Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


158 Chapter 4 Solution Stoichiometry and Types of Reactions
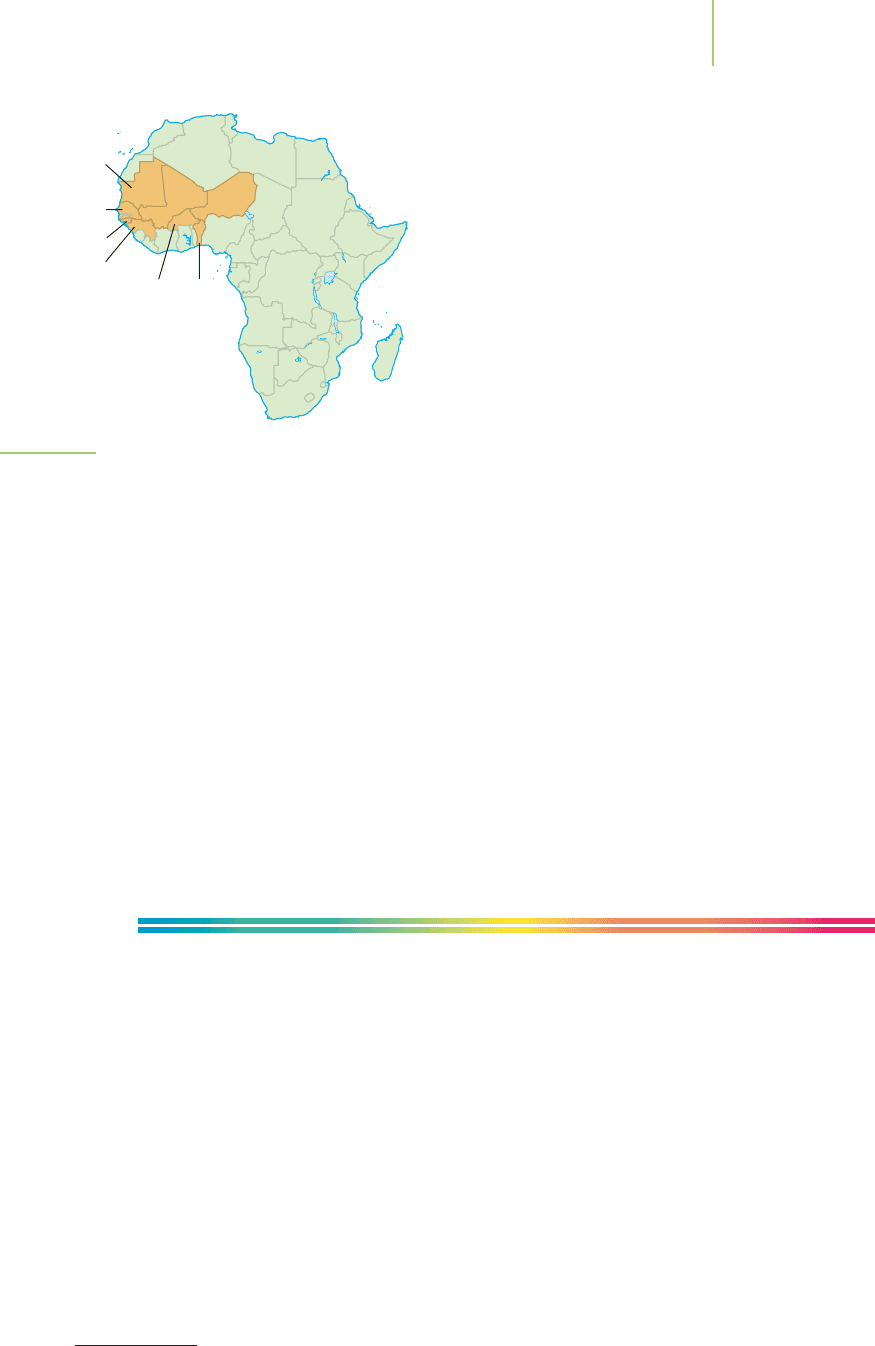
reported by the World Heath Organization in the African countries of Benin,
Burkina-Faso, Guinea, Guinea-Bissau, Mali, Mauritania, Niger, and Senegal
(Figure 4.19). In those countries that disinfect their water supply with chlorine,
however, cholera has been all but eradicated. Unfortunately, wastewater treat-
ment effluent (runoff from the cities back into lakes, streams, rivers, etc.) has
resulted in chlorine concentrations between 1 and 5 parts per million. Any effect
these levels of chlorine have on the environment has yet to be determined.
