Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


188 Chapter 5 Energy
Water as gas, liquid,
and solid.
+
This is an exothermic reaction.
Many different standard enthalpies of reactions have been defined. Common
ones include the standard enthalpy of formation and the standard enthalpy of
combustion.The
standard enthalpy of formation,
f
H° (also known as the standard
heat of formation
) of a substance is the enthalpy change for the formation of
1 mole of the substance in its standard state from its elements in their reference
forms. For example, the standard enthalpy of formation of carbon dioxide from
carbon and oxygen in their reference forms, which for carbon is graphite, can be
summarized as follows:
C(s) + O
2
(g) → CO
2
(g)
f
H° =−394 kJ/mol
We have to show just 1 mole of the product, because that value is embodied in the
definition. This can force us to use fractional amounts of moles of some of the
reactants, as in the next example, which indicates the standard enthalpy of for-
mation of water:
H
2
(g) +
1
2
O
2
(g) → H
2
O(l)
f
H° =−286 kJ/mol
We must show the oxygen in its standard state as O
2
(g). We cannot simply use O
(because O is not the reference form of oxygen), so we must indicate half a mole
of O
2
. Keep in mind that when you are writing standard enthalpy equations,
it is acceptable to leave equations with fractional stoichiometric values, rather
than multiplying by some common factor to convert all the fractions to whole
numbers.
EXERCISE 5.6 To the Moon
The combustion of gaseous hydrazine (N
2
H
4
) is a reaction that provides thrust for
rockets. This reaction is also used on the space shuttle for minor attitude changes
when the shuttle is in orbit. Write the equation that describes the standard enthalpy
of formation for this compound. Given that for hydrazine
f
H° =+95.40 kJ/mol,
calculate the change in enthalpy accompanying the formation of 125 g of hydrazine
from its elements in their reference forms.
First Thoughts
The equation that we prepare needs to describe the formation of just 1 mole of hy-
drazine. Because hydrazine contains nitrogen and hydrogen, we can show its for-
mation from those elements in their reference forms. We can then convert the num-
ber of grams into moles and use that value to determine the enthalpy for the
reaction.

5.4 Enthalpy 189
Solution
The equation for the formation of gaseous hydrazine from its elements in their ref-
erence forms is
The number of moles of hydrazine can then be used to calculate the enthalpy.
125 g N
2
H
4
×
1 mol N
2
H
4
32.05 g N
2
H
4
= 3.90 mol N
2
H
4
3.90 mol N
2
H
4
×
95.40 kJ
1 mol N
2
H
4
= 372 kJ
Further Insights
The amounts of materials that we use in reactions determine the change in en-
thalpy of the reaction. Conversely, the enthalpy change in a reaction can be used to
determine the amount of material consumed or produced in the reaction.
PRACTICE 5.6
Calculate the enthalpy change for the formation of 38.0 g of water. (Use the appen-
dix to find the standard enthalpy change for water.)
See Problems 47–50.
Elements in their reference forms are the basic starting materials for all the re-
actions associated with standard enthalpies of formation. The elements themselves
are not formed from any simpler chemicals, so the standard enthalpies of formation
of elements in their reference forms are all equal to zero. This is to say that, using
calcium as an example,
Ca(s) → Ca(s)
f
H° = 0 kJ/mol
Note that
f
H° = 0 kJ/mol does not mean that U = 0 kJ/mol. Also, recall that
only one form of an element is its reference form. For example, O
2
(g) is the refer-
ence form for oxygen. When O
2
forms the allotrope ozone, O
3
, in the atmosphere,
f
H° is not equal to 0 kJ/mol.
3
2
O
2
(g) → O
3
(g)
f
H° = 143 kJ/mol
The standard enthalpy of combustion,
c
H° (also known as the standard heat of
combustion
) of a substance is the enthalpy change when 1 mole of the substance
in its standard state is completely burned in oxygen gas. For example, consider
the combustion of propane gas (C
3
H
8
), which we first discussed in Exercise 5.5.
C
3
H
8
(g) + 5O
2
(g) → 3CO
2
(g) + 4H
2
O(l)
c
H° =−2202 kJ/mol
2H
2
(g) + N
2
(g)N
2
H
4
(g)
+
→
++
When we write the equation to illustrate the standard enthalpy of combustion,
we must show exactly 1 mole of the reactant, propane, being burned. The coeffi-
cients must be adjusted to fit that requirement, even as the mole ratios of all reac-
tants and products necessarily remain the same. Consider, as well, the equation
In some cases, we will find it useful to multiply all of the equation coefficients
by some number. Let’s see what happens when we multiply the coefficients in the
“combustion of carbon” equation by 2:
C(s) + O
2
(g) → CO
2
(g) + 394 kJ/mol
2C(s) + 2O
2
(g) → 2CO
2
(g) + 2 × (394 kJ/mol)
Note that although the mole ratios of the reactants and products remain the
same, the enthalpy change is affected by this multiplication. Remembering that
enthalpy as a product indicates a negative change in enthalpy, we note that H of
this reaction is now (2
×−394 kJ/mol) =−788 kJ/mol.
This ability to reverse equations and automatically know the corresponding
enthalpy change is a consequence of what we know as Hess’s law, which is the
subject of the next section. It enables us to determine the enthalpy changes of
reactions that might be very difficult to actually perform.
EXERCISE 5.7 How Much Heat?
A camper wishes to warm a pot of water on a propane stove. She calculates that
she’ll need to produce 209 kJ to increase the temperature of her pot of water by
50°C. How much propane, in moles, will be consumed to produce this much heat?
C
3
H
8
(g) + 5O
2
(g) → 3CO
2
(g) + 4H
2
O(l)
c
H° =−2202 kJ/mol
190 Chapter 5 Energy
FIGURE 5.18
Negative enthalpy changes can be
thought of as adding enthalpy to the
product side of the equation. Reversing
the direction of the equation changes
the sign of the enthalpy change.
C(s) + O
2
(g) → CO
2
(g) + 394 kJ/mol
c
H° =−394 kJ/mol
394 kJ/mol + CO
2
(g) → C(s) + O
2
(g) H =+394 kJ/mol
for the standard enthalpy of combustion of carbon. Note that the equation is the
same as the equation for the standard enthalpy of formation of carbon dioxide.
