Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

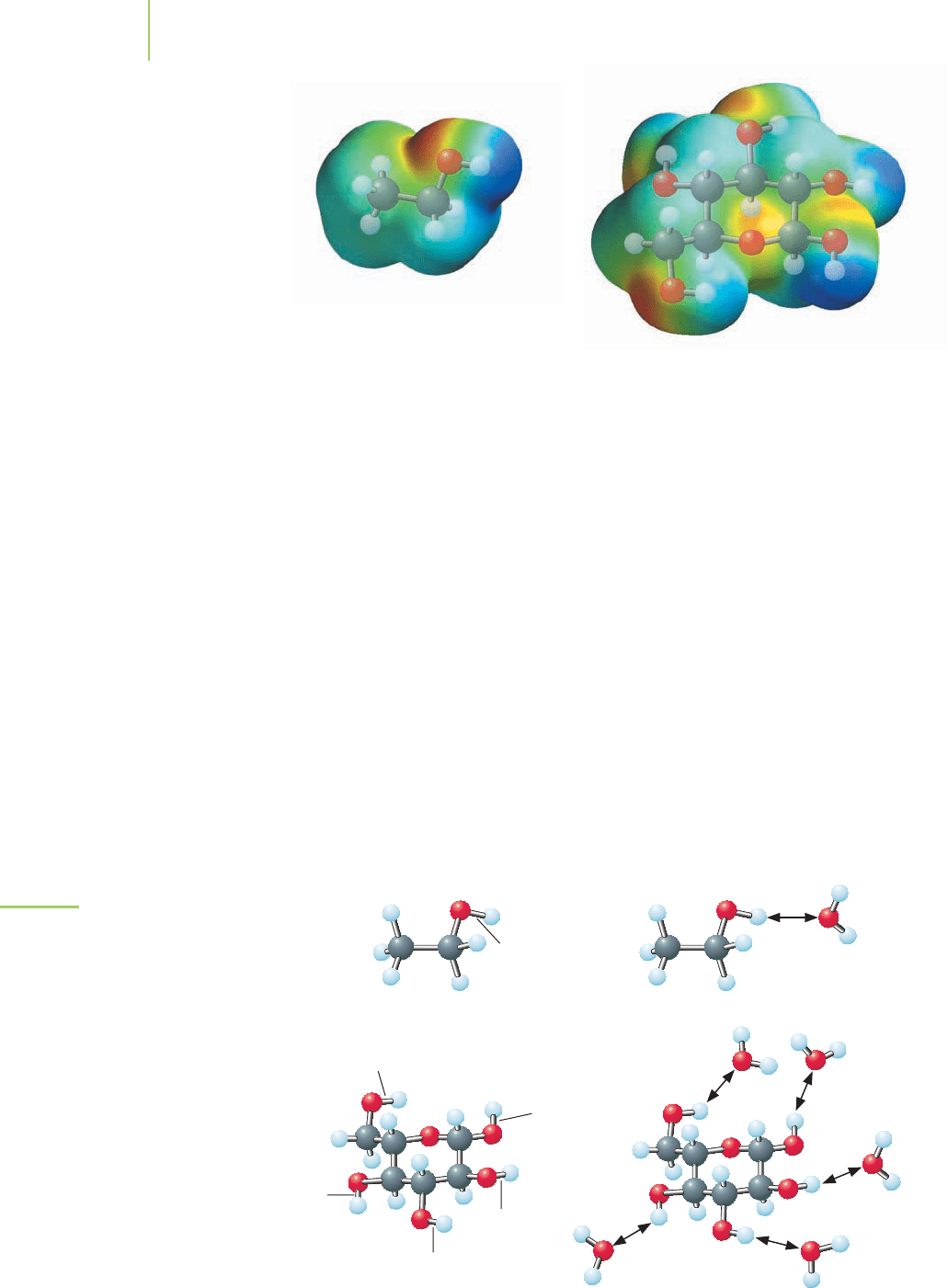
surrounded by their individual hydration spheres. We can represent the hydra-
tion sphere in a chemical equation by writing the symbol (aq) after the hydrated
molecules, ions, or atoms to specify their aqueous phase:
NaCl(s) → Na
+
(aq) + Cl
−
(aq)
There are many substances that are not made up of cations and anions. Some
of them dissolve in water, such as the nutrient molecule glucose (C
6
H
12
O
6
) and
the molecule ethanol (C
2
H
6
O) found in alcoholic beverages. Why do they dis-
solve in water? These molecules possess a feature similar to water: The electrons
are polarized toward specific regions of the molecule. This leaves a slightly posi-
tive charge and a slightly negative charge on different regions of the molecules.
These partial charges can interact with the partial charges on water molecules.
The strong interaction allows the molecules to mix easily with water and dissolve
as shown in Figure 4.5. And, as is true with ions, a cage of water molecules sur-
rounds the dissolved solute molecules.
C
6
H
12
O
6
(s) → C
6
H
12
O
6
(aq)
Acids and bases are common substances, and many of them dissolve readily in
water. For example, nitric acid, HNO
3
, dissolves in water to produce the nitrate
ion,NO
3
−
(aq),and the hydrogen ion,H
+
(aq).According to one definition of these
common substances, acids produce a hydrogen ion when dissolved in water. On
128 Chapter 4 Solution Stoichiometry and Types of Reactions
δ
–
δ
+
δ
+
Polar
bond
Polar
bond
Polar
bond
Polar
bond
Polar
bond
(a) (b)
Polar
bond
δ
+
δ
+
δ
+
δ
+
δ
–
δ
–
δ
–
δ
–
δ
–
FIGURE 4.5
The partial charges on polar covalent
bonds, such as the O—H bonds in
ethanol and glucose, can interact with
the partial charges on water molecules
and allow molecules to dissolve in water.
Ethanol
An electrostatic potential map of glucose and
ethanol. The electrostatic potential is plotted
on the surface of a computer-generated
model of the molecules. Note how the electron
density is distributed in each molecule, and
compare these models to that of water
(Figure 4.3).
