Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


198 Chapter 5 Energy
landscape. The alternative strategy of harnessing energy from nuclear processes
was eagerly adopted in many countries in the latter half of the twentieth century.
The source of this energy will be discussed fully in Chapter 21.
One of the most attractive options is to make increasing use of
renewable
sources of energy
, which can be rapidly replaced by natural processes. This is in
contrast to nonrenewable sources, particularly the fossil fuels: coal, oil, and
natural gas. Staff at the U.S. National Renewable Energy Laboratory in Colorado
have studied the feasibility of using renewable energy sources to meet all of
America’s energy needs. The United States is the largest single user of energy in
the world, accounting for approximately 50% of global consumption. A feasible
plan to meet all or most of U.S. energy needs with renewable sources of energy
would encourage countries around the world to make a similar commitment.
According to the Renewable Energy Laboratory, the main renewable energy
sources that could meet the needs of the United States are
■
Solar cells (also known as photovoltaic systems, or PVs), which convert the
energy of sunlight into electricity.
■
Solar thermal systems, which covert the energy of sunlight into heat.
■
Biomass conversion, which releases energy from the chemical conversion—
often the burning—of plants and trees that can be grown as quickly as they
are consumed.
The Hoover Dam harnesses water to gen-
erate electricity.
Windmills can convert wind into electricity.
Solar energy can be used to make electricity.

■
Hydroelectric systems, which use the power of falling water is used to turn
turbines that generate electricity.
■
Wind power, which uses turbines driven by the force of the wind to generate
electricity.
■
Geothermal systems, which involve drilling deep into the earth and exploiting
the flow of heat from the interior of the earth out toward the surface.
Each of these systems could readily make a significant contribution to power
generation in the United States. They already contribute 8%, as shown in Fig-
ure 5.25, but that could rise toward 50% or even 100% if social and political in-
centives were in place. One of the most interesting points made in the Renewable
Energy Laboratory’s report is summarized in Figure 5.26, which shows the total
area that would have to be devoted to solar cells sufficient to generate all the en-
ergy required by the United States. It is a large area, equivalent to more than 10%
of the area of the state of Nevada, but that is less than 25% of the area of the
country that is currently paved over with roads and streets. So equipped, the
United States could garner enough energy from the sun to meet all its needs.
Solar energy arrives free of charge, although providing the technology to cap-
ture, distribute, and use it entails significant costs. The energy is also very “clean”
in the sense that its use does not release anywhere near the amount of pollutants
released by the burning of fossil fuels. Some pollution would inevitably be asso-
ciated with the manufacturing processes and other activities involved in making
and maintaining the solar cells, but the overall effect of a switch to solar is likely
to be a huge reduction in pollution. One of the main challenges of the future
might be to develop the chemical systems needed to make full use of the free en-
ergy that floods down on the planet every day from the Sun.
When the possible contributions from biomass, hydroelectric, wind, and
geothermal power are added to the equation, it all amounts to a persuasive argu-
ment for continuing to explore the use of “renewables”as an energy source for the
future.
5.6 Energy Choices 199
FIGURE 5.26
Total area required for a solar cell power
plant to meet the total U.S. annual elec-
trical power demand is represented by
the square on this map.
Photovoltaic cells can be used to power almost anything.
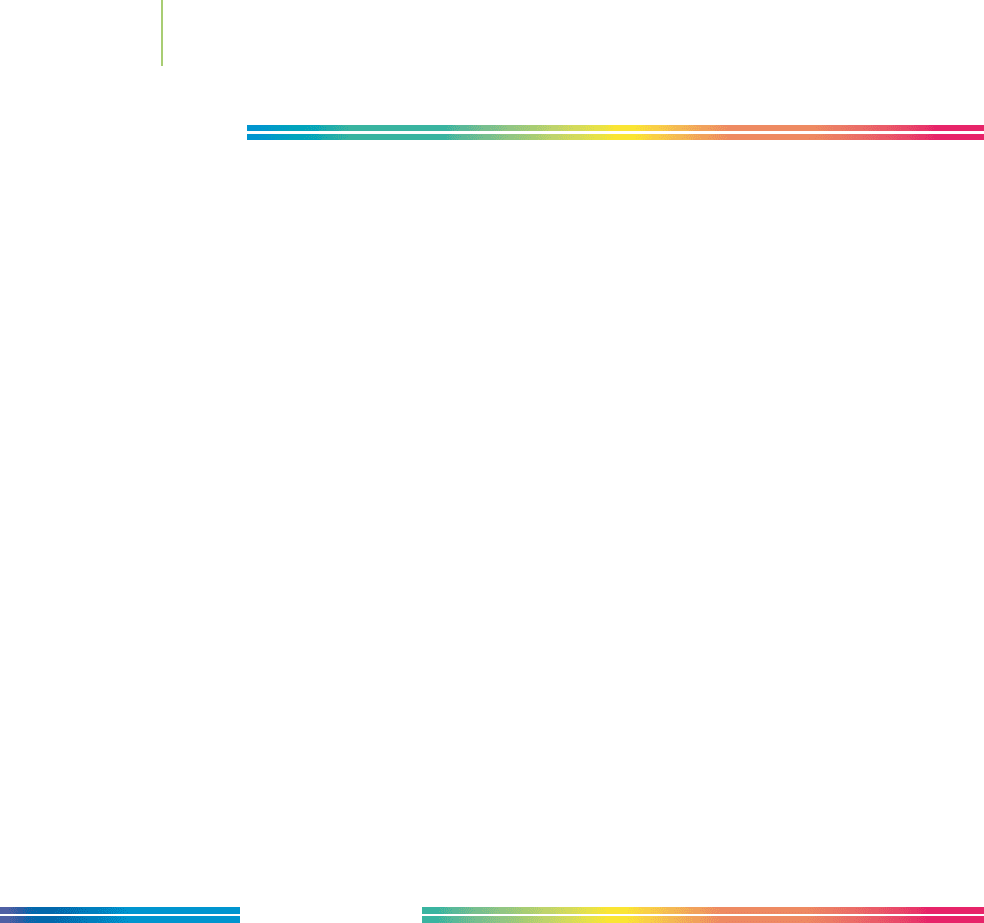
200 Chapter 5 Energy
The Bottom Line
■
Chemical changes are accompanied by the gain or
release of energy. (Section 5.1)
■
Chemicals can store huge amounts of energy and
then release it as soon as a chemical reaction begins.
