Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


EXERCISE 1.8 Powers in Dimensional Analysis
A typical can of cola contains 355 mL of liquid. How many cubic meters would be
occupied by the liquid from a 12-pack of cola?
First Thoughts
Let’s map out how we will convert from milliliters into cubic meters. The conversion
from cubic centimeters to cubic meters will be a little more involved than a straight
conversion. We’ll have to break the cm
3
term down into cm × cm × cm in order to
get those units to cancel.
cans
cans to milliliters
−−−−−−−−−→mL
mL to cm
3
−−−−−−−→cm
3
cm
3
to m
3
−−−−−−−→m
3
Solution
12 cans ×
355 mL
can
×
1cm
3
mL
×
m
100 cm
×
m
100 cm
×
m
100 cm
= 0.00426 m
3
Further Insight
Note that in converting the unit cm
3
into m
3
, we completed the conversion from cm
to m three times. In the end, the units of cm
3
= cm × cm × cm, and the resulting
unit m
3
was obtained. This practice for converting units of multiple powers can be
used in any problem.
PRACTICE 1.8
A house requires 1200 yd
2
of carpet. How many square meters of carpet will it
require? How many square inches of carpet will it require?
See Problems 70 and 81.
1.6 Uncertainty, Precision,
Accuracy, and Significant Figures
The density of the silicone tubes used in heart–lung machines is a good indicator
of the flexibility and strength of the tubing. Strong and flexible tubes typically
have relatively low densities. Judith Fairclough-Baity knows this, so she measures
densities to give an idea of the quality of a freshly made tube. Often she finds that
the density of her new tubing is quite similar to that of the tubing already in use
in hospitals. In some cases, the difference in density between good tubing and bad
tubing is very small (1.2011 g/mL versus 1.2005 g/mL). What degree of confi-
dence does she have in her measurements? How can she be sure that the numbers
she obtains for her densities are correct? The confidence that Fairclough-Baity
has in her measurements is based on the use of uncertainty and signifi-
cant figures. This ensures that the numbers she calculates for the densities are
meaningful.
Uncertainty
There are a few things in science, and in life, that we can know for certain, with no
margin of error or room for doubt. For example, if you are asked how many U.S.
presidents’ faces are carved into Mt. Rushmore, you can be positively sure that the
answer is 4. This is an
exact number, a quantity that we know without any uncer-
tainty. Indeed, all data that are determined by counting are exact.
Measurement is different from merely counting, however, because every mea-
surement is associated with a certain degree of
uncertainty. There are no measure-
ments made in which we are absolutely certain of the answer because we, and our
28 Chapter 1 The World of Chemistry

measurement instruments, are imperfect. The uncertainty in a measurement, how
“sure” we are that our measurement is correct, depends on the method used to ob-
tain the measurement, on the quality of the measuring apparatus, and on the care
taken by the person making the measurement. For example, if you are asked to
measure the mass of an orange, you might obtain 151 g or 151.2 g or 151.20467 g,
depending on the balance you use and on how well you know how to use the bal-
ance. Each measured value will be associated with a particular level of uncer-
tainty. No measurement is ever exact.
How do scientists report the uncertainty in a measurement? One way is to use
the symbol ±, which means “plus or minus,” to indicate the limits within which
we confidently expect the true value to lie. (This approach works only if we can
experimentally determine those limits.) Here are two examples:
If we write a mass of 151 ± 1 g, we mean that we are certain that the mass lies be-
tween 150 g and 152 g, but we are not certain of exactly where it lies between
these limits.
If we write a mass of 151.3 ± 0.2 g, we mean that we are certain the mass lies be-
tween 151.1 g and 151.5 g, but we are not certain of exactly where it lies between
these limits.
Even though scientists do their best to minimize the uncertainty of every
measurement, there is still a level of uncertainty in their answers. The two most
common sources of uncertainty in measurements for properly designed experi-
ments are
■
human error and variability. None of us is perfect, and we can make errors
from simple human clumsiness (spilling our reaction or improperly operating
a piece of equipment). But it is also true that our eyes and hands and brains
can manipulate labware, or read a balance, or interpret what we see only so
well.