D-glucose

the other hand, sodium hydroxide (NaOH)
is a common base that dissolves in water, re-
sulting in Na
+
(aq) and OH
−
(aq). Accord-
ing to the same definition, bases produce
hydroxide ion in solution.
Electrolytes
You may have encountered sports drinks
sold with the claim that they help maintain
a healthy electrolyte balance by supplying
ions, sugar (to supply calories), and water
in an appropriate combination. The key in-
gredients quoted on the label of a leading
brand of sports drink can be found in Fig-
ure 4.6. The sodium and potassium in the
drink are present as hydrated Na
+
and K
+
ions. They are accompanied by hydrated
anions such as Cl
−
(chloride ions) so that
the entire solution has no net electrical
charge. Ionic compounds, such as sodium chloride and potassium chloride,
which dissociate in water to release free ions, are known as
electrolytes. The name
comes from the ability of solutions containing electrolytes to conduct electricity
because of the presence of mobile ions that can carry the electric current.
Electrolytes are of great medical importance, because our blood and cells
must contain an appropriate mixture of electrolytes to maintain good health. The
presence of electrolytes in blood and cells allows the body to absorb the right
amount of water from the gut and to excrete water via the kidneys. Maintaining
the optimal balance of electrolytes is especially important after strenuous exer-
cise, in which sweat containing water, sodium ions, and potassium ions leaves the
body as part of a vital cooling mechanism. The ions of electrolytes also play a cen-
tral role in creating the nerve impulses that allow our sense organs to work, our
muscles to move, and our brains to think.
Substances such as sodium chloride, which dissociate completely into ions
when they dissolve, are called
strong electrolytes. In fact, most ionic compounds
that dissolve in water fall into this category of electrolyte. A few molecules, such
as hydrogen chloride (HCl), are also considered strong electrolytes. The equation
below shows that gaseous HCl first dissolves, and then dissociates in the water, to
form hydrated ions.
HCl(g) → HCl(aq) → H
+
(aq) + Cl
−
(aq)
Other electrolytes, such as acetic acid (vinegar, CH
3
COOH), are called weak
electrolytes
, because even though they may dissolve completely in water, the for-
mation of ions occurs to a much lesser extent, as shown in Figure 4.7. Note the dif-
ferent type of arrow,
, that defines a reaction that does not proceed completely
to products.
CH
3
COOH(l) → CH
3
COOH(aq)
H
+
(aq) + CH
3
COO
−
(aq)
Those substances, such as glucose (C
6
H
12
O
6
), that dissolve in water without
forming ions are called
nonelectrolytes. The nonelectrolytes dissolve and associate
with water to form a hydration sphere, but they do not form ions. The equation
that represents a nonelectrolyte’s addition to water does not show the dissocia-
tion step found in the previous two equations.
C
6
H
12
O
6
(s) → C
6
H
12
O
6
(aq)
4.1 Water—A Most Versatile Solvent 129
HCl
Electrostatic potential map of HCl.
Note the very low electron density
at the hydrogen end of the
molecule.
Application
C
HEMICAL
E
NCOUNTERS:
Sports Drinks and
Electrolyte Balance
FIGURE 4.6
The key ingredients of a sports drink in-
clude sodium and potassium cations.
Visualization: Electrified Pickle
Video Lesson: CIA
Demonstration: The Electric
Pickle

130 Chapter 4 Solution Stoichiometry and Types of Reactions
CH
3
COOH(aq)
Acetic acid
Acetate
Water
Water
H
3
O
+
CH
3
COOH(aq) + H
2
O(l) CH
3
COO
–
(aq) + H
3
O
+
(aq)
Acetic
acid
FIGURE 4.7
Weak electrolytes (such as acetic acid)
do not dissociate completely in water.
Defining Electrolytes
How do we know whether a compound is a strong electrolyte, a weak electrolyte, or
a nonelectrolyte?
Often the type of compound, assuming it dissolves in water, can
tell us whether it will dissociate. For example, most ionic compounds that dis-
solve in water are strong electrolytes, as are the strong acids and bases. Weak acids
and bases do not dissociate completely in aqueous solution and are therefore
weak electrolytes. Nonelectrolytes include the water-soluble but nonionic com-
pounds. Although there are many exceptions to these rules, the basic trends, sum-
marized in Table 4.1, are valid.
