Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

EXERCISE 2.2 Atomic Bookkeeping
Fill in the blanks for each neutral element in the following table, and write the ele-
mental symbols using nuclide notation.
First Thoughts
To fill in our table, we will use the relationships that define the mass number and
atomic number for an element. Specifically, we recall that
A − Z = n
A glance at the periodic table will be helpful, because the name of the element indi-
cates the atomic number of the element. On the periodic table inside the front cover
of this book, we can verify that the atomic number of silicon is in fact 14. We know
that this is equal to the number of protons in atoms of the element. In the blanks for
molybdenum, we are asked to find the number of protons and electrons present in
an atom of the neutral element. We are given the number of neutrons (n) and the
mass number (A), the sum of protons and neutrons. We can take the difference to
solve for the number of protons (Z):
A − n = Z
This is also equal to the number of electrons in the electrically neutral atom. The
key to filling in the blanks for krypton is to remember that the number of protons
equals the number of electrons in a neutral atom.
Solution
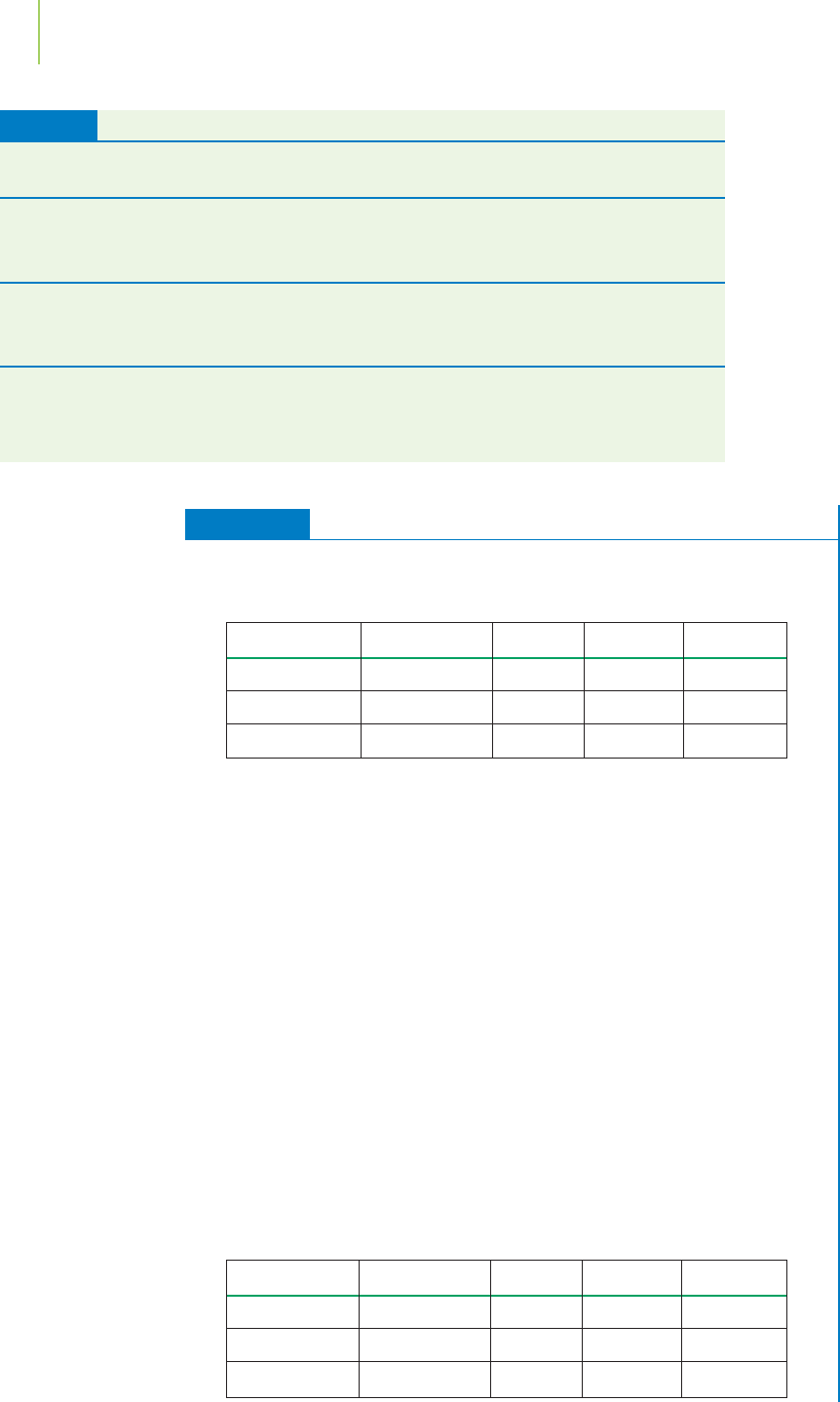
Element Mass Number Protons Neutrons Electrons
silicon 28 14 14 14
molybdenum 96 42 54 42
krypton 84 36 48 36
Element Mass Number Protons Neutrons Electrons
silicon 28 14
molybdenum 96 54
krypton 48 36
58 Chapter 2 Atoms: A Quest for Understanding
Some Isotopes of Hydrogen, Carbon, and Oxygen
Nuclide Relative Natural
Notation Abundance (%) Name of Isotope Protons Neutrons Electrons
1
1
H 99.985% hydrogen 1 0 1
2
1
H 0.015 deuterium 1 1 1
3
1
H10
−18
tritium 1 2 1
12
6
C 98.93 carbon-12 6 6 6
13
6
C 1.07 carbon-13 6 7 6
14
6
C2× 10
−10
carbon-14 6 8 6
16
8
O 99.762 oxygen-16 8 8 8
17
8
O 0.038 oxygen-17 8 9 8
18
8
O 0.200 oxygen-18 8 10 8
TABLE 2.4
Further Insights
As we go through the various numerical features of the elements, such as mass
number and numbers of protons, neutrons, and electrons, please keep in mind that
these numbers represent one of two ways in which we characterize the elements.
The other way, which ultimately led to the formation of the periodic table of the
elements, is via the physical and chemical characteristics of the elements.
PRACTICE 2.2
Indicate the number of protons, neutrons, and electrons in atoms of each of these
elements:
14
7
N
20
10
Ne
48
22
Ti
Carbon-11 Lithium-7 Phosphorus-31
See Problems 25, 26, 35–40, 45, and 46.
2.5 Atomic Mass
Plants and other organisms have a slightly greater preference for the lighter
isotopes of the carbon that they use as a raw material. When a plant absorbs car-
bon dioxide to make cell walls, it more efficiently uses the molecules that contain
the carbon-12 isotope than those that contain carbon-13 or carbon-14. The pref-
erence isn’t great at all, but the plant absorbs a slightly larger proportion of
carbon-12 than of the other carbon isotopes. When the organism dies, it leaves
behind some residue that is indicative of this preference. How does the scientist
in search of the origins of life determine the amount of carbon-12 versus the
amount of carbon-13 in a rock formation?