(Section 5.1)
■
Thermodynamics is the study of energy changes and
exchanges. (Section 5.1)
■
Energy comes in two basic forms, which we call ki-
netic energy (the energy of motion) and potential
energy (positional energy). (Section 5.1)
■
Energy is never created or destroyed; it is only trans-
ferred from place to place and converted from one
form into another. This is the law of conservation of
energy. (Section 5.1)
■
A chemical reaction that releases energy from the
chemicals involved is known as an exothermic reac-
tion, because energy is flowing out of the system and
into the surroundings. A reaction that absorbs en-
ergy into the chemicals involved is known as an en-
dothermic reaction. (Section 5.1)
■
All chemical reactions begin with an input of energy,
needed to “jolt” the chemicals into reacting. The en-
ergy required to make this happen is known as the
activation energy of the reaction. (Section 5.1)
■
The total change in the energy of a chemical system,
as it undergoes a chemical reaction, is equal to the
heat flow (q), known as the heat of reaction, and the
work done (w): U = q + w. (Section 5.1)
■
The SI unit of energy is the joule, which we can re-
late to the more familiar units of calories and (in the
context of food) Calories. (Section 5.2)
■
Each substance has a particular specific heat capacity
(c), often simply called its specific heat, which is the
amount of heat needed to raise the temperature of
1 gram of the substance by 1 degree Celsius (or 1 kel-
vin) when the pressure is constant. (Section 5.3)
■
Hess’s law states that the enthalpy change of a chem-
ical reaction is independent of the chemical path or
mechanism involved in the reaction. (Section 5.5)
■
The standard enthalpy change for a reaction can be
calculated by subtracting the sum of the enthalpies
of formation of the reactants of the reaction from
the sum of the enthalpies of formation of the prod-
ucts of the reaction. (Section 5.5)
■
Making appropriate choices about our energy
sources will be an important part of building a suc-
cessful and sustainable future for humanity. (Sec-
tion 5.6)
Key Words
activation energy The energy that is required to initiate
a chemical reaction. (p. 174)
biomass conversion The release of energy from the
chemical conversion—often simply the burning—
of plants and trees. (p. 198)
bomb calorimeter Apparatus in which a chemical reac-
tion occurs in a closed container, allowing the energy
released or absorbed to be measured. (p. 184)
Calorie (Note capital C.) Unit of energy equal to 1000
calories (1 kilocalorie) and to 4184 joules. (p. 179)
calorie (Note lowercase c.) Unit of energy equal to
4.184 joules. (p. 180)
calorimeter An apparatus in which quantities of heat
can be measured. (p. 183)
calorimetry The study of the transfer of heat in a pro-
cess. (p. 183)
chemical energy Energy that is stored in a substance as
a result of the motions and positions of its atomic
nuclei and their electrons. (p. 172)
constant-volume calorimetry A form of calorimetry in
which the reacting system is sealed within a chamber
of fixed volume, and the only way the system can re-
lease or gain energy is by the exchange of heat with
the surroundings. (p. 184)
electromagnetic radiation Energy that propagates through
space. Examples include visible light, X-rays, and
radiowaves. (p. 170)
electroweak force The fundamental force responsible for
the attraction between objects carrying opposite
electric charges, for the repulsion between objects
carrying the same electric charge, and for some trans-
formations within subatomic particles. It is also re-
sponsible for the phenomena of magnetism and light.
(p. 169)
endothermic reaction A reaction that absorbs energy
from the surroundings. (p. 175)
enthalpy A thermodynamic quantity symbolized by H
and defined as H = U + PV.(p. 186)
exothermic reaction A reaction that releases energy into
the surroundings. (p. 174)
first law of thermodynamics The total change in the
closed system’s energy in a chemical process is equal

Key Words 201
to the heat flow (q) into the system and the work
done (w) on the system:
U = q + w
Energy is neither created nor destroyed; it is only
transferred from place to place and converted from
one form into another: U
system
+ U
surroundings
= 0.
(p. 177)
geothermal systems Power-generating systems that in-
volve drilling deep into the earth and exploiting the
flow of heat from the interior of the Earth out toward
the surface. (p. 199)
gravitational force The fundamental force that causes
all objects with mass to be attracted to one another.
(p. 169)
heat (q) The energy that is exchanged between a system
and its surroundings because of a difference in tem-
perature between the two. (p. 174)
heat capacity The amount of heat needed to raise the
temperature of any particular amount of a substance
by 1°C. (p. 182)
heat of reaction The energy as heat released or absorbed
during the course of a reaction. (p. 186)
Hess’s law Thermodynamic law stating that the en-
thalpy change of a chemical reaction is independent
of the chemical path or mechanism involved in the
reaction. This enables us to determine the enthalpy
changes of reactions that might be very difficult to
actually perform. (p. 192)
hydroelectric systems Power-generating systems in which
the power of falling water is used to generate electric-
ity. (p. 199)
internal energy (U) The energy of a system defined as
the sum of the kinetic and potential energies. Ab-
solute internal energy is difficult to measure. (p. 177)
joule (J) The SI unit of energy. In terms of base units,
1 Joule = 1 kg·m
2
·s
–2
.(p. 179)
kilocalorie Unit of energy equal to 1000 calories and to
1 Calorie (note capital C). (p. 180)
kinetic energy The energy things possess as a result of
their motion. (p. 170)
law of conservation of energy Law stating that energy is
neither created nor destroyed but is only transferred
from place to place or converted from one form into
another. (p. 171)
molar heat capacity The heat capacity of one mole of a
substance. (p. 182)
photovoltaic systems (PVs) Fabricated systems that con-
vert the energy of sunlight into electricity. Also
known as solar cells. (p. 198)
potential energy The energy things possess as a result of
their positions, such as their position in a gravita-
tional or electromagnetic field. (p. 170)
reference form The most stable form of the element at
standard conditions. (p. 187)
renewable sources of energy Energy sources that can be
rapidly replaced by natural processes. (p. 198)
solar cells Fabricated systems that convert the energy of
sunlight into electricity. Also known as photovoltaic
systems or PVs. (p. 198)
solar thermal systems Fabricated systems that convert
the energy of sunlight into heat. (p. 198)
specific heat The amount of heat needed to raise the
temperature of one gram of a substance by one de-
gree Celsius (or one kelvin) when the pressure is con-
stant. Also known as the substance’s specific heat
capacity. (p. 181)
specific heat capacity (c) The amount of heat needed to
raise the temperature of one gram of a substance by
one degree Celsius (or one kelvin) when the pressure
is constant. Also known as the substance’s specific
heat. (p. 181)
standard enthalpy of combustion (
c
H°) The enthalpy
change when one mole of a substance in its standard
state is completely burned in oxygen gas.Also known as
the substance’s standard heat of combustion. (p. 189)
standard enthalpy of formation (
f
H°) The enthalpy
change for the formation of one mole of a substance
in its standard state from its elements in their refer-
ence form. Also known as the standard heat of for-
mation. (p. 188)
standard enthalpy of reaction (
rxn
H°) Enthalpy change of
a reaction in which all of the reactants and products
are in their standard states. (p. 187)
standard state The state of a chemical under a set of
standard conditions, usually at 1 atmosphere of pres-
sure and a concentration of exactly 1 molar for any
substances in solution. Standard states are often re-
ported at 25°C. (p. 187)
state function A property of a system that depends only
on its present state, not on the path by which it
reached that state. (p. 191)
strong nuclear force The force that holds protons and
neutrons together in atomic nuclei. (p. 169)
surroundings Everything in contact with a chemical sys-
tem. Energy may flow between the surroundings and
system. Strictly speaking, the surroundings of a chem-
ical system comprise the entire universe except the
system. (p. 168)
system Any set of chemicals whose energy change we
are interested in. (p. 168)
thermochemistry The study of energy exchange in chem-
ical processes. (p. 168)
thermodynamics The study of energy exchange. (p. 168)
universe The space consisting of both the system and
the surroundings. (p. 168)
wind power The use of the energy of the wind to gener-
ate electricity. (p. 199)
work (w) Force acting on an object over a distance.