The gold miners we mentioned at the start of this chapter have to be careful
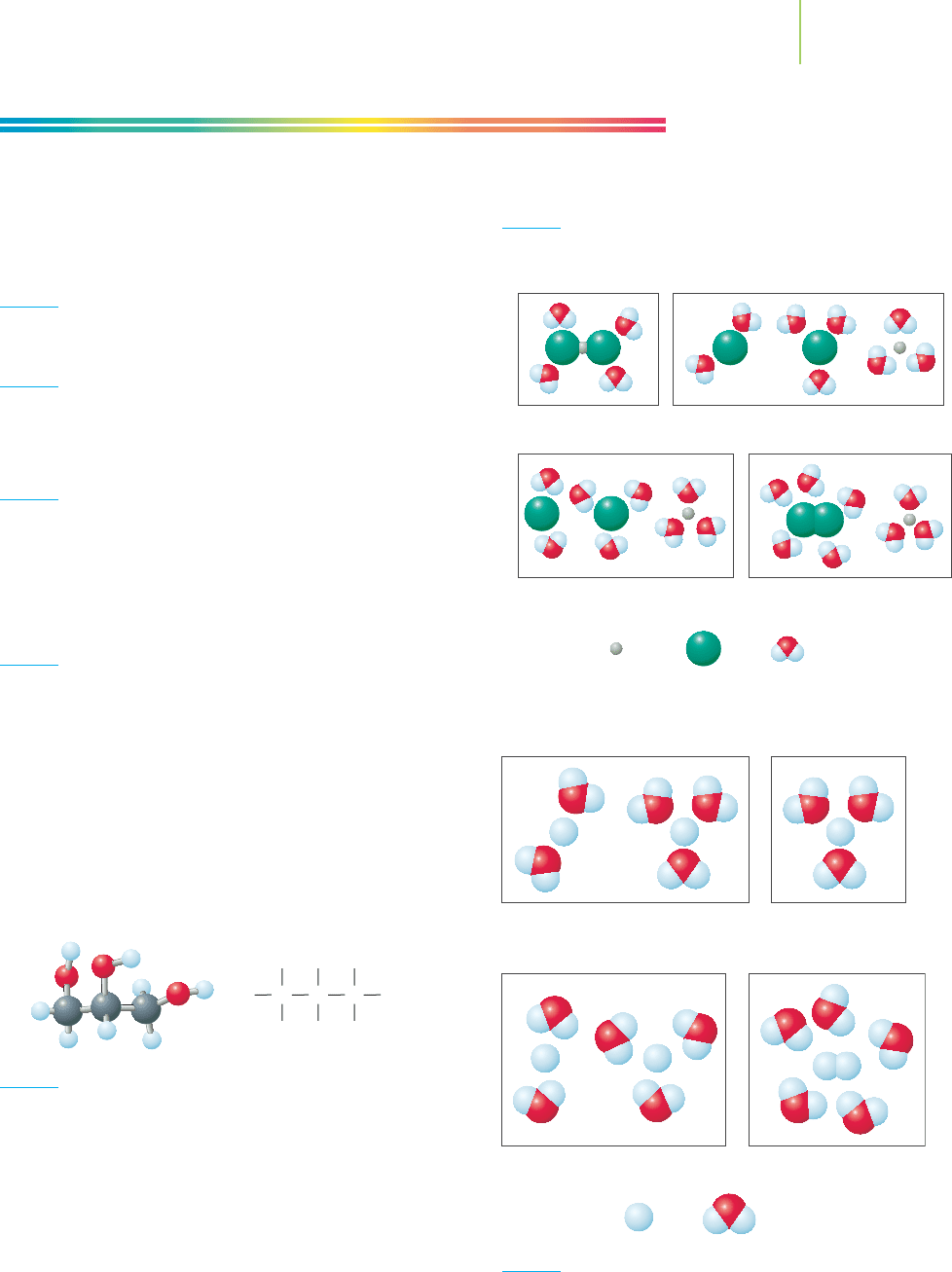
with their cyanide leaching solution, because the MCLG for CN
−
is 0.200 ppm
(200 ppb). Larger quantities of this ion in water can be very harmful to the envi-
ronment. In the winter of 2000, an accidental spill in Romania resulted in ap-
proximately 22 million gallons of cyanide waste from a gold mine flowing into
the nearby Tisza River. This river flows into the Danube River and through Bel-
grade, Yugoslavia, on its way to the Black Sea. The initial cyanide spill killed most
of the life in the Tisza River and harmed much of the life in the Danube River. To
compound the disaster, the Tisza River is home to 17 of Hungary’s 29 protected
species of fish, including the last known species of Danube sturgeon. It will be
decades before the environment recovers from this accident.
FIGURE 4.18
The quantitative chemistry of fresh water
is a vital consideration in ensuring the
safety of our water supplies and the
health of the environment.
Maximum Contaminant Level Goal for Selected Substances in Safe Water
Potential Human
MCLG Organ Damage Due to
Substance (mg/L = ppm) Exceeding the MCLG Source
As 0.010 Skin and circulatory Runoff from orchards
system and glass-manufacturing
plants
Cd 0.005 Kidney Corrosion of pipes,
discharge from used
batteries
CN
−
0.200 Thyroid and nerve Discharge from gold
mining, fertilizer, and
plastics manufacturing
Hg 0.002 Kidney Discharge from refineries
Dioxin 0.00000003 Reproductive system By-products of smelting,
(C
12
H
4
Cl
4
O
2
) bleaching, and pesticide
manufacture
TABLE 4.6
A water technician checks the chlorina-
tion process at a water treatment plant.
Application
The Danube sturgeon.
What must rivers and lakes contain in order to be healthful habitats for fresh-
water life?
They must contain sufficient oxygen, hold appropriate quantities of
nutrients (too little or too much of these can adversely affect the water quality),
and have an appropriate acid–base balance. If pollution problems are suspected
in any river or lake, chemists and biologists must analyze the water and perhaps
also the flesh and blood of fish, birds, and other organisms that live in or around
the water. Chemical water analysis includes working with measures of concentra-
tion, including parts per million, parts per billion, and molarity, and investigat-
ing each specific type of reaction that occurs in the aqueous environment.
The Bottom Line 159
■
Water is an extremely versatile solvent, partly thanks
to its polarity, which is due to the uneven distri-
bution of electrons in the molecule. (Sec-
tion 4.1)
■
When ionic compounds dissolve in water, the ions
dissociate and become surrounded by water
molecules—a process known as hydration.
(Section 4.1)
■
The concentration of a solution can be expressed
in units known as molarity. This term indicates
how many moles of the chemical concerned would
be present if we had one liter of the solution.
(Section 4.2)
Molarity =
moles of solute
liter of solution
= M
Mali
Niger
Benin
Burkina-Faso
Mauritania
Senegal
Guinea-Bissau
Guinea
FIGURE 4.19
Cholera outbreaks in 2005 were reported in many western
Africa nations.
