation
4.5 Precipitation Reactions 145
HERE’S WHAT WE KNOW SO FAR
■
Molecular equations show the formulas of all reactants and products but do
not indicate whether any of the compounds really exist as ions in solution.
■
Complete ionic equations show all of the dissolved ions present in an equation
individually, along with all other reactants and products.
■
Net ionic equations show only those dissolved ions, and other reactants and
products, that actually participate in or result from the reaction concerned.
EXERCISE 4.7 The Three Equations
Aqueous hydrochloric acid and aqueous sodium carbonate react to form aqueous
sodium chloride, water, and gaseous carbon dioxide. Write the molecular, complete
ionic, and net ionic equations for this reaction.
Solution
The molecular equation is
2HCl(aq) + Na
2
CO
3
(aq) → 2NaCl(aq) +H
2
O(l) + CO
2
(g)
In the complete ionic equation, all ionic compounds are written as separated ions in
solution:
2H
+
(aq) + 2Cl
−
(aq) + 2Na
+
(aq) + CO
3
2−
(aq)
→ 2Na
+
(aq) + 2Cl
−
(aq) + H
2
O(l) + CO
2
(g)
The net ionic equation is completed by removing the spectator ions:
2H
+
(aq) + CO
3
2−
(aq) → H
2
O(l) + CO
2
(g)
PRACTICE 4.7
Identify the molecular, complete ionic, and net ionic equations that describe the re-
action of potassium oxalate (K
2
C
2
O
4
) and nitric acid (HNO
3
) to form potassium
nitrate (KNO
3
) and oxalic acid (H
2
C
2
O
4
). Assume that oxalic acid is the only non-
electrolyte in the reaction.
See Problems 43–46, 59–62, and 91.
4.5 Precipitation Reactions
An important challenge for chemists trying to clean up industrial wastewater is to
remove the ions of “heavy metals” (often very dense metals, 5 g/mL or greater)
such as lead, mercury, and cadmium, which are toxic to humans and other life.
