Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


78 Chapter 2 Atoms: A Quest for Understanding
chemical formula A representation, in symbols, that con-
veys the relative proportions of atoms of the different
elements in a substance. (p. 68)
chemical nomenclature A system of rules used to assign
a name to a particular substance. (p. 72)
coulomb The unit of electrical charge. (p. 55)
covalent bonds Bonds that atoms form by sharing elec-
trons. (p. 70)
Dalton’s atomic theory The theory, developed by John
Dalton, that all substances are composed of indivisi-
ble atoms. (p. 50)
diatomic elements Elements whose normal state is in the
form of molecules composed of two atoms attached
together (most notably H
2
,N
2
,O
2
,F
2
,Cl
2
,Br
2
,
and I
2
). (p. 71)
electron One of the subatomic particles of which atoms
are composed. Electrons carry a charge of −1.
(p. 52)
empirical formula A chemical formula indicating the
ratio in which elements are found to be present in a
particular compound, regardless of the molecular
structure of the compound. (p. 71)
gamma ray High-energy electromagnetic radiation
emitted from the decay of a radioactive element.
(p. 54)
group One of the vertical columns in the periodic table
of the elements. (p. 64)
halogens The elements of Group VIIA of the periodic
table. (p. 66)
inert gases See noble gases. (p. 65)
ion An electrically charged entity that results when an
atom has gained or lost electrons. (p. 56)
ionic compound A compound composed of ions. (p. 68)
isotopes Forms of the same element that differ in the
number of neutrons within the nucleus. (p. 57)
law of combining volumes Scientific law stating that when
gases combine, they do so in small whole-number ra-
tios, provided that all the gases are at the same tem-
perature and pressure. (p. 50)
law of conservation of mass Scientific law stating that the
mass of chemicals present at the start of a chemical
reaction must equal the mass of chemicals present at
the end of the reaction. Although not strictly true,
this law is correct at the level of accuracy of all labo-
ratory balances. (p. 47)
law of definite composition Scientific law stating that any
particular chemical is always composed of its compo-
nents in a fixed ratio, by mass. (p. 48)
law of multiple proportions Scientific law stating that
when the same elements can produce more than one
compound, the ratio of the masses of the element
that combine with a fixed mass of another element
corresponds to a small whole number. (p. 49)
mass number (A) The total number of protons and neu-
trons in the nucleus of an atom. (p. 57)
mass spectrometer An instrument used to measure the
masses and abundances of isotopes, molecules, and
fragments of molecules. (p. 59)
mass spectrum The output of a mass spectrometer, in
the form of a chart listing the abundance and masses
of the isotopes, molecules, and fragments of mole-
cules present. (p. 60)
metalloids A small number of elements, found at the
boundary between metals and nonmetals in the peri-
odic table, that have properties intermediate between
those of metals and nonmetals. Also known as semi-
metals. (p. 65)
metals The largest category of elements in the periodic
table. Metals exhibit such characteristic properties as
ability to conduct electricity, shiny appearance, and
malleability. (p. 64)
molecular formula A chemical formula indicating the ac-
tual number of each type of atom present in one
molecule of a compound. (p. 70)
molecular ions Ions composed of two or more atoms at-
tached together. Also known as polyatomic ions.
(p. 75)
molecules Neutral compounds containing two or more
atoms attached together. (p. 70)
neutron One of the subatomic particles of which atoms
are composed, carrying no electrical charge and
found in the nucleus. (p. 54)
noble gases The unreactive elements in the rightmost
group of the periodic table (Group VIIIA). Also
known as inert gases. (p. 65)
nonmetals The elements on the right-hand side of the
periodic table. Nonmetals exhibit characteristic prop-
erties that are distinct from those of the metals.
(p. 65)
nuclide notation Shorthand notation used to represent
atoms, listing the symbol for the element, accompa-
nied by the atomic number and the mass number of
the atom. (p. 57)
period One of the horizontal rows in the periodic table
of the elements. (p. 64)