The Bottom Line
■
Chemicals present at very low levels are often mea-
sured in terms of parts per million (ppm), parts per
billion (ppb), or parts per trillion (ppt). (Section 4.2)
■
The quantitative analysis of the chemicals present
in solutions is of great importance in medicine,
industry, and environmental science. (Sections 4.3,
4.8)
■
Precipitation reactions involve an insoluble precipi-
tate forming when soluble chemical species com-
bine. (Section 4.5)
■
Acid–base reactions involve acids and bases reacting
in ways that can neutralize the acidic and basic char-
acter of each. (Section 4.6)
■
Oxidation–reduction reactions are electron transfer
processes in which some reactants lose electrons (are
oxidized), while others gain electrons (are reduced).
(Section 4.7)

160 Chapter 4 Solution Stoichiometry and Types of Reactions
Key Words
acid A compound that produces hydrogen ions (H
+
)
when dissolved in water. (p. 148)
acid–base reaction The reaction between an acid and
a base. The products are water and an ionic com-
pound. (p. 148)
aqueous Water-based; also implies that a dissolved sub-
stance has a sphere of hydration. (p. 126)
base A compound that produces hydroxide ions (OH
−
)
when dissolved in water. (p. 148)
buret A laboratory device used to precisely and accu-
rately add small known quantities of solution.
(p. 140)
complete ionic equation A chemical equation that indi-
cates all of the ions present in a reaction as individual
entities. (p. 144)
concentration An intensive property of a solution that
describes the amount of solute dissolved per volume
of solution or solvent. The typical concentration
units include molarity, ppm, and w/w. (p. 131)
diprotic Can produce 2 mol of H
+
when it dissolves.
(p. 151)
electrolyte A compound that produces ions when dis-
solved in water. (p. 129)
end point In a titration, the volume of the added reac-
tant that causes a visual change in the color of the
indicator. (p. 140)
equivalence point In a titration, the point at which all re-
actants have just been completely consumed. (p. 140)
half-reaction An incomplete equation that describes the
oxidation or reduction portion of a redox reaction.
(p. 153)
hydration sphere The shell of water molecules surround-
ing a dissolved molecule, ion, or other compound.
This shell arises because of the force of attraction be-
tween the water molecules and the solute. (p. 127)
insoluble Not capable of dissolving in a solvent to an
appreciable extent. (p. 145)
molar (M) The “shorthand” method of describing mo-
larity, as in “that is a 3 molar HCl solution.”
(p. 132)
molarity (M) A specific concentration term that reflects
the moles of solute dissolved per liter of total solu-
tion. (p. 131)
molecular equation A chemical equation that shows
complete molecules and compounds. (p. 144)
monoprotic Can produce 1 mol of H
+
when it dissoci-
ates. (p. 151)
net ionic equation A complete ionic equation written
without the spectator ions. (p. 144)
nonelectrolyte A compound that doesn’t dissociate into
ions when it dissolves. (p. 129)
oxidation The process of losing electrons. Such a sub-
stance is said to be oxidized. (p. 152)
oxidation number A “bookkeeping” number that reflects
the charge on an ion. (p. 154)
oxidation–reduction reactions Reactions that involve the
transfer of electrons from one species to another.
Also known as redox reactions. (p. 153)
oxidation state See oxidation number. (p. 154)
oxidized The species that has lost electrons in a redox
reaction. (p. 152)
parts per billion (ppb) One gram of solute per billion
grams of solution. (p. 135)
parts per million (ppm) One gram of solute per million
grams of solution. (p. 135)
parts per trillion (ppt) One gram of solute per trillion
grams of solution. (p. 135)
precipitation reaction A reaction involving the formation
of a solid that isn’t soluble in the reaction solvent.
(p. 146)
precipitate Any solid material that forms within a solu-
tion; the action describing the formation of a solid.
(p. 145)
redox reactions See oxidation–reduction reactions.
(p. 153)
reduced The species that has gained electrons in a redox
reaction. (p. 152)
reduction The process of gaining electrons. Such a sub-
stance is said to be reduced. (p. 152)
soluble The ability of a substance to dissolve within a
solution. (p. 145)
solutes Molecules, ions, or atoms that are dissolved in a
solvent to form a solution. (p. 126)
solution (Defined in Chapter 2.) A homogeneous mix-
ture of solute and solvent. (p. 126)
solvent A compound that typically makes up the ma-
jority of a homogeneous mixture of molecules, ions,
or atoms; dissolves the solute. (p. 126)
spectator ions Ions that do not participate in a reaction.
(p. 144)
strong acid An acid that completely dissociates in solu-
tion. (p. 149)
strong base A base that completely dissociates in solu-
tion. (p. 149)
strong electrolyte Any compound that completely disso-
ciates in solution. (p. 129)
standard solution A solution with a well-defined and
known concentration of solute. (p. 140)
titration The process of adding one reactant to an un-
known amount of another until the reaction is com-
plete; used to determine the concentration of an
unknown solute. (p. 140)
triprotic Can produce 3 mol of H
+
when it dissolves.
(p. 151)
weak acid An acid that partially dissociates in solution.
(p. 150)
weak base A base that partially dissociates in solution.
(p. 150)
weak electrolyte Any substance that only partially disso-
ciates in solution. (p. 129)
Focus Your Learning 161
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 4.1 Water—A Most Versatile Solvent
Skill Review
1. Explain how water molecules can dissolve both cations and
anions.
2. Why doesn’t pure water conduct an electric current?
3. Explain what is meant by the term hydration sphere?
4. Diagram, using circles for atoms, a crystal of KCl versus the
same crystal of KCl dissolved in water.
Chemical Applications and Practices
5. Earth’s oceans contain tons of dissolved sodium chloride.Yet,
when a ship develops an oil leak, almost none of the oil dis-
solves in the ocean. Explain this phenomenon.
6. When an ion dissolves, it is surrounded by a hydration
sphere. If water molecules surrounded the ion so that the hy-
drogen portion of the water was closer to the ion, would the
ion most likely be a cation or an anion?
7. Pure water does not conduct an electric current. However,
aqueous solutions of some compounds do form solutions
that conduct electricity. Explain why the presence of some
solutes converts nonconducting water into a conducting
solution.