(p. 169)
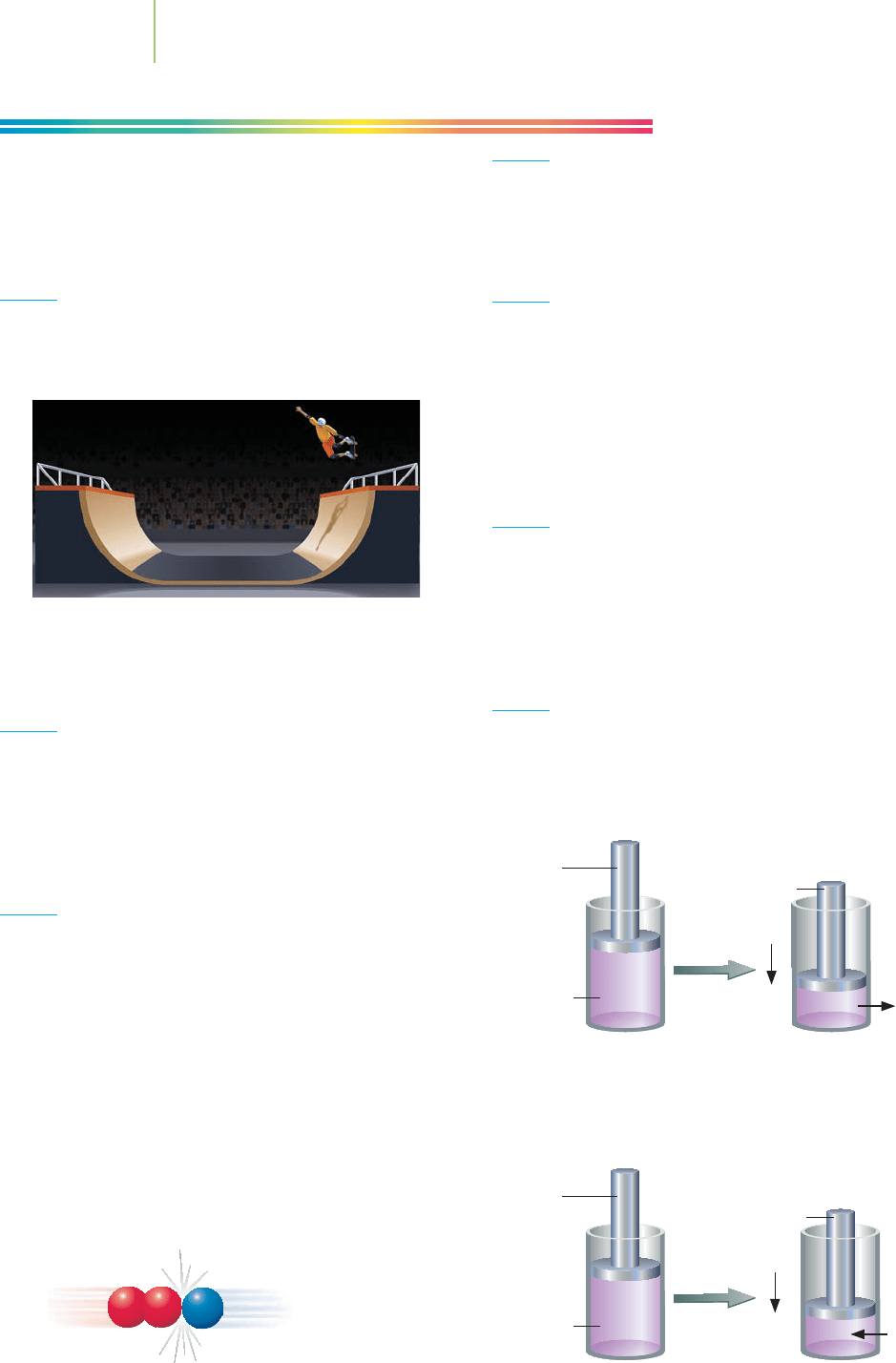
Gas in
cylinder
14.02 kJ
9.33 kJ
Piston
Piston
Piston
moves
Gas in
cylinder
67 kJ
23 kJ
Piston
Piston
Piston
moves
202 Chapter 5 Energy
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 5.1 The Concept of Energy
Skill Review
1. Imagine a skateboarder at the top of a half-pipe. Describe the
changes in potential energy and in kinetic energy as the skater
goes from the top of one side of the half-pipe to the top of the
other side.
2. Describe the changes in potential and kinetic energyof a snow-
boarder going over a hill. If there were another uphill slope
waiting at the bottom of the first hill, would the snowboarder’s
potential energy increase or decrease as she or he came down
the first hill and immediately began to climb the second?
3. Calculate the kinetic energy of the following:
a. A 185-lb chemistry professor jogging at 8.0 mi/h.
b. A 42-g hackey sack being kicked at 0.78 m/s.
c. A molecule of CO
2
moving at 560 m/s.
