Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

108 Chapter 3 Introducing Quantitative Chemistry
This is useful information for a chemist who wants to make aspirin. For ex-
ample, the equation indicates that it would be appropriate to add 102.088 g of
ethanoic anhydride for every 138.118 g of salicylic acid to prepare 180.154 g
of aspirin. That corresponds to the simple mole ratio of 1 mol of ethanoic anhy-
dride per 1 mol of salicylic acid, as indicated by the equation. In the context of a
large chemical process plant, in which millions of aspirin tablets are made daily,
the reactions may require megagram quantities.
3.7 Working with Equations
The formation of ethanoic anhydride and water from ethanoic (acetic) acid can
be used to explore further how we put equations to work, using them to calculate
things we want to know. Remember that in addition to indicating what chemicals
are involved in the reaction, the equation establishes the mole ratios among all
the chemicals. We found that the balanced chemical equation indicates that 2 mol
of ethanoic acid (C
2
H
4
O
2
) produce 1 mol of ethanoic anhydride (C
4
H
6
O
3
) and
1 mol of water (H
2
O).
2C
2
H
4
O
2
→ C
4
H
6
O
3
+ H
2
O
2 mol → 1 mol + 1 mol
The mole ratios that indicate how compounds react and form products are
known as
stoichiometric ratios after the Greek stoicheon for “element” and metron
for “measure.” The stoichiometric ratios are vital when we want to answer ques-
tions about the quantity of reactant that reacts or the quantity of product that
forms. This process of asking and answering mathematical questions based on
balanced chemical equations is an aspect of
stoichiometry, which is essentially the
study and use of quantitative relationships in chemical processes.
For instance, the equation relating the formation of ethanoic anhydride de-
scribes not only the compounds involved in the reaction but also the stoichio-
metric ratios of those compounds. We can write all the mole ratios in the form of
conversion factors that will allow us to convert among the numbers of moles of
the chemicals concerned:
2 mol ethanoic acid
1 mol ethanoic anhydride
1 mol ethanoic anhydride
2 mol ethanoic acid
2 mol ethanoic acid
1 mol water
1 mol water
2 mol ethanoic acid
1 mol ethanoic anhydride
1 mol water
1 mol water
1 mol ethanoic anhydride
Note that the ratios can be written either “right side up” or “upside down.”
For example, let’s assume we wish to know the maximum mass of ethanoic
anhydride that could be formed from 300.0 kg of ethanoic acid. The molar mass
of ethanoic acid is 60.05 g and that of ethanoic anhydride is 102.1 g. We can ob-
tain the answer using the appropriate conversion factors. Note that this follows
our flowchart diagram in converting mass to moles, then moles to moles, then
moles to mass.
kg C
2
H
4
O
2
1000 g
kg
−−−−−→ gC
2
H
4
O
2
mol C
2
H
4
O
2
gC
2
H
4
O
2
−−−−−−→ mol C
2
H
4
O
2
mol C
4
H
6
O
3
mol C
2
H
4
O
2
−−−−−−→
mol
C
4
H
6
O
3
gC
4
H
6
O
3
mol C
4
H
6
O
3
−−−−−−→gC
4
H
6
O
3
Video Lesson: Stoichiometry
and Chemical Equations
Tutorial: Introduction to
Stoichiometry
Video Lesson: CIA
Demonstration: Magnesium
and Dry Ice
First Thoughts
The key strategy in solving problems such as this is to establish the conversion factor
that describes how many moles of the product are formed from how many moles of
reactant. In this case, the mole ratio is one-to-one, based on the balanced equation.
That is,
1 mol stearic acid
1 mol hydrogen
Our strategy for the conversion is
gH
2
mol H
2
gH
2
−−−−−→mol H
2
mol C
18
H
36
O
2
mol H
2
−−−−−−−→mol C
18
H
36
O
2
gC
18
H
36
O
2
mol C
18
H
36
O
2
−−−−−−−→gC
18
H
36
O
2
Solution
425 g H
2
×
1 mol H
2
2.016 g H
2
×
1 mol C
18
H
36
O
2
1 mol H
2
×
284.5gC
18
H
36
O
2
1 mol C
18
H
36
O
2
= 6.00 × 10
4
g C
18
H
36
O
2
3.7 Working with Equations 109
×
102.1 g ethanoic anhydride
1 mol ethanoic anhydride
= 255,000 g (255.0 kg) ethanoic anhydride
Three hundred kilograms of ethanoic acid can produce 255,000 g (255.0 kg) of
ethanoic anhydride by this reaction.