8. Glycerin can be produced as a by-product in soap making.
The compound dissolves so easily in water that it absorbs
water from the air. This latter characteristic is why glycerin
is often found in many skin lotions. As glycerin absorbs
the water, the skin can be kept moist. Glycerin’s structure is
shown below. What aspects of glycerin’s structure contribute
most to its ease of dissolving in water?
9. A conductivity-testing apparatus, such as the one shown in
this chapter, possesses a light bulb whose brightness is related
to how much current is flowing through it (and also through
the solution.) A small, but measurable, amount of current
must be present before the bulb becomes visibly brighter.
What effect would this characteristic of the apparatus have
on the classification of solutions containing strong elec-
trolytes, containing weak electrolytes, and containing non-
electrolytes?
10. When dissolved in water, which of the following would you
expect to cause a conductivity tester, shown in the chapter, to
produce a very bright light? (Assume that 0.50 mole of each
is placed in 1.0 L of solution.)
a. C
2
H
5
OH (ethanol) d. KCl
b. NaOH e. BaSO
4
c. Na
2
CO
3
Section 4.2 The Concentration of Solutions
Skill Review
11. Which of the following would best represent MgCl
2
dissolved
in water?
12. Which of the following would best represent H
2
dissolved in
water?
13. If 0.100 mol MgCl
2
is dissolved in water, how many total
moles of ions are there in the resulting solution?
14. If 0.100 mol H
2
is dissolved in water, how many total moles of
ions are there in the resulting solution?
CHH
H
OH
C
H
OH
C
H
OH
Gl
y
cerin
(a) (b)
(c) (d)
Mg
2+
H
2
OCl
–
(a) (b)
(c) (d)
H
2
OH

162 Chapter 4 Solution Stoichiometry and Types of Reactions
25. Determine the concentration, in ppm, for each of the follow-
ing solutions.
a. 2.5 mg NaCl per 1000.0 L solution
b. 5.25 mg Cu
2+
ions per 500.0 mL solution
c. 12.5 mg Cl
−
ions per 300.0 mL solution
26. Determine the concentration, in ppm, for each of the follow-
ing solutions.
a. 1.20 mg KCl per 1000.0 mL solution
b. 0.100 g Co
2+
ions per 500.0 mL solution
c. 1.00 micrograms CN
−
ions per 450.0 mL solution
27. Perform the indicated conversions.
a. 1.20 × 10
−4
M NaCl to ppm
b. 5.33 ppm CN
−
to ppb
c. 170 ppm NaOH to mass percent
28. Perform the indicated conversions.
a. 0.00250 M CuCl
2
to mass percent
b. 19 ppm CN
−
to M
c. 0.0011% NaOH by mass to M
Chemical Applications and Practices
29. When growing certain bacteria, biologists must often control
the acidity level of the growth medium. To do this, a variety
of compounds, referred to as buffer systems, may be added in
specific amounts. Which of the following has the greater
molarity?
a. 42.0 g of KH
2
PO
4
dissolved in 250.0 mL of solution
b. 21.0 g of KH
2
PO
4
dissolved in 125.0 mL of solution
30. If a student seeking to prepare a 1.0 M solution of KOH
added 56 g of solid KOH to exactly 1.0 L of water, would the
solution be greater or less than 1.0 M? Explain the basis of
your conclusion.
31. Adding antifreeze to aqueous automobile cooling systems
lowers the freezing point of the resulting solution. The main
ingredient in most antifreeze products is ethylene glycol
(C
2
H
6
O
2
). How many kilograms of ethylene glycol are dis-
solved in a 10.0-liter solution that is 16.0 M?
32. Oxygen dissolved in water is critical for aquatic life. The level
of dissolved oxygen is often used to report the quality of
water in the environment. If 0.0090 g of O
2
is dissolved per
liter of solution, what would be reported as (a) the molarity,
and (b) the concentration in ppm, of dissolved oxygen?
33. Occasionally students enjoy a cup of coffee or tea while
studying chemistry. The amount of caffeine in those bever-
ages varies greatly. At a temperature of 65°C, the maximum
amount of caffeine (C
8
H
10
N
4
O
2
) that can be dissolved in
water is 455g/L. What would be the maximum molarity of
caffeine in a 425-mL cup of 65°C coffee?
34. The compound potassium permanganate forms intensely
purple solutions that are used to react with other solutions
that contain iron. How many grams of potassium perman-
ganate (KMnO
4
) would you need to dissolve into 0.500 L of
solution if you wanted to prepare a 0.00100 M solution of
KMnO
4
?
35. The pesticide atrazine (C
8
H
14
N
5
Cl) is so slightly soluble in
water that its concentration is not usually reported in molar-
ity. Health advisory warnings are issued if the atrazine con-
centration is higher than 3.0 parts per billion. How many
15. The formula of vitamin C is C
6
H
8
O
6
. Calculate the molarity
of each of the following vitamin C solutions:
a. 0.150 g of vitamin C dissolved in enough water to produce
a 1.50 L solution
b. 0.250 g of vitamin C dissolved in enough water to produce
a 0.500 L solution
c. 3.50 g of vitamin C dissolved in enough water to produce
a 2.0 L solution
16. The formula of ethanol is C
2
H
6
O. Calculate the molarity of
each of the following ethanol solutions:
a. 0.150 g of ethanol dissolved in enough water to produce a
1.50 L solution
b. 25 g of ethanol dissolved in enough water to produce a
0.500 L solution
c. 100 g of ethanol dissolved in enough water to produce a
2.0 L solution
17. Determine the mass of glycine (C
2
H
5
NO
2
) in each of the
following solutions:
a. 100.0 mL of 0.015 M glycine
b. 125.0 mL of 0.0145 M glycine
c. 74.6 mL of 1.44 M glycine
18. Determine the volume of solution needed to provide the
following amounts:
a. 4.50 g ethanol (C
2
H
6
O) from a 2.50 M ethanol solution
b. 63.7 g HCl from a 6.0 M HCl solution
c. 3.0 g glucose (C
6
H
12
O
6
) from a 0.150 M solution
19. Calculate the molarity of
a. calcium ions in 1.00 L of solution containing 24.55 g of
calcium chloride
b. chloride ions in 1.00 L of solution containing 24.55 g of
calcium chloride
c. water in pure water
20. Calculate the molarity of
a. HBr if 45.0 g is dissolved in 1.0 L.
b. NaOH if 32.5 g is dissolved in 500 mL
c. chloride ions in a 5.0 L solution containing 2.00 g NaCl.