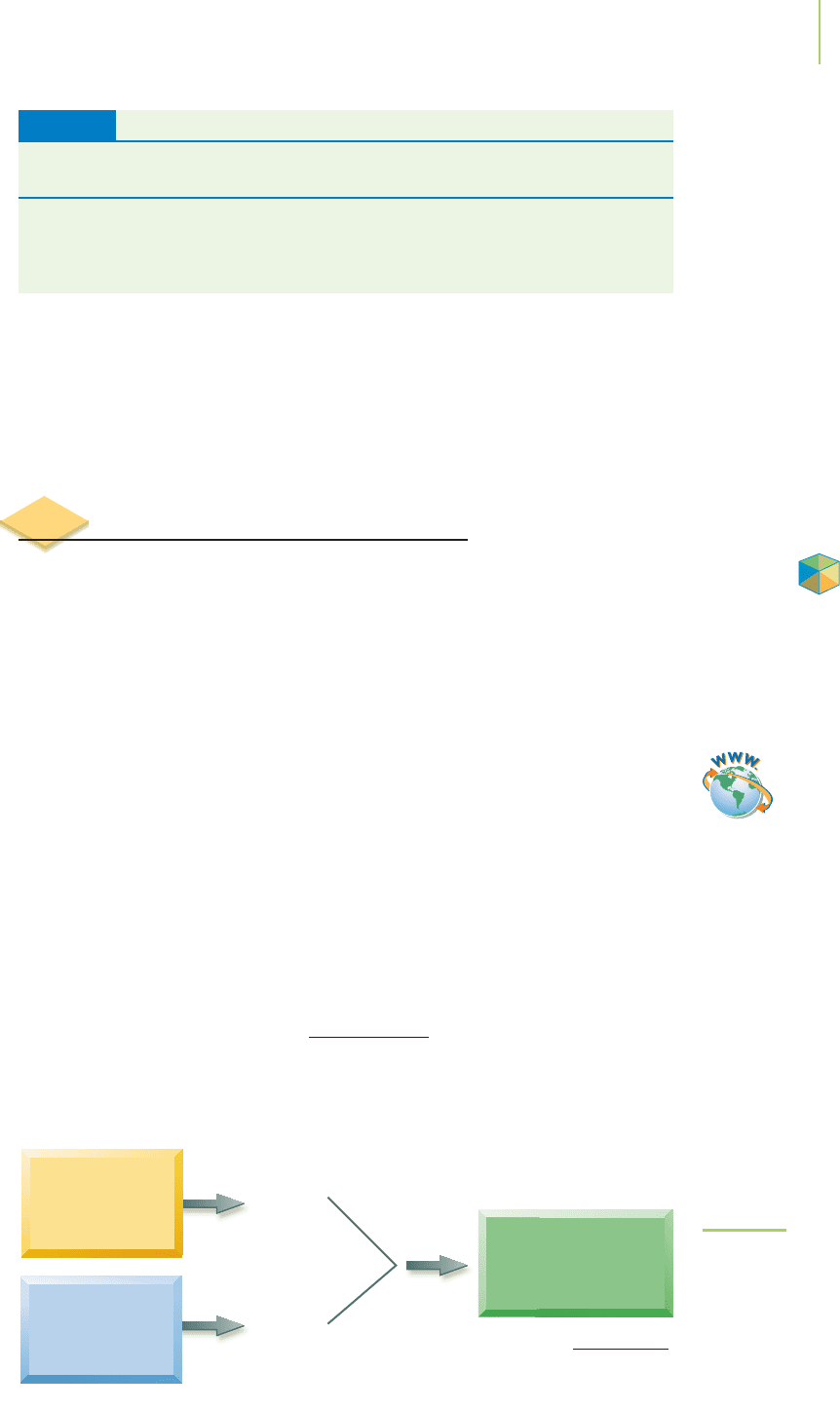
Experimentation can provide evidence as to whether a particular solution
contains a strong electrolyte, a weak electrolyte, or a nonelectrolyte. One such
experiment is shown in Figure 4.8. Using a solution as the connector in a light
bulb circuit, we can measure the ability of the solution to conduct electricity. The
FIGURE 4.8
The effect that each type of electrolyte
has on the conductivity of a solution as
measured by the brightness of a light
bulb. From left to right are solutions of
sodium chloride, acetic acid, and glucose.
(a) (b) (c)
Visualization: Electrolyte
Behavior
Visualization: Electrolytes
4.2 The Concentration of Solutions 131
Electrolytes and Types of Compounds
Ionic Strong Weak Molecular
Compounds Acids/Bases Acids/Bases Compounds
Strong Electrolyte Yes Yes No Sometimes
Weak Electrolyte Sometimes No Yes Sometimes
Nonelectrolyte No No No Sometimes
TABLE 4.1
Strong and weak acids and bases are described in Section 4.6.
brightness of a light bulb demonstrates the type of electrolyte in solution. What’s
happening in Figure 4.8? Because ions can “carry” an electrical charge, the solu-
tion containing the greatest number of ions will have the brightest light bulb.
4.2 The Concentration of Solutions
Hyponatremia is a serious condition characterized by a low sodium level in the
blood. The organization USA Track and Field recently released new guidelines
calling for long-distance runners to avoid drinking excessive amounts of water
during long runs, because doing so could lead to excessively diluted blood and a
severely reduced sodium level. The result, hyponatremia, gives rise to a high fever,
nausea, and, ultimately, heat stroke. Hyponatremia occurs when the sodium con-
centration is less than 130 mmol (mmol = 10
−3
mol) of sodium ions per liter of
blood.
The
concentration, the amount of solute per volume of solution, is often
quoted in moles of solute per liter of solution, in grams of solute per liter of
solution, or in various other ways. For example, long-distance runners should
consume sports drinks instead of water. A typical sports drink has 110 mg Na
+
per 8-ounce serving, which won’t reduce the sodium level in your blood. An
8-ounce serving of this drink contains 110 mg sodium ions. A larger quantity of
the drink—say, 1 quart (32 ounces)—contains 440 mg Na
+
, but the concentration
remains the same: 110 mg Na
+
per 8-ounce serving. Concentration is an intensive
property (see Section 1.4) because it doesn’t change with the volume of solution.
A useful concentration unit is known as
molarity. This unit specifically indi-
cates the moles of solute per liter of solution, as illustrated in Figure 4.9.
Molarity =
moles of solute
liter of solution
= M
Application
Moles of
solute
Liters of
solvent
Solution with
known concentration
in molarity
Molarity =
moles solute
liters solution
Compound
with a
known mass
Solvent
with a
known volume
FIGURE 4.9
The basic concepts of determining concen-
tration, measured in moles per liter.