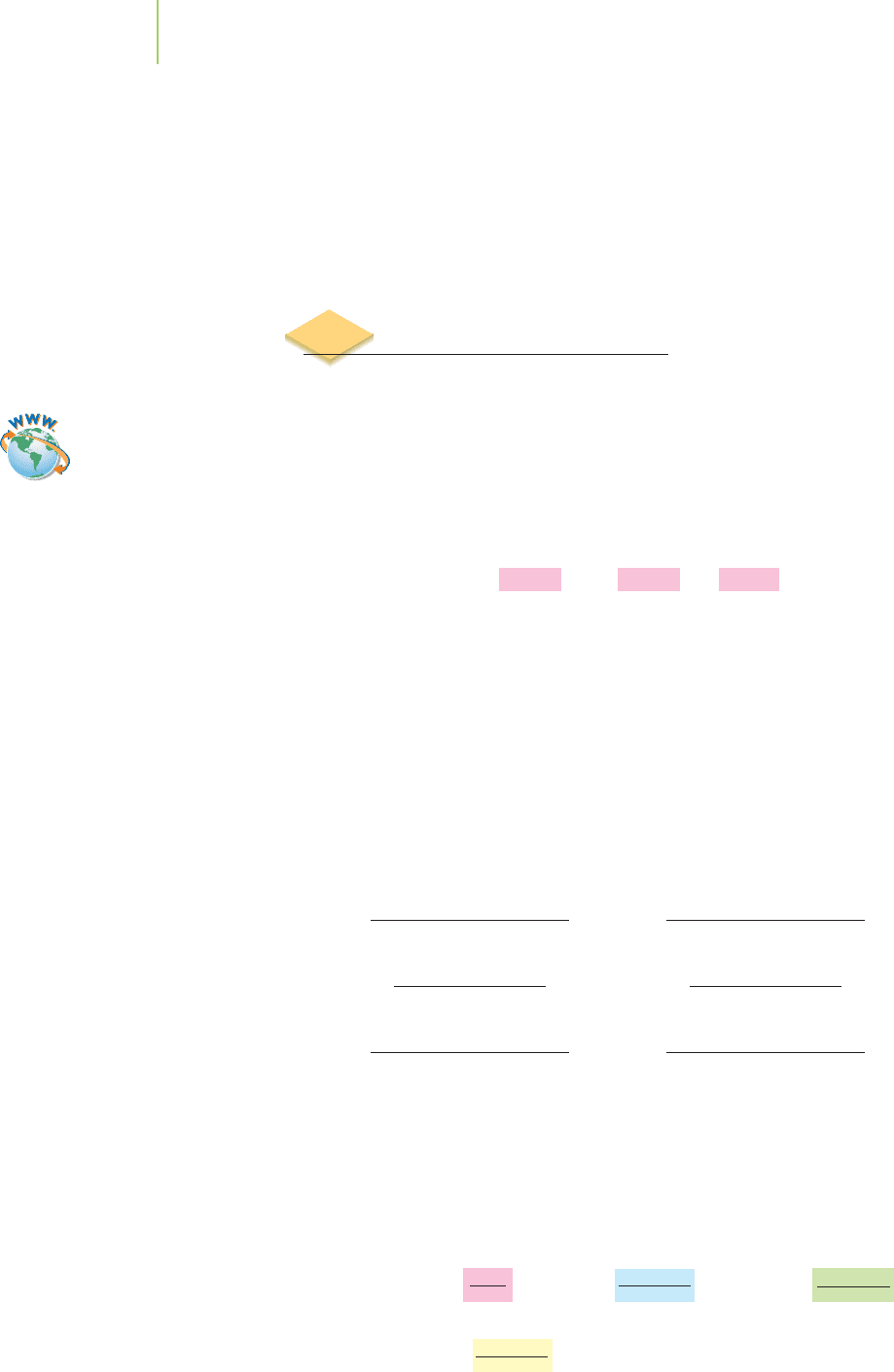
EXERCISE 3.11 Determining Products from Reactants
Hydrogen gas is used extensively in the food industry in a process called hydro-
genation, in which the gas is added to compounds called unsaturated fatty acids.
This hardens the fatty acids of vegetable oils, making them solids at room tempera-
ture, like the fats found in butter. It also converts the fatty acids into forms that are
less prone to the “oxidation” processes that give them an unpleasant rancid taste and
odor. An example of hydrogenation is the reaction of hydrogen gas with the fatty
acid called oleic acid:
C
18
H
34
O
2(s)
+ H
2(g)
→ C
18
H
36
O
2(s)
Oleic acid Stearic acid
What is the maximum amount of stearic acid that can be produced from the reac-
tion of 425 g of hydrogen gas with an excess of oleic acid?
Oleic acid H
2
Stearic acid
300.0 kg ethanoic acid ×
1000 g
1kg
×
1 mol ethanoic acid
60.05 g ethanoic acid
×
1 mol ethanoic anhydride
2 mol ethanoic acid
110 Chapter 3 Introducing Quantitative Chemistry
Further Insights
Demand for hydrogen gas is increasing rapidly. By the year 2007, almost 15 trillion
ft
3
of H
2
gas is expected to be used in the chemical industry, with over 93% used in
the production of ammonia-based agricultural fertilizers and “petrochemicals,”
chemicals derived from petroleum and natural gas. Although food and drink pro-
cessing, including hydrogenation reactions, make up a relatively small percentage
of the total demand, their percentage is rising as a consequence of increasing con-
sumer demand for products containing hydrogenated fatty acids.
PRACTICE 3.11
How many grams of oleic acid are needed to produce 72.55 g of stearic acid?
See Problems 65–68.
EXERCISE 3.12 Determining Products from Reactants: Focus on Mole Ratios
The most common route used for the industrial synthesis of hydrogen is the reac-
tion of water as steam with hydrocarbons—substances that contain only hydrogen
and carbon, such as ethane (C
2
H
6
), as in Exercise 3.10, or propane (C
3
H
8
). The re-
actants are passed over a catalyst such as nickel, a substance that greatly speeds the
process. The production of hydrogen from the reaction of steam with propane is as
follows:
C
3
H
8
(g) + 3H
2
O(g)
Ni(700-900
◦
C)
−−−−−−→
3CO(g) + 7H
2
(g)
How much hydrogen can be produced from the reaction of 30.0 g of propane with
excess steam?