periodic table of the elements A table presenting all the
elements, in the form of horizontal periods and verti-
cal groups; shown on the inside front cover of this
book. (p. 56)
polyatomic ions Ions composed of two or more atoms
covalently attached together. Also known as molecu-
lar ions. (p. 75)
products The materials that are produced in a chemical
reaction. (p. 47)
proton One of the subatomic particles of which atoms
are composed, carrying an electrical charge of +1
and found in the nucleus. (p. 53)
radiation The particles and/or energy emitted during
radioactive decay. (p. 54)
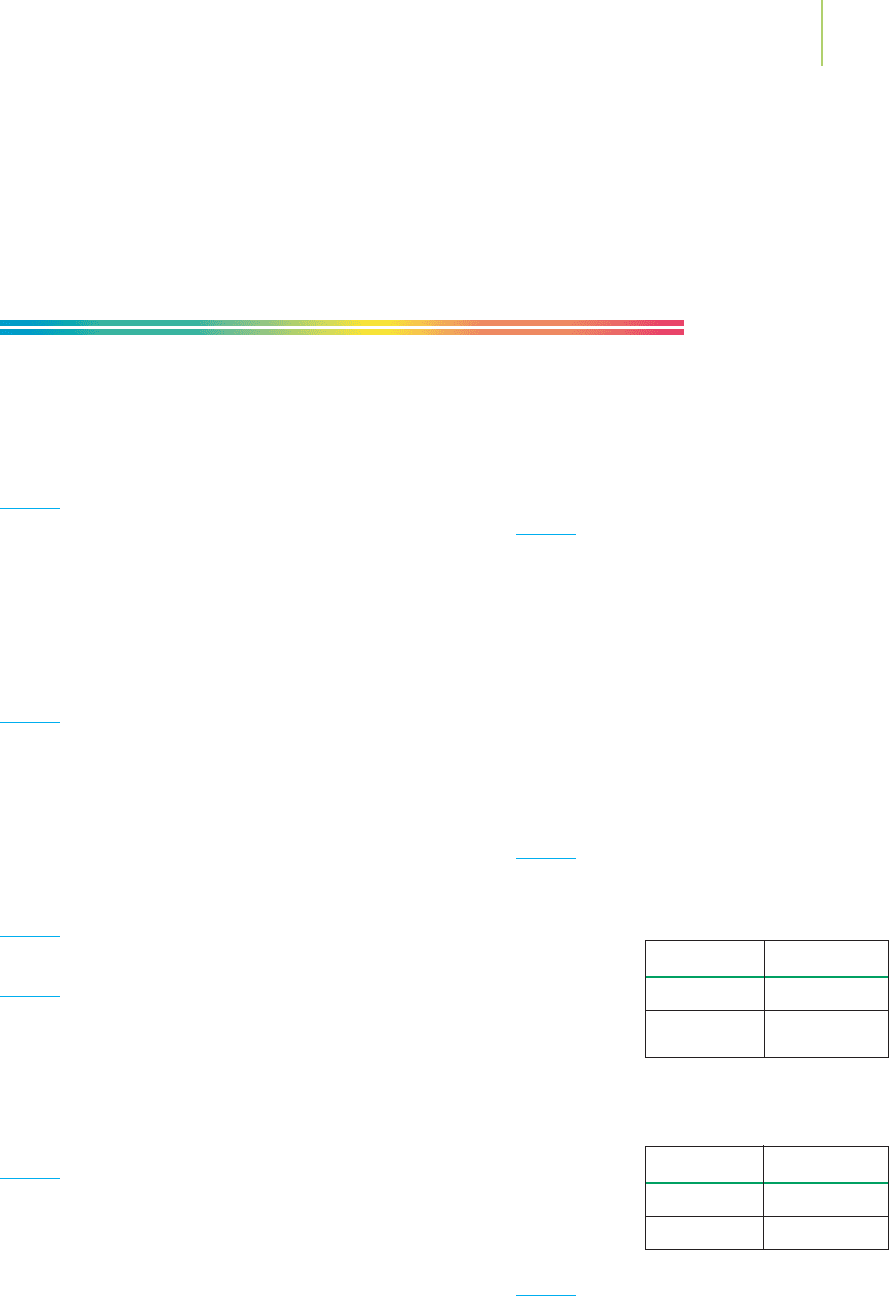
radioactive decay The process by which an unstable nu-
cleus becomes more stable via the emission or ab-
sorption of particles and accompanying energy.
(p. 54)
radioactivity The emission of radioactive particles
and/or energy. (p. 54)
Focus Your Learning 79
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 2.1 Early Attempts to Explain Matter
Skill Review
1. Exploring the unseen world often relies on indirect reason-
ing. Useful deductions led early philosophers and scientists
to make conclusions about the possible existence of atoms.
How would the formation of ice crystals on the branches of a
bush support the ideas of Democritus?
2. Ancient practices, and some modern ones, involve burning
incense. How would entering a room in which incense had
recently been burned lead you to consider the world to be
made of small, invisible particles?
3. The scientists who first used atoms to explain chemical
changes assumed that the small, unseen pieces of matter
could continually be rearranged into new combinations with
new properties. Show how that same principle operates when
the letters in the word dormitory are used to form several
other words.
4. Taking the analogy in Problem 3 a bit further, explain what
would have to happen in order for the exercise to illustrate
the law of conservation of mass.
5. In your own words, explain the law of conservation of mass.
6. In your own words, explain the law of definite composition.
7. Modern science is based on asking questions. In what way
does a researcher determining the age of Earth ask the same
basic questions posed by Democritus?
8. What connection exists between the exploration and obser-
vations of the United States space program and Democritus’
questions?
Chemical Applications and Practices
9. Some compounds absorb water from the atmosphere in such
a way that the water is chemically combined with the com-
pound. The absorbed water is called water of hydration, and
the compounds with absorbed water are known as hydrates.
If 14.7 g of calcium chloride hydrate, when heated, loses 3.6 g
of water, how many grams of water are lost when a 23.4-g
sample is heated? To which law(s) did you refer in obtaining
these results?
10. The white appearance of several magnesium-containing
compounds causes an apparent resemblance among the
compounds. One such compound was shown to contain
60.0% magnesium. Another sample of a magnesium-
containing compound was found. What quantity of magne-
sium, in grams, would have to be present in a 34.6-g sample
of the second compound in order for us to claim that it is the
same compound as the first sample?
Section 2.2 Dalton’s Atomic Theory and Beyond
Skill Review
11. Lavoisier used carefully determined masses to make discover-
ies about changes that occurred in chemical reactions.
a. Which fundamental law did his work lead him to discover?
b. Suppose Lavoisier evaporated the water from 250.0 g of
seawater. If the remaining salt had a mass of 2.2 g, what
would you calculate as the mass of the water that had
evaporated?
12. A 200.0-g sample of a pure substance was composed of
132.9 g of copper and 67.1 g of sulfur. Another sample of the
same substance, this time with a mass of 150.0 g, was brought
to Joseph Proust to analyze. How many grams of copper and
sulfur would you expect to find in the sample? What basic
law in chemistry are you employing in order to make your
determination?
13. Fill in the missing information for a compound of copper
and oxygen, assuming that it is composed of a fixed ratio of
copper to oxygen of 3.97:1.00.
14. Fill in the missing information for a compound consisting of
sodium and oxygen, assuming that is composed of a fixed
ratio of sodium to oxygen of 1.44:1.00.