■
instrument error and variability. Measurement devices are not perfect. Be-
cause of imperfections in their manufacture, faults, or limitations, instru-
ments themselves can contribute to the error in a measurement.
Accuracy and Precision
As we noted in the previous section, the quality of the balance we use to measure
mass is important in a measurement. More broadly, what is a high-quality mea-
surement? We define this via the accuracy and precision of the measurements
we make. To a chemist like Fairclough-Baity, these two terms mean completely
different things.
Accuracy relates how close a measurement is to its true value.
Precision relates how close repeated measurements are to each other, whether they
are accurate or not. A set of measurements can be accurate, can be precise, can ex-
hibit both of these properties, or can offer neither (see Figure 1.29). Fine instru-
ments tend to yield measurements that are both accurate and precise.
1.6 Uncertainty, Precision, Accuracy, and Significant Figures 29
FIGURE 1.29
The application of precision and accuracy to a
game of darts.
Neither precise nor accurate Precise, but not accurate Accurate, but not precise Precise and accurate
Video Lesson: Precision and
Accuracy
Video Lesson: CIA
Demonstration: Precision and
Accuracy with Glassware
Suppose four students need to measure the mass of a sample of orange juice
prior to determining its vitamin C content. Let’s assume that the mass of the or-
ange juice is known to be 1.4683 g. The students all use the same sophisticated
balance that provides four figures after the decimal point, but they forget that the
balance must be set to zero before they use it. These students might get these
results:
Average mass 1.5674 g
Tr ue mass 1.4683 g
These are very precise answers because the masses are in close agreement
and are reported to four decimal places. Unfortunately, however, they are not
accurate answers, either individually or when averaged, because they all contain
the same instrument error, due to the students’ forgetfulness in not zeroing the
balance before they used it.
Now another group of students measures the mass of the orange juice sample,
and they remember to re-zero the balance before each use. Let’s assume that they
obtain these results:
Average mass 1.4684 g
True mass 1.4683 g
The results obtained by Group 2 are both precise and accurate. Each value
reported is in close agreement with the average, and all the values are accurate
because they are very close to the true value for the mass.
A third group of students remembers to zero the balance before use, but they
are less careful in making their measurements. This makes their measurements
subject to human error. Let’s assume this group of students obtains these results:
Average mass 1.4683 g
True mass 1.4683 g
The results they obtain are less precise than those of either Group 1 or Group2, be-
cause they show a greater degree of variability. Each result is individually less ac-
curate than the individual results obtained by Group 2, but they yield an average
Student Group 3
Zeb 1.4673 g
Jeff 1.4691 g
Elena 1.4675 g
Alice 1.4692 g
Student Group 2
Rebecca 1.4682 g
Maggie 1.4684 g
Winston 1.4685 g
Rob 1.4684 g
Student Group 1
Amy 1.5674 g
Jorge 1.5673 g
Barb 1.5673 g
Mike 1.5675 g
30 Chapter 1 The World of Chemistry
Application

value that is accurate. Taking the average value of a set of results subject to ran-
dom errors, such as many human errors, can yield a very accurate result. How-
ever, systematic instrument errors (caused by, for example, a balance that is
tilted) cannot be overcome by averaging, because all the results suffer from the
same instrument error.
What combination of precision and accuracy would the careless members of
Group 3 have produced if they had also forgotten to zero the balance? They
would have produced results that, compared to those of Group 2, were both
imprecise and inaccurate.
Why is it important for us to know about uncertainties in our measurements and
to worry about precision and accuracy?
Lives and jobs and a lot of money can be at
risk if our measurements don’t do what we want them to do. The dosage of drugs
must be measured carefully, within known limits, to avoid doing harm instead of
good. The amount of fuel loaded onto a plane before takeoff is critical if the plane
is to make it to its destination. Impure water puts lives at risk. And an accurate
and precise measurement of the density of heart–lung machine tubing could
mean the creation of a tube that doesn’t crack or break apart during a long
surgery.