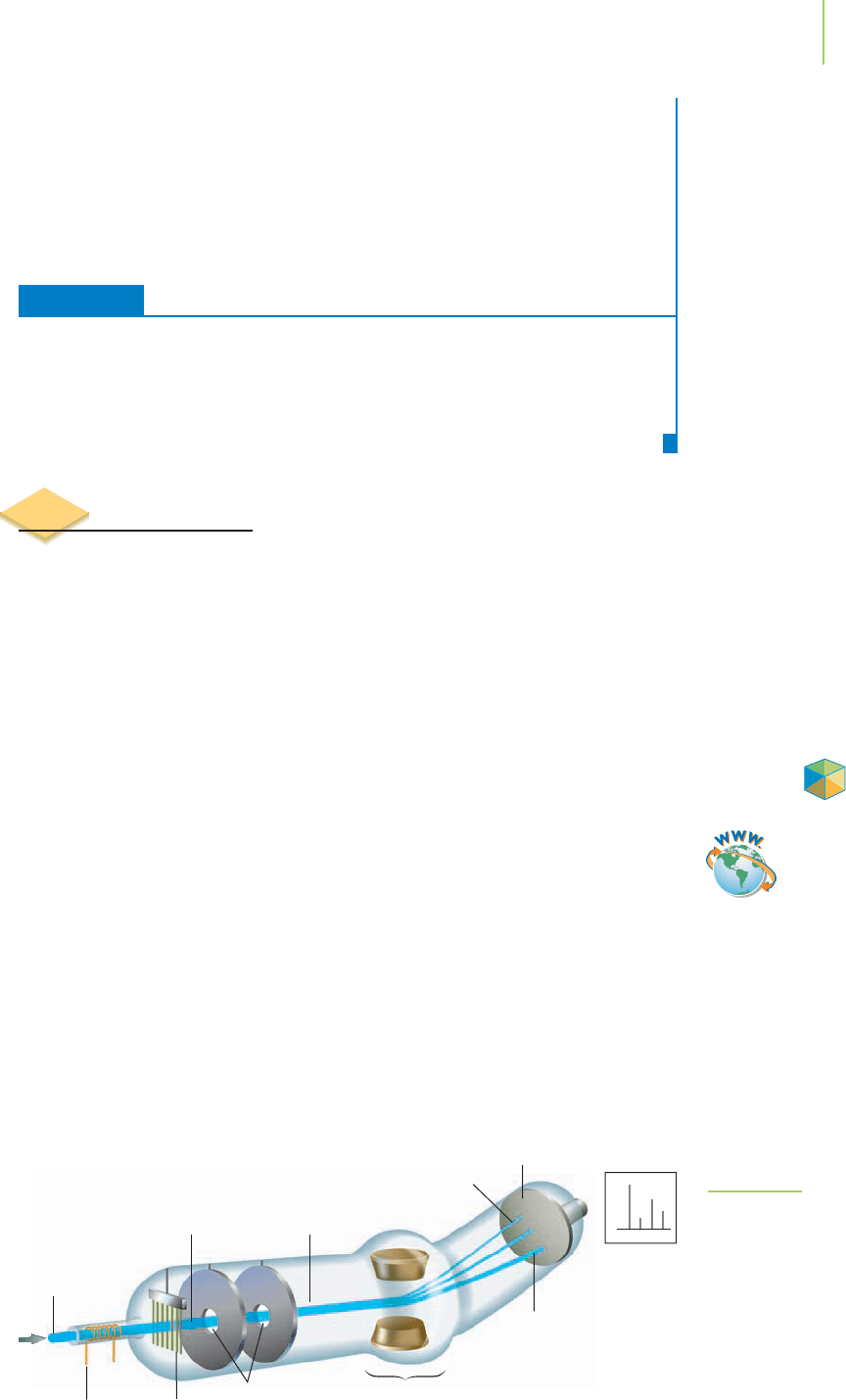
The distribution of isotopes in a sample of an element can be found using an
instrument called a
mass spectrometer. There are different kinds of mass spec-
trometers, but the simplest kind is illustrated in Figure 2.21. In the instrument, a
sample is vaporized and then converted into positive ions when a beam of fast-
moving electrons knocks some of the electrons out of the atoms of the sample.
The ions that are formed are accelerated by their attraction to charged plates.
Holes in these plates allow some of the ions to be projected through and into a
magnetic field. The interaction between the charge on the ions and the magnetic
field causes the ions to be deflected from their original path. The extent of this
deflection depends on the masses and charges of the ions. The more massive an ion
is, the less deflection it experiences, because of the greater momentum it possesses
when heading out in its original straight-line path (much as a very heavy adult
would not be blown so far off course by a fierce,gusty wind as a small child would).
Some atoms have two of their electrons knocked away by the beam that
strikes them, converting them into cations with a +2 charge. These ions deflect
2.5 Atomic Mass 59
Sample
Positive
ions
Electron
beam
Sample is
vaporized
Accelerated
ion beam
Magnetic field
Detector
plate
Least
massive ions
Most
massive ions
Slits
FIGURE 2.21
The mass spectrometer separates ions by
exploiting their deflection to different
extents in a magnetic field.
Application
Video Lesson: Mass
Spectrometry: Determining
Atomic Masses
60 Chapter 2 Atoms: A Quest for Understanding
34
35
Cl
37
Cl
80
60
40
20
0
100
35 36 37 38
Atomic weight (amu)
Relative intensity
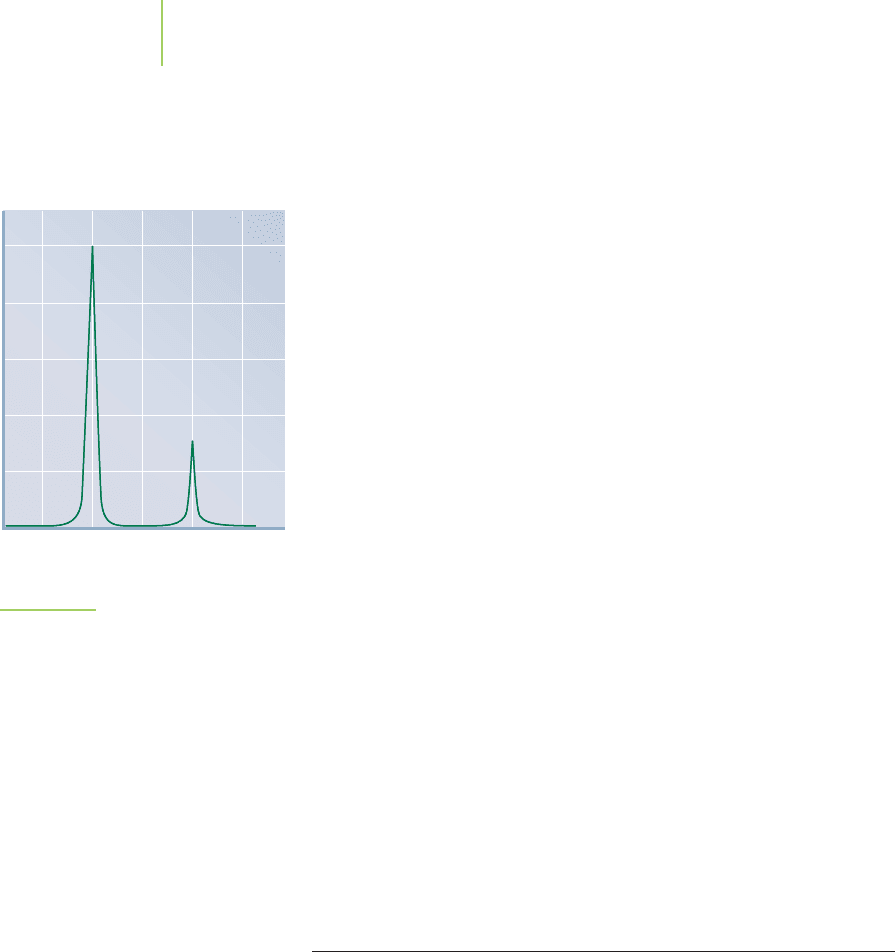
FIGURE 2.22
The mass spectrum of chlorine. From this
spectrum we can determine the relative
abundances of
35
CI (75.77%) and
37
CI
(24.23%).
more than otherwise identical ions with a single positive charge because of the
greater interaction between their charge and the magnetic field. Within a very
short distance, the original coherent beam of ions spreads out into beams of dis-
tinct ions, each with a different mass-to-charge ratio.