Solution
In this case, our goal is to find grams of hydrogen from propane, so the pertinent
conversion factor is
7 mol H
2
1 mol C
3
H
8
Note that because the conversion factor was determined by counting the number of
moles of each compound, the resulting factor has an infinite number of significant
digits. The strategy can be diagrammed as follows:
gC
3
H
8
mol C
3
H
8
gC
3
H
8
−−−−−→mol C
3
H
8
mol H
2
mol C
3
H
8
−−−−−→mol H
2
gH
2
mol H
2
−−−−−→gH
2
30.0 g C
3
H
8
×
1 mol C
3
H
8
44.09 g C
3
H
8
×
7 mol H
2
1 mol C
3
H
8
×
2.016 g H
2
1 mol H
2
= 9.60 g H
2
Even though hydrogen is a very light gas, the 7-to-1 mole ratio of hydrogen to
propane means that a good deal of hydrogen will be produced from the reaction of
propane with steam.
PRACTICE 3.12
Using the equation in Exercise 3.11, determine how many kilograms of stearic acid
can be formed from the reaction of 673 kg of oleic acid with excess hydrogen gas.
See Problems 69a, 69b, 69c, 70a, and 71.
Pro
p
ane
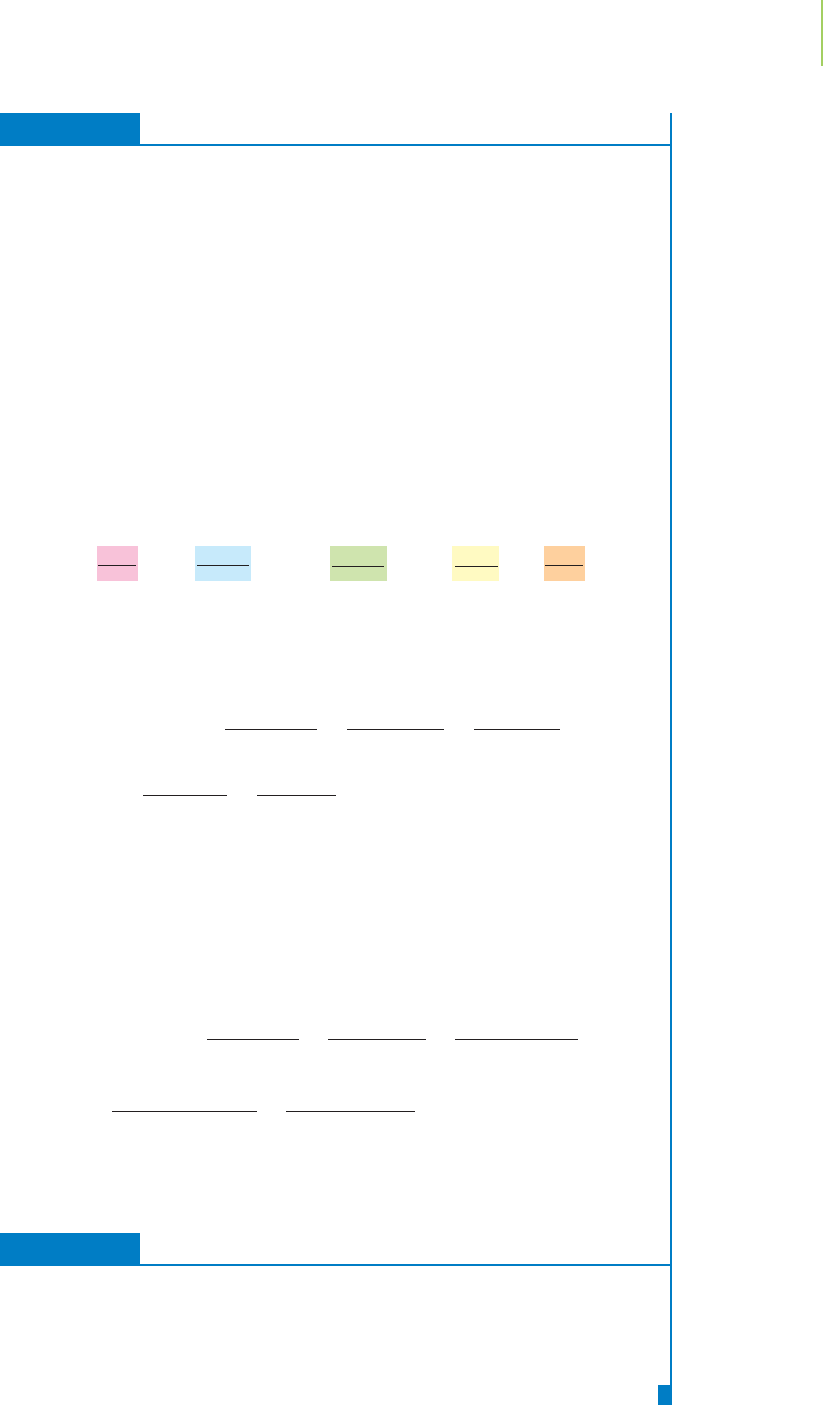
EXERCISE 3.13 Calculations Using Equations
Photosynthesis in plants generates sugars such as glucose (C
6
H
12
O
6
) and oxygen
from carbon dioxide and water. How many kilograms of oxygen are produced from
the photosynthesis of 330 kg of carbon dioxide?
First Thoughts
We need to obtain a balanced equation before performing any stoichiometric calcu-
lations in chemistry. For photosynthesis, the information given in the question en-
ables us to work out that the equation is unbalanced:
CO
2
(g) + H
2
O(l) → C
6
H
12
O
6
(s) + O
2
(g)
After balancing the equation, we get
6CO
2
(g) + 6H
2
O(l) → C
6
H
12
O
6
(s) + 6O
2
(g)
One aspect of good problem solving is to know what you are given, what you want,
and how to get there. We are starting with kilograms of CO
2
and want to find kilo-
grams of O
2
. We can navigate from one to the other in this way:
kg CO
2
1000 g
1kg
−−→gCO
2
mol CO
2
gCO
2
−−−−→mol CO
2
mol O
2
mol CO
2
−−−−→mol O
2
gO
2
mol O
2
−−−→gO
2
1kg
1000 g
−−→kg O
2
Solution
In our equation we have a 1-to-1 mole ratio of CO
2
to O
2
.