C(s) + O
2
(g) → CO
2
(g)
c
H° =−394 kJ/mol =
f
H°(CO
2
)
Manipulating Enthalpies
When we know the value for any standard enthalpy change of a reaction, we also
automatically know the value for the enthalpy change of the reverse reaction. For
example, converting carbon dioxide gas back into solid carbon and oxygen gas
would be the reverse of the forward reaction, the combustion of carbon to form
CO
2
. It should therefore be accompanied by an enthalpy change that is equal to
that of the forward reaction but opposite in sign, as shown in Figure 5.18. To help
us understand what the signs on the enthalpy terms mean, we can consider this
manipulation as though the enthalpy change were part of the equation. Let’s
examine the following equations and see how this applies to the end result. The
enthalpy of combustion is negative, so it must be released from the reaction and,
hence, appear on the same side as the products in this exothermic process.
C(s) + O
2
(g) → CO
2
(g) + 394 kJ/mol
The reverse of this reaction is
CO
2
(g) + 394 kJ/mol → C(s) + O
2
(g)
which shows that the enthalpy is gained by the reaction.
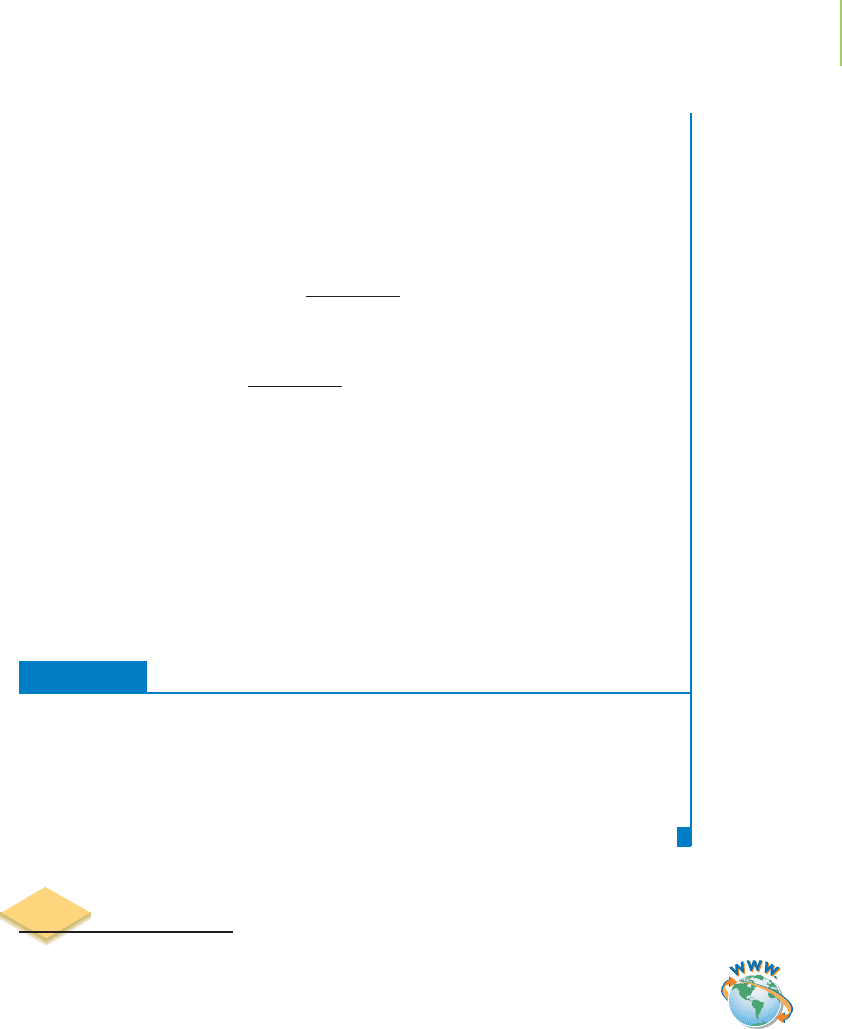
First Thoughts
We must incorporate the enthalpy of combustion into the equation and determine
the stoichiometric relationship between moles of propane (C
3
H
8
(g)) and the
change in enthalpy. Accordingly, we can write
1 mol C
3
H
8
(g) =−2202 kJ
or
1 mol C
3
H
8
2202 kJ
Solution
209 kJ ×
1 mol C
3
H
8
2202 kJ
= 0.0949 mol C
3
H
8
(g )
Note that we drop the negative sign from the enthalpy change because the amount
of heat released from the system is the amount of heat needed to warm the water.
Further Insights
The thermodynamic sign conventions can be a little misleading and confusing at
first. In the present example, the quantity of enthalpy released (209 kJ) by the com-
bustion reaction (the system) is equal to the quantity of enthalpy gained by the sur-
roundings (209 kJ), assumed here to be the pot of water, so although the quantities
are equal, the signs are opposite: H
system
=−209 kJ; H
surround
=+209 kJ.
PRACTICE 5.7
Calculate how many moles of the compound printed in boldface type will be
needed to produce a change in enthalpy of 250 kJ for each of these reactions.
a. 2NH
3
(g) + 3N
2
O(g) → 4N
2
(g) + 3H
2
O(l) H =−1012 kJ
b. 2N
2
O(g) → O
2
(g) + 2N
2
(g) H =−164 kJ
See Problems 34, 35, 71b, 71c, 72b, 72c, 76, and 99b.
5.5 Hess’s Law
The search for alternative fuel sources to supplement what we currently use is a
high priority in all fields of transportation, including commercial aviation, per-
sonal automobile use, and even rocketry. The search for such fuels could involve
the examination of lots of new reactions, some of which might never have been
performed before. Fortunately, one of the features of enthalpy change values
works to our benefit.We can calculate the energy or enthalpy changes of reactions
without having to perform them. The reason for this ease of calculation lies in the
fact that energy (U) and enthalpy (H) are state functions. A
state function is a
property of a system that depends only on its present state, not on how it got there.