Significant Figures
As we have just seen, it is useful to be able to assess the precision and accuracy of
different values. Furthermore, we cannot claim that our answers are more precise
than any of the measurements we used to obtain the answer. In practice, the re-
sults of calculations must contain at least as much uncertainty as is present in the
measured values we used to obtain these results. This means that when we per-
form calculations, we cannot accept all of the numbers shown on the calculator
display, because doing so would imply that we know the result to a degree of cer-
tainty that is not justified. A temperature of 325 K can be divided by 3 on a cal-
culator, which might display an answer of 108.33333333333. However, we do not
know the temperature to 14 digits, only to 3. Some of the numbers in the calcu-
lator’s display are “not significant,” in the sense that we simply cannot trust them;
in other words, some of the numbers in the display have no meaning, so we
should not use them. We can deal with this problem through the use of
significant
figures
,specific numbers in a measurement whose values we can trust.
Let’s follow Judith Fairclough-Baity as she measures the mass of a new piece
of tubing she prepared. She uses a rough balance to obtain a mass of the little
piece of tube. The balance’s display says 5 g. What is the quality of that measure-
ment? Specifically, the number means that the mass is closer to 5 g than it is to 4 g
or to 6 g. This value has just one significant figure. Fairclough-Baity then uses a
higher-quality balance to measure the mass. This balance says the mass of the
tubing is 5.125 g. This number has four significant figures. The balance’s dis-
play indicates that the mass of our sample is closer to 5.125 g than it is to either
5.124 g or 5.126 g. Some uncertainty about the value still exists, but there is less
uncertainty than before. The more significant figures there are in any measure-
ment, the less uncertainty there is about the measurement.
When Fairclough-Baity writes down any value in her notebook, all of the
numbers other than zero are always implied to be significant. When a zero is en-
countered in a measurement, determining its significance is less straightforward.
All zeros that come before the nonzero digits in a number are used to locate the
position of the decimal point, so they do not count as significant figures. For ex-
ample, 0.005 has only one significant figure, not four, because the zeros on the left
just hold the position of the decimal point. She clears up any ambiguity by writ-
ing the value in scientific notation. For example, she writes 0.005 as 5 × 10
–3
to
show that there is only one significant figure.
Zeros that lie between nonzero digits, which are called “captive zeros,” are al-
ways significant. Therefore, the number 505, for example, has three significant
1.6 Uncertainty, Precision, Accuracy, and Significant Figures 31
When we divide 5.125 by 4.6 on a
calculator, not all of the digits in its
answer are significant.
Video Lesson: Significant
Figures
Video Lesson: Scientific
(Exponential) Notation

figures. The number 0.00505 also has only three significant figures. We can write
the latter of these two numbers in exponential notation as 5.05 × 10
–3
.
Zeros that come at the end of a number, which are sometimes called “trailing
zeros,” are truly ambiguous. For instance, if a technician reports a mass of 200 g,
we don’t know whether he or she means 2 × 10
2
or 2.0 × 10
2
or 2.00 × 10
2
.We
can take the most careful possible view and say the value is closer to 200 g than it
is to either 300 g or 100 g. That’s a fairly wide range of uncertainty, but we have no
evidence to allow us to assume that the measurement is more precise. The only
way to resolve the ambiguity is to write a measurement with ambiguous trailing
zeros in scientific notation. If the technician records the value as 2.0 × 10
2
, then
the zero is after the decimal point, which makes it significant. This measurement
unambiguously has two significant figures. If the value is written as 2.00 × 10
2
,
then the measurement has three significant figures. In this case, we would know
that the actual value is between 199 g and 201 g. Table 1.7 summarizes the rules
for determining the number of significant figures in a measured number.
Finally, note that counting numbers (such as the number of people in a room)
and certain defined conversion factors and equalities (such as 4 qt = 1 gal) are
considered to have an infinite number of significant figures. They are exact.