21. One important source of bromine, in the form of Br
−
(aq), is
seawater. If the molarity of Br
−
in seawater is approximately
0.00081 M, how many liters of seawater would be required to
obtain 1 mole of Br
−
?
22. Chloride can be obtained from seawater. If the molarity of
Cl
−
in seawater is approximately 0.522 M, how many liters of
seawater would be required to obtain 1 mole of Cl
−
?
23. Determine the volume of a 0.125 M solution of MgCl
2
needed to provide
a. 0.10 mol MgCl
2
b. 0.10 mol Mg
2+
c. 2.33 mol Cl
−
24. Determine the volume of a 0.0357 M solution of Na
2
SO
4
needed to provide
a. 0.10 mol Na
2
SO
4
b. 0.10 mol Na
+
c. 4.30 mol SO
4
2−
Focus Your Learning 163
43. First complete the following reaction using words rather than
chemical symbols. Then write the molecular, complete ionic,
and net ionic equations that describe this aqueous solution
reaction.
Potassium hydroxide + hydrochloric acid →
44. List the spectator ions for the following reaction:
Sulfuric acid + potassium hydroxide →
45. Write the molecular, complete ionic, and net ionic equations
for the following reaction:
Sodium chloride + calcium nitrate
→ sodium nitrate +calcium chloride
46. Write the molecular, complete ionic, and net ionic equations
for the following reaction:
Magnesium chloride + sodium bromide
→ sodium chloride +magnesium bromide
Chemical Applications and Practices
47. Using the stoichiometry found in the chapter for the vita-
min C and I
2
reaction, determine which of the following two
drinks contains the greater concentration of vitamin C.
Which sample contains the greater amount of vitamin C?
a. Brand A: 250.0 mL solution required 10.5 mL of
0.0855 M I
2
b. Brand B: 300.0 mL solution required 12.0 mL of
0.0855 M I
2
48. Using the stoichiometry found in the chapter for the vita-
min C and I
2
reaction, determine which of the following two
drinks contains the greater concentration of vitamin C.
Which sample contains the greater amount of vitamin C?
a. Brand A: 150.0 mL solution required 8.5 mL of
0.0650 M I
2
b. Brand B: 100.0 mL solution required 12.5 mL of
0.0250 M I
2
49. One of the more common agents for standardizing solutions
of sodium hydroxide (NaOH) is potassium acid phthalate
(KHC
8
H
4
O
4
), or, more accurately, potassium hydrogen
phthalate. The compound is often abbreviated KHP, where
the P designates the phthalate ion (C
8
H
4
O
4
2−
), not the phos-
phorus atom. From the following data, determine the average
molarity of a NaOH solution that was standardized with
KHP.
Trial A: 45.12 mL of NaOH solution neutralized 0.5467 g
of KHP
Trial B: 44.89 mL of NaOH solution neutralized 0.5475 g
of KHP
Trial C: 46.50 mL of NaOH solution neutralized 0.5501 g
of KHP
50. One of the more common agents for standardizing solutions
of potassium hydroxide (KOH) is potassium acid phthalate
(KHC
8
H
4
O
4
), or, more accurately, potassium hydrogen
phthalate. From the following data, determine the average
molarity of a KOH solution that was standardized with KHP.
Trial A: 45.12 mL of KOH solution neutralized 0.2573 g
of KHP
Trial B: 48.89 mL of KOH solution neutralized 0.2250 g
of KHP
Trial C: 45.50 mL of KOH solution neutralized 0.2502 g
of KHP
grams of atrazine would you expect to find in 1 L of water
that is 3.0 ppb in atrazine? What would be the molarity of the
solution?
36. A can of nondiet soda may contain 45 g of sucrose
(C
12
H
22
O
11
) per 450 g of soda. What is the sugar concentra-
tion reported as ppm? Approximately how many grams of
water would you have to add to the soda to reduce this sugar
concentration to only 1 part per million?
37. A certain sports drink has a NaCl concentration of 0.20 M.If
the container held 250 mL of the liquid, how many moles of
Na
+
would you consume when swallowing 50.0 mL of the
drink?
38. One method used to detect the presence of the toxic heavy
metal barium is to precipitate the barium +2 ion as barium
sulfate. If a 10.0-mL sample produced 0.565 g of barium sul-
fate (and assuming that no more Ba
2+
remained dissolved)
what was the original molarity of Ba
2+
in the solution?
Section 4.3 Stoichiometric Analysis of Solutions
Skill Review
39. Assume 1:1 reaction stoichiometry in each of the following:
a. 25.0 mL of HCl required 35.0 mL of 0.155 M NaOH to
neutralize. What is the molarity of the HCl solution?
b. 50.0 mL of Sr(OH)
2
required 35.0 mL of 0.0200 M sulfuric
acid to neutralize. What is the molarity of the Sr(OH)
2
solution?
c. 40.5 mL of niric acid (HNO
3
) required 25.0 mL of 0.35 M
KOH to neutralize. What is the molarity of the HNO
3
solution?