State functions are usually represented by an italic capital letter.
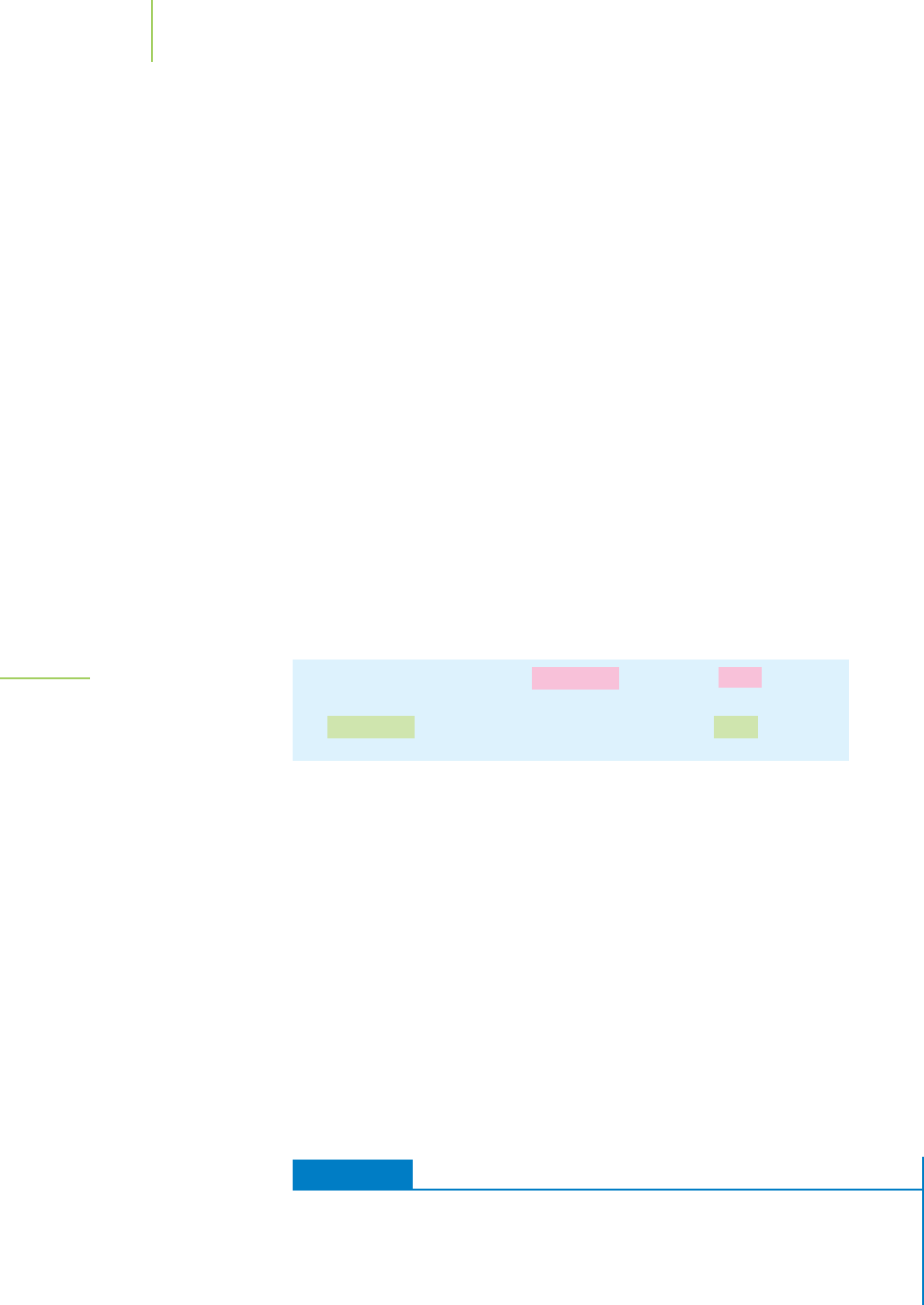
For an illustration of this idea, consider the different paths that the space
shuttle might take to reach the international space station, orbiting at an altitude
of 240 miles above sea level, as graphically shown in Figure 5.19. One shuttle mis-
sion may be launched directly up to a 240-mile-high orbit, ready to rendezvous
with the space station. A later shuttle mission might first go into a higher orbit to
release a satellite and then come back down to the 240-mile-high orbit of the
space station. The eventual altitude of each shuttle is the same: 240 miles above
sea level. In short, the final altitude is independent of the path taken. The dis-
tances traveled by the two shuttles in reaching their final altitude are very
different, however, because one took the direct route and the other took a more
circuitous route. Altitude is a state function; distance traveled is not.
5.5 Hess’s Law 191
Video Lesson: Hess’s Law
Video Lesson: Enthalpies of
Formation
Tutorial: Hess’s Law
192 Chapter 5 Energy
Higher
orbit
route
Direct
route
FIGURE 5.19
Two shuttles visiting the International
Space Station will end up at the same al-
titude, regardless of the route taken. In
this example, altitude is a state function;
its value depends only on the initial and
final states, not on the route taken.
A
A
C then:
B
B
CC
(b)
(a)
∆H
2
∆H
1
AC
B
∆H
2
∆H
1
∆H
3
B
FIGURE 5.20
A diagrammatic summary of Hess’s law. (a) If the distance down the
mountain from A to B and the distance up to the top from A to C are
known, the distance from B to C can be determined. (b) The enthalpy
change for a series of reactions is analogous to this. If the enthalpy of
reaction from A to B and from A to C is known, the enthalpy of reac-
tion from B to C can be calculated.
Products
Progress of reaction
Energy
Reactants
100 J
Forward
reaction
releases
100 J
Reverse
reaction
absorbs
100 J
FIGURE 5.21
A forward reaction has an enthalpy that is equal, but op-
posite in sign, to that of its reverse reaction.
The change in the value of any state function depends only on the initial and
final states between which it is changing. In the case of enthalpy,
H = H
final
− H
initial
If a chemical reaction is carried out by two different chemical paths, the overall
enthalpy change associated with each path will be the same. That basic principle
is known as
Hess’s law and is summarized diagrammatically in Figure 5.20.