Chemical Applications and Practices
15. Copper and oxygen combine in more than one ratio. In one
such compound it is found that 65 grams of copper combine
with 16 grams of oxygen. If you assumed, as John Dalton
might have, that the combination represented one atom of
copper combining with one atom of oxygen, what would you
predict as the copper-to-oxygen ratio in another compound
between the two?
Sodium Oxygen
6.0 grams grams
grams 50.0 grams
Copper Oxygen
17.0 grams grams
grams 11.5 grams
reactants The materials that combine in a chemical re-
action. (p. 47)
semimetals A small number of elements found at the
boundary between metals and nonmetals in the peri-
odic table. Also known as metalloids. (p. 65)
80 Chapter 2 Atoms: A Quest for Understanding
16. Using the first mass ratio presented in Problem 15 and your
answer for the other ratio, determine how many grams of
oxygen would combine with 10.0 g of copper in both
compounds.
17. When the calcium is properly extracted, you can expect to
obtain 40 g of calcium from 100.0 g of calcium carbonate. A
10.0-g sample of pure calcium chloride yields 6.40 g of chlo-
rine. Which of these two compounds is a better source of
calcium?
18. Three magnesium compounds have the following percent-
ages of magnesium: Compound A: 28.6% magnesium;
Compound B: 60.0% magnesium; Compound C: 41.4%
magnesium. Why, according to Dalton, can there be such a
great difference in the percentages of magnesium?
19. Water and hydrogen peroxide are both clear liquids that are
made up of only hydrogen and oxygen. Water contains 2 g of
hydrogen for every 16 g of oxygen in water. In hydrogen
peroxide there are 2 g of hydrogen for every 32 g of oxygen.
Show how these data illustrate the law of multiple
proportions.
20. Oxygen exists naturally in two combined forms. The di-
atomic oxygen that we utilize in breathing consists of two
atoms combined into one unit.Ozone consists of three atoms
of oxygen joined into one unit. The relative masses of the oxy-
gen and ozone units are 32 and 48. Does this agree with the
law of multiple proportions? Explain.
Section 2.3 The Structure of the Atom
Skill Review
21. If the discoverers of the particles that make up the atom had
determined the charge on the electron to be −2, what would
be the charge on the neutron and proton?
22. Describe what might have been the results of Rutherford’s
experiment if the charge on the electron had been reversed
(that is, if it were +1 instead of −1).
23. Draw diagrams of the models of the atom that J. J. Thomson
and Rutherford, respectively, would have come up with if the
electron had a +1 charge.
24. What are two objections to the explanation that positive pro-
tons compose a nucleus that is surrounded by negative
electrons.
+1 electron
– charged nucleus
O
3
O
2
Section 2.4 Atoms and Isotopes
Skill Review
25. Fill in the information missing from the following table:
26. Fill in the information missing from the following table:
27. Indicate whether each of these phrases describes the proton,
neutron, or electron. (You may need to assign more than one
term to each phrase.)
a. Determines the identity of an atom.
b. Has about the same mass as a proton.
c. The number of particles can be changed without changing
the identity of the atom.
28. Indicate whether each of these phrases refers to protons, neu-
trons, and/or electrons. (You may need to assign more than
one term to each phrase.)
a. Is represented by the mass number.
b. Is represented by the atomic number.
c. Are always present in the same quantity in an atom with
0 charge.
29. What would you calculate for the mass, in grams, of an atom
that contained 1 proton and 1 electron? . . . 1 proton, 1 neu-
tron, and 1 electron?
30. What would you calculate for the mass, in grams, of an atom
that contained 2 protons, 2 neutrons, and 2 electrons? . . .
1 proton, 1 neutron, and 2 electrons?
31. What would be the charge on each of the particles listed in
Problem 29?
32. What would be the charge on each of the particles listed in
Problem 30?
33. Assume that an atom contained 1 proton and 1 electron.
By what percentage would the mass of this atom increase if
10 electrons were added to the atom?
34. Assume that an atom contained 1 proton and 1 electron.
By what percentage would the mass of this atom increase if
10 neutrons were added to the atom?
35. Which of the most abundant isotope in each pair contains
the greater number of neutrons? Assume the mass number of
the most abundant isotope can be found by rounding the av-
erage atomic mass to the nearest whole number. (Use the pe-
riodic table of the elements inside the front cover of your
textbook.)
a. iron or chromium c. cobalt or nickel
b. tellurium or iodine d. helium or neon
Isotope Protons Neutrons Electrons Charge
calcium-40 20 18
14 14 0
sulfur-32 16 14
Isotope Protons Neutrons Electrons Charge
carbon-12 6 6 0
13 14 10
chlorine-35 18 −1
Focus Your Learning 81
36. Which of the most abundant isotope in each pair contains
the greater number of neutrons? Round the average atomic
mass to the nearest whole number to obtain the mass num-
ber of the most abundant isotope. (Use the periodic table of
the elements inside the front cover of your textbook.)
a. zinc or sodium c. phosphorus or sulfur
b. hydrogen or sodium d. bromine or selenium
37. Arrange the following atoms in order of their quantity of
protons (least number to greatest number): iron, hydrogen,
calcium, fluorine, aluminum, boron.
38. Arrange these atoms in order of their quantity of protons
(least number to greatest number): sodium, magnesium,
copper, iodine, tin, carbon, lithium.
39. Arrange these atoms in order of their quantity of electrons
(least number to greatest number): iron, hydrogen, calcium,
fluorine, aluminum, boron.
40. Arrange these atoms in order of their quantity of electrons
(least number to greatest number): sodium, magnesium,
copper, iodine, tin, carbon, lithium.