4. Calculate the kinetic energy of the following:
a. A 2-ton truck lumbering along at 60.0 mi/h.
b. A 200-g apple falling from a tree at 20 mi/h.
c. A 0.25-kg ball moving at 1.75 m/s.
5. Describe the following atomic phenomena as primarily a
function of the potential or kinetic energy of the system.
a. The attractive force between an electron in a hydrogen
atom and its nucleus.
b. The vibration of two hydrogen atoms bonded in a
molecule of H
2
.
c. The movement of a hydrogen molecule inside a balloon.
6. Describe the following atomic phenomena as primarily a
function of the potential or kinetic energy of the system.
a. The attraction of a hydrogen atom’s electron to oxygen in
water.
b. The repulsion of a hydrogen atom’s electron from the
other hydrogen electron in H
2
.
c. The collision of two molecules as they react to generate a
product.
7. Review the definition of heat. What is being transferred when
heat moves between a system and its surroundings? How is
this transfer accomplished?
8. What are the three main forms in which energy can be trans-
ferred between a system and its surroundings? Give an exam-
ple of each.
9. When gasoline in an automobile engine undergoes combus-
tion, new compounds and heat are produced. Identify the
system and the surroundings in this process. Is the system
gaining or losing energy in this process? What is the sign on
w for this process?
10. When a cold pack is applied to a sports injury, the athlete
may remark that the pack feels very cold. Identify the system
and surroundings associated with the cold pack. Is the system
gaining or losing energy in this process? What is the sign on q
for this process?
11. In a chemical reaction, the value of q was determined to
be +24 joules (J). The work function was determined to be
+12 J. What is the total energy change for this system? Did
the surroundings gain or lose energy?
12. In a chemical reaction, the value of q was determined to
be −200 joules (J). The work function was determined to be
+158 J. What is the total energy change for this system? Did
the surroundings gain or lose energy?
13. Calculate the total energychange for each of thefollowing sys-
tems.Do the surroundings gain or lose energyin each process?
a. q =+45 J; w =+45 J
b. q =−266 J; w = 1.2 kJ
c. q = 23.4 kJ; w =−14 kJ
d.
14. Calculate the total energy change for each of the following
systems. Does the system gain or lose energy in each process?
a. q =−23 J; w =−37 J
b. q =−88 J; w =+36 J
c. q =+105 J; w =−133 J
d.
Focus Your Learning 203
Chemical Applications and Practices
15. Burning hydrocarbon fuels and digesting carbohydrates both
involve the formation of CO
2
molecules and the release of
chemical energy. Which type of compounds—hydrocarbons,
carbohydrates, or carbon dioxide—would release the least
amount of energy? Explain how you came to this conclusion.
16. During photosynthesis, green plants take in CO
2
and com-
bine it with water to make carbohydrates and other com-
pounds. This process is driven by energy from the Sun. When
these food molecules are digested, energy is released. (This
energy can be used to maintain healthy body temperature and
to run other reactions.) Is the energy released via digestion the
same as the energy that absorbed from the Sun? Explain.
17. On a surface excursion during Apollo 14, Alan Shephard hit a
few golf balls. While he originally stated that the balls flew for
miles and miles, he later and more accurately estimated the
distance as 200 to 400 yards. How would the amount of force
needed to drive a ball 300 yards on the Moon differ from that
needed to drive a ball the same distance on Earth? Base your
explanation on the differences between the environments.
18. There are many forces at work in our everyday commute to
work. Identify the typical forces that you’d expect to see
evidence of as you drive an automobile down the street.
19. In the catabolic biochemical pathways in cells, adenosine
triphosphate (ATP) is produced as part of an endothermic
reaction. What is the sign for q in the reaction that represents
the production of ATP? Later, the ATP can be used to help the
cells do work on the surroundings. What is the sign for w in
the reaction that represents that work?
20. In each of the following situations, supply the correct sign for
q and w in the reaction that represents the process.
a. In order to make soft drinks have fizz, or become
carbonated, gaseous CO
2
at room temperature must be
pumped, under pressure, into an aqueous solution at
lowered temperatures. The system may be considered to be
the resulting carbonated solution.
b. Water placed in a microwave oven can be made to boil
away into steam. Consider the water in its container to be
the system.
Section 5.2 Keeping Track of Energy
Skill Review
21. Suppose a 275-g apple and a 175-g orange are moving with
the same kinetic energy. If the apple is moving at 15 m/s, how
fast is the orange moving? (Maybe we can compare apples
and oranges after all!)
22. Some of the best tennis players may serve a 56.9-g tennis ball
at a velocity of approximately 115 mi/h. What is the kinetic
energy of the tennis ball in such a serve?
23. A molecule of N
2
moving through a room may have a veloc-
ity of approximately 420 m/s. What is the kinetic energy of a
molecule of N
2
?
24. An automobile is moving at a rate of 31 m/s. At this rate it
has a kinetic energy of 436 kJ. What is the mass of this
automobile?
25. A low-fat popcorn snack advertises that one serving provides
90 Cal. One serving is considered to be 34 g. What is the
energy provided in Calories per gram? What is the energy
provided in calories per gram? What is the energy provided
in joules per gram?
26. A different food item advertises that one serving provides
320 Cal. One serving is considered to be 80 g. What is the en-
ergy provided in Calories per gram? What is the energy pro-
vided in calories per gram? What is the energy provided in
joules per gram?
27. How much energy as heat is needed to raise 50.0 g of water
from 23.0°C to 37.0°C?
28. How much energy as heat is required to raise 77.0 g of water
from 18.0°C to 25.0°C?
29. The British thermal unit is used on some heating and cooling
devices. The BTU is defined as the amount of energy as heat
that will raise one pound of water from 58.5°F to 59.5°F.
Express that amount of heat in joules.
30. A popular instant coffee-flavored drink provides 25 food
calories per serving. Express that quantity in calories, joules,
and kilojoules.
Chemical Applications and Practices
31. One of the reactions used in some hand-warmer packets is
the oxidation of iron to form rust. The formation of 1 mole
of rust produces approximately 410 kJ of heat.
a. If this heat were absorbed by 2000.0 g of water at 22.0°C in
a calorimeter, how hot could the water be made?
b. If only 0.10 mole of rust were formed, how hot could
200.0 g of water at 22.0°C become?
32. Ammonium nitrate can sometimes be used in the chemical
cold pack applied to some sports injuries. If 10.0 g of ammo-
nium nitrate were placed in a coffee cup calorimeter that
contained 100.0 g of water and the temperature changed
from 25.0°C to 18.0°C, what was the heat transferred in
kilojoules?