Enthalpy is a state function, which also justifies our earlier statement that the
enthalpy change for any reaction will have the same value as the enthalpy change
for the reverse reaction but will be opposite in sign. If the forward reaction has an
enthalpy change of
−100 joules, the reverse reaction will have an enthalpy change
of
+100 joules. This is summarized diagrammatically in Figure 5.21.
Visualization: Hess’s Law
Using Hess’s Law
On the Salt River-Pima Maricopa Indian Community in Arizona, methane (CH
4
)
collected from a 10-million-ton garbage landfill is used to fuel a“green” (environ-
mentally benign) power plant that provides electricity for 2000 homes. Methane
is a chemically very simple and convenient fuel, arising from the biological degra-
dation of foodstuffs and other wastes in the landfill. It also makes up about 95%
of the “natural gas” found above oil deposits, is burned to provide energy for
domestic cooking and heating, and is as a source of heat in many industries.
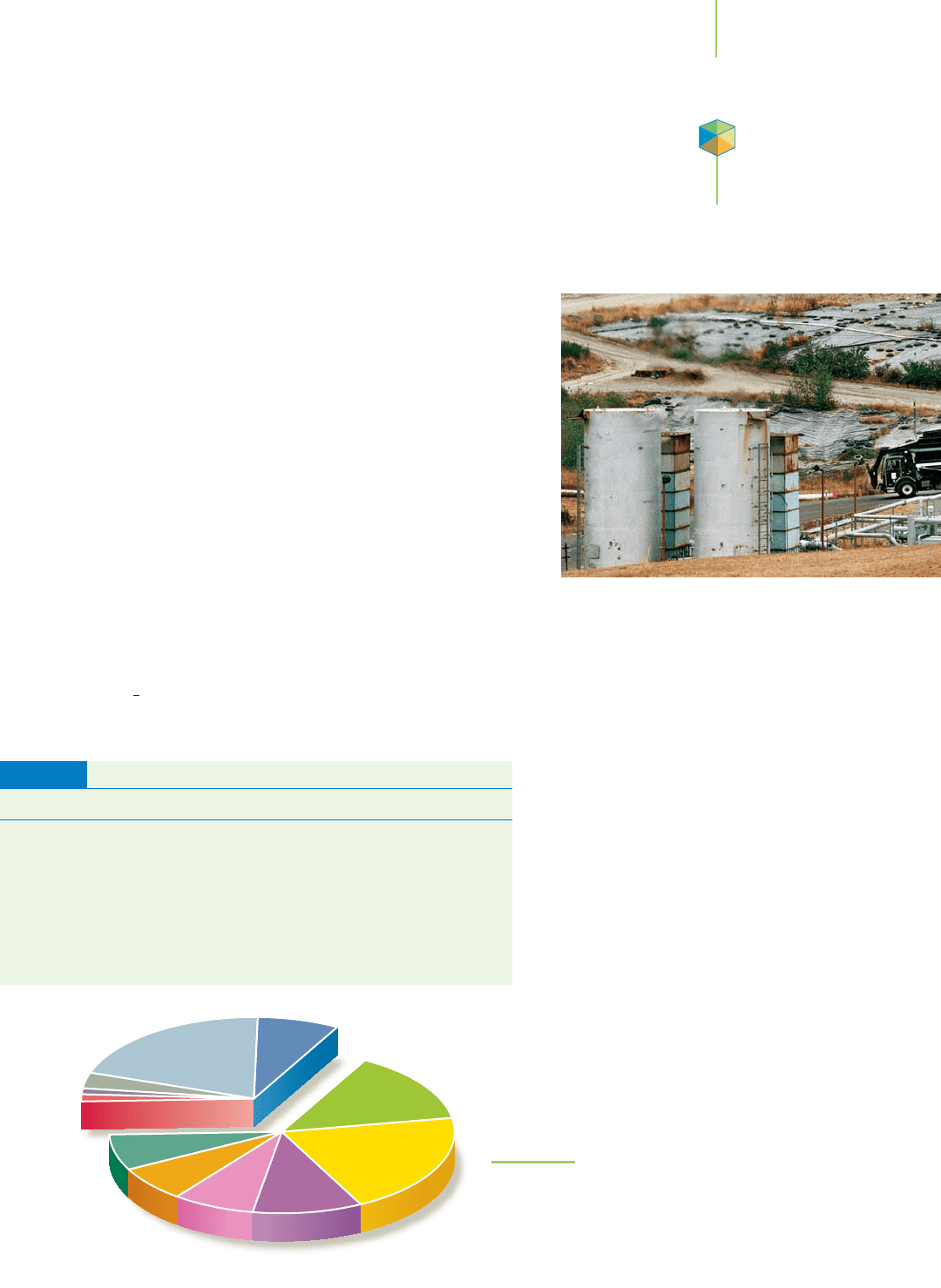
Table 5.3 and Figure 5.22 list the important sources of methane emis-
sions into the atmosphere. The data in the table suggest that it is not pos-
sible to accurately quantify the production of a gas that is emitted every
day from so many sources. The numbers should be seen as rough projec-
tions of quantities that cannot be measured with great certainty. Nonethe-
less, harvesting some of this methane, as at the Salt River Project in Ari-
zona, could represent an important addition to the world’s energy supply.
Although methane found in natural gas is formed in a variety of dif-
ferent biological and chemical ways, it can be described by the following
chemical equation:
C(s) + 2H
2
(g) → CH
4
(g)
If we use Hess’s law, we can calculate the standard enthalpy change for this
reaction without actually performing the reaction. To complete the calcu-
lation, we need to know the standard enthalpy changes of other reactions
that will allow us to consider forming methane indirectly. We use reference
tables to obtain these reactions and their corresponding standard en-
thalpies. These values are
C(s) + O
2
(g) → CO
2
(g) H° =−394 kJ/mol
H
2
(g) +
1
2
O
2
(g) → H
2
O(l) H° =−286 kJ/mol
CH
4
(g) + 2O
2
(g) → CO
2
(g) + 2H
2
O(l) H° =−890 kJ/mol
5.5 Hess’s Law 193
Projected U.S. Methane Emissions in the Year 2020
Source Teragrams (1 Tg = 10
12
g) of CH
4
Landfills 7.0
Coal mining 5.5
Natural gas/oil drilling 7.0
Livestock manure 4.5
Livestock digestion releases (“enteric fermentation”) 6.5
Other 0.5
TOTAL 31.0
TABLE 5.3
Methane gas can be harvested from landfills.