This is particularly important in mining operations, where aqueous waste
streams from the mine can contain abnormally high levels of heavy metals. One
way to clean up the wastewater is to treat it with sulfide ions (S
2−
), because most
nonalkali metal ions will combine with sulfide ions to form a solid “precipitate.”
The word
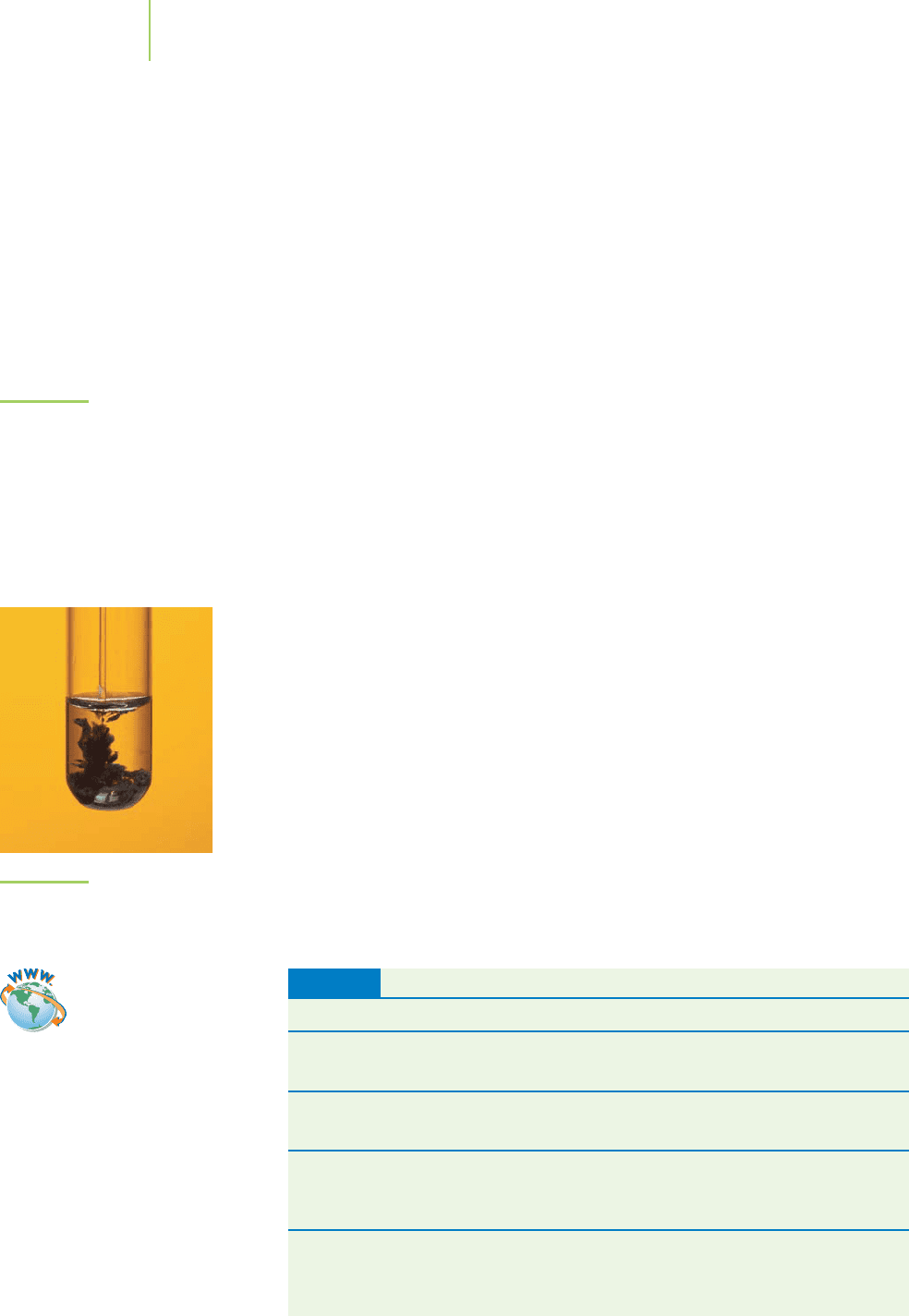
precipitate is both a noun and a verb. It is used as a noun to refer to any
solid material that forms within a solution, and as a verb to describe that process
in action. We can say that metal sulfides form a precipitate (noun). Alternatively,
we can say that metal sulfides will precipitate (verb) out of solution as soon as
they form. We say that a precipitate is
insoluble because not very much of it can
dissolve into the solvent. On the other hand, a relatively large amount of a
soluble
substance can dissolve into the solvent.About 2 kg of sucrose, table sugar, can dis-
solve in a liter of water at 25°C. That is highly soluble compared to silver chloride,
which has a solubility of about 2 mg per liter of water at 25°C.
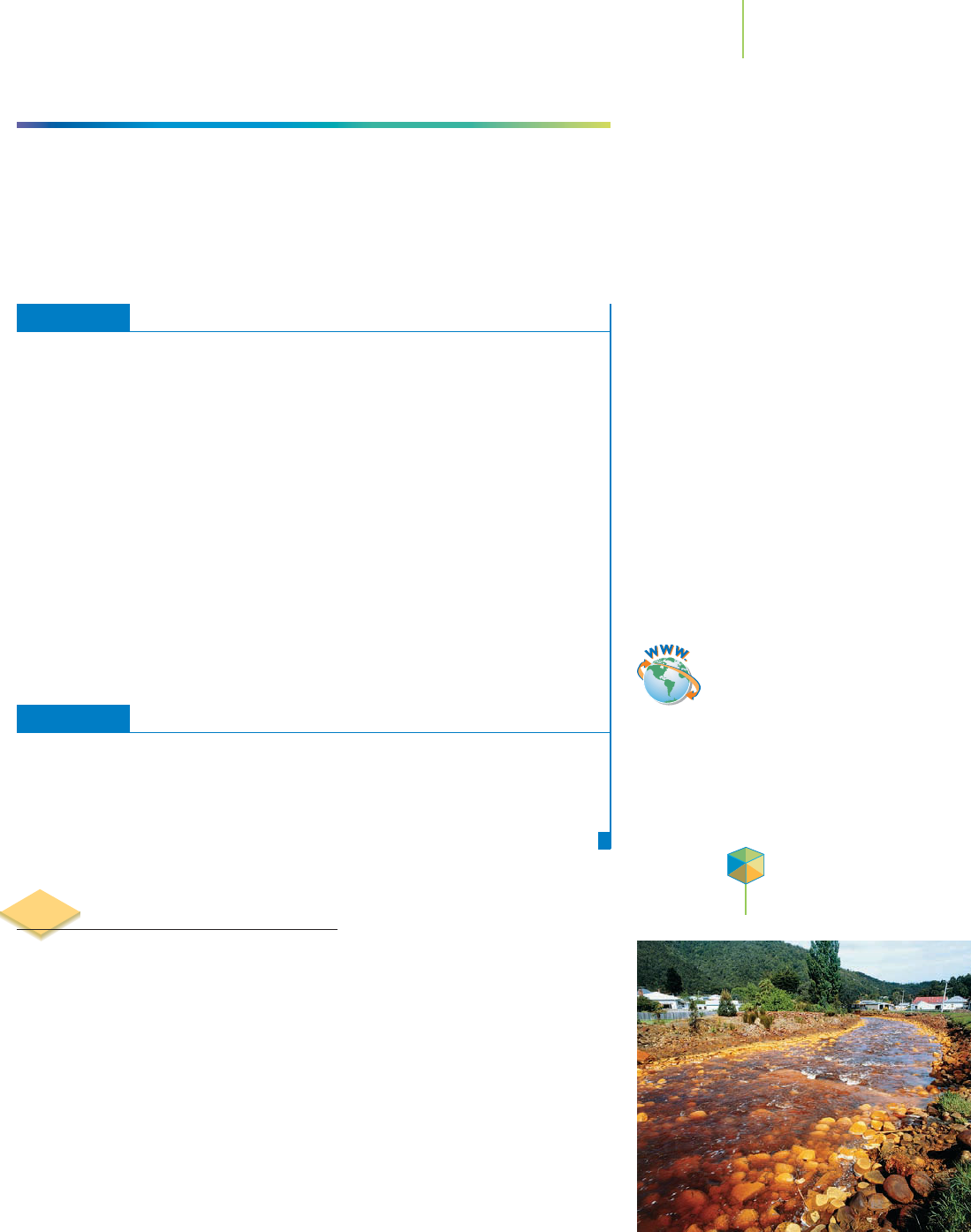
Water drainage from a mine is often tainted with
high levels of sulfide, sulfate, and other ions.
Application
C
HEMICAL ENCOUNTERS:
Focus on Lead Sulfide
Video Lesson: Precipitation
Reactions
Tutorial: Precipitation Reactions
Visualization: Precipitation
Reactions
Industrial wastewater can be treated with sulfate-reducing bacteria. These
bacteria convert sulfate ions into sulfide ions as part of their natural biological ac-
tivity (Figure 4.15). In the presence of heavy metals, the sulfide generated by the
bacteria can help to reduce the amount of soluble heavy metals by making insol-
uble precipitates. For example, the net ionic equation for the reaction of lead(II)