33. Ethanol (C
2
H
5
OH) is being blended with gasoline mixtures
to produce a fuel for automobiles called gasohol. If the
combustion of 10.0 g of ethanol produces 268 kJ of heat,
how much heat will be produced in burning 1 mole of
ethanol?
34. If the combustion of 48.8 g of methane (CH
4
) produces
855 kJ of heat, how much heat will be produced in burning
1 mole of methane?
204 Chapter 5 Energy
44. A different bomb calorimeter was calibrated by combusting a
2.250-g sample of methanol (CH
4
O). The calorimeter tem-
perature rose by 0.522°C. What is the heat capacity of this
calorimeter? The molar energy of combustion for methanol
is known to be approximately −890.3 kJ.
45. a. The heat capacity of a certain bomb calorimeter was cali-
brated at 28.9
kJ
◦
C
. When 1.500 g of an unknown sugar was
combusted in the calorimeter, the temperature rose by
2.56°C. What was the energy of combustion of the sugar?
b. What additional information would we need in order to
report the molar heat of combustion for the sugar?
46. A student constructs a crude “coffee cup” calorimeter that
contains 94.1 g of water, at 22.0°C, in a double cup set up
with a thermometer and cork cover. When an 85.8-g piece
of copper at a temperature of 100.0°C was placed in the
calorimeter, the temperature was noted to equilibrate at
28.0°C. The specific heat of copper is approximately
0.386
J
g·
◦
C
.
a. Calculate the heat gained by just the water.
b. Determine the heat capacity, in J/°C, for the empty
calorimeter.
47. The molar heat of combustion of propane is −2.2 × 10
3
kJ.
How many grams of propane would have to be combusted to
raise 1.0 kg of water (the amount you might boil to prepare a
tasty macaroni and cheese dinner) from 22.0°C to 100.0°C?
Assume that no heat is absorbed by the container or the air.
48. The specific heat of water is 4.184 J/g°C. How many grams
of water at 85.0°C would have to be added to raise 1.00 kg of
water from 25.0°C to 50.0°C? Assume that no heat is ab-
sorbed by the container or the air.
49. The heat of combustion for acetylene (C
2
H
2
) is −1300 kJ/
mol. Methane has a heat of combustion of −890 kJ/mol.
Decide, by calculation, which provides more energy as heat
per gram.
50. The heat of combustion for glucose (C
6
H
12
O
6
) is
−2803 kJ/mol. Tristearin (C
57
H
110
O
6
), a typical fat, has a
heat of combustion of −37,760 kJ/mol. Decide, by calcula-
tion, which provides more energy as heat per gram.
51. One way to test a metallic sample to see whether it is made of
gold is to heat it up and place it in a calorimeter to determine
the specific heat of the sample. The specific heat of gold
is approximately 0.13
J
g·K
. What temperature change would
prove that the metal was gold, if 15.0 g of the sample at
99.0°C were placed in a calorimeter initially at 25.0°C? The
heat capacity of the calorimeter is 25.0
J
K
.
52. In the chapter, a bomb calorimeter was discussed that had a
heat capacity of 32.4
kJ
◦
C
. If 0.550 g of benzoic acid (C
7
H
6
O
2
)
were combusted in the calorimeter, what would be the
expected change in temperature? (The molar energy of com-
bustion for benzoic acid is −3227 kJ/mol.)
Section 5.3 Specific Heat Capacity and Heat Capacity
Skill Review
35. Most of the definitions that you have learned in chemistry
have very specific terminology. Explain why the definition of
specific heat allows you to report the value in either °C or K.
36. In order to clarify the differences among specific heat, heat
capacity, and molar heat capacity, provide a brief definition
of each.
37. Which of the following involves the greater amount of heat
transfer?
a. 10.0 kg of water in an automobile cooling system changes
from 45.0°C to 10.0°C as it cools overnight.
b. In preparation for the annual “chili cook-off,” 8.0 L of
water is heated from 22.0°C to 99.0°C.
38. If the heat capacity of a metal coffee pot filled with water
were known to be 5.87
kJ
°C
, what would you calculate to be the
amount of heat transferred from a campfire when the tem-
perature of the coffee pot changes from 22.0°C to 95.0°C?
39. Fill in the values missing from the following table.
Chemical Applications and Practices
41. Some research is being done on the production of nickel
compact discs. These disks could store tremendous amounts
of information and last an unusually long time. Like most
metals, nickel has a low specific heat. If 35.0 g of nickel
absorbs 311 J, it increases in temperature by 20.0°C. What is
the specific heat of nickel?
42. If 50.0 g of a metal alloy absorbs 471 J, it increases in temper-
ature by 30.0°C. What is the specific heat of this metal alloy?
43. In order to calibrate a bomb calorimeter, a 2.000-g sample of
benzoic acid (C
6
H
5
COOH; molar mass = 122.12) was com-
busted. The calorimeter temperature rose by 1.978°C. What
is the heat capacity of this calorimeter? The molar energy of
combustion for benzoic acid is known to be approximately
−3227 kJ.
Heat, q Specific Heat
J
g·
◦
C
Mass (g) T (°C)
10.0 joules 4.184 10.0
0.115 10.0 5.0
15.5 joules 42.5 15.0
Heat, q Specific Heat
J
g·
◦
C
Mass (g) T (°C)