330 kg CO
2
×
1000 g CO
2
1kgCO
2
×
1 mol CO
2
44.01 g CO
2
×
6 mol O
2
6 mol CO
2
×
32.00 g O
2
1 mol O
2
×
1kgO
2
1000 g O
2
= 240 kg O
2
Further Insights
One mole of glucose (C
6
H
12
O
6
) is produced for every 6 mol of carbon dioxide
(CO
2
) consumed in the reaction. If we wanted to calculate the mass of glucose pro-
duced to accompany the calculated amount of oxygen, the problem-solving strategy
would be the same, except we would use glucose and its mole-to-mole ratio to car-
bon dioxide instead of that of oxygen to carbon dioxide.
330 kg CO
2
×
1000 g CO
2
1kgCO
2
×
1 mol CO
2
44.01 g CO
2
×
1 mol C
6
H
12
O
6
6 mol CO
2
×
180.16 g C
6
H
12
O
6
1 mol C
6
H
12
O
6
×
1kgC
6
H
12
O
6
1000 g C
6
H
12
O
6
= 230 kg C
6
H
12
O
6
Keep in mind the key parts of stoichiometry problem solving: the balanced equa-
tion, where you start, where you want to end up and, how you get there.
PRACTICE 3.13
What mass of CO
2
gas is released when 26 g of methane (CH
4
) burns in oxygen (O
2
)
to generate CO
2
and water (H
2
O)? To solve this question, you need to use several of
the techniques presented in this chapter—specifically, working out molar masses,
writing balanced equations, and performing a stoichiometric calculation.
See Problems 75a, 76a, and 85.
3.7 Working with Equations 111
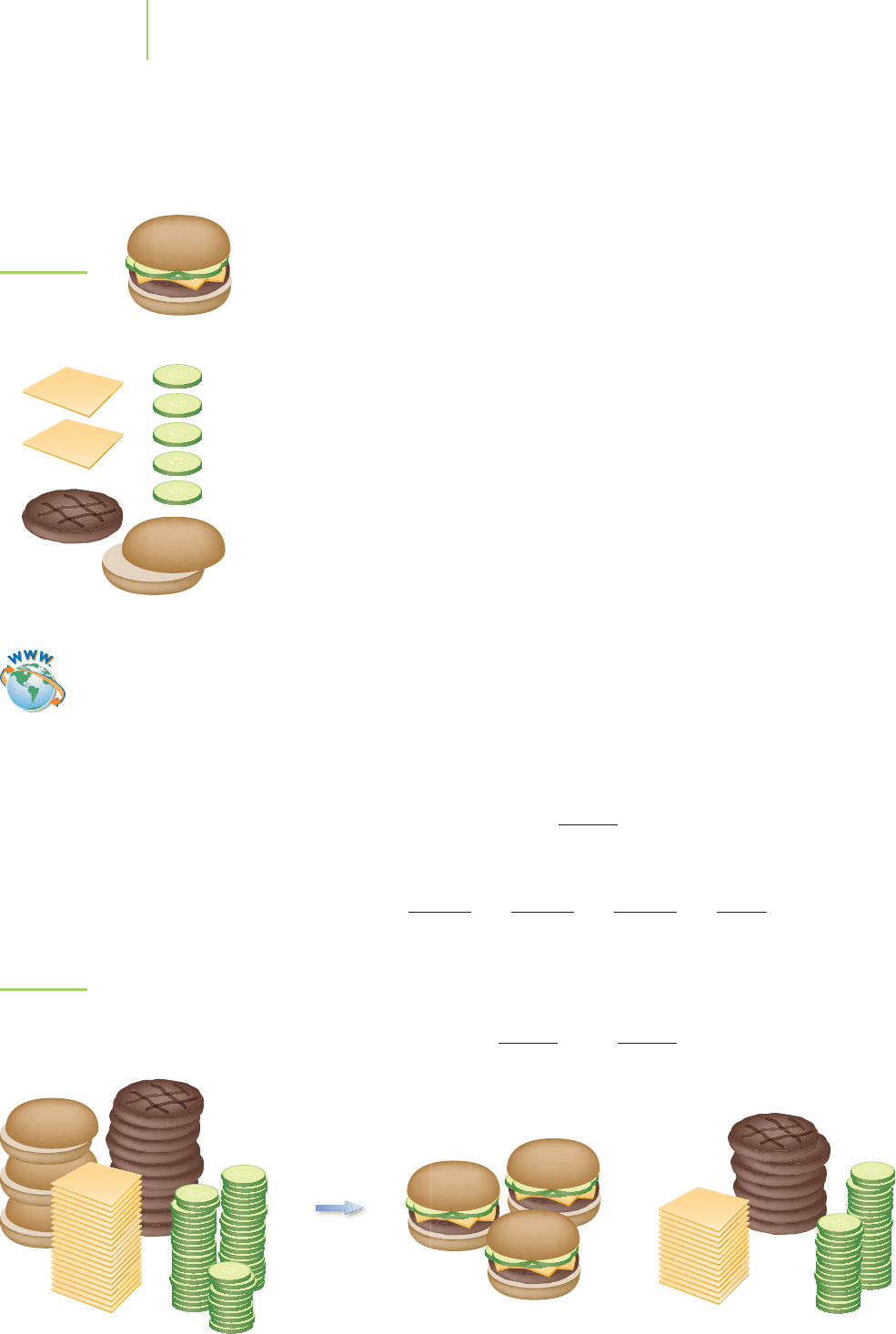
FIGURE 3.14
Analogy of limiting reagent to limiting
ingredient in the construction of a
sandwich.