Calculations Involving Significant Figures
Judith Fairclough-Baity’s mass for her piece of tubing can be used to determine
the density of the tubing. She places the tubing into a graduated cylinder and
notes the displacement of water. In the end, she determines that the volume dis-
placed by the 5.125-g piece of tubing was 4.6 mL. She then divides the mass by the
volume to obtain the density, which she reads from the calculator display as
1.1141304 g/mL.
Density =
mass
volume
=
5.125 g
4.6mL
= 1.1141304
g/mL
How many of the digits displayed on her calculator are significant? The rules
we use to determine this (see Table 1.8), depend on whether the number is the
result of addition, subtraction, multiplication, or division. These rules ensure
that Fairclough-Baity’s answer is no more precise than either of her two
measurements.
Using these rules, Fairclough-Baity writes 1.1 g/mL in her notebook. This an-
swer contains only two significant figures and is just as precise as the volume
measurement that she obtained. If she needed to measure the density to four sig-
nificant figures, she would have to measure more precisely the volume of water
displaced by the piece of tubing, or she could use a different strategy for deter-
mining the density.
32 Chapter 1 The World of Chemistry
Rules for Significant Figures
Nonzero digits are always significant. 123 has three significant figures.
Zeros before the nonzero digits are never significant. 0.0123 has three significant figures.
Zeros between nonzero digits are always significant. 1.023 has four significant figures.
Zeros at the end of a number that does not contain 1230 has three significant figures.
a decimal point are ambiguous.
When in doubt, write the number using 1230 written as 1.230 10
3
has four
exponential notation. significant figures.
Counting numbers have infinite significant figures. 200 pencils is an exact number because it came
from counting rather than measuring.
TABLE 1.7

When to Round Off
If you are performing a series of calculations on a calculator, you should carry all
the extra digits through until you obtain the final answer and then do the round-
ing off at the end. You should not round off at intermediate stages, because in doing
so you might lose figures that affect the accuracy of the final result. This rule can
sometimes cause problems—for example, if you perform in one step a calcula-
tion that a textbook or multimedia package has performed in two or more steps.
If you get answers that differ very slightly from those in a textbook or multime-
dia package’s solutions, don’t automatically assume it is wrong. The problem may
lie in the different approaches taken or in rounding off at different stages.
We’ll emphasize this important rule about not rounding off during a calcula-
tion throughout this textbook. For instance, if we are working on a problem that
involves many steps before the answer is obtained, we will not drop the next in-
significant figure. Instead, the first insignificant figure will be highlighted in color,
or otherwise noted at the end of the number. This will serve as a reminder that
one shouldn’t round off until the end of the calculation.
For example, let’s consider the calculation of the density of a piece of metal. If
we measured the volume of water initially as 25.25 mL and then added a 53.375-g
chunk of metal, we note that the volume of water changes to 32.1 mL. To solve
this problem, we determine the difference in the volumes and divide that into the
mass of the metal. Mathematically, here are the steps we take:
Density = 53.375 g ÷ (32.1 mL − 25.25 mL)
= 53.375 g ÷ (6.85 mL)
At this step, we’ve highlighted the insignificant digit in the subtraction so that we
can easily count the number of significant figures for our next step. We haven’t
rounded anything yet because we are not finished with the calculation.
53.375 g ÷ (6.85 mL) = 7.79197 g/mL
= 7.8 g/mL
Here, approaching our final answer, we highlighted the insignificant figures, and
then, because this was the final calculation, we rounded the final answer.
1.6 Uncertainty, Precision, Accuracy, and Significant Figures 33
Calculations Using Significant Figures
Addition/ The result has the same number of digits after 21.3 1.045 22.3
subtraction the decimal point as the measurement with the
least precision.
Multiplication/ The result contains the same number of 1.2 4.2613 5.1
division significant figures as the measurement with the
smallest number of significant figures.