4.184 100.0 40.0
450 joules 0.315 15.0
48.5 joules 48.5 15.0
40. Fill in the values missing from the following table.
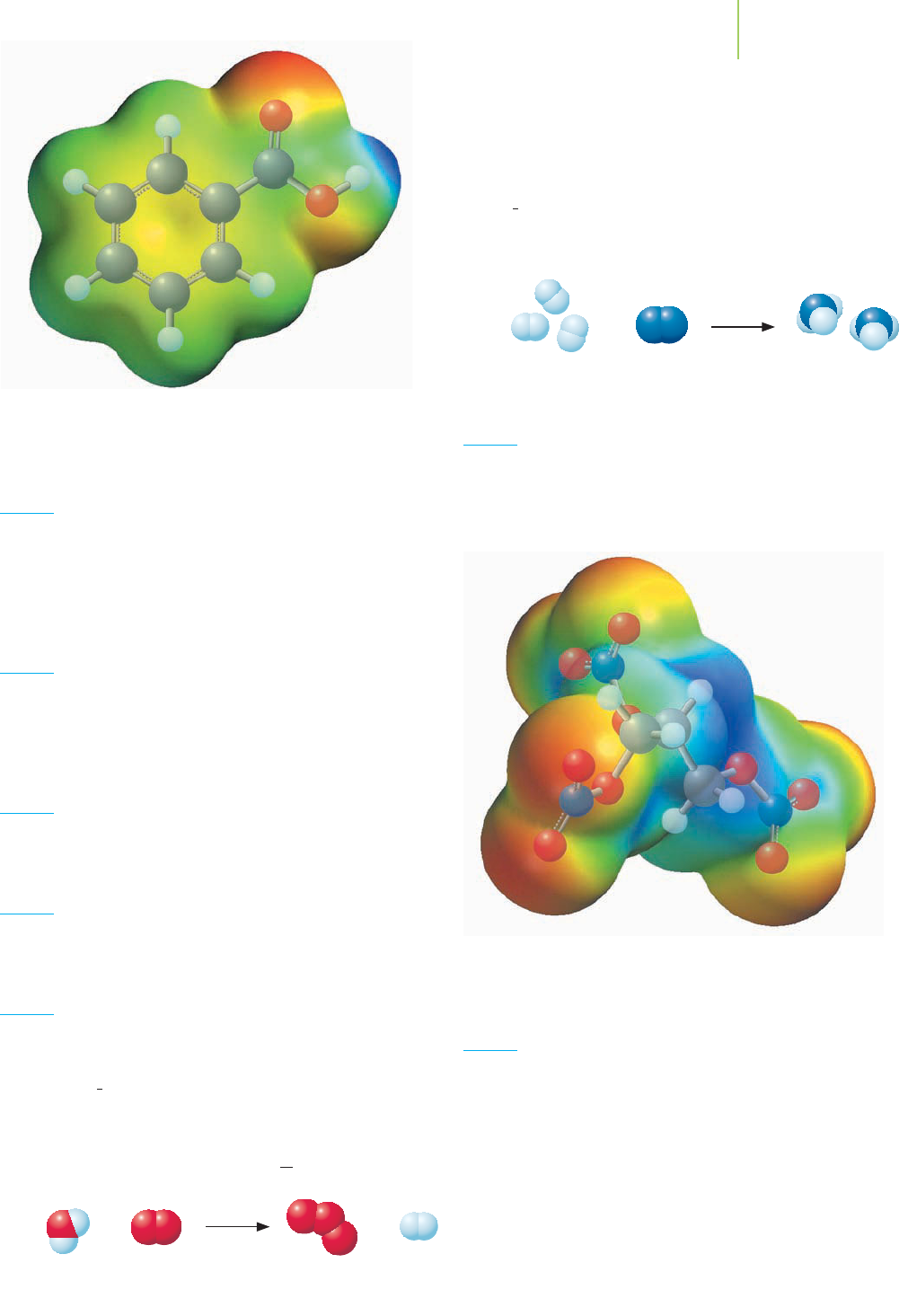
Chemical Applications and Practices
63. Explain why the values of H and U for reactions such as
the explosion of nitroglycerin, represented here, could vary
significantly.
4C
3
H
5
N
3
O
9
(l) → 12CO
2
(g) + 10H
2
O(g) + 6N
2
(g) + O
2
(g)
Focus Your Learning
205
62. Of the following, which would not be considered appropriate
equations to represent “standard heat of formation” pro-
cesses? Explain the reasons for your choices.
a. C(s) + O
2
(g) → CO
2
(g)
b. H
2
(g) + O
2
(g) → H
2
O
2
(g)
c.
1
2
H
2
(g) + O
2
(g) → H
2
O(g)
d. CH
4
(g) + 2O
2
(g) → CO
2
(g) + 2H
2
O(g)
e. 2H(g) + O(g) → H
2
O(l)
Section 5.4 Enthalpy
Skill Review
53. Explain how chemical reactions that generate gases can be
thought of as constant-pressure situations but the volume of
the products can be thought of expanding?
54. When the equation U = q
p
+ w is recast as U = q
p
−
PV, the sign between the heat and work function changes.
Explain why it is now proper to express the PV with a
negative sign.
55. Without using any symbols or numbers, define the term
enthalpy as it is applied in chemistry.
56. What is the chief thermodynamic difference among the
expressions known as “heat of reaction,” “standard heat of
reaction,”“standard heat of formation,”and “standard heat of
combustion”?
57. In an exothermic reaction, does the system or the surround-
ings gain energy as heat?
58. What sign would characterize the value of H for the reverse
of an exothermic reaction?
59. What are the conditions being described when a substance is
taken to be in its standard state?
60. What is the physical state of carbon dioxide when it is in
standard state conditions?
61. Of the following, which would not be considered appropriate
equations to represent “standard heat of formation”
processes? Explain the reasons for your choices.
a. C(s) +
1
2
O
2
(g) → CO(g)
b. N
2
(g) + 3H
2
(g) → 2NH
3
(g)
c. CS
2
(g) → CS
2
(l)
d. C(g) + 4H(g) → CH
4
(g)
e. 4CO
2
(g) + 5H
2
O(l) → C
4
H
10
(g) +
13
2
O
2
(g)
Benzoic acid
64. Consider the reaction for nitroglycerin decomposition
shown in Problem 63. Cite two reasons why the reverse of the
reaction would not qualify as the standard heat of formation
for nitroglycerin.
65. a. Write out the balanced chemical equation for the standard
formation of carbon monoxide.
b. If the value for the standard heat of formation for carbon
monoxide were −110.5 kJ/mol, would you consider the
reaction endothermic or exothermic?
c. What would be the H value for the reverse of the reaction?
66. a. Write out the balanced chemical equation for the standard
formation of gaseous hydrogen peroxide (H
2
O
2
).
b. If the value for the standard heat of formation for hydro-
gen peroxide were −136.1 kJ/mol, would you consider the
reaction endothermic or exothermic?
c. What would be the H value for the reverse of the reaction?
+
(gas) (gas) (gas) (gas)
+
+
(gas) (gas) (gas)
Nitroglycerin
f.
f.