40. Assume 1:1 reaction stoichiometry in each of the following:
a. 33.0 mL of HCl required 17.6 mL of 2.50 M NaOH to
neutralize. What is the molarity of the HCl solution?
b. 10.25 mL of Sr(OH)
2
required 15.56 mL of 0.00150 M
sulfuric acid to neutralize. What is the molarity of the
Sr(OH)
2
solution?
c. 100.0 mL of nitric acid (HNO
3
) required 84.30 mL of
1.562 × 10
−4
M KOH to neutralize. What is the molarity
of the HNO
3
solution?
41. The label on a solution was partially obscured. It was either
0.10 M CuCl or 0.10 M CuCl
2
. When the entire 25.0 mL of
the solution was evaporated to dryness, 0.336 g of solid re-
mained. What should the label read?
42. The label on a solution was partially obscured. It was either
0.10 M FeCl
2
or 0.10 M FeCl
3
. If the solution was actually
0.10 M FeCl
2
, how many grams of FeCl
2
would be obtained if
100.0 mL of the solution were evaporated to dryness?
CH
3
CH
Cl
CH
3
CH
2
CH
3
H
N
HN
N N
N
Atrazine

51. Hydrochloric acid has many industrial uses. The steel indus-
try uses hydrochloric acid in a process known as “pickling
steel.” This is done to steel prior to galvanizing. To analyze
the concentration of the “pickle liquor,” a titration with
sodium hydroxide may be used. Write the net ionic reaction
between hydrochloric acid and sodium hydroxide, and then
determine the concentration of the HCl in the pickling
liquor if a 10.00 mL sample of the hydrochloric acid re-
quired 45.55 mL of a 0.9876 M solution of NaOH to com-
pletely react.
52. Nitrogen for increased yields on farms can come from a vari-
ety of sources. One common nitrogen-containing fertilizer
is ammonium sulfate, (NH
4
)
2
SO
4
. The reaction between
ammonia (NH
3
) and sulfuric acid (H
2
SO
4
) produces this
important fertilizer.
a. Balance the reaction.
b. How many liters of a 1.55 M solution of sulfuric acid
would be needed to completely react with 1.00 kg of
ammonia?
53. One method that field geologists use to test for the presence
of carbonates is to drip hydrochloric acid on a sample and
note the formation of carbon dioxide gas bubbles.
a. Balance the reaction between calcium carbonate (CaCO
3
)
and hydrochloric acid (HCl.)
b. If 5.00 mL of 0.500 M HCl completely reacted with
calcium carbonate in a rock sample, how many grams
of calcium carbonate did the sample contain?
54. Hydrogen peroxide, in very dilute concentrations, can be
used as a disinfectant. The concentration of hydrogen perox-
ide (H
2
O
2
) can be determined in a titration experiment using
potassium permanganate (KMnO
4
) as shown in the follow-
ing balanced equation:
2MnO
4
−
(aq) + 5H
2
O
2
(aq) + 6H
+
(aq)
→ 5O
2
(g) + 2Mn
2+
(aq) + 8H
2
O(l)
If a 25.0 mL sample of hydrogen peroxide required 25.2 mL
of 0.353 M KMnO
4
solution to react with all the hydrogen
peroxide, what was the molarity of the hydrogen peroxide
solution?
Section 4.4 Types of Chemical Reactions
Skill Review
55. Predict the products of the following incomplete reactions.
After balancing the reaction, label each as a precipitation,
acid–base, or redox reaction. For those reactions where it is
possible, write the net ionic equation.
a. C
4
H
10
(g) + O
2
(g) →
b. Ca(OH)
2
(aq) + HNO
3
(aq) →
c. Pb(NO
3
)
2
(aq) + NaCl(aq) →
56. Predict the products of the following incomplete reactions.
After balancing the reaction, label each as a precipitation,
acid–base, or redox reaction. For those reactions where it is
possible, write the net ionic equation:
a. HCl(g) + Ca(OH)
2
(aq) →
b. CH
4
(g) + O
2
(aq) →
c. Ba(ClO
4
)
2
(aq) + Na
2
S(aq) →
164 Chapter 4 Solution Stoichiometry and Types of Reactions
Section 4.5 Precipitation Reactions
Skill Review
57. Which of the following salts would qualify as soluble in
water?
a. CuCO
3
d. KOH
b. NiS e. lead acetate
c. (NH
4
)
2
CO
3
58. Which of the following salts would qualify as soluble in
water?
a. NaNO
3
d. PbBr
2
b. Ba(OH)
2
e. AgI
c. MgSO
4
59. Predict the products and write the net ionic equations for
each of the following:
a. BaCl
2
(aq) + NaNO
3
(aq) →
b. Fe(NO
3
)
3
(aq) + (NH
4
)
2
SO
4
(aq) →
c. CaCl
2
(aq) + K
2
SO
4
(aq) →
60. Predict the products and write the net ionic equations for
each of the following:
a. MgCl
2
(aq) + KNO
3
(aq) →
b. AgNO
3
(aq) + NH
4
Cl(aq) →
c. CaCl
2
(aq) + NaOH(aq) →
61. Write out the formulas of each of the following reactants.
Then predict the result of mixing the aqueous solutions for
each situation. Finally, report the net ionic equation for each.
a. Copper(II) nitrate + potassium hydroxide →
b. Sodium carbonate + aluminum chloride →
c. Ammonium phosphate + zinc chloride →
62. Write out the formulas of each of the following reactants.
Then predict the result of mixing the aqueous solutions for
each situation. Finally, report the net ionic equation for each.
a. Barium nitrate + sodium hydroxide →
b. Ammonium carbonate + aluminum bromide →
c. Silver nitrate + zinc bromide →
63. Name two soluble ionic compounds that could be used to
produce each of the following insoluble salts:
a. BaS b. Cu(OH)
2
c. PbSO
4
64. Name two soluble ionic compounds that could be used to
produce each of the following insoluble salts:
a. AgBr b. Fe(OH)
2
c. PbS
Chemical Applications and Practices
65. Suppose you have a water sample that is to be analyzed. You
know the sample contains Cu
2+
,Ba
2+
, and Ag
+
ions. Suggest
a sequence for adding other aqueous electrolyte solutions
that could separate each of these by selective precipitation.