41. Using the periodic table, determine the first element that
does not have the same number of protons as neutrons.
Round the average atomic mass to the nearest whole number
to obtain the mass number of the most abundant isotope.
42. Using the periodic table, indicate two atoms that have the
same number of protons as neutrons. Round the average
atomic mass to the nearest whole number to obtain the mass
number of the most abundant isotope.
43. In a neutral atom, the number of electrons must equal the
number of protons. However, if an atom had two more pro-
tons than electrons, what would you report as its charge?
Would this ion be called an anion or a cation?
44. If an atom had two more electrons than protons, what would
you report as its charge? Would this ion be called an anion or
a cation?
Chemical Applications and Practices
45. Several radioactive isotopes have medical applications. Using
the nuclide representation shown here, determine the num-
ber of protons, electrons, and neutrons in an atom of each of
these elements.
Radioactive sodium is used in blood circulation studies:
24
11
Na
Radioactive iodine can be used to examine the thyroid gland:
131
53
I
Radioactive cobalt can be used to kill cancer cells:
60
27
Co
Radioactive chromium can be used to measure total blood
volume:
51
24
Cr
Radioactive phosphorus is used in antileukemia therapy:
32
15
P
46. Write nuclide notation for each of the following isotopes:
carbon-12, carbon-13, phosphorus-32, chlorine-35,
chlorine-37, iron-55.
Section 2.5 Atomic Mass
Skill Review
47. If the atomic mass of
12
C had been set equal to 6.000000 amu,
what would be the mass of
16
O? . . . the mass of
1
H?
48. If the atomic mass of oxygen-16 were set equal to 4.000000
amu, what would be the mass of carbon-12? . . . the mass of
copper-63?
49. Calculate the mass, in grams, of 1 atom of carbon-12. . . . of
nitrogen-14. . . . of fluorine-19. (Assume that the mass num-
ber of these isotopes is equal to the atomic mass, rounded to
the nearest whole number.)
50. Calculate the mass, in grams, of a gross (144) of atoms of
carbon-12. . . . of nitrogen-14. . . . of fluorine-19. (Assume
that the mass number of these isotopes is equal to the atomic
mass, rounded to the nearest whole number.)
Chemical Applications and Practices
51. The metal indium is used to make heat-conducting alloys. In-
dium typically is found with two isotopes. Based on the fol-
lowing information, what would you calculate as the percent
relative abundance of the two isotopes? Indium-113 has a
mass of 112.9043, and indium-115 has a mass of 114.9041.
52. The element neon is widely used in brightly colored elec-
tronic advertising signs. There are three common isotopes of
neon with masses of 21.99, 20.99, and 19.99. One of the three
isotopes makes up approximately 90% of the atoms in a neon
sample. Which isotope is most likely to be responsible for the
majority of neon’s atomic mass reported on the periodic
table? Sketch a diagram of the probable appearance of the
mass spectrum for neon that shows the masses and an ap-
proximation of the percentage abundance.
53. The sulfur-containing amino acids that help us make certain
proteins are critically important to our diet. Sulfur samples
typically consist of four isotopes. Use the following table to
determine the average atomic mass of sulfur.
Sketch a mass spectrum that illustrates the masses observed
for a natural sample of sulfur.
Isotope Atomic Mass Abundance
S-32 31.97 95.0%
S-33 32.97 0.76%
S-34 33.97 4.22%
S-36 35.97 0.014%
Neon gas discharge tube.
82 Chapter 2 Atoms: A Quest for Understanding
54. Silver has many uses in our world, only one of which is as a
precious metal in coinage. Use the following table to deter-
mine the average atomic mass of silver.
Sketch a mass spectrum that illustrates the masses observed
for a natural sample of silver.
55. Cerium has four naturally occurring isotopes. Use the fol-
lowing table to determine the average atomic mass of cerium.
Sketch a mass spectrum that illustrates the masses observed
for a natural sample of cerium.
56. A hypothetical element was just discovered to have three
isotopes. Use the following table to determine the average
atomic mass of this hypothetical element.
Sketch a mass spectrum that illustrates the masses observed
for a natural sample of the element.
Section 2.6 The Periodic Table
Skill Review
57. Use the descriptions given in this chapter of the organization
of the periodic table to identify each of these elements.
a. The element that is in the second column and in the
second period.
b. The transition element that is in the seventh group and in
the fourth row.
c. The third most massive noble gas.
58. Use the descriptions given in this chapter of the organization
of the periodic table to identify each of these elements.
a. The lightest alkali metal.
b. The semimetal that is in the third group.
c. The halogen that is a liquid at room temperature.
59. How many elements are classified as halogens?
60. Which column contains the alkali metals? . . . the chalcogens?
61. Identify the name of the group for each of these elements.
sulfur iodine helium
beryllium francium
Isotope Atomic Mass Abundance
A-100 99.754 35.25%
A-102 101.688 25.75%
A-103 102.599 39.00%
Isotope Atomic Mass Abundance
Ce-136 135.907 0.19%
Ce-138 137.906 0.25%
Ce-140 139.905 88.48%
Ce-142 141.909 11.08%
Isotope Atomic Mass Abundance
Ag-107 106.9051 51.84%
Ag-109 108.9048 48.16%
62. Identify the name of the group for each of these elements.
lithium barium neon
oxygen chlorine
63. Which of these elements would you predict to be shiny?
gold silver lead
silicon carbon iodine
64. Which of these elements would you predict to be brittle?
gold silver lead
silicon carbon iodine
Section 2.7 Ionic Compounds
Skill Review
65. One of the first considerations in writing ionic formulas is
to determine which element is to be the cation and which
is to be the anion. Decide which element in each of these
pairs is more likely to become the positive ion.