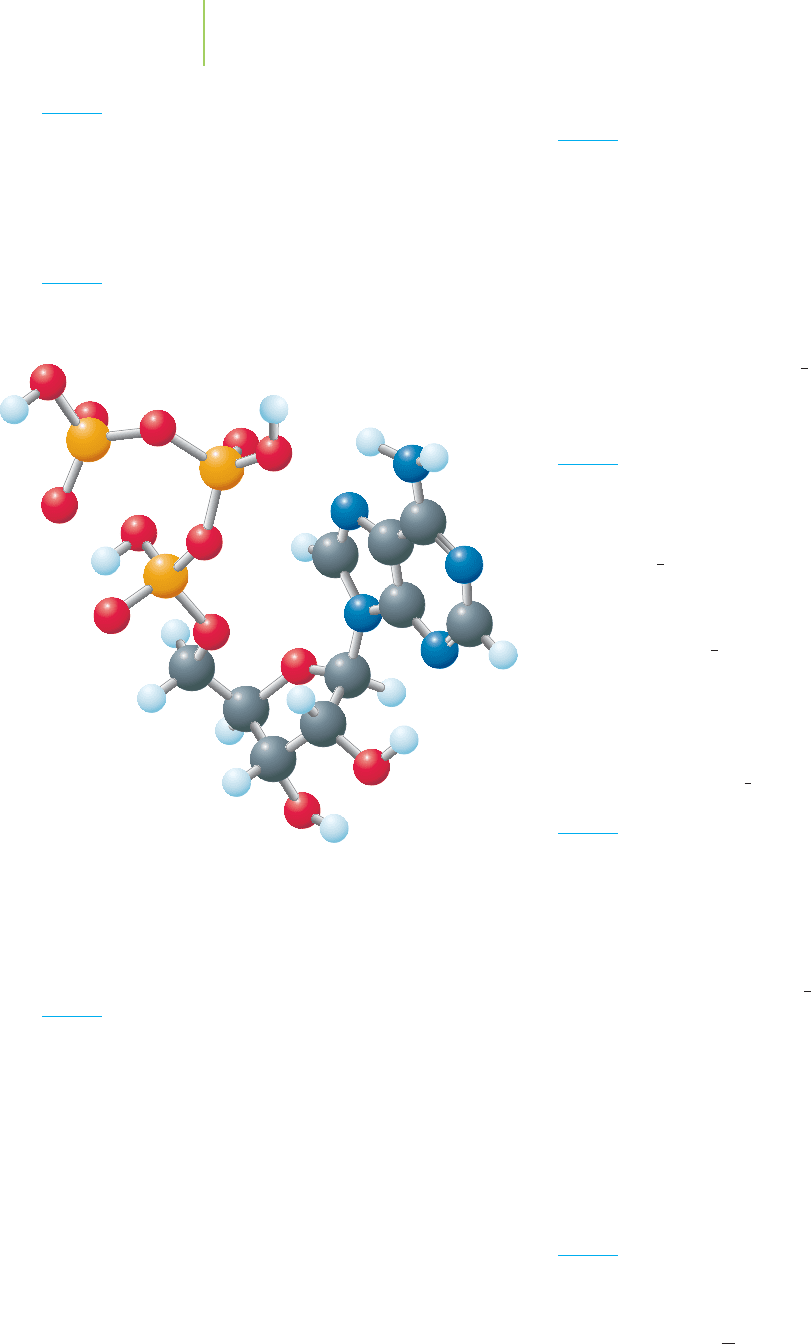
206 Chapter 5 Energy
ATP
Chemical Applications and Practices
73. The fuel used in many rural settings is propane (C
3
H
8
). Write
out the heat of formation reaction for this important fuel.
Now use the combustion reaction sequence shown in the text
to develop a Hess’s law scheme that would allow you to cal-
culate the H of formation for propane.
74. The sugar arabinose (C
5
H
10
O
5
) is obtained from plants with
the polysaccharide gum arabic. In wheat plants, this sugar
helps form important cell wall structures. The heat of forma-
tion reaction for arabinose is
5C(s) + 5H
2
(g) +
5
2
O
2
(g) → C
5
H
10
O
5
(s)
Without using actual kJ values, show the reactions that you
could use, and the way you would use them, to obtain the
arabinose formation reaction.
75. Given the following reactions and H values:
B
2
O
3
(s) + 3H
2
O(g) → B
2
H
6
(g) + 3O
2
(g) H =+2035 kJ
2H
2
O(l) → 2H
2
O(g) H =+88 kJ
H
2
(g) +
1
2
O
2
(g) → H
2
O(l) H =−286 kJ
2B(s) + 3H
2
(g) → B
2
H
6
(g) H =+36 kJ
Calculate H for
2B(s) +
3
2
O
2
(g) → B
2
O
3
(s) H = ?
76. Use the heat of formation reaction data for water and carbon
dioxide and the following reaction for the combustion of
acetylene, employed in high-temperature welding applica-
tions, to find the heat of formation of acetylene (C
2
H
2
).
C
2
H
2
(g) +
5
2
O
2
(g) → 2CO
2
(g) + H
2
O(l)
c
H =−1300 kJ/mol
77. a. Ethanol (C
2
H
5
OH) is being added to gasoline to create the
fuel “gasohol.”Using heat of formation data and Hess’s law,
determine the heat of combustion, in kJ/mol, for ethanol.
Combustion of ethanol:
C
2
H
5
OH(l) + 3O
2
(g) → 2CO
2
(g) + 3H
2
O(l)
c
H = ?
Formation of ethanol:
2C (graphite) + 3H
2
(g) +
1
2
O
2
(g) → C
2
H
5
OH(l)
H =−278 kJ
b. Using your answer from part a, determine the heat
released when 100.0 g of pure ethanol is combusted.
78. The oxidation of sulfur has many important environmental
connections. Notably, acid rain is formed from sulfur oxides
reacting with moisture in the air. Use the following two reac-
tions to determine the enthalpy change when sulfur dioxide
reacts with oxygen to form sulfur trioxide.
a. S(s) + O
2
(g) → SO
2
(g) H° =−296.8 kJ
b. 2S(s) + 3O
2
(g) → 2SO
3
(g) H° =−791.4 kJ
2SO
2
(g) + O
2
(g) → 2SO(g) H° = ?
79. The octane rating on gasoline is a method of comparing fuel
energy values. What is the value for the heat of combustion of
octane? (
f
H°(C
8
H
18
) =−249.9 kJ/mol)
C
8
H
18
(l) +
25
2
O
2
(g) → 8CO
2
(g) + 9H
2
O(l)
80. A typical componentof gasoline is pentane (C
5
H
12
).The stan-
dard enthalpy of combustion of pentane is −3537 kJ/mol.
Write the combustion reaction for pentane, and determine
the standard enthalpy of formation for pentane.
70. Glucose (C
6
H
12
O
6
) is used by our bodies as an energy source.
Write out the standard heat of formation reaction for this
important molecule.