Rounding The final answer is rounded to the nearest 5.35 2.68 1.996 2.00
significant digit. The digit is rounded down if the 8.26 rounds to 8.3
next digit is less than 5; it is rounded up if the next 8.32 rounds to 8.3
digit is greater than 5. If the last significant digit is 8.25 rounds to 8.2
odd and the next digit is exactly 5, the digit is 8.15 rounds to 8.2
rounded up; if the last significant digit is even and 8.251 rounds to 8.3
the next digit is exactly 5, the digit is not rounded. 8.151 rounds to 8.2
Final answer The final answer is the only number in a (3.9684167 1.8)
calculation that is rounded. 7.14315
Intermediate calculations are never rounded. 1.50 4.7621 4.8
TABLE 1.8
EXERCISE 1.9 Calculations
Provide the answer to this calculation. Be sure to indicate the appropriate number
of significant figures.
288 ×1.445
7.9 ×10
2
− 0.064 =
Solution
To start, let’s complete the multiplication on the numerator. We retain at least one
insignificant figure (the 2 in 416.2) in the intermediate calculation:
288 × 1.445 = 416.2
Then we’ll do the division. If this were our final computation, we would keep only
two significant figures. But because we have more calculating to do, we retain a
third, insignificant figure, highlighted in red:
416.2
7.9 ×10
2
= 0.527
Finally, we’ll subtract the two numbers. We don’t round at all until all calculations
are finished. The presence of the highlighted digit represents all of the insignificant
figures we retain.
0.527 − 0.064 = 0.463, which rounds to 0.46
Our answer only has two significant figures. If we had rounded at the previous step,
0.527 would have become 0.53. This would have led to an incorrect answer in the
final step (0.53 − 0.064 = 0.466), because 0.466 rounds to 0.47. Remember, never
round until you are completely done with a calculation.
PRACTICE 1.9
How many significant figures are there in each of these numbers?
a. 1.3090 b. 3450 c. 0.0020 d. 2.000
How many significant figures will there be in the answer to this problem?
2.3 +0.88
79.4
=
See Problems 87–98.
1.7 The Chemical Challenges of the Future
The chemist’s ability to raise questions about everything around us, to design ex-
periments, to make measurements, and to derive theories and laws via this
process we call the scientific method has taught us a great deal about our world
and the role of chemistry in all of its changes. The future is built by these changes,
and they present many chemical challenges to us. Some of these challenges are
problems caused by our use of chemistry in the past; others involve our continu-
ing efforts to make life better, more enjoyable, and more fulfilling. Some infor-
mation about a few of the future challenges for chemists follows.
■
The frontiers of medicine. Chemists will continue to search for new and more
effective medicines to treat cancer, AIDS, heart disease, Alzheimer’s disease,
rheumatoid arthritis, and many other diseases. They will also be urgently try-
ing to create new antibiotics that enable us to “stay ahead” of the pace at which
disease-causing organisms become resistant to existing antibiotics.
34 Chapter 1 The World of Chemistry
Application

1.7 The Chemical Challenges of the Future 35
■
New challenges in agriculture. Chemists have already transformed agriculture
once, through their development of artificial fertilizers and pesticides. They
are now heavily involved in a new and controversial transformation involving
the creation of genetically modified crops. This may enable us to create new
“engineered” plants with larger and more reliable yields. Nevertheless, many
people are concerned about the legal, ethical, and safety issues associated with
genetically modified crops.
■
The challenge of pollution. Oil spills, smog, and pollution in general are nega-
tive aspects of our use of chemical processes—aspects that often predominate
in public perceptions of chemistry. Pollution of our environment has been the
price we have paid for many of the comforts and conveniences of modern life.
Chemists are working hard to reduce that price by developing alternative
“green” technologies and by learning how to use chemistry more effectively to
clean up the problems that still arise.