Ca or Br S or Al Cl or Al
Sr or N I or Be
66. Use the information from the previous question to deter-
mine what formula is most likely to result from the combina-
tion of each of these pairs of elements.
Ca and Br S and Al Cl and Al
Sr and N I and Be
67. Write the formulas of the ionic compounds that form when
chlorine combines individually with lithium, beryllium,
sodium, calcium, and aluminum.
68. Write the formulas of the ionic compounds that form
when oxygen combines individually with lithium, beryllium,
sodium, calcium, and aluminum.
69. Ionic compounds typically dissociate into ions as they dis-
solve in water. For each of these compounds, predict the ions,
including their charges, that will be produced when the ionic
compound dissolves.
MgBr
2
FeCl
3
KI Na
2
S
70. Ionic compounds typically dissociate into ions as they dis-
solve in water. For each of these compounds, predict the ions,
including their charges, that will be produced when the ionic
compound dissolves.
CaCl
2
TiO
2
ZnO NiCl
2
Section 2.8 Molecules
Skill Review
71. The two major classifications of compounds are ionic and
molecular. Label the descriptions given below as characteris-
tic of an ionic compounds or of a molecule
a. Results from a metal combining with a nonmetal.
b. Persists as a complete entity when dissolved in water.
c. Formula can often be determined by the identity of the
atoms that combine.
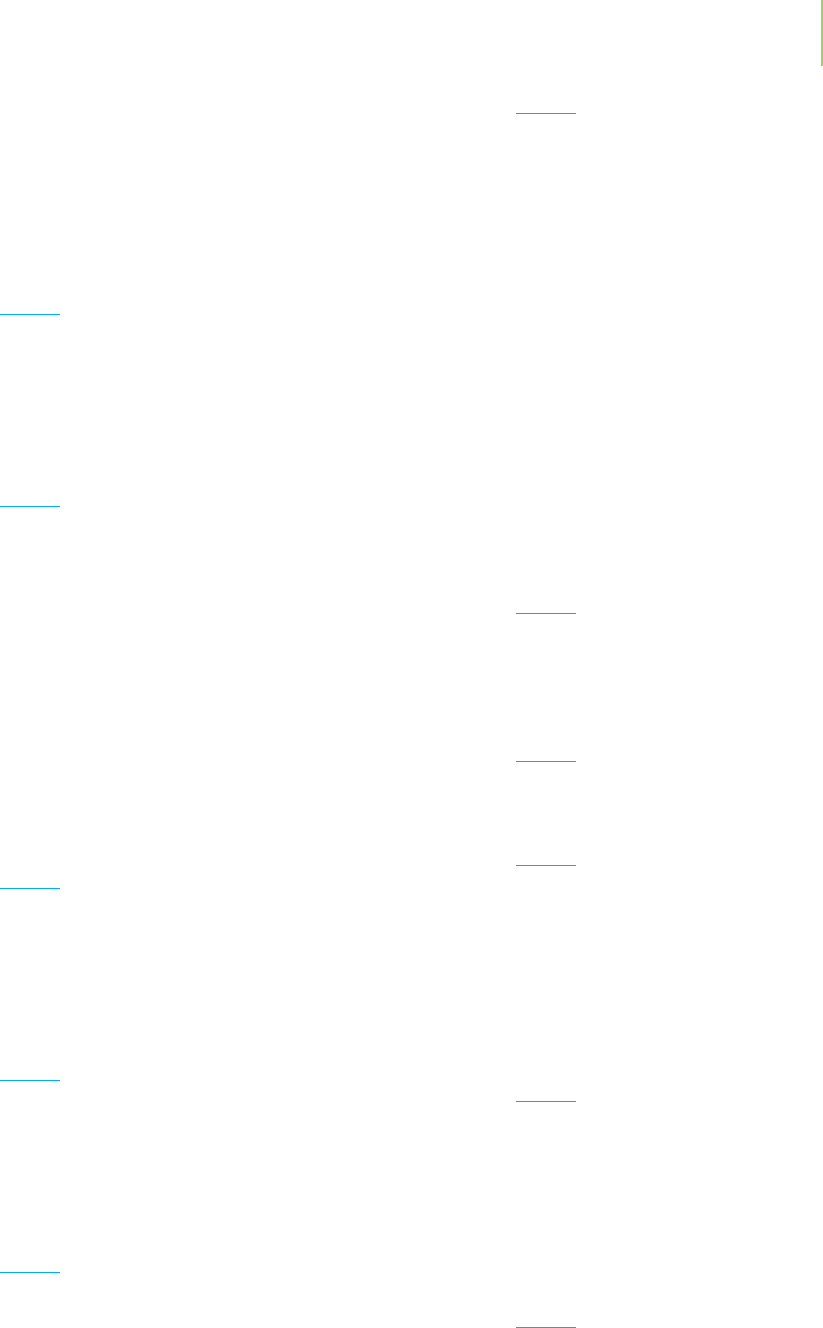
Focus Your Learning 83
72. The two major classifications of compounds are ionic and
molecular. Label the descriptions given below as characteris-
tic of an ionic compounds or of a molecule.
a. Results as atoms share their electrons.
b. Formula represents a ratio of ions involved rather than
actual numbers of atoms combined.
c. Results from the combination of two nonmetals.
d. Separates into individual charged particles when dissolved
in water.