At the end of the mass spectrometer, the beams of ions are detected. The re-
sults are displayed in a
mass spectrum, as shown in Figure 2.22. Each line on the
spectrum indicates the relative quantity of the different ion beams that result
from the original sample. Using a standard that produces an ion beam with a
known mass, we can reproduce the scale on the bottom of the mass spectrum.
In much the same way, we use a standard to determine the masses of the
atoms in the periodic table. Which element is used as the standard? The most
abundant isotope of carbon,
12
C, is defined as exactly 12 atomic mass units (amu).
12
C = 12.000000000 amu
An atomic mass unit, an arbitrary unit used to set the masses reported in the
periodic table, is currently defined as follows:
1 amu = 1.66053873 × 10
−27
kg 1 kg = 6.02214198 × 10
26
amu
1 g = 6.02214198 × 10
23
amu
Strictly speaking, the amu is not an SI unit, but its use remains sufficiently com-
mon among scientists that we will use it in this textbook.
A detailed look at the mass of individual isotopes shows that they are typically
not integer values. That is, the isotope
63
Cu does not have a mass of exactly 63.
Only
12
C is defined with an exact mass. The isotope
63
Cu has a mass number of 63
but a mass of 62.9296011 amu. The isotope
96
Mo has a mass of 95.9046789 amu.
Precise measurements have shown that neither a proton (roughly 1.0073 amu)
nor a neutron (roughly 1.0087 amu) has a mass of 1 amu. Therefore, the isotope
238
U, which has an atomic mass of 238.0508 amu, has an atomic mass lower than
the sum of the protons and neutrons, which have a total mass of 239.9418 amu,
as shown.
Mass of 92 individual protons of
238
U
= 92 × 1.0073 amu = 92.6716 amu
Mass of 146 individual neutrons of
238
U
= 146 × 1.0087 amu = 147.2702 amu
Total mass of individual protons and neutrons of
238
U = 239.9418 amu
Where did the rest of the mass go? When the nuclei of elements formed from
the individual neutrons and protons, some of the mass—a different amount for
each isotope of each element—was released as energy (see Section 5.7).
Unfortunately, the individual masses don’t translate accurately into masses
that we can measure for a macroscopic quantity of an element found on a labo-
ratory shelf. The presence of isotopes complicates the mass even more. To illus-
trate this, assume we have a bottle of carbon powder. What mass should we use
for the typical carbon atom found in this bottle, given that three different iso-
topes of carbon exist: carbon-12, carbon-13, and carbon-14? Fortunately, in na-
ture, the relative abundance of each isotope remains largely constant. In addition,
the relative natural abundances of each of the isotopes of the elements have been
determined by scientists using mass spectrometers. Knowing the percentages of
each of the isotopes (and their masses), scientists have calculated the weighted
average mass of each element, and that is the atomic mass listed for it in the
periodic table. For our bottle of carbon powder, we would use 12.011 amu as the
average mass.
To better understand the concept of weighted average, we can draw an
analogy to a student grade point average (GPA). Let’s look at an “A student” who
completed 10 credits of coursework in a semester. If 5 credits were A grades (4.0,
on a scale of 0.0 to 4.0), 3 credits were B (3.0), and 2 credits were C (2.0), then her
2.5 Atomic Mass 61
overall GPA would be the weighted average of the individual course grades. The
A grade has the greatest weight because fully half the credits were in that course.
The C grade would (thankfully) have the least weight, because that course repre-
sented only 20%, or 0.20, of the whole course load. The weighted average (here,
the GPA) would be
(0.50 × 4.0) + (0.30 × 3.0) + (0.20 × 2.0) = 3.3
contribution of: A grades B grades C grades
Does the answer make sense? Even though the student received one grade each of
A, B, and C, the A was present in much greater abundance—5 credits—than the B
or the C. It therefore makes sense that the GPA should be a little higher than 3.0,
a straight B average. We can use the same strategy to calculate the atomic mass of
an element. For example, mass spectrometry reveals what we have noted previ-
ously: that carbon-12 accounts for 98.93% of all carbon atoms. Carbon-13 makes
up 1.07% of the carbon atoms, and carbon-14’s contribution is negligible—
about 2
× 10
−
10
%. The average mass of carbon-12 is then
(12.00000 amu × 0.9893) + (13.00335 amu × 0.0107) = 12.011 amu
carbon-12 carbon-13
This agrees very nicely with the mass reported in the periodic table. The fact that
any sample of carbon has a mass greater than 12 reflects the increased mass con-
tribution made by the small proportion of carbon-13.
EXERCISE 2.3 Calculating the Atomic Mass Value
Naturally occurring chlorine is composed of two isotopes: chlorine-35 and
chlorine-37. As the spectrum shown in Figure 2.22 reveals, the lighter isotope is sub-
stantially more abundant than the heavier one. Use the relative abundances given in
the figure, along with the mass of each isotope, to calculate the atomic mass for
chlorine. Compare the mass you calculated with what is reported in the periodic
table of the elements.
First Thoughts
Analogies to common situations and experiences often help us visualize difficult
concepts. Make use of the analogy to the grade point average that we discussed in
the text. It is precisely the same calculation, except that the abundance of each iso-
tope replaces the abundance of each grade.
Solution
Atomic mass = (34.968852 × 0.7577) + (36.965903 × 0.2423) = 35.45
There was more chlorine-35 than chlorine-37 present, so it makes sense that the
mass should be closer to 35 than to 37. This value agrees with the value reported in
the periodic table.
Further Insights
In addition to determining the masses of the isotopes of atoms, mass spectrometry
can be used to reveal the masses of large and small molecules and to identify the
presence of specific molecules. Some of the most important applications of mass
spectrometry are found in biology and medicine, where it can be used to determine
the masses of protein molecules or to analyze blood samples. One example—
showing how mass spectrometry plays a role in testing for illegal drug use in
athletics—is illustrated in Figure 2.23.