ions with sulfide ions is
Pb
2+
(aq) + S
2−
(aq) → PbS(s)
The ions are initially in the aqueous phase and so are dissolved in water. When
they meet and combine, they form the insoluble solid precipitate lead(II) sulfide.
This is an example of a
precipitation reaction, a reaction in which an insoluble pre-
cipitate is formed from soluble reactants. We can precipitate lead(II) sulfide in the
laboratory by mixing a solution of a soluble lead(II) compound with a solution
of a soluble sulfide. This is illustrated in Figure 4.16, which shows a solution of
ammonium sulfide being added to a lead(II) nitrate solution. The visible precip-
itate forms via the following molecular equation:
(NH
4
)
2
S(aq) + Pb(NO
3
)
2
(aq) → 2NH
4
NO
3
(aq) + PbS(s)
The lead(II) sulfide is a solid that essentially doesn’t dissolve or ionize, so we note
this in all written forms of the equation. For example, the complete ionic equa-
tion is
2NH
4
+
(aq)+S
2−
(aq)+Pb
2+
(aq)+2NO
3
−
(aq)→2NH
4
+
(aq)+2NO
3
−
(aq)+PbS(s)
The net ionic equation more clearly shows this precipitation reaction. The am-
monium and nitrate ions are spectator ions, so the net ionic equation reduces to
Pb
2+
(aq) + S
2−
(aq) → PbS(s)
which is simply the precipitation of lead(II) sulfide.
How can we predict when a precipitation reaction will occur? For most com-
mon ions, the answer has been determined experimentally and can be summa-
rizedbyasetofsolubility rules. Table 4.3 illustrates these rules in detail. Com-
pounds generally follow these rules with a few exceptions, some of which are also
noted in the table. We can use this information to write and balance the molecu-
lar equation for a reaction, determine whether any of the compounds form insol-
uble precipitates, and write the complete ionic and net ionic equations.