Video Lesson: Concentrations
of Solutions
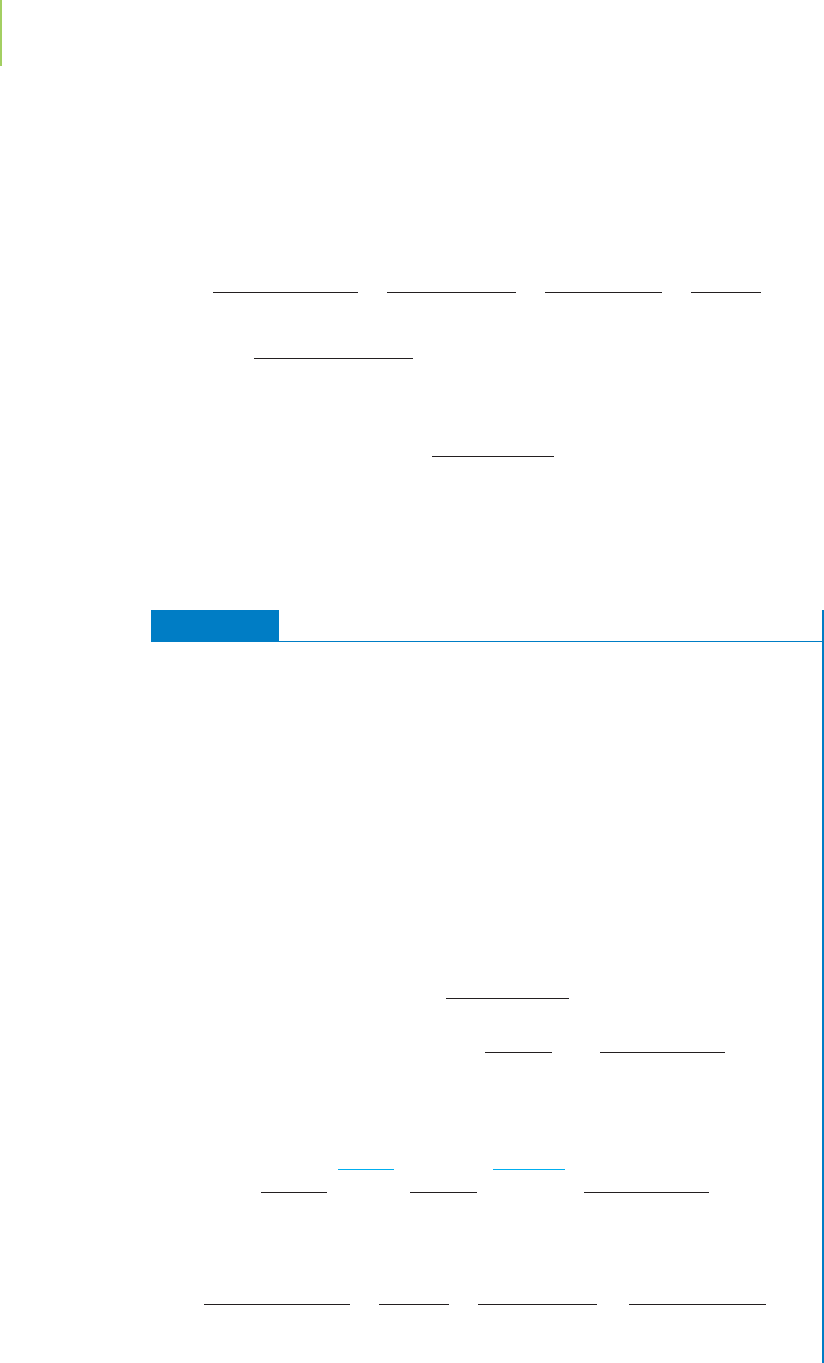
For example, 600.0 mg of aspirin in a soluble aspirin tablet might be dissolved in
orange juice to form 150.0 mL (150.0 cm
3
) of solution. We can calculate the con-
centration of the aspirin solution once we have calculated the number of moles
of aspirin and converted the volume of the juice into liters. We can use dimen-
sional analysis to convert the units of milligrams of aspirin per milliliter of solu-
tion into moles of aspirin per liter of solution:
600.0 mg aspirin
150.0 mL solution
×
1 g aspirin
1000 mg aspirin
×
1 mol aspirin
180.2 g aspirin
×
1000 mL
1L
=
0.02220 mol aspirin
L solution
The molarity is 0.02220 mole of aspirin per liter, or 0.02220 molar (written
0.02220 M).
The relationship
Molarity =
moles of solute
liter of solution
can be used to calculate the
number of moles of a solute if you know the molarity and the volume. In fact, this
relationship can be used in many ways as a dimensional analysis factor. The exer-
cises and practices that follow will give you an opportunity to use this new factor
to answer questions.
EXERCISE 4.1 Calculating Molarity
Sodium hydroxide (NaOH) is one of the most important substances used in the
chemical industry, with over 9.5 billion kg manufactured in 2004, ranking it among
the top ten chemicals produced in the United States. It is used in chemical manu-
facturing processes, such as in making soaps and detergents. It is also used to break
down the lignin that holds cellulose together in wood so that the cellulose can be
made into paper. Dilute solutions of NaOH are often used in industry to verify the
quality of medicines produced.
a. What is the molarity of NaOH in a solution that contains 3.48 g of sodium
hydroxide in 500.0 mL of solution?
b. How many grams of NaOH are required to prepare 617 mL of 1.200 M NaOH
solution?
Solution
a. Molarity is expressed in units of
moles of solute
liter of solution
. We can solve for the
molarity by converting the units of
g NaOH
mL soln
into
moles of NaOH
liter of solution
using
dimensional analysis. First, we construct a flowchart such as we introduced
in Chapter 1:
1000 mL
1L
mol NaOH
g NaOH
g NaOH
mL soln
−−−−−→
g NaOH
L soln
−−−−−−−→
moles of NaOH
liter of solution
Then we complete the calculation:
3.48 g NaOH
500.0 mL solution
×
1000 mL
1L
×
1 mol NaOH
40.00 g NaOH
=
0.174 mol NaOH
L
= 0.174 M NaOH
132 Chapter 4 Solution Stoichiometry and Types of Reactions
b. Given the molarity and the volume, we can solve for moles of NaOH and then
change to grams of NaOH using the molar mass of the compound. Again, we’ll
do it all in one step by converting units.
L soln
mL soln
mol NaOH
L soln
g NaOH
mol NaOH
mL soln −−−−−−−→L soln −−−−−−−→mol NaOH −−−−−−−→g NaOH
Then we complete the calculation:
617 mL solution×
1L
1000 mL
×
1.200 mol NaOH
1 L solution
×
40.00 g NaOH
mol NaOH
= 29.6gNaOH
PRACTICE 4.1
a. What is the molarity of K
+
in the typical muscle cell, which contains 6.5 g K
+
per liter of solution?
b. What is the molarity of Na
+
in a sports drink that contains 110 mg Na
+
per
8-ounce serving? (1 ounce = 29.6 mL)
c. How many grams of ethanol (C
2
H
6
O) do we need to prepare 600.0 mL of a
1.200 molar ethanol solution?