FIGURE 3.13
A hamburger.
112 Chapter 3 Introducing Quantitative Chemistry
Limiting Reagent
We have seen that equations indicate how many moles of each reactant will react
together, but chemists do not usually combine reactants in the exact proportions
shown in the chemical equation. Instead, they generally use an excess amount of
one or more of the reactants, perhaps the least expensive one, in order to encour-
age as much of the other reactants as possible to react. In this situation, the reac-
tant that will be used up first is called the
limiting reagent, or limiting reactant,
because it is the quantity of this reagent that imposes a limit on how much prod-
uct can be formed.
An analogy that is helpful in understanding what we mean by a limiting
reagent is the preparation of hamburgers for a frozen food company. Each ham-
burger must be like every other because the company’s customers expect a
consistent product. Each hamburger, shown in Figure 3.13 both in parts and
completed, contains only
1 patty
5 pickles
2 slices of cheese
1 bun
In this analogy, we can write the preparation of the product from the “reactants”
as follows:
1 patty + 5 pickles + 2 cheese + 1 bun → 1 hamburger
Our quota for each hour of work is 10 completed hamburgers. We have on the
shelf 10 patties, 50 pickles, 20 slices of cheese, but only 3 buns. How many ham-
burgers can we make? As Figure 3.14 shows, we can only prepare 3 complete
hamburgers, because the number of buns limits our production. The buns are the
limiting reagent in this case. How many slices of cheese are in excess—that is, how
many slices of cheese are left over? In a manner analogous to expressing a mole
ratio, we can say that according to the equation for sandwich preparation, each
bun requires 2 slices of cheese, giving us a ratio of
1bun
2 cheese
We can write also other valid reactant “mole ratios,” such as
1bun
5 pickles
5 pickles
1 patty
2 cheese
5 pickles
1 patty
1bun
We asked how many slices of cheese are left over from our original stack of
20 slices if we use 3 buns. First, we can ask, “How many cheese slices are actually
used to combine with 3 buns?” The ratio of buns to cheese is
1bun
2 cheese
or
2 cheese
1bun
+
Video Lesson: Finding Limiting
Reagents
Visualization: Limiting Reactant
Video Lesson: CIA
Demonstration: Self-Inflating
Hydrogen Balloons
The number of cheese slices used, then, is
3 buns ×
2 cheese
1bun
= 6 cheese
The number of cheese slices in excess is the number we had on the shelf (20 cheese
slices) minus the number we used (6 cheese slices).
20 cheese – 6 cheese = 14 slices of cheese in excess
This process of determining how much of a reagent is used and how much is left
over is about the same as the one we use with chemical systems. The only differ-
ence is that we think in terms of moles rather than slices of cheese, patties, pick-
les, or buns. Let’s carefully build on our understanding of the concept by using
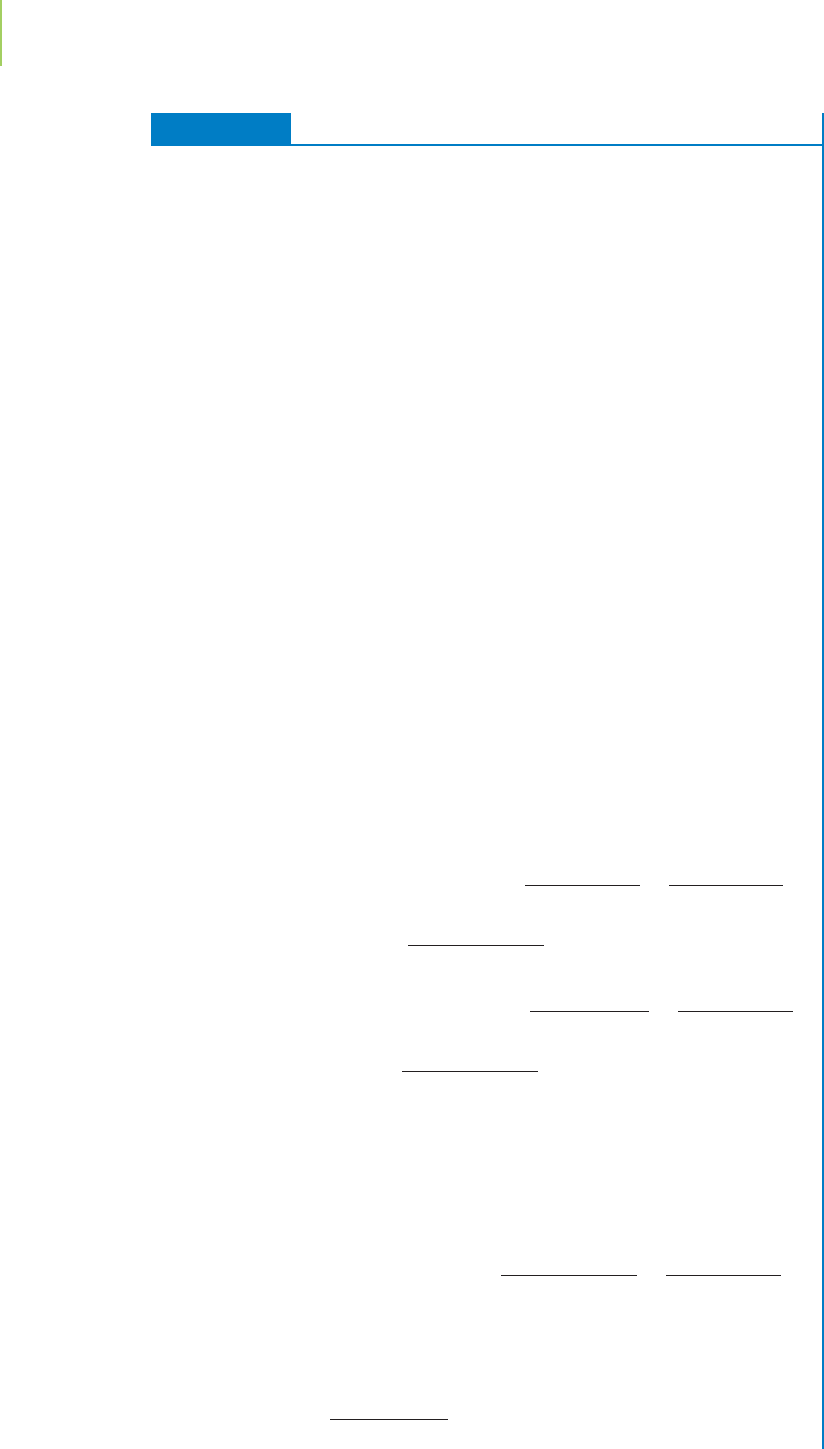
plastic models to assemble a water molecule, as shown in Figure 3.15.