Isotope Mass Abundance
chlorine-35 34.968852 75.77%
chlorine-37 36.965903 24.23%
Application
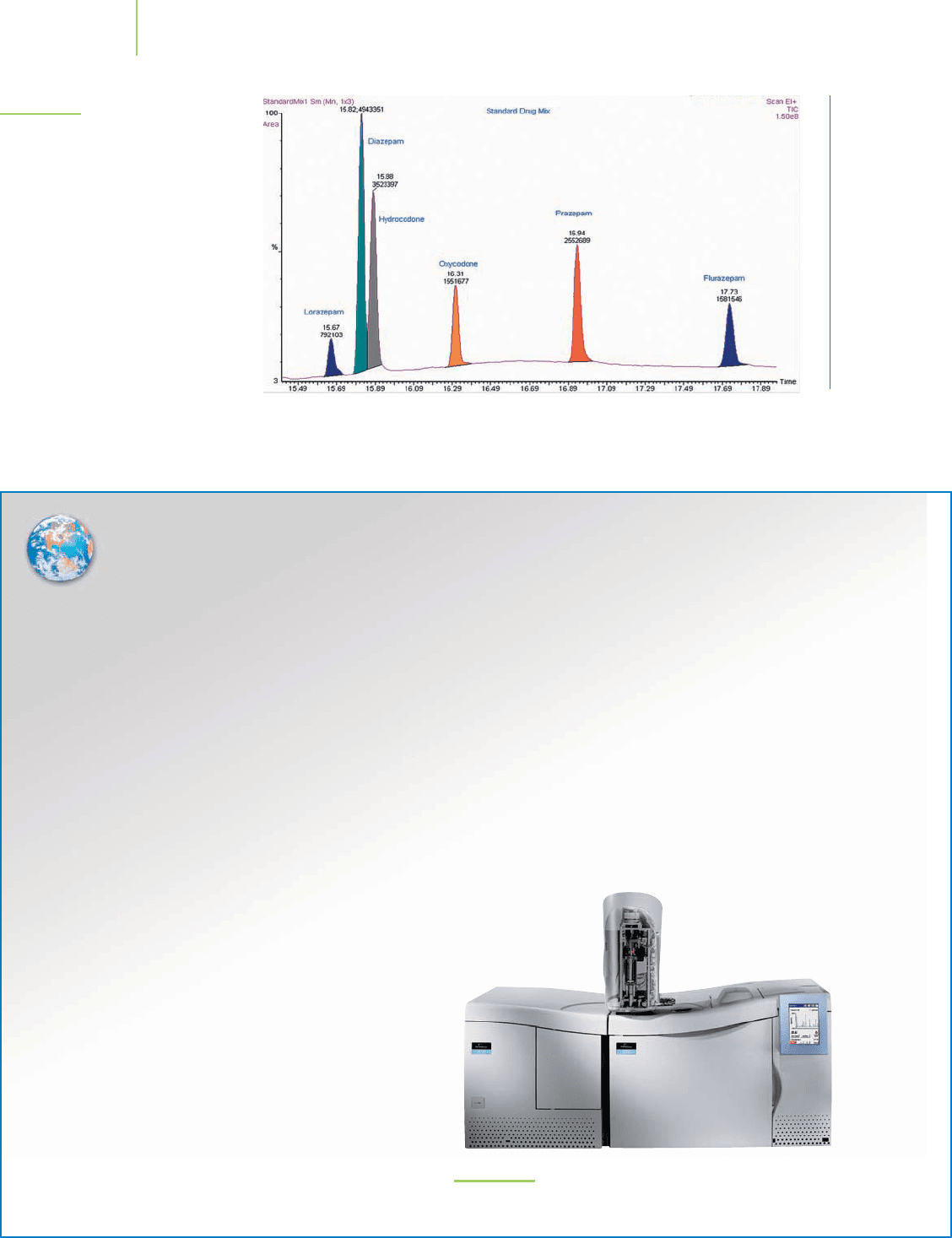
62 Chapter 2 Atoms: A Quest for Understanding
FIGURE 2.23
Drugs of abuse can be separated using a
gas chromatograph and identified with
a mass spectrometer.
“Organic” foods are big business. Accord-
ing to the U.S. Department of Agriculture,
such foods are “(1) produced and handled without the
use of synthetic chemicals and (2) not produced on land
to which any prohibited substances, including synthetic
chemicals, have been applied during the 3 years immedi-
ately preceding the harvest of agricultural products.” Un-
like organic foods, many of the other foods that we con-
sume contain industrially produced chemical additives
that increase their shelf life, lend them a particular tex-
ture, or enhance their flavor. Because of this, many con-
sumers want products that not only are organically grown
but also contain only natural additives. One useful way
of characterizing additives as natural or synthetic is by
looking at the atomic masses of their atoms with a mass
spectrometer.
The key to the analysis, pioneered by Randy Culp
and coworkers at the Center for Applied Isotope Studies
at the University of Georgia, is that synthetic flavorings
are typically made from organic chemicals derived from
fossil fuels (“crude oil”). “Natural” flavorings are made
from organic chemicals that have the same structure but
are derived from plants. Vanilla flavoring, for example,
can either come from vanilla beans or be made by com-
bining individual compounds in an industrial process.
Other than the method by which they are made, there is
no difference between these two vanilla flavorings. Or is
there?
Culp recognizes that plants were alive until they were
harvested for the flavorings. Living things contain a con-
stant and small amount of radioactive
14
C, which de-
creases slowly after they die. Fossil fuels, composed of
formerly living materialthat has been chemically modified
for tens of millions of years, contain essentially no
14
C.
NanoWorld / MacroWorld
Big effects of the very small: Flavor analysis
Measurement of the radiation that is particular to
14
C
indicates whether the chemicals in the flavoring are from
fossil fuels or plants.
Once it is established that a flavoring was prepared