See Problems 15–17, 19–20, 29, 31, 32, and 34.
EXERCISE 4.2 Calculating Moles
a. How many moles of calcium hydroxide, Ca(OH)
2
, are found in 250 mL of a
0.800 molar solution of this compound?
b. If we assume that Ca(OH)
2
is a strong electrolyte, how many moles of Ca
2+
ions will be present in 250 mL of 0.800 M Ca(OH)
2
? How many moles of
OH
−
ions will be present?
First Thoughts
This problem is different from the previous one because it asks for the number of
moles in a solution that is already prepared, rather than asking for the concentration
of a solution (part a of Exercise 4.1) or the mass of a reagent required to prepare
a solution (part b). Still, the problem-solving approach is similar. We note what
we start with, what we want, and how we get there. You can use a flowchart to help
you stay on track.
Solution
a. Again, we use dimensional analysis to solve the problem. Note that we must
convert the milliliters of solution into liters so that we can use the molarity
term. Our flowchart for this calculation is constructed first:
1L
1000 mL
mol Ca(OH)
2
L soln
mL soln −−−−−−−→L soln −−−−−−−→mol Ca(OH)
2
Then
250 mL solution ×
1L
1000 mL
×
0.800 mol Ca(OH)
2
1 L solution
= 0.20 mol Ca(OH)
2
b. We start by writing the balanced equation that describes what happens to
Ca(OH)
2
when it is added to water.
Ca(OH)
2
(s) → Ca
2+
(aq) + 2OH
−
(aq)
4.2 The Concentration of Solutions 133
Then we can calculate the answer by performing a dimensional analysis. Our
calculation can be accomplished using the mole ratio relating the number of
moles of Ca(OH)
2
to the number of moles of Ca
2
+
. Our flowchart is written
first:
1L
1000 mL
mol Ca(OH)
2
L soln
mol Ca
2+
mol Ca(OH)
2
mL soln −−−−−→L soln −−−−−−−→mol Ca(OH)
2
−−−−−−−−→mol Ca
2+
Then
250 mL solution ×
1L
1000 mL
×
0.800 mol Ca(OH)
2
1 L solution
×
1 mol Ca
2+
1 mol Ca(OH)
2
= 0.20 mol Ca
2+
Again, we need to use the mole ratio to indicate the relationship between
Ca(OH)
2
and OH
−
. We’ll use the same flowchart for the Ca
2+
determination,
but we’ll modify the last step to show the mole ratio between Ca(OH)
2
and
OH
−
. The calculation is
250 mL solution ×
1L
1000 mL
×
0.800 mol Ca(OH)
2
1 L solution
×
2 mol OH
−
1 mol Ca(OH)
2
= 0.40 mol OH
−
Further Insights
Although it might seem like “busy work” to include the mole ratio in a calculation
that can be determined by counting the number of ions in the formula, it is very
important to include mole ratios along with the equation that shows the dissolution
process. If we avoid this step now, we will find it harder to solve more complex prob-
lems in later chapters.
PRACTICE 4.2
How many moles of the strong electrolyte sodium phosphate (Na
3
PO
4
) will be in
5.00 L of a 0.77 M solution? How many moles of hydrated sodium ions will be in the
solution?
See Problems 13, 14, 37, and 38.
EXERCISE 4.3 Calculating Volumes
What volume of a 0.150 M solution of ethanol (C
2
H
6
O) will contain 12.5 moles of
ethanol?
First Thoughts
This is another unit conversion problem that we can solve using dimensional analy-
sis. However, in this case, we’re using the molarity in a different way: to determine
the volume rather than moles, grams, or molarity, as in previous problems.
Solution
First, we write our flowchart:
L soln
mol ethanol
mol ethanol −−−−−−−→L ethanol
Then
12.5 mol ethanol ×
L solution
0.150 mol ethanol
= 83.3 L solution
134 Chapter 4 Solution Stoichiometry and Types of Reactions
Further Insights
In questions like this, the identity of the solute is irrelevant provided we are dealing
with moles, rather than masses. For example, the answer we obtained for the volume
of 0.150 M ethanol containing 12.5 moles of ethanol would be equally valid for the
volume of a 0.150 M solution of glucose containing 12.5 moles of glucose. Both re-
quire a volume of 83.3 L. In a similar way, if we were asked to calculate the number
of moles of a compound in a given volume of solution, we would not need to know
the identity of the compound if we were given the molarity of the solution. How-
ever, if we were asked about specific numbers of moles of ions of a strong electrolyte, we
would need the formula of the compound to determine the number of times the
ions or atoms appear in the formula.
PRACTICE 4.3
a. What volume of a 3.40 M solution of copper sulfate will contain 4.76 moles of
copper sulfate?
b. What volume of a 2.25 M solution of Ca(NO
3
)
2
will contain 5.5 moles of
calcium nitrate? What volume will contain 5.5 moles of nitrate ions?
See Problems 18, 21, and 22–24.
Parts per Million, Parts per Billion, and so on
Drinking water standards, which indicate the maximum permissible level of
harmful contaminants in the water that we consume, are dictated in the United
States by the Environmental Protection Agency (EPA). Other countries have their
own agencies responsible for water standards, such as La Secretaría de Medio
Ambiente y Recursos Naturales (SEMERNAT, The Federal Agency of the Envi-
ronment and Natural Resources) in Mexico. If water in the United States has
more than these maximum levels, the EPA declares it unsafe to drink. For exam-
ple, as of January 23, 2006, the maximum allowed level of arsenic in drinking
water is 1.3 × 10
−7
M. We can use molarity to discuss the concentrations of pol-
lutants such as arsenic, but the resulting numbers are, in many cases, very small.