73. Which of these compounds are not molecular compounds?
a. CO d. PCl
3
b. BrO
3
e. WO
2
c. K
2
O
74. Which of these compounds are molecular compounds?
a. SO
3
d. Na
2
S
b. NiO e. SiF
4
c. BCl
3
75. In these examples, the molecular and empirical formulas of
several compounds are listed. In each case, decide which is
the empirical formula.
a. Hydrogen peroxide is a useful disinfectant. (H
2
O
2
or HO)
b. Butane is used as a heating fuel. (C
2
H
5
or C
4
H
10
)
c. Teflon is made from tetrafluoroethylene. (C
2
F
4
or CF
2
)
d. Glucose is a sweet-tasting molecule. (CH
2
O or C
6
H
12
O
6
)
76. In each of these examples, determine the correct empirical
formula.
a. Acetic acid is found in vinegar: C
2
H
4
O
2
.
b. Cane sugar is called sucrose: C
12
H
22
O
11
.
c. Paradichlorobenzene is used as a moth repellent: C
6
H
4
Cl
2
.
d. Benzene is a component of gasoline: C
6
H
6
.
Section 2.9 Naming Compounds
Skill Review
77. Provide the correct name for each of these binary molecular
compounds.
SO
2
N
2
O
5
Cl
2
O
PCl
3
CCl
4
78. Provide the correct name for each of these binary molecular
compounds.
NO
2
PBr
5
OF
2
N
2
OP
2
O
5
79. Provide the correct name for each of these binary ionic
compounds.
K
2
O CaBr
2
Li
3
N
AlCl
3
BaS
80. Provide the correct name for each of these binary ionic
compounds.
CaO MgCl
2
Na
2
S
Al
2
O
3
LiF
81. Provide the correct name for each of these ionic compounds.
CaBr
2
Fe(NO
3
)
3
CaSO
4
NH
4
Cl NaCl
82. Provide the correct name for each of these ionic compounds.
KHCO
3
Ca(CN)
2
Co(OH)
2
Cu(NO
3
)
2
MgSO
3
83. Provide the correct formula for each of these compounds.
copper(II) hydroxide
chromium(III) oxide
sulfur hexachloride
carbon tetraiodide
aluminum hydroxide
magnesium sulfate
sodium sulfite
ammonium hydroxide
boron tribromide
sodium acetate
84. Provide the correct formula for each of these compounds.
hydrogen chloride
cobalt(VI) fluoride
dinitrogen tetroxide
calcium perchlorate
barium nitrate
lead(IV) chloride
potassium dichromate
sodium bicarbonate
lithium hydroxide
titanium(IV) carbonate
85. Using the metal ion Mn
5+
, write the correct formula for a
combination with each of the following: sulfate; chloride;
nitrite; carbonate; and bisulfite.
86. Using the metal ion Cr
6+
, write the correct formula for a
combination with each of the following: sulfate; chloride;
nitrite; carbonate; and bisulfite.
87. Write the correct formula for oxalate compounds of the
following metals: Mn
2+
;Cu
+
;Fe
3+
;Mn
5+
; and Ti
4+
.
88. Write the correct formula for nitrate compounds of the
following metals: Mn
2+
;Cu
+
;Fe
3+
;Mn
5+
; and Ti
4+
.
89. Name these compounds.
(NH
4
)
2
CO
3
NaHCO
3
Cu(HSO
3
)
2
Ca(OH)
2
KMnO
4
Na
3
PO
4
Mg(CN)
2
LiClO
3
90. Name these compounds.
Zn(CN)
2
Na
2
CO
3
Na
2
CrO
4
K
2
HPO
4
Li
2
Cr
2
O
7
Ba(NO
2
)
2
SrSO
4
KClO
91. Determine the charge on the metal cation in these
compounds.
V(NO
3
)
5
TiSO
4
W(C
2
O
4
)
3
AgOH Ru(HCO
3
)
3
92. Determine the charge on the metal cation in these
compounds.
Cr(NO
3
)
3
MnO
2
Pd(C
2
O
4
)
2
AuCl
3
Co(OH)
3
Chemical Applications and Practices
93. A lab accident caused part of the label on a bottle of a chem-
ical to be obscured. All that is visible is “Fe(NO
3
.” Is this
enough information to name the compound? What possible
suggestions could you make to determine the identity of the
compound?

84 Chapter 2 Atoms: A Quest for Understanding
97. When hydrogen gas combines with chlorine gas, a corrosive
gas known as hydrogen chloride forms. According to
Dalton, the volume ratio between the reactants and prod-
ucts should be 1:1:1. However, Gay-Lussac would claim a
ratio of 1:1:2 (assuming that all gases are at the same tem-
perature and pressure). Gay-Lussac’s claim was proved to be
valid. Which law is being used here? Explain why this expla-
nation works better than Dalton’s.
98. If the mass of a metal nut were 35.0 g and the mass of a
matching threaded bolt were 7.0 g, a combined nut and bolt
would have a mass of 42.0 g.
a. What would be the mass of the threaded bolts needed to
attach to 175.0 g of nuts?
b. If someone decided to attach two bolts to a nut, what
would be the resulting mass of the unit?
c. What would be the mass of the threaded bolts needed to
attach to 175.0 g of nuts under the conditions stated in
part b?
d. What is the mass ratio between bolts connected to nuts in
the first unit (part a) and bolts connected to nuts in the
second unit (part b)?
99. Robert Millikan determined the electrical charge for an
electron, and its mass was easily calculated by using the
charge-to-mass ratio. The following analogy applies
roughly the same logic: Suppose you were buying a box of
golf balls. What information would you need in order to
know the price of each golf ball?
100. A tiny oil droplet is given an electrical charge by an electric
current. The charge causes the oil droplet to be attracted to
an oppositely charged metal plate above it. What two forces
must be equal in order for an oil droplet to be suspended
(neither rising nor falling) in the apparatus?
94. The same lab accident caused part of the label on another
bottle to be obscured as well. The visible part reads “NH
4
C.”