■
Global warming and ozone depletion. Our desire for comforts and conveniences
has created two major problems in the chemistry of the atmosphere. A rise in
levels of carbon dioxide and other greenhouse gases has been implicated in
global warming. And the destruction of the protective ozone layer by chemi-
cals called chlorofluorocarbons, or CFCs, has contributed to increases in skin
cancer. Chemists will continue to research both issues to determine the causes
of, and solutions to, these environmental concerns. In addition, the develop-
ment of new chemical technologies will help us learn more about the hazards
that caused these problems, with the goal of reducing or eliminating them.
■
Better materials. Materials are the chemicals we use to make things, such as
clothes, cars, aircraft, homes, televisions, and computers. Every material has its
own set of advantages and limitations for a given application. Chemists are
continually trying to enhance the advantages, such as durability, flexibility,
and efficiency as electrical conductors or insulators. They are also working to
overcome the limitations of some materials, such as susceptibility to corro-
sion, high cost, inability to recycle and reuse, and so on. That work will con-
tinue indefinitely, hopefully yielding a steady supply of new and more versatile
materials to make the things we need and want.
■
New ways to supply energy. The modern world is sustained by huge supplies of
materials—such as coal, oil, and gas—that can provide usable energy. Unfor-
tunately, the chemical reserves of energy are limited. Moreover, their use
contributes to environmental pollution and global warming. Chemists are
learning how to get the energy we need in more sustainable and less polluting
ways. Many alternative ways of supplying energy already exist, such as solar
and wind power. But these ways need to be made more efficient, cost-effective,
and powerful in order to find use in today’s energy-hungry world.
The frontiers of medicine involve finding
the viruses that cause disease and identi-
fying the compounds that make them.
Pollution in Mexico City.
■
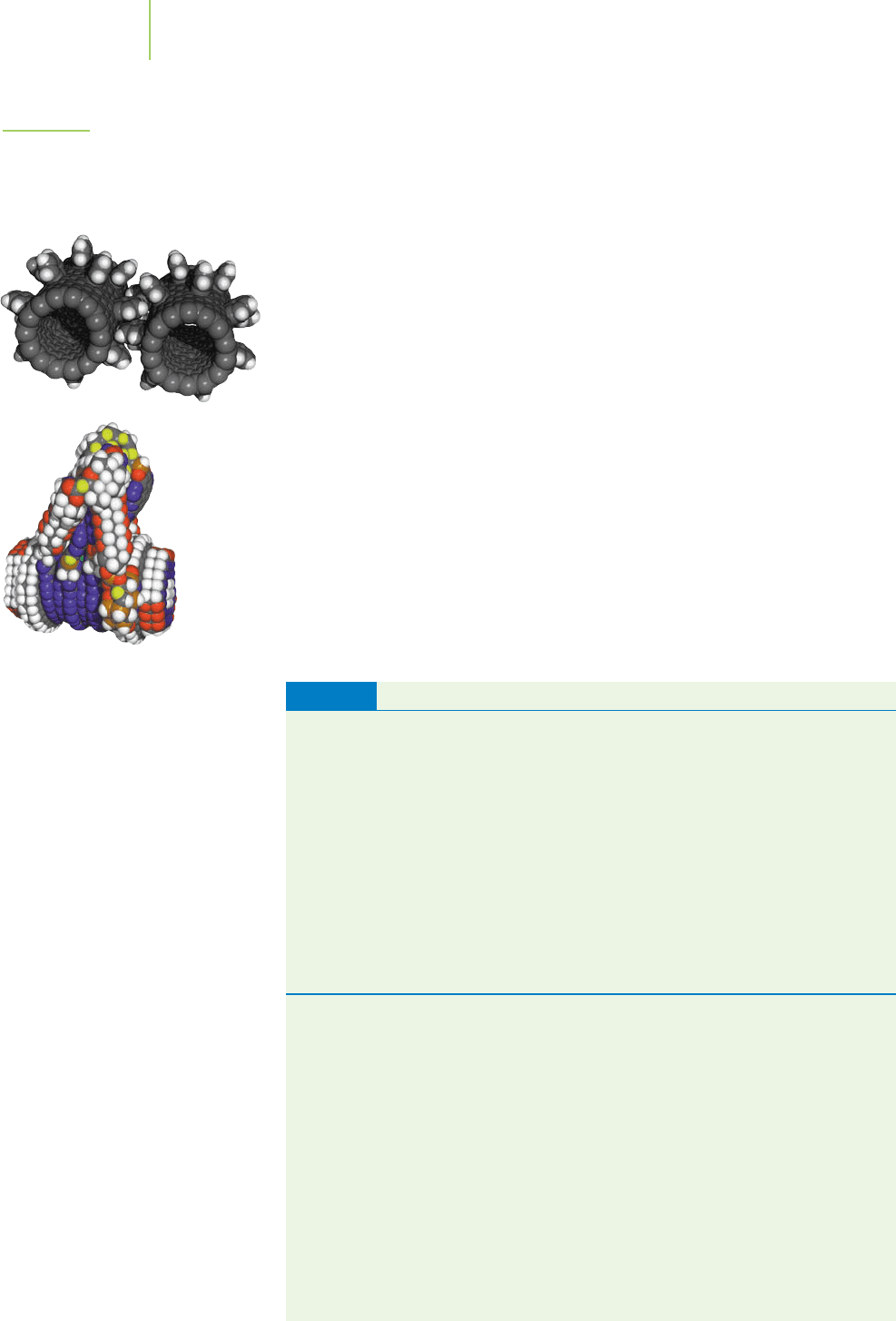
Nanotechnology. In the 1970s people began to use the term nanotechnology to
describe the manipulation and machining of matter at very small scales. Some