Application
C
HEMICAL
ENCOUNTERS:
Focus on Methane
Natural
occurrences
Anthropogenic
influences
Ruminants
Termites
Wetlands
Ocean water
Fresh water
Rice paddies
Biomass
burning
Landfills
Coal
mining
Gas
production
Methane
hydrate
FIGURE 5.22
Major sources of atmospheric methane as reported by the
U.S. Department of Energy. Sources can be broken down into
those that occur naturally and those that occur through an-
thropogenic influences (that is, through human activity).
194 Chapter 5 Energy
890 kJ/mol
CH
4
(g) + 2O
2
(g)
–76 kJ/mol
–394 kJ/mol
–286 kJ/mol
–286 kJ/mol
C(s) + O
2
(g)
H
2
(g) + O
2
(g)
H
2
O(l )
H
2
O(l )
H
2
(g) + O
2
(g)
CO
2
(g)
1
–
2
1
–
2
FIGURE 5.23
A diagrammatic representation of how
Hess’s law can be used to calculate the
enthalpy of formation of methane.
We arrange these reactions in such a way that they allow us to calculate the enthalpy
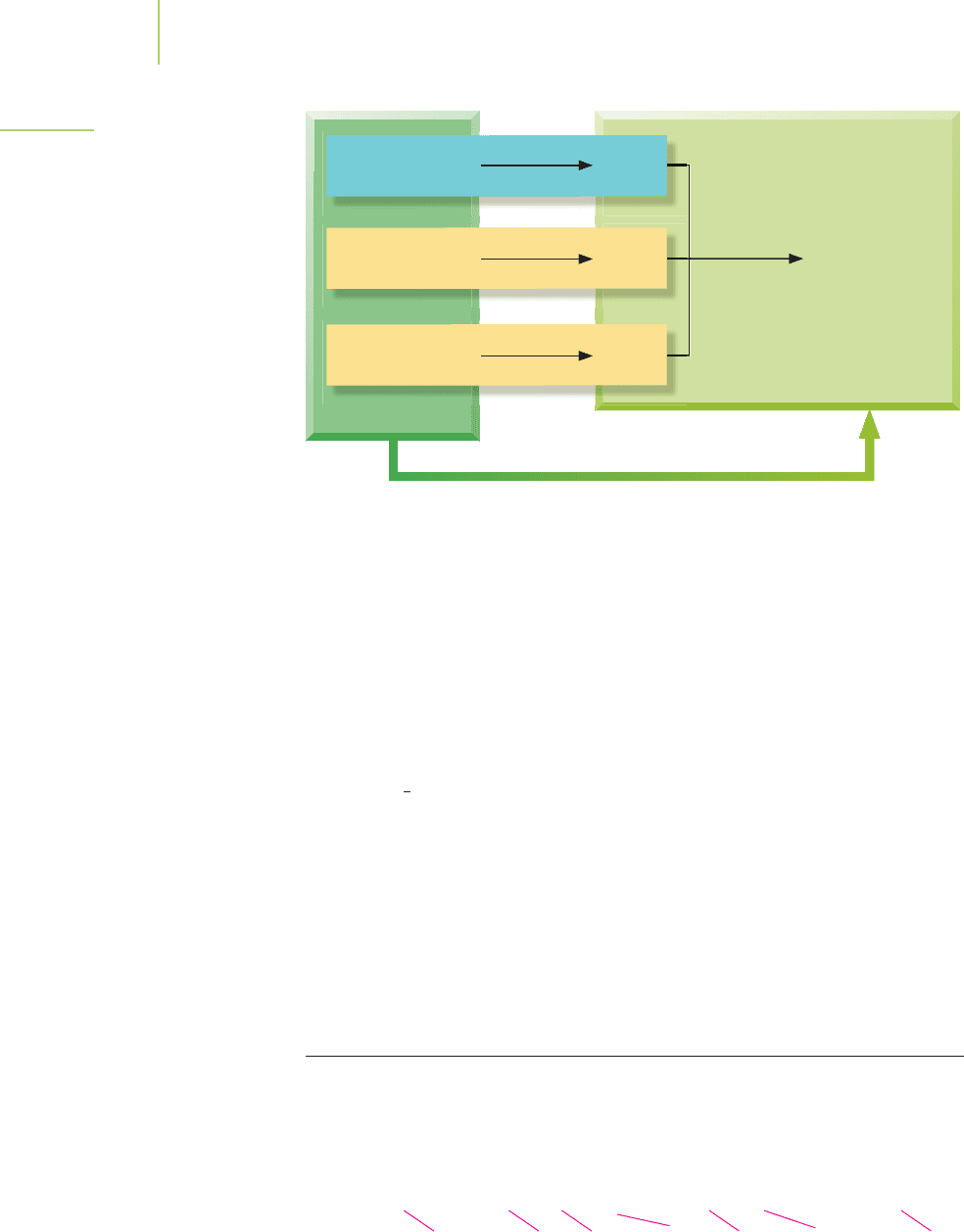
of formation of methane. This is shown diagrammatically in Figure 5.23. Alterna-
tively, we can rearrange the chemical reactions to mathematically arrive at the
same answer. The underlying logic to Hess’s law calculations is that we can mul-
tiply, and/or reverse, and then combine any chemical equations in whatever way
will achieve the overall chemical change in which we are interested. For example,
to calculate the enthalpy of formation of methane, we can manipulate the three
combustion equations as follows:
■
C(s) + O
2
(g) → CO
2
(g) H° =−394 kJ/mol
■
2 × [H
2
(g) +
1
2
O
2
(g) → H
2
O(l)] 2 × [H° =−286 kJ/mol]
= 2H
2
(g) + O
2
(g) → 2H
2
O(l) =−572 kJ
■
−1 × [CH
4
(g) + 2O
2
(g) → CO
2
(g) + 2H
2
O(l)] −1 × [H° =−890 kJ/mol]
= CO
2
(g) + 2H
2
O(l) → CH
4
(g) + 2O
2
(g) =+890 kJ
Adding the three equations together, we calculate the overall enthalpy change:
C(s) + O
2
(g) → CO
2
(g) H° =−394 kJ
2H
2
(g) + O
2
(g) → 2H
2
O(l) H° =−572 kJ
CO
2
(g) + 2H
2
O(l) → CH
4
(g) + 2O
2
(g) H° =+890 kJ
C + O
2
+ 2H
2
+ O
2
+ CO
2
+ 2H
2
O → CO
2
+ 2H
2
O + CH
4
+ 2O
2
H° =−76 kJ
When we cancel the chemicals that appear in equal amounts on both sides of the
equation (O
2
,CO
2
,H
2
O), this simplifies to the equation for the formation of
methane:
C + O
2
+ 2H
2
+ O
2
+ CO
2
+ 2H
2
O → CO
2
+ 2H
2
O + CH
4
+ 2O
2
C(s) + 2H
2
(g) → CH
4
(g)
H° =−76 kJ/mol
Whichever way we decide to calculate the change in enthalpy for the reaction
of interest, we should arrive at the same correct answer. In either case, we don’t
have to perform the final reaction to be able to calculate its enthalpy change.