from natural sources rather than fossil fuels, how can
Culp’s group tell whether the sample is made from a
vanilla bean, say, or synthetically prepared from com-
pounds derived from wood pulp? Each type of plant is
unique in the way in which it processes carbon in photo-
synthesis, so the ratio of
13
C to
12
C distinguishes differ-
ent sources of compounds. That is where a gas chromato-
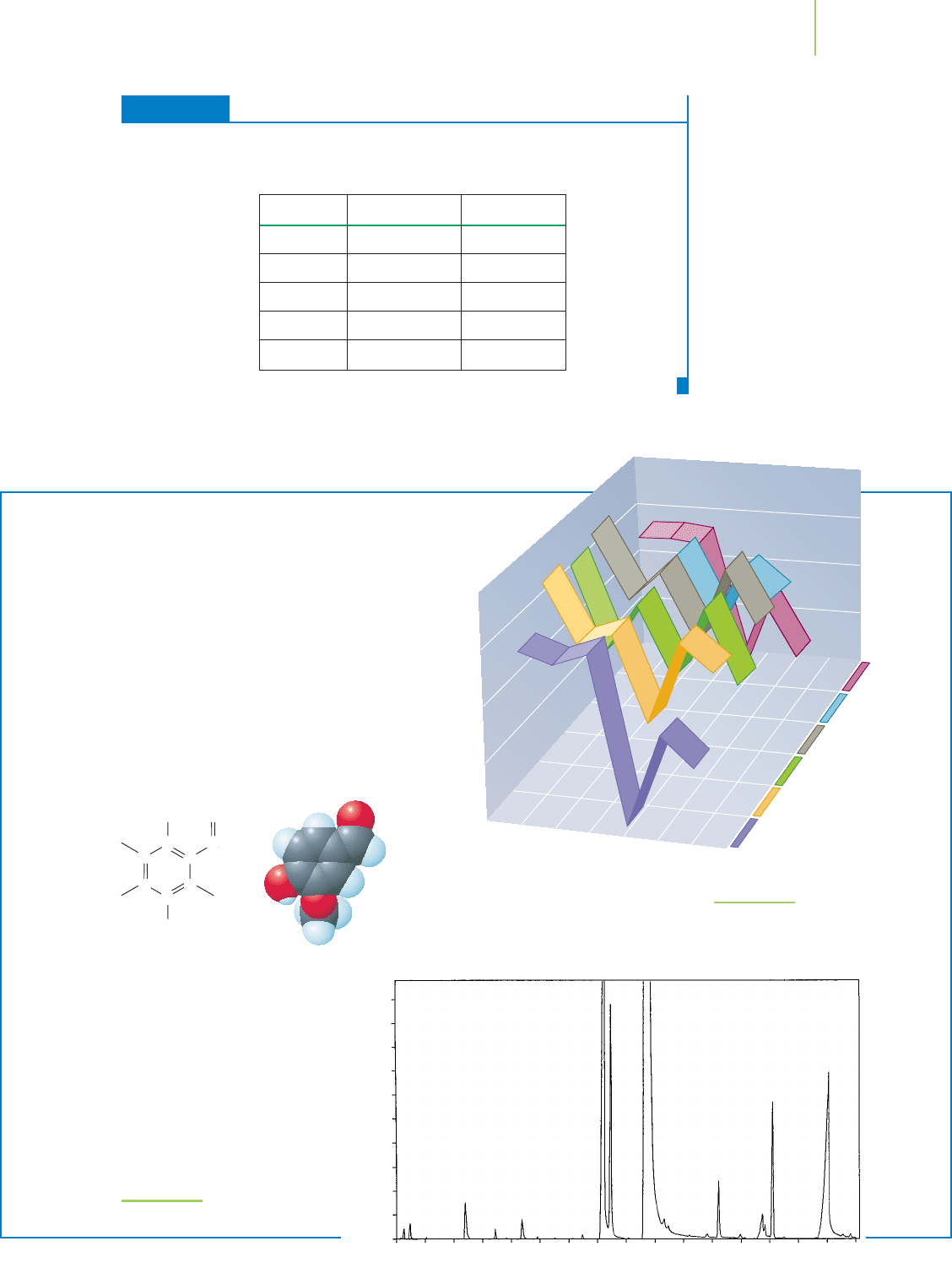
graph (GC) and mass spectrometer (MS), shown in
Figure 2.24, prove useful. The GC is a device that chemi-
cally separates the compounds in the vanilla flavor on
the basis of their mass and structural properties. A sepa-
ration of a vanilla sample is shown in Figure 2.25. After
FIGURE 2.24
A modern gas chromatograph/mass spectrometer, a PerkinElmer
®
Clarus
®
500 GC/MS.
PRACTICE 2.3
Zinc has five isotopes, which have the masses (in amu) and the abundances given
below. What is the average atomic mass of zinc?
See Problems 51–56 and 98.
Isotope Mass Abundance
zinc-64 63.9296011 48.63%
zinc-66 65.9260368 27.90%
zinc-67 66.9271309 4.10%
zinc-68 67.9248476 18.75%
zinc-70 69.925325 0.62%
2.5 Atomic Mass 63
FIGURE 2.25
GC/MS analysis of vanilla sample.
C C
CHHO
H
C
C CH
C
H
OCH
3
O
Vanillin
separation, the individual components are con-
verted into carbon dioxide and fed to the MS,
where the ratio of
13
C to
12
C is measured.
Figure 2.26 shows the analysis of the
ratio of
13
C to
12
C of compounds in sev-
eral samples of vanilla. Note that one
compound, vanillin, is present in rela-
tively large amounts. It is a “character-
istic compound” of vanilla, and we
associate its smell with the product.
Peppermint, citrus, and other oils have
similar characteristic compounds.
–32
p-OH-Benzaldehyde
o-vanillin
Vanillin
Syringaldehyde
p-OH-Benzoic acid
Vanillic acid
–28
Tonga-5269
Mexico-6234
Mexico-6233
Comore-6232
Comore-6231
Comore-5271
–24
–20
–16
Delta Carbon-13
Component
Source
FIGURE 2.26
The ratio of
13
C to
12
C reveals the
origin of the sample.
6
0
5000
10000
15000
20000
25000
30000
ρ-hydroxy benzaldehyde
ρ-hydroxy benzoic acid
Vanillin
Vanillic acid
35000
40000
45000
50000
8 101214161820
Time (minutes)
Abundance
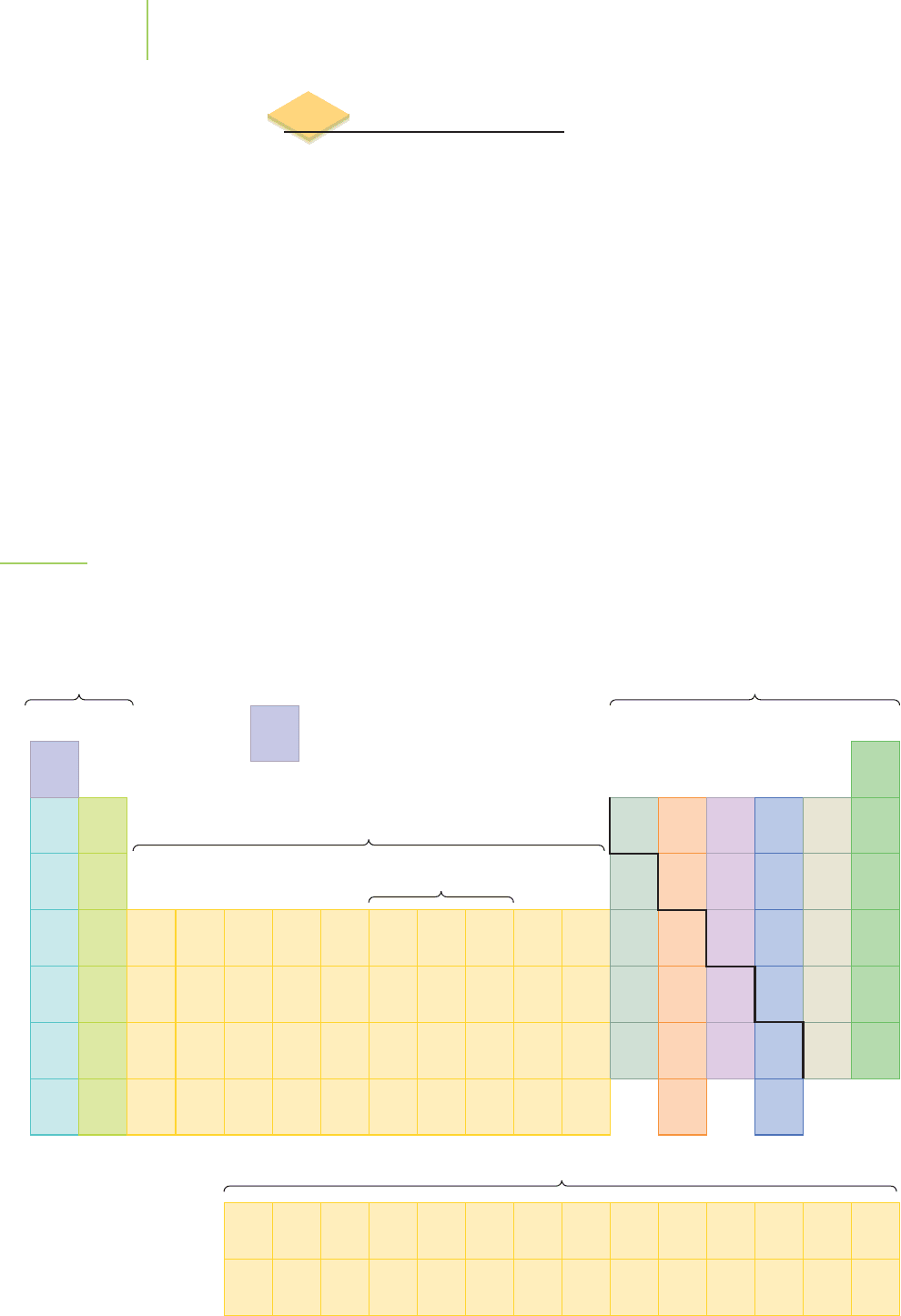
FIGURE 2.27
The elements are arranged in the
periodic table in columns that represent
similar properties. Elements with similar
properties are also color-coded.