When the concentrations get this small, we often find it easier to use an alterna-
tive measure of concentration. Specifically, we can talk about the concentration
of arsenic in terms of
parts per million (ppm),or parts per billion (ppb),or even
parts per trillion (ppt).
We are already familiar with the related, but larger, unit “percent.” What does
percent mean? In a compound that is “1 percent nitrogen by mass,” the nitrogen
contributes 1 gram out of every 100 grams to the total mass. In the
same way, a level of one part per million (1 ppm), means the chem-
ical contributes 1 gram out of every million grams of the total mass.
Similarly, one part per billion (1 ppb) corresponds to a level of
1 gram out of every billion grams of the total. One part per trillion
(ppt) corresponds to 1 gram out of every trillion grams.
These concentration units correspond to very small levels in-
deed. We can get an idea of exactly how small from the following
approximate comparisons, as illustrated in Figure 4.10:
■
One part per million is roughly equivalent to a drop of ink in a
12-gallon bucket of water.
■
One part per billion is roughly equivalent to a drop of ink in a
large tanker truck full of water.
■
One part per trillion is roughly equivalent to a drop of ink in a
12-million-gallon reservoir of water.
4.2 The Concentration of Solutions 135
Application
C
HEMICAL
ENCOUNTERS:
Maximum Levels
of Chemicals in
Drinking Water
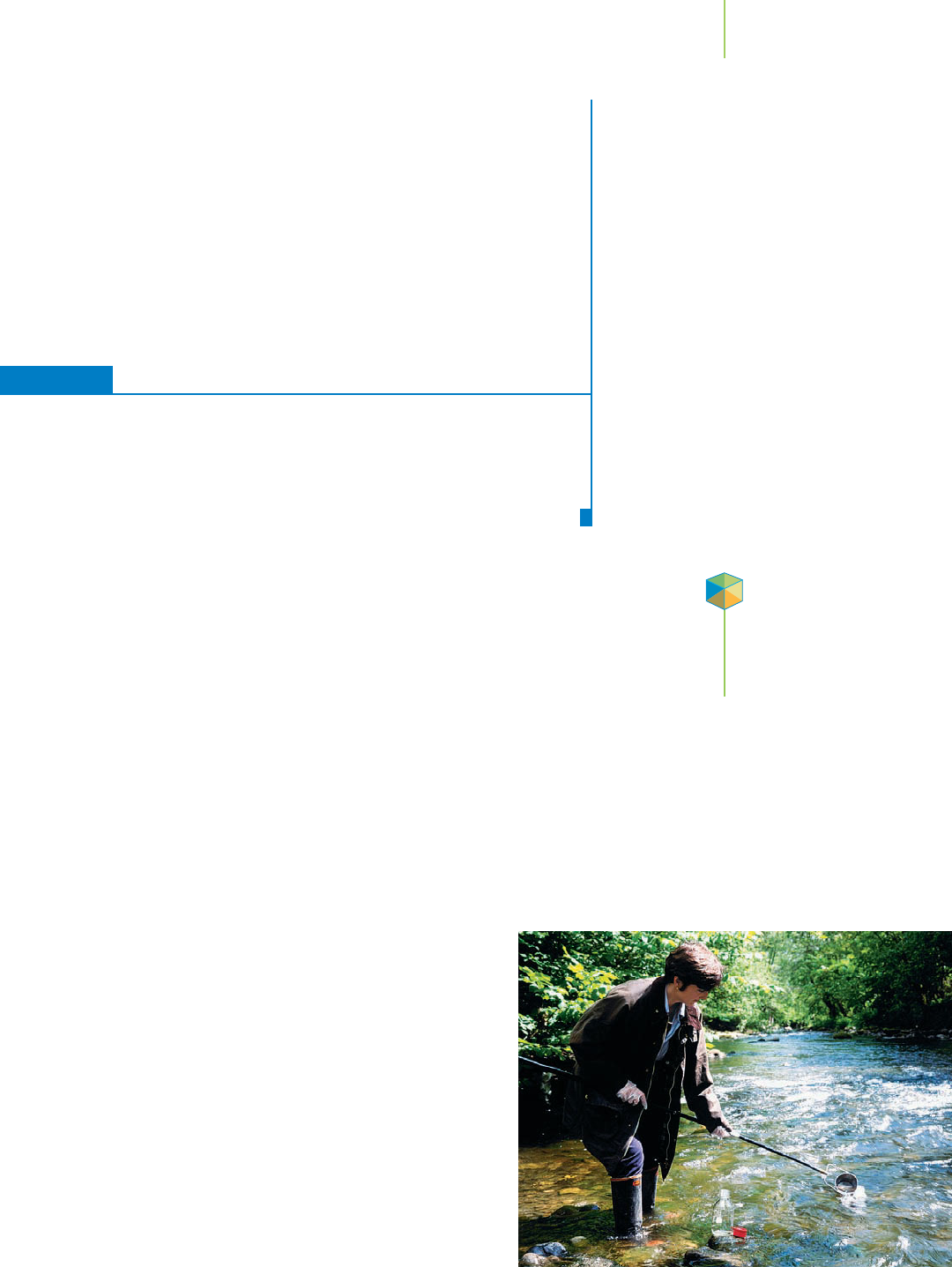
An environmental chemist sampling the
water in a river.