146 Chapter 4 Solution Stoichiometry and Types of Reactions
FIGURE 4.15
Sulfate-reducing bacteria. Spring water
in the floodplain of the Prairie Dog Town
Fork of the Red River that flows through
the panhandle of Texas has exposed
black mud. The black sediment is formed
from the interaction of dissolved metal
ions and sulfide produced from sulfate-
reducing bacteria.
Solubility Rules for Ionic Compounds
Soluble Insoluble
Group IA and ammonium Most carbonates are insoluble except
compounds are soluble. Group IA carbonates and (NH
4
)
2
CO
3
.
Acetate, chlorate, perchlorate, and Most phosphates are insoluble except
nitrate compounds are soluble. Group IA phosphates and (NH
4
)
3
PO
4
.
Most chlorides, bromides, and iodides Most sulfides are insoluble except
are soluble except those of Ag
+
,Hg
2
2+
, Group IA sulfides and (NH
4
)
2
S.
and Pb
2+
.
Most sulfates are soluble except those Most hydroxides are insoluble except
of Ca
2+
,Sr
2+
,Ba
2+
,Ag
+
,Hg
2
2+
, Group IA hydroxides and Ca(OH)
2
,
and Pb
2+
. Sr(OH)
2
, and Ba(OH)
2
.
TABLE 4.3
FIGURE 4.16
Lead sulfide precipitates from the
combination of its soluble ions.
Visualization: Solubility Rules

EXERCISE 4.8 Identifying Precipitation Reactions
Which combinations of the following aqueous solutions will produce precipitates:
aluminum bromide, barium hydroxide, magnesium sulfate, and nickel(II) iodide?
Use the solubility rules to guide you in your decision.
First Thoughts
You need to consider all the possible combinations of positive and negative ions in
order to identify those that will produce an insoluble product. The four solutions in
this question contain Al
3+
,Br
−
,Ba
2+
,OH
−
,Mg
2+
,SO
4
2−
,Ni
2+
, and I
−
.
Solution
Table 4.3 reveals the following solubilities for all the possible combinations of ions:
aluminum hydroxide—insoluble aluminum sulfate—soluble
aluminum iodide—soluble barium bromide—soluble
barium sulfate—insoluble barium iodide—soluble
magnesium bromide—soluble magnesium hydroxide—insoluble
magnesium iodide—soluble nickel(II) bromide—soluble
nickel(II) hydroxide—insoluble nickel(II) sulfate—soluble
The solubility rules indicate that we could make four insoluble precipitates, in the
following precipitation reactions:
❚ Add aluminum bromide solution to barium hydroxide solution, to form a
precipitate of aluminum hydroxide.
Al
3+
(aq) + 3OH
−
(aq) →Al(OH)
3
(s)
❚ Add barium hydroxide solution to magnesium sulfate solution, to form a
precipitate of barium sulfate, mixed with a precipitate of magnesium hydroxide.
Mg
2+
(aq) +2OH
−
(aq) →Mg(OH)
2
(s) and Ba
2+
(aq) +SO
4
2−
(aq) →BaSO
4
(s)
❚ Add nickel(II) iodide solution to barium hydroxide solution, to form a precipitate
of nickel(II) hydroxide.
Ni
2+
(aq) + 2OH
−
(aq) → Ni(OH)
2
(s)
Further Insights
The result when we add a barium hydroxide solution to a magnesium sulfate solu-
tion reveals a complication. Precipitation reactions may produce a mixture of pre-
cipitates if the cations of each dissolved compound form insoluble products with
the anions of the other compound with which they are mixed. When we prepare a
compound by precipitation, we must ensure that the only precipitate that forms is
the desired one. We will discuss ways of doing this in Chapter 18.
PRACTICE 4.8
Which combination of the following solutions will produce a precipitate:
AgNO
3
(aq), NaCl(aq), Na
2
S(aq), ZnSO
4
(aq)?
See Problems 57–66, 89, and 90.
EXERCISE 4.9 The Stoichiometry of a Precipitation Reaction
Calculate the mass of the precipitate that is formed when 1.30 L of 0.0200 M AlBr
3
solution is added to 3.00 L of 0.0350 M NaOH solution.
First Thoughts
First we map out our plan of attack. We will do the following steps:
1. Write the balanced molecular equation (we will not need to write the net ionic
equation).
4.5 Precipitation Reactions 147