The formulafor water is H
2
O,so we need 2 model hydrogen atoms and 1 model
oxygen atom per water molecule. If we have available a pile of 10 oxygen atoms
and a pile of 60 hydrogen atoms, we will be able to assemble only 10 water mole-
cules before we run out of oxygen atoms. In this situation oxygen is the limiting
reagent because the number of oxygen atoms present limits to 10 the number of
water molecules we can form. Hydrogen atoms, on the other hand, are“in excess,”
so some of them are left over once all the oxygen has been used up.
We can apply our understanding of limiting reagents to a chemical system in
which ensuring that the correct reactant is the limiting one is literally a matter of
life or death. In a “manned” spacecraft, it is vital that the carbon dioxide gas
breathed out by the astronauts not be allowed to accumulate in the cabin. If it
were to accumulate, it would soon rise to levels that would cause the occupants to
become dizzy and confused and eventually to slip into unconsciousness. One
process that has been used by the U.S. space program to remove carbon dioxide
involves the reaction of the gas with lithium hydroxide (LiOH) as it is drawn
through special filters:
2LiOH(s) + CO
2
(g) → Li
2
CO
3
(s) + H
2
O(l)
In this application, it is essential that there be more than enough lithium hydrox-
ide available to react with all of the carbon dioxide that the astronauts will pro-
duce. Therefore, it is vital that carbon dioxide be the limiting reagent and that
lithium hydroxide be present in excess. Will 5.0 kg of lithium hydroxide be suffi-
cient to remove the carbon dioxide released by one astronaut per day (typically
1.0 kg of CO
2
)?
We can answer the question by determining the limiting reagent in these cir-
cumstances. Here is the basic information we need:
2LiOH(s) + CO
2
(g) → Li
2
CO
3
(s) + H
2
O(l)
2 moles + 1 mole
23.95 g/mol 44.01 g/mol
We can calculate the amount of lithium hydroxide needed to react with the aver-
age 1.0 kg (1.0
× 10
3
g) of carbon dioxide released per day as follows. Remember
our flowchart diagram:
1.0 ×10
3
gCO
2
×
1 mol CO
2
44.01 g CO
2
×
2 mol LiOH
1 mol CO
2
×
23.95 g LiOH
1 mol LiOH
= 1100 g LiOH per 1.0 × 10
3
gCO
2
If we have 5.0 kg (5.0 × 10
3
g) of LiOH available per day, the carbon dioxide is
going to be the limiting reagent, as we wish, with a big margin of error to be on
the safe side. The LiOH will be in excess, and there will be plenty of LiOH left over
at the end of the day.
3.7 Working with Equations 113
FIGURE 3.15
Model atoms typically used in teaching
laboratories can be used to illustrate the
principle of the limiting reagent.
Application
C
HEMICAL ENCOUNTERS
:
Cleaning the Air on
Manned Spacecraft
114 Chapter 3 Introducing Quantitative Chemistry
EXERCISE 3.14 Calculating Yield and Limiting Reagent
Sodium hydroxide reacts with phosphoric acid to give sodium phosphate and water.
Assume that 17.80 g of NaOH is mixed with 15.40 g of H
3
PO
4
.
a. How many grams of Na
3
PO
4
can be formed?
b. How many grams of the excess reactant remain unreacted?
First Thoughts
We must first write a balanced chemical equation for this reaction:
3NaOH(aq) + H
3
PO
4
(aq) → Na
3
PO
4
(aq) + 3H
2
O(l)
In part (a), as in all limiting-reagent problems, the basic question to be addressed is
Which compound limits the amount of product formed? One useful strategy is to
calculate the amount of product that would be formed by each reactant if it were
fully consumed. The reactant that forms the least product is limiting, and that total
amount of product will be formed, theoretically:
g of reactant
molar mass
−−−−−→moles of reactant
mole ratio
−−−−−→
moles of product
molar mass
−−−−−→gofproduct
Given your understanding of mole-to-mole relationships, you might see several
ways of dealing with this problem, including not going all the way to grams but,
rather, making your decision on the basis of moles. We present here one of several
ways of solving this problem. In part (b), to determine grams of excess reactant, you
must calculate how many moles of excess reactant were actually used.