EXERCISE 5.8 Hess’s Law
Given the following data, calculate the value of H for the reaction shown below.
P
4
(s) + 10Cl
2
(g) → 4PCl
5
(g) H =−2139 kJ
PCl
3
(g) + Cl
2
(g) → PCl
5
(g) H =−155 kJ
P
4
(s) + 6Cl
2
(g) → 4PCl
3
(g) H = ?? kJ
Solution
1 × [P
4
(s) + 10 Cl
2
(g) → 4PCl
5
(g)] 1 × H =−2139 kJ
−4 × [PCl
3
(g) + Cl
2
(g) → PCl
5
(g)] −4 × H =+620 kJ
P
4
(s) + 6Cl
2
(g) → 4PCl
3
(g) H =−2139 kJ + 620 kJ =−1519 kJ
PRACTICE 5.8
Given the following data, calculate the value of H for the reaction shown below.
6Fe(s) + 4O
2
(g) → 2Fe
3
O
4
(s) H =−1787 kJ
2Fe
3
O
4
(s) +
1
2
O
2
(g) → 3Fe
2
O
3
(s) H =−186 kJ
3Fe
2
O
3
(s) → 6Fe(s) +
9
2
O
2
(g) H = ?? kJ
See Problems 75–78, 81, and 82.
Reaction Enthalpies from Enthalpies of Formation
The energy needed to maneuver the space shuttle when it is in orbit is provided
by the reaction between methylhydrazine (CH
3
NHNH
2
) and dinitrogen tetrox-
ide (N
2
O
4
):
4CH
3
NHNH
2
(l) + 5N
2
O
4
(l) → 4CO
2
(g) + 9N
2
(g) + 12H
2
O(l)
One of the requirements for a chemical reaction that can be used as part of an
effective propulsion system is that it have a large negative H value, indicating
that it releases a lot of energy. How can we determine the value of H for this
reaction?
One answer, based on our earlier discussion, is to perform the reaction in a
calorimeter—something that we may not wish to do, especially if we are studying
rocket fuel. Alternatively, we could use Hess’s law to calculate the enthalpy of the
reaction. However, it is often difficult to find all of the enthalpies that are needed
to complete the circuitous route from reactants to products. Instead, there is an-
other way to determine the enthalpy of the reaction. We can calculate H of the
reaction by means of a useful general rule about enthalpies of formation if other
appropriate values of H are known:
The standard enthalpy change for a reaction can be calculated by subtracting
the sum of the enthalpies of formation of the reactants from the sum of the
enthalpies of formation of the products.
This can be expressed mathematically as follows:
H° reaction =
n
p
f
H
◦
(products) −
n
r
f
H
◦
(reactants)
where the Greek symbol
(sigma) means “the sum of the following terms.”
n
p
= the number of moles of each product in the reaction equation
n
r
= the number of moles of each reactant in the reaction equation
5.5 Hess’s Law 195
Methylhydrazine
196 Chapter 5 Energy
4CH
3
NHNH
2
(l)
+
4C(s)
4N
2
(g)
12H
2
(g)
5N
2
(g)
4CO
2
(g)
9N
2
(g)
12H
2
O(l)
10O
2
(g)
5N
2
O
4
(l)
∆H = 4 × (–55 kJ/mol)
= –220 kJ/mol
∆H = 4 × (–394 kJ/mol)
= –1576 kJ/mol
∆H = 12 × (–286 kJ/mol)
= –3432 kJ/mol
∆H = 0.0 kJ/mol
∆H = 5 × (20.0 kJ/mol)
= 100 kJ/mol
∆H = –220 kJ/mol + 100 kJ/mol
= –120 kJ/mol
∆H = –120 kJ/mol + –5008 kJ/mol
= –5128 kJ/mol
Reactants to elements
∆H = –1576 kJ/mol + 0.0 kJ/mol + –3432 kJ/mol
= –5008 kJ/mol
Elements to products
Reactants to products
FIGURE 5.24
We can consider performing a reaction
by taking the indirect route, via the ele-
ments in their reference forms. Then, by
Hess’s law, the reactions shown here can
allow us to determine the enthalpy of
the reaction.