64 Chapter 2 Atoms: A Quest for Understanding
2.6 The Periodic Table
The periodic table of the elements is the most significant document summarizing
what we know about atoms. The table, shown in Figure 2.27 and on the inside
front cover of this book, is the reference chart that shows the hierarchy of atomic
structure. The layout of the table summarizes some of the most crucial ideas and
discoveries of chemistry.
The periodic table is arranged in
periods (rows) and groups (columns). If you
read from left to right along the first period, then left to right along the second
period, and so on, you will find the atomic numbers building up one proton at a
time. The atoms generally get heavier as you move along the periods. The table
seems to have a strange structure in places, with unexplained spaces we must
jump over as we read. There are excellent reasons for the shape of the table, and
we will discuss them in Chapter 6.
The arrangement of elements in the periodic table is linked to each element’s
chemical properties in ways that enable us to make many useful predictions. One
of the specific arrangements is indicated by the heavy stair-step line on the right-
hand side of the table. This line serves as the boundary between metal and non-
metal elements. The
metals, located to the left of the boundary, share common
characteristics. They are lustrous (shiny), malleable (can be shaped or bent), and
ductile (can be stretched into wires), and they act as conductors of heat and elec-
tricity. Many of these metals are commonplace in everyday life, such as iron (Fe),
the main component of steel; copper (Cu), used to make electric wiring; and the
silver (Ag) and gold (Au) used widely in jewelry.
1
H
1.00794
1
H
1.00794
3
Li
6.941
11
Na
22.989770
19
K
39.0983
37
Rb
85.4678
55
Cs
132.90545
87
Fr
(223)
4
Be
9.012182
12
Mg
24.3050
20
Ca
40.078
38
Sr
87.62
56
Ba
137.327
88
Ra
(226)
21
Sc
44.955910
39
Y
88.90585
57
La*
138.9055
89
Ac**
(227)
22
Ti
47.867
40
Zr
91.224
72
Hf
178.49
104
Rf
(261)
23
V
50.9415
41
Nb
92.90638
73
Ta
180.9479
105
Db
(262)
24
Cr
51.9961
42
Mo
95.94
74
W
183.84
106
Sg
(266)
25
Mn
54.938049
43
Tc
(98)
75
Re
186.207
107
Bh
(264)
26
Fe
55.845
44
Ru
101.07
76
Os
190.23
108
Hs
(277)
27
Co
58.933200
45
Rh
102.90550
77
Ir
192.217
109
Mt
(268)
28
Ni
58.6934
46
Pd
106.42
78
Pt
195.078
110
Ds
(281)
111
Uuu
(272)
112
Uub
(285)
114
Uuq
(289)
116
Uuh
(292)
29
Cu
63.546
47
Ag
107.8682
79
Au
196.96655
30
Zn
65.409
48
Cd
112.411
80
Hg
200.59
5
B
10.811
13
Al
26.981538
31
Ga
69.723
49
In
114.818
81
Tl
204.3833
6
C
12.0107
14
Si
28.0855
32
Ge
72.64
50
Sn
118.710
82
Pb
207.2
7
N
14.0067
15
P
30.973761
33
As
74.92160
51
Sb
121.760
83
Bi
208.98038
8
O
15.9994
16
S
32.065
34
Se
78.96
52
Te
127.60
84
Po
(209)
9
F
18.9984032
17
Cl
35.453
35
Br
79.904
53
I
126.90447
85
At
(210)
2
He
4.002602
10
Ne
20.1797
18
Ar
39.948
36
Kr
83.798
54
Xe
131.293
86
Rn
(222)
58
Ce
140.116
90
Th
232.0381
59
Pr
140.90765
91
Pa
231.03588
60
Nd
144.24
92
U
238.02891
61
Pm
(145)
93
Np
(237)
62
Sm
150.36
94
Pu
(244)
63
Eu
151.964
95
Am
(243)
64
Gd
157.25
96
Cm
(247)
65
Tb
158.92534
97
Bk
(247)
66
Dy
162.500
98
Cf
(251)
67
Ho
164.93032
99
Es
(252)
68
Er
167.259
100
Fm
(257)
69
Tm
168.93421
101
Md
(258)
70
Yb
173.04
102
No
(259)
71
Lu
174.967
103
Lr
(262)
1
IA
1
2
3
4
Period
5
6
7
2
IIA
3
IIIB
4
IVB
5
VB
6
VIB
7
VIIB
*Lanthanides
**Actinides
11
IB
12
IIB
13
IIIA
14
IVA
15
VA
16
VIA
17
VIIA
18
VIIIA
9
VIIIB
810
Transition metals
Atomic number
Symbol
Atomic weight
Inner transition metals
Main-group elements Main-group elements
2.6 The Periodic Table 65
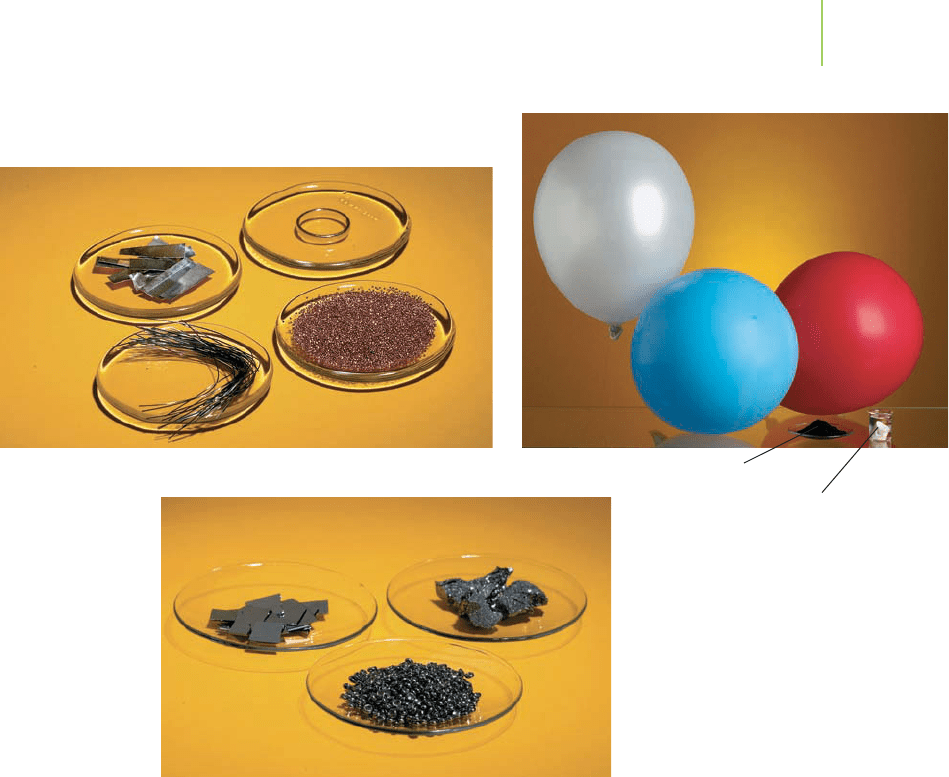
The nonmetals, located to the right of the boundary, also share some common
properties that are quite different from those of the metals. Typically, they are not
lustrous, break easily, and act as insulators to heat and electricity. The nonmetals
are a much smaller set of elements than the metals, but they include some of the
most common elements found in living things. For example, the carbon (C),
hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P) atoms that form
DNA are all nonmetals.