developments in this field can be described as “molecular manufacturing,” in-
volving attempts to build tiny machines, materials, and medical devices by
manipulating very small assemblies of matter, even molecule by molecule or
atom by atom. Traditional chemistry works with huge numbers of particles.
The twenty-first century might see a new industrial revolution, in which the
precise manipulation of tiny numbers of atoms, molecules, and ions becomes
a routine part of our technology. Many of the first steps toward that new rev-
olution have already been taken (Figure 1.30).
■
Understanding life. We understand a great deal about the chemistry of life, but
many processes are not fully understood. Indeed, the chemistry of what we re-
ally are—the chemistry of consciousness—is still a complete mystery to us.
We have uncovered the general chemical mechanisms at the heart of all life,
but there are endless intricate details still to be learned. As we learn these de-
tails, and therefore learn more about the workings of life, we will be in a much
better position to fix things when the chemistry of life goes wrong and makes
us ill.
These are just a few of the “hot” chemical topics that will tap the creative en-
ergy of chemists tomorrow and into the distant future. Chemists will also be
involved in the operation of oil refineries, plastics factories, pharmaceutical com-
panies, food manufacturers, cosmetics companies, makers of paints, glues, and
varnish, semiconductor plants, and much, much more (see Table 1.9). Their op-
eration depends on the application of chemical knowledge, continuously, day
and night, to keep the modern world running.
36 Chapter 1 The World of Chemistry
Some Career Opportunities in Chemistry
TABLE 1.9
Agronomist
Anesthesiologist
Biochemist
Ceramics engineer
Chemical engineer
Consumer protection
specialist
Dentist
Dietitian
Educator
Food and drug analyst
Food technologist
Geneticist
Geologist
Industrial health
engineer
Internist
Laboratory analyst
Medicinal chemist
Metallurgist
Nuclear scientist
Patent examiner
Pharmacist
Pharmacologist
Pharmacologist sales
representative
Physician
Professor
Sanitarian
Science technician
Technical writer
Toxicologist
Wood scientist
FIGURE 1.30
Nanotubes, nanospheres, nanogears, and
nanolevers. Such things might be the
building blocks of a new industrial
revolution in “nanotechnology.”