66. The presence of metal ions in aqueous systems can often
cause environmental complications. Suppose it was neces-
sary to remove Cu
2+
ions from a sample of water. Suggest at
least two reagents that you could add to the aqueous system
to remove the copper ions.
67. A traditional method for the analysis of dissolved ions is
called gravimetric analysis. This name is derived from the
process in which the ion to be analyzed is precipitated and
filtered as gravity separates the liquid from the precipitate.
Suppose that you found that all the silver ion (Ag
+
) in a solu-
tion, present as AgNO
3
(aq), reacted with 25.0 mL of 0.242 M
NaCl to form solid AgCl.
a. Write the molecular and net ionic equations for the
precipitate formation.
b. Determine the grams of precipitate formed and the
grams of silver present in the original solution.
68. One method of preparing the compound AgBr, used in pho-
tographic films, is to precipitate it from a solution of KBr.
After first writing and balancing the molecular and net ionic
equations, calculate many grams of AgBr precipitate when
100.0 mL of 2.00 M KBr is mixed with 100.0 mL of 1.00 M
AgNO
3
.
Section 4.6 Acid–Base Reactions
Skill Review
69. What is the net ionic reaction of the following acid–base re-
action? You’ll have to provide the balanced molecular equa-
tion in order to begin this problem.
Hydrogen bromide + magnesium hydroxide
→ water + magnesium bromide
70. What acid–base reaction would produce each of the follow-
ing salts? Write the balanced chemical equation in each case.
a. NaCl b. K
2
CO
3
c. Na
2
SO
4
d. Al(NO
3
)
3
71. a. What would be the molarity of a KOH solution if, during a
titration with 0.50 M HCl, 34.5 mL of the HCl neutralized
22.4 mL of the KOH solution?
b. How much of a 0.50 M solution of H
2
SO
4
would be
needed to neutralize the same amount of the KOH
solution?
72. If a student mixes 25.0 mL of 0.255 M H
2
SO
4
with 50.0 mL of
0.115 M KOH (assume the volumes are additive), which
reagent will be in excess? What will be the concentration of
the excess reagent?
Chemical Applications and Practices
73. Oxalic acid can be found in rhubarb plants. If a 0.255-g sam-
ple of purified oxalic acid required 25.7 mL of NaOH to neu-
tralize, what would you report as the molarity of the NaOH
solution? (Oxalic acid: H
2
C
2
O
4
)
74. Phosphoric acid is a very versatile acid with widespread uses,
from making fertilizer to soft drink ingredients.
a. Balance the reaction between phosphoric acid (H
3
PO
4
)
and ammonium hydroxide (NH
4
OH).
b. How many milliliters of 0.459 M ammonium hydroxide
would be needed to neutralize 33.5 mL of 0.100 M
phosphoric acid?
75. Carbonic acid is formed when gaseous carbon dioxide is
pumped into soft drinks to establish their carbonation. Sup-
pose you are employed at the new “ChemCola” beverage
company. You must titrate the carbonic acid in the soft drink
using 0.1445 M NaOH.
Focus Your Learning
165
a. Write the molecular and net ionic equations.
b. What is the molarity of carbonic acid if a 50.00 mL sample
required 38.98 mL of 0.1445 M NaOH?
76. Acetic acid is the ingredient in vinegar that gives it its vine-
gary taste and smell. If a particular brand claims to be 5.00%,
by mass, acetic acid (HC
2
H
3
O
2
), how many milliliters of
0.255 M NaOH would be required to neutralize the acetic
acid in a 25.0 mL vinegar sample?
77. Stomach acid (HCl) is neutralized by a variety of commercial
antacids. For each of the following, determine how many
grams of active antacid ingredient would be necessary to
neutralize the HCl in 50.00 mL of 0.0100 M HCl (an approx-
imation of the concentration of HCl in the stomach).
a. Al(OH)
3
b. Mg(OH)
2
c. CaCO
3
78. Sulfuric acid is the acid ingredient in automobile battery acid
solutions. The sulfuric acid content of such a solution can be
determined through a lab analysis by reacting it in a titration
with potassium hydroxide. The unbalanced reaction is
H
2
SO
4
(aq) + KOH(aq) → K
2
SO
4
(aq) + H
2
O(l)
Use the balanced reaction to fill in the missing data in the
following table.
Molarity Volume Molarity Volume
of H
2
SO
4
of H
2
SO
4
of KOH KOH
(M) (mL) (M) (mL)
0.25 75.0 28.6
28.9 0.36 35.8
0.88 1.11 27.5
1.76 22.0 49.7
Section 4.7 Oxidation–Reduction Reactions
Skill Review
79. In a compound made up only of each of the following pairs
of elements, select the atom that is more likely to carry a neg-
ative or slightly negative charge.
a. C, H d. P, Fe
b. O, F e. Ca, O
c. Na, O
80. In a compound made up only of each of the following pairs
of elements, select the atom that is more likely to carry a neg-
ative or slightly negative charge.
a. K, H d. N, Ca
b. Li, F e. N, Mg
c. Na, S
81. Assign oxidation numbers to all the elements in each of the
following molecules or ions:
a. N
2
O
5
d. N
2
b. PO
4
3−
e. H
2
SO
3
c. CuCO
3
Carbonic acid

82. Assign oxidation numbers to all the elements in each of the
following molecules or ions:
a. CH
4
c. KHCO
3
e. KMnO
4
b. SO
4
2−
d. Na
2
Cr
2
O
7
Chemical Applications and Practices
83. The following balanced equation depicts a reaction that can
be used for the determination of iron in a steel sample. Is this
a redox reaction? Prove it by showing which elements change
oxidation state.