The elements that form the stair-step boundary between metals and non-
metals are called
semimetals or metalloids. This group of elements exhibits some
properties associated with metals and other properties associated with non-
metals. Some of their properties are intermediate between the two. For example,
they are lustrous but are often brittle. The semimetal silicon (Si) is called a semi-
conductor because its ability to conduct electricity is part way between that of a
conductor and that of an insulator.
The elements are also located in another pattern that indicates similar prop-
erties (Figure 2.28). Each vertical column in the periodic table contains a group
of elements with similar chemical and physical properties—hence the name
group. For instance, the elements of a group may combine with other elements in
a certain way, or they may have similar abilities to conduct or not conduct elec-
tricity. All of the elements in Group VIIIA, for example, are very stable gases that
are unreactive except under extreme conditions. They are known as the
noble
gases
—noble being used in the sense of having regal behavior. They are often
referred to as the
inert gases, although for those in the lower part of the group,
this is not, strictly speaking, correct. The helium used to keep dirigibles (blimps)
aloft and the neon used in neon lighting tubes are two common examples
of noble gases. All of the elements in Group IA are highly reactive metals known
Metals.
Metalloids. The metalloids silicon and
boron have properties that are
intermediate to the metals (see above)
and the nonmetals (such as selenium).
Nonmetals.
Gold
Copper
Silver
Iron
Hydrogen
Nitrogen
Oxygen
Phosphorus
Carbon
Silicon
Boron
Selenium
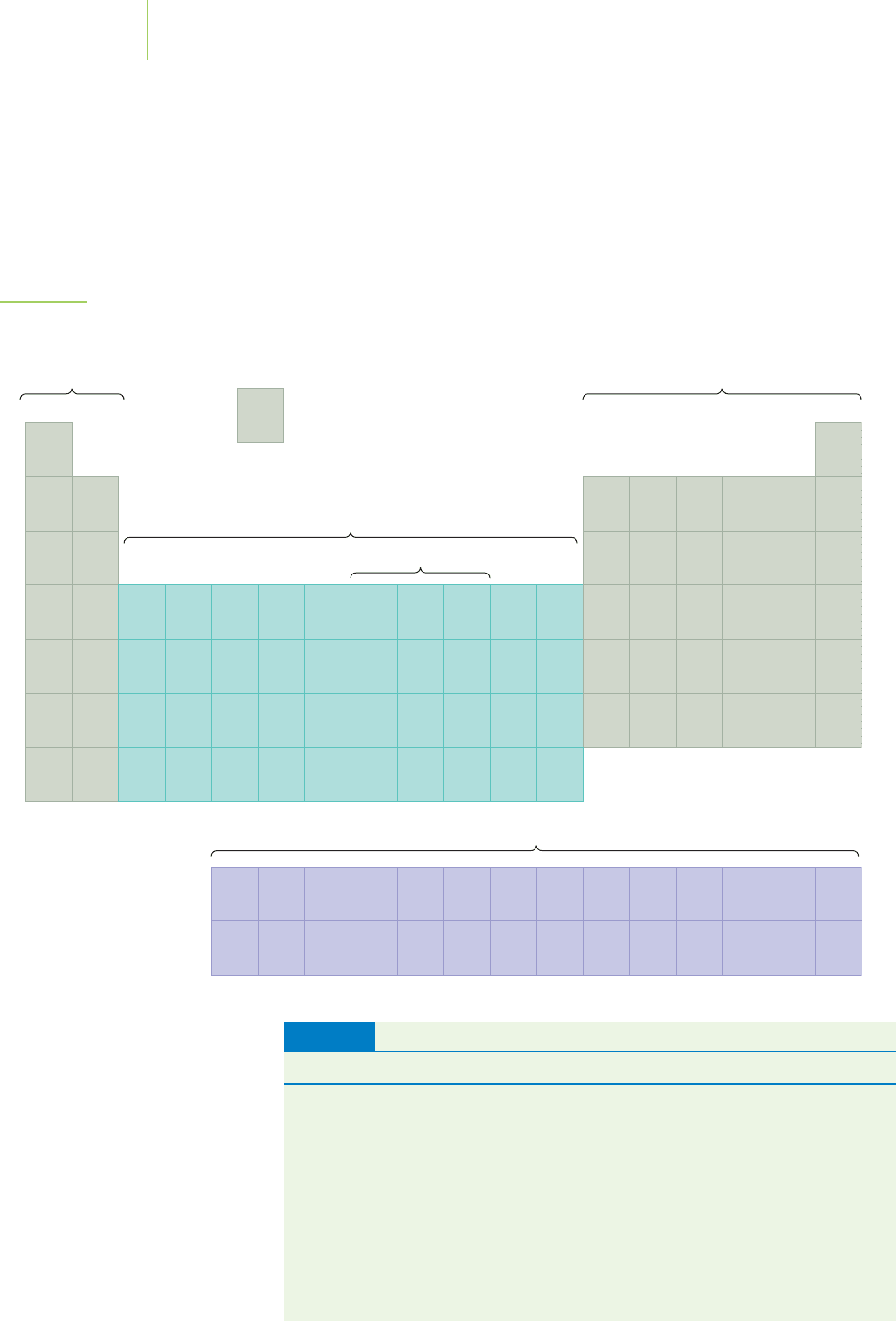
FIGURE 2.28
The main-group, transition, and inner
transition elements.
66 Chapter 2 Atoms: A Quest for Understanding
1
H
1
H
3
Li
11
Na
19
K
37
Rb
55
Cs
87
Fr
4
Be
12
Mg
20
Ca
38
Sr
56
Ba
88
Ra
21
Sc
39
Y
57
La*
89
Ac**
22
Ti
40
Zr
72
Hf
104
Rf
23
V
41
Nb
73
Ta
105
Db
24
Cr
42
Mo
74
W
106
Sg
25
Mn
43
Tc
75
Re
107
Bh
26
Fe
44
Ru
76
Os
108
Hs
27
Co
45
Rh
77
Ir
109
Mt
28
Ni
46
Pd
78
Pt
110
Ds
111
Uuu
112
Uub
29
Cu
47
Ag
79
Au
30
Zn
48
Cd
80
Hg
5
B
13
Al
31
Ga
49
In
81
Tl
6
C
14
Si
32
Ge
50
Sn
82
Pb
7
N
15
P
33
As
51
Sb
83
Bi
8
O
16
S
34
Se
52
Te
84
Po
9
F
17
Cl
35
Br
53
I
85
At
2
He
10
Ne
18
Ar
36
Kr
54
Xe
86
Rn
58
Ce
90
Th
59
Pr
91
Pa
60
Nd
92
U
61
Pm
93
Np
62
Sm
94
Pu
63
Eu
95
Am
64
Gd
96
Cm
65
Tb
97
Bk
66
Dy
98
Cf
67
Ho
99
Es
68
Er
100
Fm
69
Tm
101
Md
70
Yb
102
No
71
Lu
103
Lr
IA
1
2
3
4
Period
5
6
7
IIA
IIIB IVB VB VIB VIIB
*Lanthanides
**Actinides
IB IIB
IIIA IVA VA VIA VIIA
VIIIA
VIIIB
Transition metals
Atomic number
Symbol
Inner transition metals
Main-group elements Main-group elements
as the alkali metals because they form compounds called alkalis when left in con-
tact with water. Table 2.5 summarizes the titles most commonly used for some of
the groups in the periodic table.