Which of the following would you suggest as the most likely
name of the compound in this bottle? Explain why you reject
the others: ammonium carbonate, ammonium oxalate,
ammonium chloride, ammonium copper(II).
Comprehensive Problems
95. What were the major objections to Dalton’s atomic theory?
Rewrite Dalton’s atomic theory to take into account our cur-
rent understanding of matter.
96. Match each chemical statement on the left-hand side of the
following list with a correlating statement from Dalton’s
atomic theory on the right.
Chemistry Atomic Theory
(1) Elements are made up of
tiny particles called atoms.
(2) Atoms of a given element
are identical; atoms of
different elements differ in
some fundamental way.
(3) Compounds are formed
when atoms combine; each
compound always has the
same numbers and types of
atoms.
(4) Chemical reactions consist
of the reorganization of
the atoms involved; the
atoms themselves are not
changed.
a. Graphite and
diamond have
different properties,
but both are made
of only atoms of
carbon.
b. 2H
2
+ O
2
→ 2H
2
O
c. It takes 6.02 ×10
23
atoms of iron to make
55.85 g.
d. It takes 6.02 × 10
23
atoms of gold to
make 197 g.
Charged
plate (+)
Charged oil drop
Charged
plate (–)
Window
Viewing chamber
Microscope
Oil
Problem 100
then measured, and the weights were found to be as follows
(assume these are exact weights):
32 oz 28 oz
18 oz 22 oz
36 oz 14 oz
a. What is the mass of each bag in grams?
b. Judging on the basis of the masses recorded in ounces, and
the simplifying assumption that the tomatoes are the same
size, what would you estimate as the weight of just one
tomato?
c. Another bag had an exact weight of 20 oz; how does that
influence your answer to part a? What is the chemical law
that you are using to answer this question?
d. Describe the process that you could follow to identify the
volume of each of the tomatoes.
107. In this chapter, we discussed determining the age of objects
using radioactive isotopes. Scientists are constantly refining
these techniques to correct for the differences between con-
ditions long ago and those at the present time. What condi-
tions might have changed that could require correcting? In
each case, what would be the nature of the correction (that
is, whether the real age is greater or less) and why?
108. Scientists now think that much of the universe is composed
of “dark matter.” Investigate some scientific journals, such as
Scientific American, to learn enough about dark matter to
describe it. On the basis of your research, discuss the exper-
iments that compelled scientists to draw this conclusion.
Why is dark matter useful to understand?
2.2
2.0
1.8
1.6
1.4
1.2
1.0
0 10,000 20,000 30,000 40,000
Age (years before present)
Atmospheric C-14/C-12 ratio
relative to present day
101. How much mass was lost from the combination of protons,
neutrons, and electrons when
63
Cu (62.9296011 amu) was
formed? What happened to this mass?
102. If the atoms in a 2.500 g sample of aluminum were lined up
end to end, how long would the line of iron be in inches?
(Assume the aluminum atoms are spheres with a radius of
143 pm.)
103. If a sample of carbon atoms contains 7.45 10
15
atoms,
how many gross of atoms are there in the sample (1 gross
144 atoms)? If each of those atoms is 154 pm in diameter,
would they stretch to 1 mile if they were lined up so that
they were touching end to end?
104. During the analysis of an unknown sample of metal, a
researcher places a 16.07 g chunk of the metal into a gradu-
ated cylinder containing 20.3 mL of water. The volume of
the mixture was then recorded to be 22.3 mL. What is the
density of the metal?
105. A new isotope of beryllium is created such that it contains
9 neutrons.
a. How many protons and electrons would an atom of this
isotope contain?
b. What is the mass number for this new isotope?
c. Use the information in Table 2.3 to determine the mass of
the isotope in kilograms. (Assume that no mass is lost in
the creation of the new isotope from the individual parts.)
Thinking Beyond the Calculation
106. In the produce section of a grocery store, bags of small
tomatoes were being packaged. The weight of each bag was
Problem 107
Focus Your Learning
85

Introducing
Quantitative
Chemistry
The use of quantitative chemical
equipment is important in the
biomedical field.
86
Contents and Selected Applications
3.1 Formula Masses
Chemical Encounters: Adrenaline in the Body
3.2 Counting by Weighing
3.3 Working with Moles
Chemical Encounters: Cholesterol in the Blood
3.4 Percentages by Mass
Chemical Encounters: Nitrogen in Fertilizers
3.5 Finding the Formula
Chemical Encounters: A Focus on Chocolate
3.6 Chemical Equations
Chemical Encounters: Preparation of Aspirin
3.7 Working with Equations
Chemical Encounters: Cleaning the Air on Manned Spacecraft
Go to college.hmco.com/pic/kelterMEE for online learning resources.
A sudden, loud noise, the shock of some
dramatic twist in a horror film, losing control of
your car on an icy road—any one of these can cause the
rapid physical responses we attribute to the release of
adrenaline, including increasing heart rate, stronger heartbeat,
sweating, and shifting of some blood flow from the skin to the
internal organs. Adrenaline, also known as epinephrine (Figure 3.1),
is normally present in your blood at approximately
2 × 10
–8
g/L and is secreted from the adrenal glands
near your kidneys. In times of extreme stress, your
adrenaline level increases a thousandfold. The powerful
effects of adrenaline demonstrate the vital significance of
changing quantities of chemicals. The chemistry of the
body provides many other examples. For instance, taking
600 mg of aspirin can effectively relieve pain. Taking
100 times that amount, 60 g, would kill you. Quantities in
chemistry are crucial.
Up to this point, we have been dealing with individual
atoms and molecules. However, in the lab we typically
measure quantities in liters, grams, and other “macro-sized”
units. What is the relationship between “nanoworld” and
“macroworld” quantities? Specific questions arise: How
many molecules of adrenaline will be in each liter of blood?