6Fe
2+
(aq) + 14H
+
(aq) + Cr
2
O
7
2−
(aq)
→ 6Fe
3+
(aq) + 2Cr
3+
(aq) + 7H
2
O(l)
84. The following reaction depicts one of the steps in obtaining
the important steel alloying ingredient titanium.
TiCl
4
+ 2Mg → Ti + 2MgCl
2
a. Identify the substance being oxidized.
b. Identify the substance being reduced.
Comprehensive Problems
85. Why is water called the universal solvent?
86. An interesting demonstration often used by chemistry teach-
ers utilizes several of the principles that you have been reading
about in this chapter. First, a solution of barium hydroxide is
shown to conduct electricity by having the electrodes from a
conductivity tester immersed in the solution and observing
that a light begins to shine. Then a solution of sulfuric acid is
slowly added.A white precipitate begins to form,and the light
bulb dims. Eventually, the addition of the sulfuric acid causes
the bulb to go out. Finally, continued addition of the sulfuric
acid solution brings the bulb back on to bright light.
a. What is the identity of the white precipitate?
b. What is the net ionic equation for the reaction between
barium hydroxide and sulfuric acid?
c. What is the significance of the point at which the bulb
goes out completely?
d. Explain why continued addition of the sulfuric acid
causes the light to come back on.
87. Some chemical reactions are best done in solution. If you had
100.0 mL of 0.230 M NaOH, how many milliliters of 0.530 M
HCl would you need to have the same number of moles as
found in the NaOH solution?
88. Extreme ozone pollution is described as any concentration
greater than 0.28 ppm ozone, C, O
3
. If the density of a sample
of air containing that concentration of ozone were 1.30 g/L,
what would be the molarity of ozone in the sample? How
many molecules of ozone would be in 1 L of the air?
89. Using Table 4.3, determine the identity and formula of any
and all precipitates that are likely to form when a solution
containing NaOH and (NH
4
)
2
CO
3
is mixed with a solution
that contains CuNO
3
and BaCl
2
.
90. a. An unlabeled solution may contain either Ag
+
ions or Al
3+
ions. Using Table 4.3, determine a suitable anion solution
that, through selective precipitation, could be added to
identify the cation present in the solution.
b. Another unlabeled solution contains either nitrate ions
or sulfate ions. Using Table 4.3, determine a solution that
166 Chapter 4 Solution Stoichiometry and Types of Reactions
contains a cation that could be used in a selective precipi-
tation to determine the identity of the anion in the unla-
beled solution.
91. Barium-containing “milkshakes” are often used to obtain
X-rays of patients suffering from intestinal problems. Bar-
ium compounds can also be toxic. Barium sulfate is insoluble
in water, so it can be given to patients without concern that it
would be absorbed. It is also opaque to X-rays. Write the
molecular and ionic balanced equations for the formation of
BaSO
4
(s) from Ba(NO
3
)
2
(aq) and Na
2
SO
4
(aq). How many
grams could be obtained from mixing 125.0 mL of 0.567 M
Na
2
SO
4
(aq) and 75.0 mL of 0.786 M Ba(NO
3
)
2
(aq)?
92. The reaction of gaseous dinitrogen trioxide with water can
provide aqueous nitrous acid as the product.
a. Write the balanced chemical reaction.
b. If 12.5 grams of dinitrogen trioxide treated with excess
water produced 12.5 grams of nitrous acid, what is the
percent yield of the reaction?
c. To make the nitrous acid as described in part b, the
dinitrogen trioxide was bubbled into 1.55 gallons of
water. Assuming that the amount of water remains
constant during the reaction, what is the concentration
of nitrous acid after the reaction?
93. A chef wishes to make a very lightly sweetened tea by dissolv-
ing 1.00 g fructose (C
6
H
12
O
6
) in 12 fluid ounces of tea.
a. What is the concentration of fructose in the tea in
molarity?
b. What is the concentration of fructose in ppm?
c. How many carbon atoms are there in 1.00 g of fructose?
d. How many water molecules are in the serving of tea?
Thinking Beyond the Calculation
94. Oxalic acid can be found in a variety of plants. This com-
pound is considered toxic. Therefore, it is often important
to determine the quantity in a sample. This can be done
through a redox titration.
5C
2
O
4
2−
(aq) + 16H
+
(aq) + 2MnO
4
−
(aq)
→ 2Mn
2+
(aq) + 10CO
2
(g) + 8H
2
O(l)
a. Judging on the basis of the
structure of oxalic acid shown
here, would you expect it to be
soluble or insoluble in water?
b. Write equations that illustrate the
dissolution and dissociation of
oxalic acid in water.
c. Which of the species in the bal-
anced redox reaction above is
more oxidized, oxalate (C
2
O
4
2−
)
or carbon dioxide?
d. Which of the species in the balanced redox reaction is
more oxidized, permanganate or manganese ions?
e. How many electrons are being transferred among the
reactants in the balanced redox reaction?
f. Determine which species is oxidized, and which is re-
duced, in the balanced redox reaction.
g. If a properly prepared plant sample required 33.5 mL of a
0.00976 M KMnO
4
solution to react, calculate the
number of grams of oxalic acid (H
2
C
2
O
4
) present.
Oxalic acid

167
Energy
The space shuttle lifts off from its
launch pad in Florida. The tremen-
dous amount of energy released by
the chemical reactions in the solid
rocket boosters and main engine
results in a force strong enough to
oppose the force of gravity acting
on the shuttle.
167
Contents and Selected Applications
Chemical Encounters: Setting the Stage with the Space Shuttle
5.1 The Concept of Energy
5.2 Keeping Track of Energy
Chemical Encounters: Energy in Foods
5.3 Specific Heat Capacity and Heat Capacity
5.4 Enthalpy
5.5 Hess’s Law
Chemical Encounters: Focus on Methane
5.6 Energy Choices
Chemical Encounters: Energy Choices
Go to college.hmco.com/pic/kelterMEE for online learning resources.