Elements in the A groups of the periodic table are historically known as the
main-group elements, and those in the B groups are called transition elements or
transition metals (Figure 2.28). There are two rows of elements separated from
the main table known as the inner transition elements. We will have much more to
say about the historical and chemical organization of the periodic table in future
chapters. At this point, however, we have the background to begin learning about
how the elements combine to form more complex structures, including compounds
and molecules.
Some Groups in the Periodic Table with Characteristic Names
Group Common Name Members
IA Alkali metals Li; Na; K; Rb; Cs; Fr
IIA Alkaline earth metals Be; Mg; Ca; Sr; Ba; Ra
IB Coinage metals Cu, Ag, Au
VIA Chalcogens O; S; Se; Te; Po
(“chalk formers”)
VIIA Halogens F; Cl; Br; I; At
(“salt formers”)
VIIIA Noble gases He; Ne; Ar; Kr; Xe; Rn
(inert gases; “do not react easily”)
TABLE 2.5
2.7 Ionic Compounds 67
2.7 Ionic Compounds
Chemical reactions consist of the rearrangement of atoms to make different sub-
stances. Although the nucleus of an atom remains unchanged in the course of a
chemical reaction, the number of electrons surrounding that nucleus can change.
When electrons are added to or subtracted from a neutral atom or group of
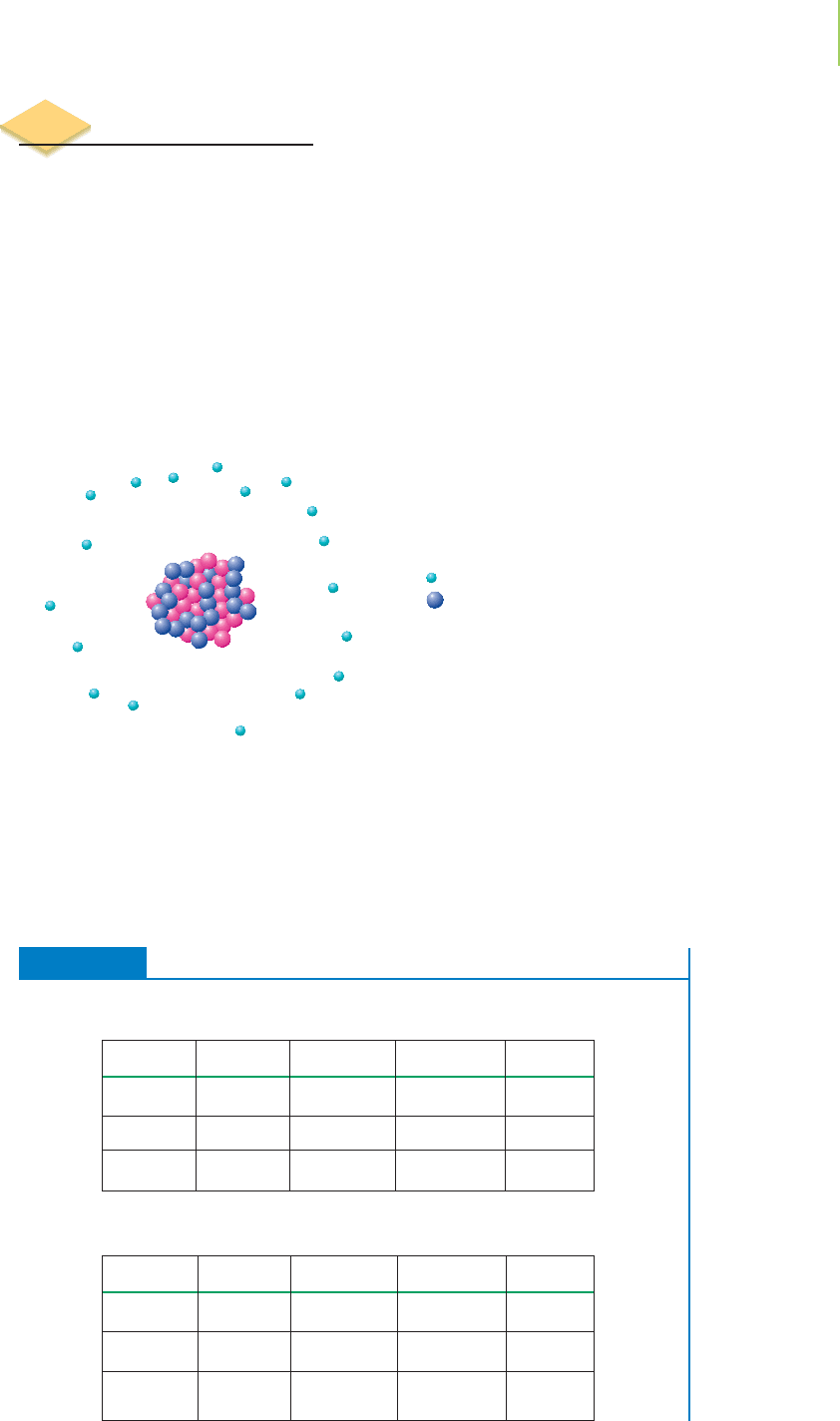
atoms, a charged species known as an ion forms. For example, the neutral cal-
cium-40 atom has 20 protons, 20 neutrons, and 20 electrons, and we denote this
using the nuclide notation
40
20
Ca. When calcium combines with oxygen, it loses
two electrons, which the oxygen atom gains. We can write the nuclide notation
for the resulting calcium ion, which now has 20 protons and only 18 electrons
(two more positive than negative charges) as
40
20
Ca
2+
Ca
2+
18 = 18 electrons = –18 charge
20 = 20 protons = +20 charge
Net charge = +2
Because we are typically interested only in the charge on the ion, and the number
of protons is implicit in the symbol (that is, calcium must have 20 protons if it is
to be called calcium), we simplify the notation even further and write Ca
2+
. Using
the same simplified notation, we denote the oxygen ion, which contains 8 protons
and 10 electrons, as O
2−
.
EXERCISE 2.4 Ions
Fill in the numbers missing from the following table.
Solution
Symbol Protons Neutrons Electrons Charge
32
16
S
2−
16 16 18 −2
137
56
Ba
2+
56 81 54 +2
37
17
Cl
17 20 18 1
Symbol Protons Neutrons Electrons Charge
32
16
S
2−
56 81 54
Cl
20 1