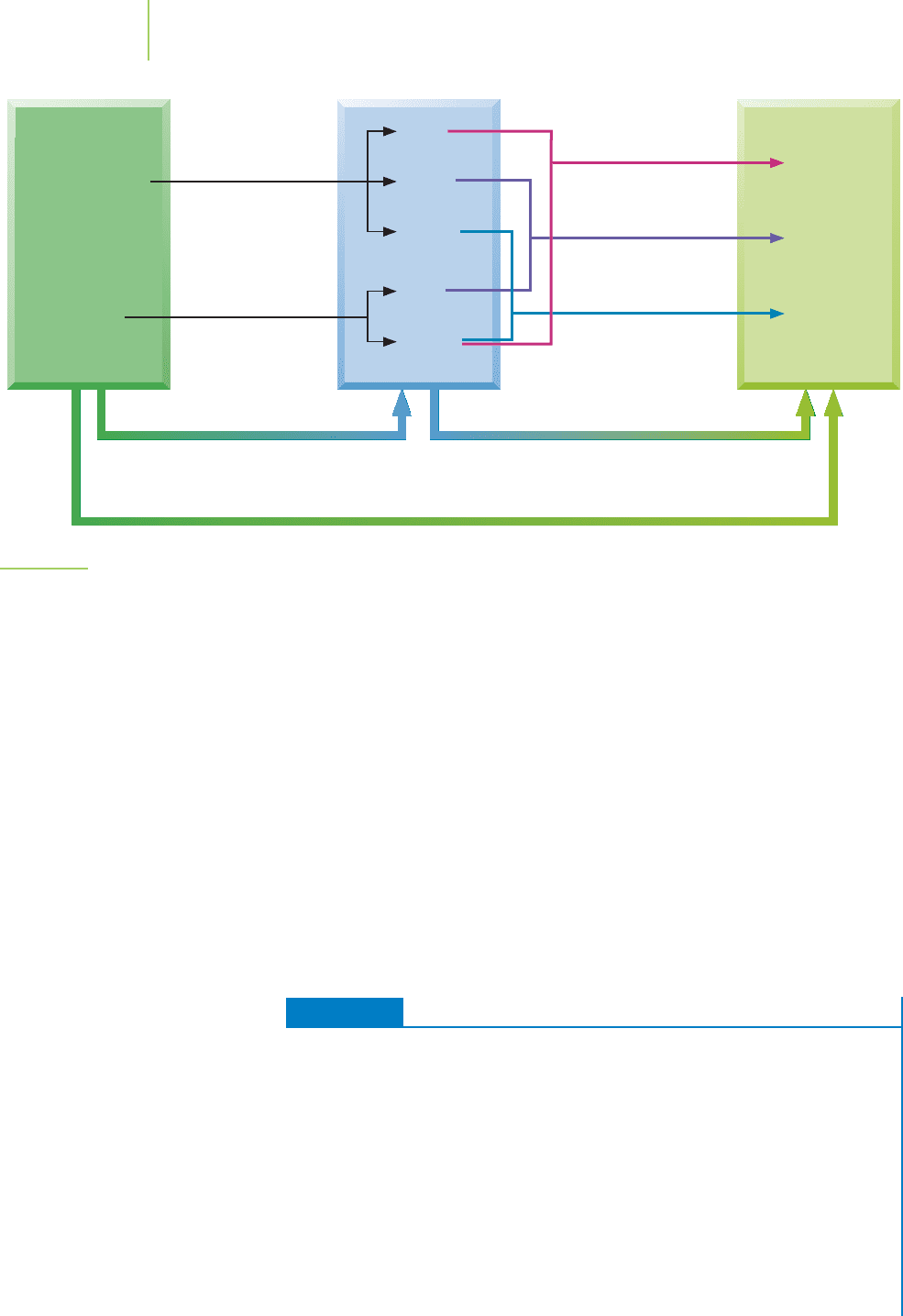
Figure 5.24 clarifies why this procedure works.
f
H°(CO
2
(g)) =−394 kJ/mol
f
H°(N
2
(g)) = 0.0 kJ/mol
f
H°(H
2
O(l)) =−286 kJ/mol
n
p
f
H
◦
(products) = [4 ×−394] + [9 × 0.0] + [12 ×−286] =−5008 kJ
f
H°(CH
3
NHNH
2
(l)) =+55 kJ/mol
f
H°(N
2
O
4
(g)) =−20.0 kJ/mol
n
r
f
H°(reactants) = [4 ×+55] + [5 ×−20.0] =+120 kJ
H°reaction =
n
p
f
H
◦
(products) –
n
r
f
H
◦
(reactants)
= (−5008 kJ) − (120 kJ) =−5128 kJ
We find, as expected, that this reaction used to provide propulsion to maneuver
the space shuttle in orbit has a relatively large negative H value equal to
−5128 kJ/mol.
EXERCISE 5.9 Thermite in Space
One reaction that may prove useful for the welding and brazing that are necessary
when building platforms in space is the “thermite reaction” between aluminum and
iron oxide:
2Al(s) + Fe
2
O
3
(s) → 2Fe(s) +Al
2
O
3
(s)
Calculate the standard enthalpy change of this reaction, given the following
enthalpies of formation:
f
H°(Fe
2
O
3
) =−824 kJ/mol;
f
H°(Al
2
O
3
) =−1676 kJ/mol
First Thoughts
Why don’t reference tables provide heat of formation values for aluminum and
iron? They are in their reference forms, and by definition, their heat of formation is
zero. This means that the enthalpy change of the reaction (remember, it’s a state
The thermite reaction in action. This
photo shows Austrian railroad workers
in 1910 using the thermite reaction.
The molten iron produced by the reaction
drips between the sections of rail and
welds the track together.
function!) is equal to the heat of formation of the aluminum oxide (Al
2
O
3
) minus
the heat of formation of the iron oxide (Fe
2
O
3
).
Solution
H°reaction =
n
p
f
H
◦
(products) − n
r
f
H°(reactants)
H°reaction = (−1676 + 0) − (−824 + 0) =−852 kJ/mol
Further Insights
In “manned” space flight, many other, less dramatic reactions occur some exother-
mic and some endothermic. In particular, the reaction of molecular hydrogen and
oxygen gases on board the space shuttle to form water and usable electricity is
exothermic, whereas many of the scientific experiments, such as growing plants,
require the input of energy, rather than its release, to run.
PRACTICE 5.9
Calculate the enthalpy change for the reaction
4NH
3
(g) + 3O
2
(g) → 2N
2
(g) + 6H
2
O(l)
given the following reactions and H values:
a. 2NH
3
(g) + 3N
2
O(g) → 4N
2
(g) + 3H
2
O(l) H =−1012 kJ
b. 2N
2
O(g) → O
2
(g) + 2N
2
(g) H =−164 kJ
See Problems 75–78, 81, and 82.