# moles excess = # moles original − # moles used
Then convert moles to grams.
Solution
a. Determination of limiting reagent
g Na
3
PO
4
from NaOH = 17.80 g NaOH
×
1 mol NaOH
40.00 g NaOH
×
1 mol Na
3
PO
4
3 mol NaOH
×
163.94 g Na
3
PO
4
1 mol Na
3
PO
4
= 24.32 g Na
3
PO
4
from NaOH
gNa
3
PO
4
from H
3
PO
4
= 15.40 g H
3
PO
4
×
1 mol H
3
PO
4
97.99 g H
3
PO
4
×
1 mol Na
3
PO
4
1 mol H
3
PO
4
×
163.94 g Na
3
PO
4
1 mol Na
3
PO
4
= 25.76 g Na
3
PO
4
from H
3
PO
4
Therefore, NaOH is the limiting reagent, and 24.32 g of Na
3
PO
4
are formed.
b. Determination of grams of excess reactant
H
3
PO
4
is in excess. If 24.32 g of Na
3
PO
4
is formed, the moles of H
3
PO
4
used is
given by
mol H
3
PO
4
used = 24.32 g Na
3
PO
4
×
1 mol Na
3
PO
4
163.94 g Na
3
PO
4
×
1 mol H
3
PO
4
1 mol Na
3
PO
4
= 0.1483 mol H
3
PO
4
used
The number of moles of H
3
PO
4
originally present is
15.40 g H
3
PO
4
×
1 mol H
3
PO
4
97.99 g H
3
PO
4
= 0.1572 mol H
3
PO
4
originally present

mol H
3
PO
4
excess = mol H
3
PO
4
originally present − mol H
3
PO
4
used
= 0.1572 mol − 0.1483 mol
= 0.0089 mol H
3
PO
4
excess
g H
3
PO
4
excess = 0.0089 mol H
3
PO
4
×
97.99 g H
3
PO
4
1 mol H
3
PO
4
= 0.87 g H
3
PO
4
excess
Further Insights
We often choose to perform a chemical reaction with one reactant in excess. Yet
there is an important class of reactions, “titrations,” in which the goal is to add pre-
cisely enough moles of one reactant to just consume the other. Using titrations, we
can determine the amount of many types of substances, including acids, bases, and
metals. We will learn more about titrations in the next chapter, and we will take an
in-depth look at the technique in Chapters 16–18.
PRACTICE 3.14
Calcium hydroxide reacts with hydrogen chloride to produce water and calcium
chloride. If 12.33 g of calcium hydroxide is placed in a flask with 32.15 g of hydro-
gen chloride, how many grams of calcium chloride will be formed?
See Problems 70, 73, 74, 77, and 80.
Chemical reactions are rarely 100% efficient, because there are always losses
due to unwanted side reactions, to some of the reactants remaining unreacted,
and/or to some of the products being converted back into reactants once they are
formed. Chemists designing a synthesis reaction can compare the actual masses
obtained under various conditions with the masses predicted from the equation.
Doing so enables them to calculate the percentage yield of a reaction and to
adjust the conditions until the maximum percentage yield is obtained. The max-
imum amount of any chemical that could be produced in a chemical reaction,
which can be calculated from the equation for the reaction, is called the
theoretical
yield
. The amount of product obtained experimentally is called the actual yield.
The
percentage yield of a reaction equals the actual yield expressed as a percent-
age of the theoretical yield:
Percentage yield =
actual yield
theoretical yield
× 100%
This first look at the uses of equations should help you realize that equations
provide useful information that chemists must have if they are to do their jobs of
predicting and producing desired products, whether on a relatively small scale in a
laboratory or on an industrial process scale in a huge manufacturing facility.
EXERCISE 3.15 Percentage Yield
In the previous exercise, we determined that 24.32 g of sodium phosphate could
theoretically be prepared under the conditions given. If only 20.07 g were obtained
from the reaction, what would be the percentage yield of the reaction?
Solution
Percentage yield =
actual yield
theoretical yield
× 100% =
20.07 g
24.32 g
× 100% = 82.52% yield
3.7 Working with Equations 115
Video Lesson: Theoretical Yield
and Percent Yield

PRACTICE 3.15
Assuming that the yield of sodium phosphate from sodium hydroxide and phos-
phoric acid can never be greater than 82.52%, how many grams of sodium hydrox-
ide would be needed to produce 100 g of sodium phosphate?
See Problems 69d, 75b, 76b, 81, 82b, 84, and 86.
116 Chapter 3 Introducing Quantitative Chemistry
In everyday life, we are bombarded by
conflicting messages from different sides
of debates about quantitative chemistry. We may not
realize that quantitative chemistry is at the heart of these
debates, but it is. In considering our diet, we often pose
questions such as: How much food should we eat each
day? How many grams of fat should be in our diet? What
levels of each vitamin are too low for good health (see
Figure 3.16), and what levels are dangerously high? We
know that too much alcohol in beverages is bad for us,
but are small, regular amounts beneficial, or is total
abstinence the most healthful choice?
These issues are complex because most chemicals
have more than one effect on the body. In other words,
a single chemical can participate in several different
chemical reactions within the body. Some of these reac-
tions may bring clear benefits, whereas others can pose
Issues and Controversies
Everyday controversies of quantitative chemistry
dangers. These are all issues of quantitative chemistry,
revolving around what quantities of a chemical are re-
quired to produce a certain level of benefit and what
quantities produce a given level of harm. The benefits
and the dangers are all caused, directly or indirectly, by
the stoichiometry of the chemical reactions in which the
chemicals participate.