How can we actually measure the level of adrenaline or any other chemical in
blood? These are the kinds of quantitative issues we explore in this chapter.
3.1 Formula Masses
In addition to being naturally present in the body, adrenaline is administered as a drug to
stimulate the heart, to alleviate allergic reactions, and even to help break up fat cells during
liposuction. As you might expect, control over the amounts administered is vital.
To make, use, or detect specific quantities of adrenaline, we need information about the
mass of its molecules, so we may know how many molecules are in any given mass that is
administered to a patient.
In Chapter 2 we looked at the method for determining the masses of atoms, and we saw
that each element has a given mass (expressed in atomic mass units), which is the average
mass of one atom of the element. We can work out the average
molecular mass (or molecular
weight) of a molecule, such as adrenaline, by adding together the average atomic masses of all
the atoms present in the molecule. To simplify our calculations, we will use atomic masses
rounded to two decimals, except for hydrogen, which we will round to three decimals.
Formula of each adrenaline molecule = C
9
H
13
NO
3
In the formula we have
9 carbon atoms @ 12.01 amu = 108.09
13 hydrogen atoms @ 1.008 amu = 13.104
1 nitrogen atom @ 14.01 amu = 14.01
3 oxygen atoms @ 16.00 amu = 48.00
Average molecular mass of adrenaline = 183.204 = 183.2 amu
87
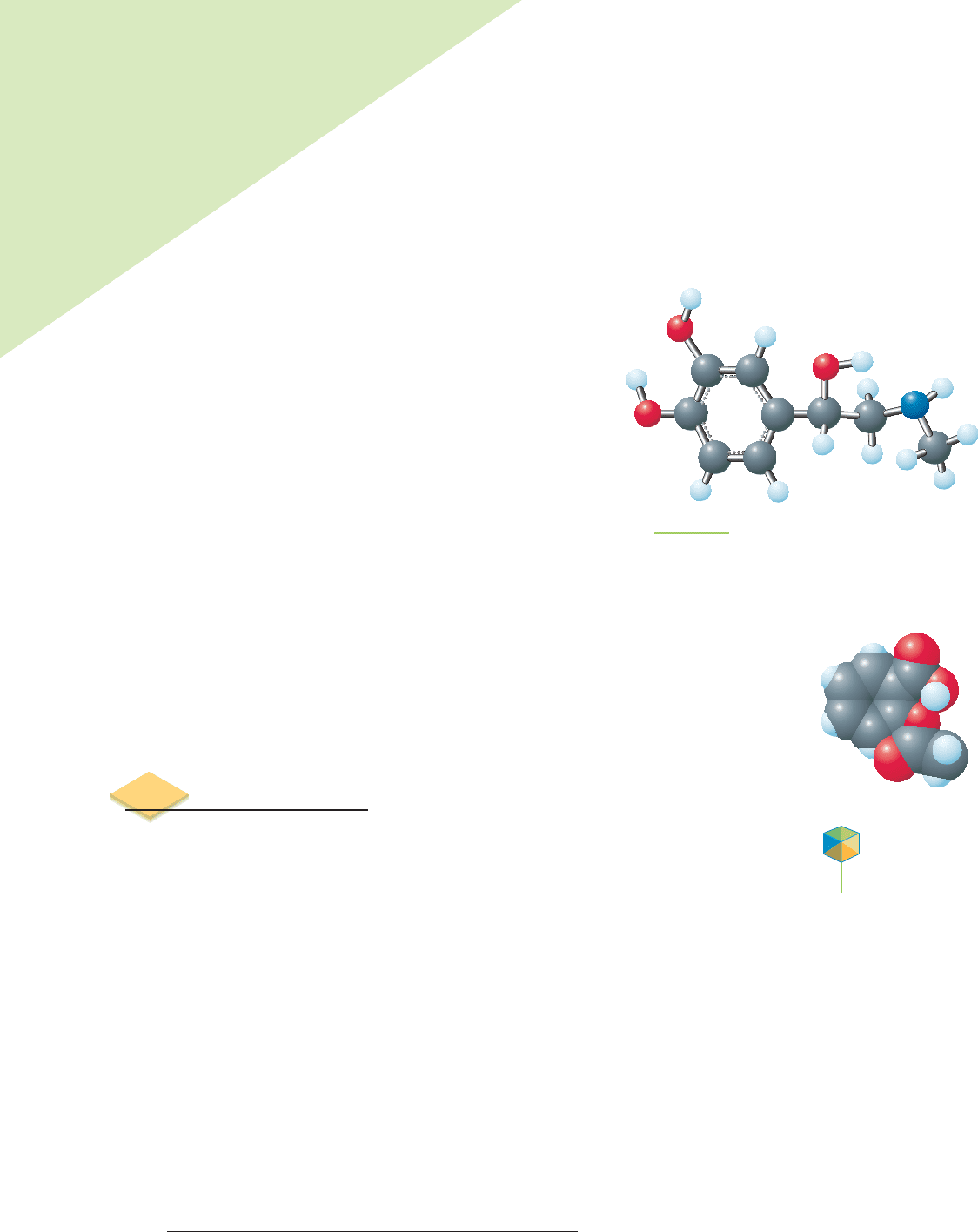
FIGURE 3.1
Adrenaline (epinephrine) has the chemical formula
C
9
H
13
NO
3
. In this “ball-and-stick” representation,
carbon atoms are charcoal gray, hydrogen atoms are
light blue, oxygen atoms are red, and nitrogen atoms
are dark blue.
Application
C
HEMICAL ENCOUNTERS:
Adrenaline in the Body
Aspirin