5.6 Energy Choices
Humans have always had a variety of energy sources to choose from, and choices
based on energy issues have always been important. In ancient times, people could
keep warm by burning wood, moving south to sunnier climes, or minimizing
energy losses from their bodies by wrapping themselves in animal skins and furs.
Ancient people also learned to make use of wind energy to power great journeys by
boat acrossseas and oceans.Nowadays,the energychoicesfacing us are much more
complex,and the possible effects of making unwise choices are much greater.
The development of modern civilization was powered largely by burning
things to release heat. This heat could keep people warm and could also be used
to boil water, generating the steam to power steam engines and steam turbines.
The engines and turbines were used to power machinery, trains, and ships and
eventually to make electricity. The greatest leaps forward in technol-
ogy came with the discovery of the fossil fuels—coal, oil, and natural
gas—that could be harvested from the Earth in seemingly limitless
quantities and burned with few obvious disadvantages, apart from the
annoyance of a bit of smoke and the loss of lives in the process of
extracting these natural resources. Today, the burning of fossil fuels
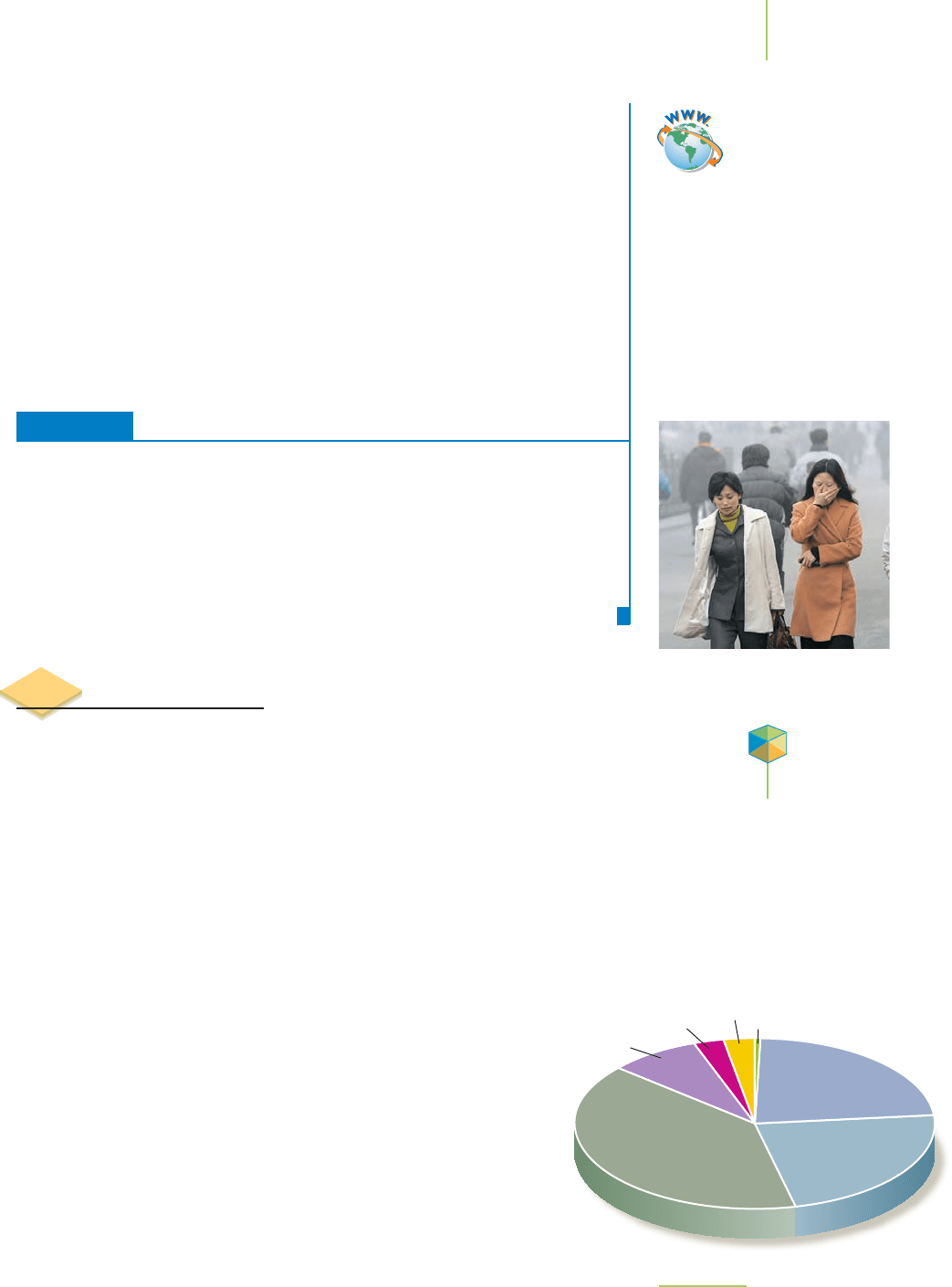
provides about 70% of the energy needed to sustain a complex in-
dustrialized country such as the United States, as shown in Figure
5.25. However, we now know that the supplies of fossil fuels are not
limitless and that burning them to provide energy also generates
problems. The most significant problem might be the apparent
warming of the planet, the “global warming” effect, caused in part by
the carbon dioxide gas that is a by-product of burning these fuels.
Because fossil reserves are finite, the burning of these fuels causes
environmental problems, and their processing and distribution have
had a significant impact on the world’s economic and political
5.6 Energy Choices 197
Application
C
HEMICAL ENCOUNTERS:
Energy Choices
Natural gas,
22.9%
Coal,
22.9%
Geothermal,
solar, wind,
0.5%
Alcohol,
2.8%
Hydropower,
2.7%
Nuclear,
8.2%
Oil,
39.9%
FIGURE 5.25
U.S. Energy use in 2004.
Smog is a serious urban pollutant in
almost every major city in the world.
Video Lesson: CIA
Demonstration: The Thermite
Reaction
Visualization: Thermite Reaction