Vitamin C is an excellent example. We know that we
must consume a certain amount of this vitamin, which is
normally obtained from fresh fruits and vegetables, for
good health. A deficiency of vitamin C causes a variety
of problems known collectively as “scurvy,” which is
characterized by fatigue, sore joints and muscles, hemor-
rhaging, and anemia. The minimum level of vitamin C
needed to prevent these problems can be determined by
the quantitative analysis of people’s diets and by the as-
sociation between these diets and the appearance of
symptoms of vitamin C deficiency. Even this issue, how-
ever, is linked to controversy, because different countries
use different recommended levels, and nutritionists reg-
ularly debate whether the levels should be revised up-
ward or downward. The current recommended dietary
allowance (RDA) of vitamin C in the United States is
60 mg per day for adults. Go into a drugstore, however,
and you will easily find tablets available that each deliver
a massive 1000-mg (1-g) dose of vitamin C. These are
sold because some scientists, most notably Linus Paul-
ing, have promoted the view that huge “megadoses” of
vitamin C can protect against colds and even cancer.
Others caution, however, that excessive consumption of
vitamin C may cause problems such as nausea, diarrhea,
iron overload—and possibly even cancer.
The debate about the appropriate amount of vitamin
C to consume is mirrored by similar debates about every
other vitamin and about many
other components of our diet.
One of the most important activ-
ities in the attempts to resolve
these debates is the careful quan-
titative analysis of the many ef-
fects each chemical can have on
the chemistry of life.
FIGURE 3.16
The quantities of chemicals we consume are
important—and what quantities are ideal is the
subject of much debate.
Oranges are a good source of Vitamin C.
Video Lesson: A Problem Using
the Combined Concepts of
Stoichiometry
Key Words 117
The Bottom Line
■
Quantities in chemistry are crucial. Different
amounts of the same chemical can have very differ-
ent effects on chemical systems such as living things,
environmental systems, and industrial processes.
(Chapter opening)
■
The formula mass (formula weight) of a chemical is
the total mass of all the atoms present in its formula,
in atomic mass units (amu) or in grams per mole.
(Section 3.1)
■
The average molecular mass (molecular weight) of a
molecule is the total mass of the molecule, in atomic
mass units (amu) or in grams per mole. (Section 3.1)
■
The mole is the basic counting unit of chemistry—
the chemist’s“dozen”—and 1 mol of any chemical
contains Avogadro’s number (6.022 × 10
23
) of mole-
cules, atoms, or formula units (which entity to use
depends on the chemical concerned). (Section 3.2)
■
We use the mole to convert between molecules and
grams of a substance. (Section 3.3)
■
The percent, by mass, of an element in a compound
is called its mass percent. (Section 3.4)
■
The empirical formula for a compound indicates the
simplest whole-number ratio in which its component
atoms are present. The molecular formula indicates
the actual number of each type of atom in one mole-
cule of the compound. (Section 3.5)
■
A chemical equation uses chemical formulas to indi-
cate the reactants and products of a reaction and
uses numbers before the formulas to indicate the
proportions in which the chemicals involved react
together and are formed. (Section 3.6)
■
Stoichiometry is the study and use of quantitative
relationships in chemical processes. (Section 3.7)
■
The limiting reagent in a reaction is the one that is
consumed first, causing the reaction to cease despite
the fact that the other reactants remain “in excess.”
(Section 3.7)
■
The percentage yield of a reaction equals the actual
yield expressed as a percentage of the theoretical yield:
Percentage yield =
actual yield
theoretical yield
× 100%
(Section 3.7)
■
Chemistry is a quantitative science. The practice
of chemistry in the real world demands mastery of
the quantitative skills introduced in this chapter.
(Issues and Controversies)
Key Words
actual yield The experimental quantity, in grams, of
product obtained in a reaction. (p. 115)
Avogadro’s number (N
A
) The number of particles
(6.022 × 10
23
) of a substance in 1 mol of that
substance. By definition, Avogadro’s number is
equal to the number of carbon-12 atoms in exactly
12.0000 g of carbon-12. (p. 91)
balanced Appropriate coefficients have been added such
that the number of atoms of each element are the
same in both reactants and products. (p. 103)
chemical equation A precise quantitative description of
a reaction. (p. 103)
coefficients The numbers placed in front of the
substances in a chemical equation that reflect the
specific numbers of units of those substances
required to balance the equation. (p. 105)
formula mass The total mass of all the atoms present in
the formula of an ionic compound, in atomic mass
units (amu), or the mass of one mole of formula
units in grams per mole. (p. 88)
limiting reagent The reactant that is consumed first,
causing the reaction to cease despite the fact that the
other reactants remain “in excess.” Also known as the
limiting reactant. (p. 112)
mass percent The percent of a component by mass.
(p. 97)
Mass percent =
total mass component
total mass whole substance
× 100%
molar mass The total mass of all the atoms present in
the formula of a molecule, in atomic mass units
(amu) or in grams per mole. (p. 92)
mole The quantity represented by 6.022 × 10
23
particles. (p. 92)
molecular mass The mass of one molecule, expressed in
atomic mass units (amu), or the mass of one mole of
molecules in grams per mole. (p. 87)
percentage yield The actual yield of a reaction divided
by the theoretical yield and then multiplied by 100%.
(p. 115)
phase A part of matter that is chemically and physically
homogeneous. (p. 107)
products The substances located on the right-hand side
of a chemical equation. (p. 103)