Section 5.5 Hess’s Law
Skill Review
71. The H value for the following reaction is −1012 kJ.
2NH
3
(g) + 3N
2
O(g) → 4N
2
(g) + 3H
2
O(l)
a. What is the value of H for the reverse of the reaction?
b. What is the value of H for 1 mole of NH
3
reacting?
c. What is the value of H of 4 moles of NH
3
reacting?
72. The H value for the following reaction is +284.6 kJ.
3O
2
(g) → 2O
3
(g)
a. What is the value of H for the reverse of the reaction?
b. What is the value of H for 1 mole of O
2
reacting?
c. What is the value of H of 4 moles of O
2
reacting?
67. The hydrocarbon fuel butane (C
4
H
10
) is used in small
portable lighters. Write the reaction for the standard heat of
combustion for this reaction. (Recall that the two products of
hydrocarbon combustion are carbon dioxide and water.)
68. Pentane (C
5
H
12
) can be blended with other hydrocarbons
and additives to form gasoline. Write the reaction that de-
picts the standard heat of formation of pentane.
69. The primary energy molecule in cells is ATP (adenosine
triphosphate, C
10
H
15
N
5
O
13
P
3
). Write out the standard heat
of formation reaction for this important biomolecule.

Focus Your Learning 207
81. Calcium metal will react in water to form calcium hydroxide,
Ca(OH)
2
.Use the thermochemical data that follow and Hess’s
law to calculate the value of H° for the reaction
Ca(s) + 2H
2
O(l) → Ca(OH)
2
(s) + H
2
(g)
a. H
2
(g) +
1
2
O
2
(g) → H
2
O(l) H° =−286 kJ
b. CaO(s) + H
2
O(l) → Ca(OH)
2
(s) H° =−64 kJ
c. Ca(s) +
1
2
O
2
(g) → CaO(s) H° =−635 kJ
82. Ozone is reduced by hydrogen to produce water. Use the
thermochemical data that follow and Hess’s law to calculate
the value of H° for the reaction
3H
2
(g) + O
3
(g) → 3H
2
O(g)
a. H
2
(g) +
1
2
O
2
(g) → H
2
O(l) H° =−286 kJ
b. 2H
2
(g) + O
2
(g) → 2H
2
O(g) H° =−483.6 kJ
c. 3O
2
(g) → 2O
3
(g) H° =+284.6 kJ
Section 5.6 Energy Choices
Chemical Applications and Practices
83. Most of the world’s energy consumption for power is based
on fossil fuels. This source, however, is considered nonre-
newable. Look back at the list of renewable energy sources
and cite one advantage, in addition to this renewability,
that each would have over the petroleum-based fuels used
today.
84. Assuming that the fossil fuels are completely depleted at
some point in the future, the world will need to depend on
renewable sources of energy. Look back at the list of renew-
able energy sources and describe one disadvantage of using
each source as the sole source of energy for a country.
Comprehensive Problems
85. Individual atoms and molecules are so small that they have
very low values of kinetic energy. However, given their mass
and velocity, it is possible to calculate the value. What is the
kinetic energy of an oxygen molecule (O
2
) in air that you are
breathing if its velocity is 460 m/s? Would you expect a
nitrogen molecule (N
2
) moving at the same speed to have
more or less kinetic energy than the oxygen molecule?
Explain.
86. Distinguish between the two terms in each of the following
pairs:
a. Heat and temperature
b. System and surroundings
c. Exothermic and endothermic
d. q and U
87. What is the role of activation energy in a chemical process? Is
it possible for the activation energy of a reaction to be greater
than the overall change in energy for a chemical reaction?
Explain.
88. The average temperature of a healthy human is approxi-
mately 37°C. The average room temperature may be about
25°C. Explain how we are able to keep our body temperature
so much higher than that of our environment.
89. A 400-mL glass beaker contains 250 g of water at room
temperature. As several NaOH pellets are placed in the water
and begin to dissolve, you notice a warming sensation in the
hand in which you are holding the beaker. Answer the fol-
lowing questions.
a. If the NaOH and the water make up the system, would you
consider the process endothermic or exothermic? Explain.
b. Is the beaker part of the system or part of the
surroundings?
c. During this process, is energy flowing into or out of the
system?
d. During this process, has the kinetic energy of the water
molecules been raised or lowered?
e. What work is being done during this process?
90. A serving of Italian rice—risotto—provides 150 food Calo-
ries. What would this value be in kilojoules? Assume that this
quantity of energy would be available to do the work of lift-
ing 2.5-kg chemistry textbooks from the floor to a height of
1.5 m. How many such chemistry textbooks could, theoreti-
cally, be lifted through that height? (And later, of course, after
that work, brought to class.)
91. Express the energy content of a 2.00-oz. candy bar that con-
tains 247 Calories in Calories per gram, in joules per gram,
and in kilojoules per gram. If this energy were efficiently
used to provide the kinetic energy to move a 1.5-pound
chemistry book, how fast, in meters per second, could the
book move?
92. One of the main reasons for eating is to obtain a supply
of energy. A low-fat apple muffin may provide 170 food
Calories for each 50-g muffin. How many muffins (or frac-
tions of muffins) are needed to provide 1 joule?
93. a. Suppose you are heating water (225 g) in a mug that you
have placed in a microwave oven. As you wait to add the in-
stant hot chocolate, please calculate, from the following
data, the amount of energy as heat that the water has ab-
sorbed: The original water temperature was 15.0°C. When
you remove the mug of hot water, you find that the tem-
perature has risen to 98°C.
b. What additional information would you need in order to
determine the heat absorbed by the mug?
94. You have just removed a hot cheese pizza from the oven, and
all of the ingredients are presumably at the same tempera-
ture. Without waiting for it to cool, you take a bite of the
pizza. As you bite the pizza, the bread is hot on your tongue
but does not burn. However, as you continue to bite, pizza
sauce (mostly tomatoes and water) squeezes out and burns
the roof of your mouth. Which has the higher specific heat,
the bread or the sauce? Explain the basis of your answer using
q = m × c × T.
95. One way to determine the heat capacity of a constant-vol-
ume calorimeter is to burn measured amounts of pure car-
bon in the presence of oxygen gas to form carbon dioxide.
From the following experimental data, determine the heat
capacity of the calorimeter. A 0.200-g sample of carbon,
when completely combusted, raised the temperature of the
water and the contents of the entire calorimeter from 24.0°C
to 25.5°C. It is known that under these conditions, the heat
released from the complete combustion of 1 mole of carbon
is 392 kJ.