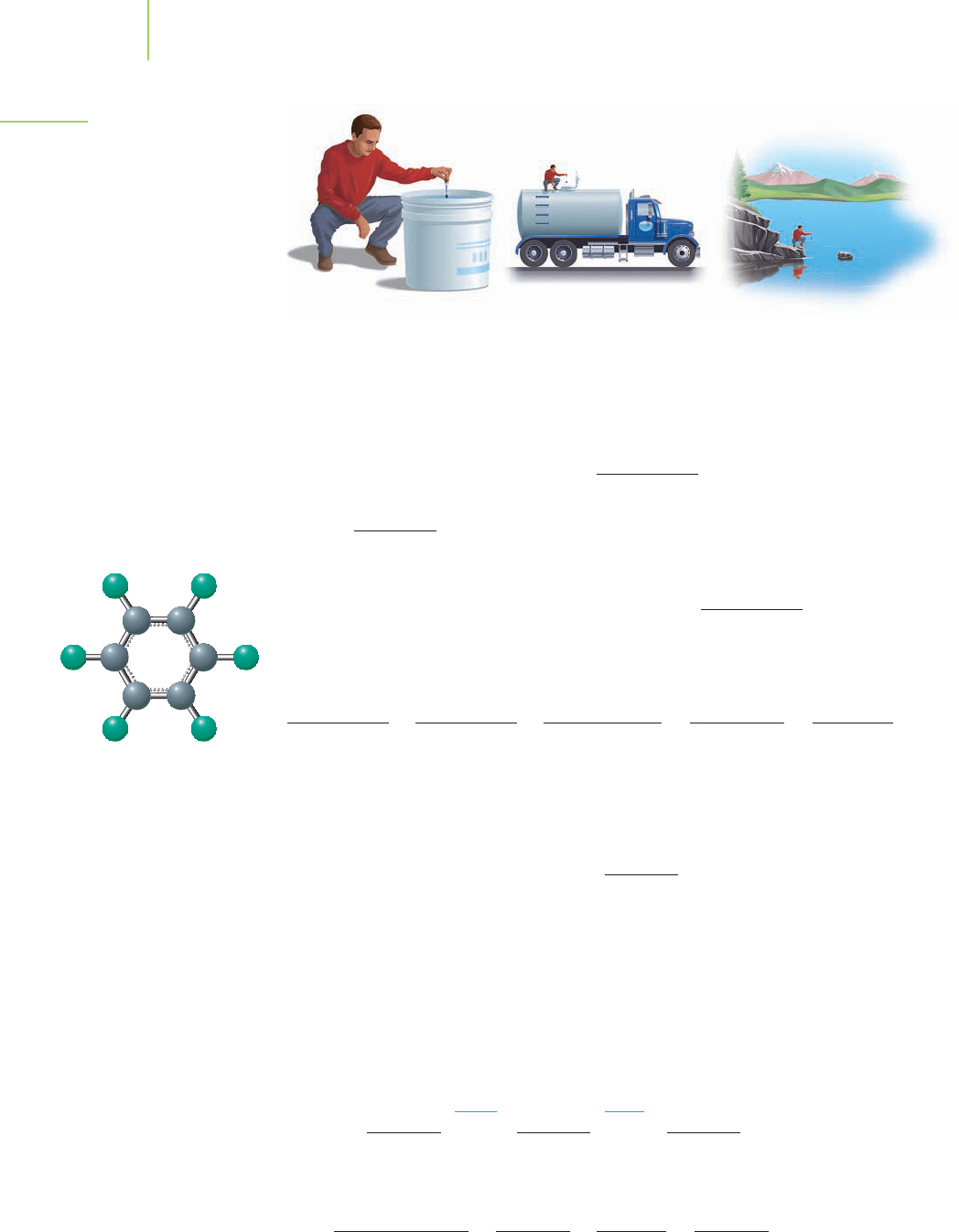
Let’s look at these concentration units in a slightly different way.As an example,
the EPA maximum level for hexachlorobenzene (C
6
Cl
6
), a pesticide used on wheat
in the United States until 1965, is 1 ppb. This means that the maximum allowable
level in drinking water should be 1 gram of C
6
Cl
6
per billion grams of solution.
1ppbC
6
Cl
6
=
1gC
6
Cl
6
10
9
g solution
If we use
1 g water
1 mL water
as the density of water and make the key assumption that the
solution is so dilute that its density is about equal to that of water, then
Density of very dilute aqueous solutions
=
1 g solution
1 mL solution
This means that we can express the concentration of hexachlorobenzene as the
number of grams of C
6
Cl
6
per liter of solution:
1gC
6
Cl
6
10
9
g solution
×
1 g solution
1 mL solution
×
10
3
mL solution
1 L solution
=
10
−6
gC
6
Cl
6
L solution
=
1 µgC
6
Cl
6
L solution
↑↑ ↑
(one ppb C
6
Cl
6
) (density of the solution) (convert mL to L)
The bottom line is that in dilute aqueous solutions, we can express parts per
billion as
ppb solute =
µg solute
L solution
This relationship, the number of grams of solute per liter of solution, is more
conceptually understandable than the actual definition of parts per billion. For
our hexachlorobenzene example, 1 liter of solution that contains 1.0 µg hexa-
chlorobenzene is said to have a concentration of 1.0 ppb hexachlorobenzene. We
can even use this definition to convert from other concentrations to ppb. What is
the maximum level of arsenic, in ppb, if the molarity of a solution at this maxi-
mum level is 1.3 × 10
−7
M arsenic?
Our flowchart looks like this:
gAs
mol As
1g
10
−6
g
mol As
L solution
−−−−−→
gAs
L solution
−−−−−→
µgAs
L solution
= ppb As
Then
1.3 ×10
−7
mol As
1 L solution
×
74.92 g As
1 mol As
×
1 µgAs
10
−6
gAs
=
9.7 µgAs
L solution
= 9.7ppb
Note the relationships among parts per million, parts per billion, and parts per
trillion in Table 4.2.
136 Chapter 4 Solution Stoichiometry and Types of Reactions
Hexachlorobenzene
(a) (b) (c)
FIGURE 4.10
To visualize the idea of parts per million,
per billion, and per trillion, consider that
one drop of ink could be placed in these
quantities of water. (a) Placing that drop
of ink in a 12-gallon bucket results in
1 ppm. (b) Placing it in a tanker truck
results in 1 ppb. (c) Placing it in a
12-million-gallon reservoir results in
1 ppt.