Beverage companies
Centers for Disease Control
Chemical companies
Chemical manufacturing firms
Colleges and universities
Cosmetics companies
Department of Agriculture
Department of Defense
Engineering firms
Environmental Protection Agency
Food companies
Food and Drug Administration
Hospitals
Journals
Local government
Medical laboratories
Medical libraries
Medical supply companies
Mining companies
National Institutes of Health
Newspapers and magazines
Petroleum refineries
Pharmaceutical companies
Research firms
State and federal government
Technical libraries
Textile manufacturers
Possible Employers of Chemistry Majors
Key Words 37
The Bottom Line
■
Our universe and everything in it are made of chem-
icals. Chemicals are involved in all of the changes
that affect and sustain us. (Section 1.1)
■
Atoms are the most fundamental particles of the
chemical world. A substance containing only one
kind of atom is called an element. The particles of
chemistry—atoms and molecules—are incredibly
tiny compared to the objects we see in the everyday
world. (Section 1.2)
■
Molecules are composed of two or more atoms
chemically bonded together. A chemical compound
is any substance that contains different elements
chemically bonded together. (Section 1.2)
■
Chemical changes occur when chemicals undergo
reactions in which new chemical products are
formed from the initial chemical reactants. Physical
changes occur when chemicals undergo changes in
their state. (Section 1.2)
■
Scientists find out about nature, and learn how to
change it, using the scientific method. (Section 1.3)
■
The International System (SI) defines the fundamen-
tal base units, and a variety of derived units, that are
used to measure physical quantities. (Section 1.4)
■
The calculations in chemistry include the conversion
of units by using factors that relate identical quanti-
ties. This can be done via a method known as dimen-
sional analysis. (Section 1.5)
■
Precision and accuracy are important to the discus-
sion of chemistry. They relate the uncertainties in
measurements. The number of significant digits a
number contains is related to the precision of that
number. (Section 1.6)
■
Our understanding of chemistry can be used to
solve problems in many fields, including medicine,
agriculture, pollution control, and nanotechnology.
In addition, we can broaden our understanding of
many issues, including global warming, materials,
meeting our energy needs, and life itself.
(Section 1.7)
Key Words
accuracy The closeness of a measurement to the actual
value. (p. 29)
ampere (A) The SI base unit of electric current. (p. 20)
atoms The smallest identifying unit of an element.
(p. 5)
base units The set of seven fundamental units of the In-
ternational System (SI). (p. 17)
candela (cd) The SI base unit of luminous intensity.
(p. 20)
chemical See matter.
chemical changes Changes that involve chemical reac-
tions in which existing chemicals, the reactants, are
changed into different chemicals, the products. (p. 6)
chemical property A characteristic of a substance that
can be determined as it undergoes a change in its
chemical composition. (p. 6)
chemical reaction The combination or reorganization of
chemicals to produce different chemicals. (p. 6)
chemistry The systematic study of the composition,
structure, and properties of the matter of our uni-
verse. (p. 4)
composition The relative proportions of the elements in
a compound. (p. 5)
compound A substance containing different elements
chemically bonded together. (p. 5)
conversion factor A mathematical expression of the ratio
of one unit to another, used to convert quantities
from one system of units to another. (p. 25)
cubic decimeter (dm
3
) The derived unit commonly
used to measure volumes in the laboratory. See liter.
(p. 22)
cubic meter (m
3
) The derived SI unit used for volume.
(p. 22)
degree Celsius (°C) The unit of temperature on the Cel-
sius scale. (p. 19)
degree Fahrenheit (°F) The unit of temperature on the
Fahrenheit scale. (pp. 18–19)
density The mass of a substance that is present in a
given volume of the substance. The SI unit for mea-
suring density is kilograms per cubic meter (kg/m
3
).
(p. 22)
derived units Units formed by the combination of SI
base units. (p. 22)
desalination The process that removes dissolved salts
from seawater to make potable water. (p. 10)
dimensional analysis An extremely useful method for
performing calculations by using appropriate con-
version factors and allowing units (dimensions) to
cancel out, leaving only the desired answer in the de-
sired units. (p. 26)