µ
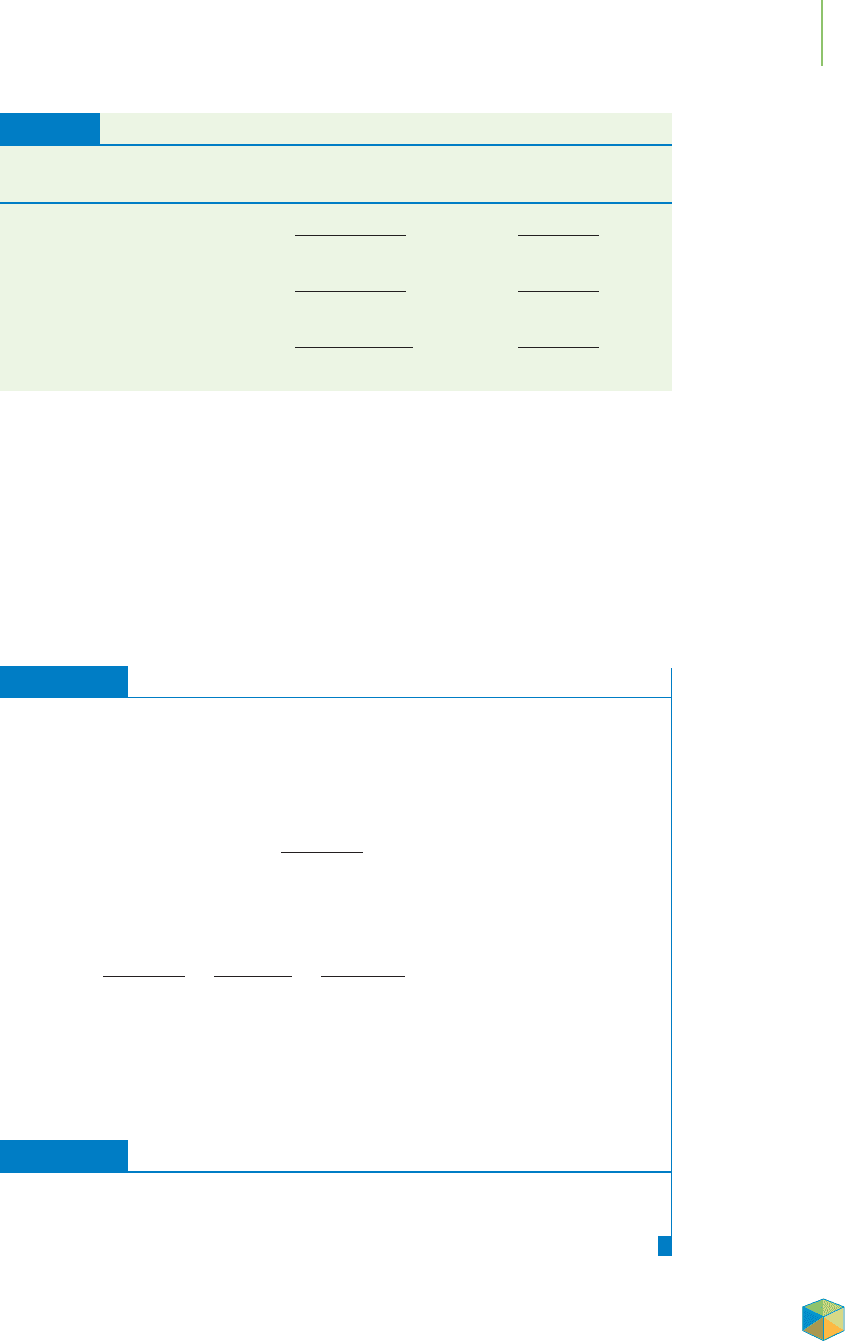
4.2 The Concentration of Solutions 137
Although we more often hear about how small concentrations of compounds
in water can be harmful, very small levels of some chemicals in drinking water
can actually be helpful to human health. For example, the presence of tiny
amounts of fluoride (F
−
) in drinking water is recognized as beneficial in the pre-
vention of tooth decay. The U.S. Public Health Service recommends a level of be-
tween 0.7 and 1.2 ppm F
−
. The effects of such small levels in preventing tooth
decay have been reported as “striking” by the American Dental Association.
EXERCISE 4.4 Converting ppm to Molarity
An acceptable midrange value for fluoride ion (F
−
) in drinking water is 1.0 ppm. To
what concentration of fluoride, in M, does this correspond?
First Thoughts
Using the mass-per-volume relationship we established in Table 4.2 enables us to
solve the problem with
1.0 ppm =
1.0mgF
−
L solution
.
Solution
Remember to construct the flowchart before you start the calculation.
1.0mgF
−
L solution
×
10
−3
gF
−
1mgF
−
×
1 mol F
−
19.00 g F
−
= 5.3 ×10
−5
M F
−
Further Insights
High concentrations of fluoride ion can be very harmful to your health. In fact,
some would argue that fluoride at any level is harmful. The debate surrounding
fluoridation of drinking water continues.
PRACTICE 4.4
Cyanide ion (CN
−
) concentrations of 0.200 ppm in drinking water are considered
the upper limit for human consumption. What is this concentration in M?
See Problems 25–28, 32, 35, and 36.
Dilution
How do municipal water treatment plant workers prepare water so that it contains
a fluoride ion concentration of about 1 part per million?
They use a concentrated
source of fluorine, hydrofluorosilicic acid, H
2
SiF
6
(“HFSA”), which they then di-
lute in the drinking water. HFSA reacts with water in a fairly complex way that re-
leases fluoride ion into the water. In Ireland, a company in the town of New Ross,
Common Units Used in Expressing “Parts per” Concentration of “X”
Mass-to-Mass Mass-to-Volume
Unit Relationship Relationship
parts per million (ppm)
gX
10
6
g solution
mg X
L solution
parts per billion (ppb)
gX
10
9
g solution
µgX
L solution
parts per trillion (ppt)
gX
10
12
g solution
ng X
L solution
Mass-to-volume relationship assumes a density of 1g/mL.
TABLE 4.2
Application
