Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

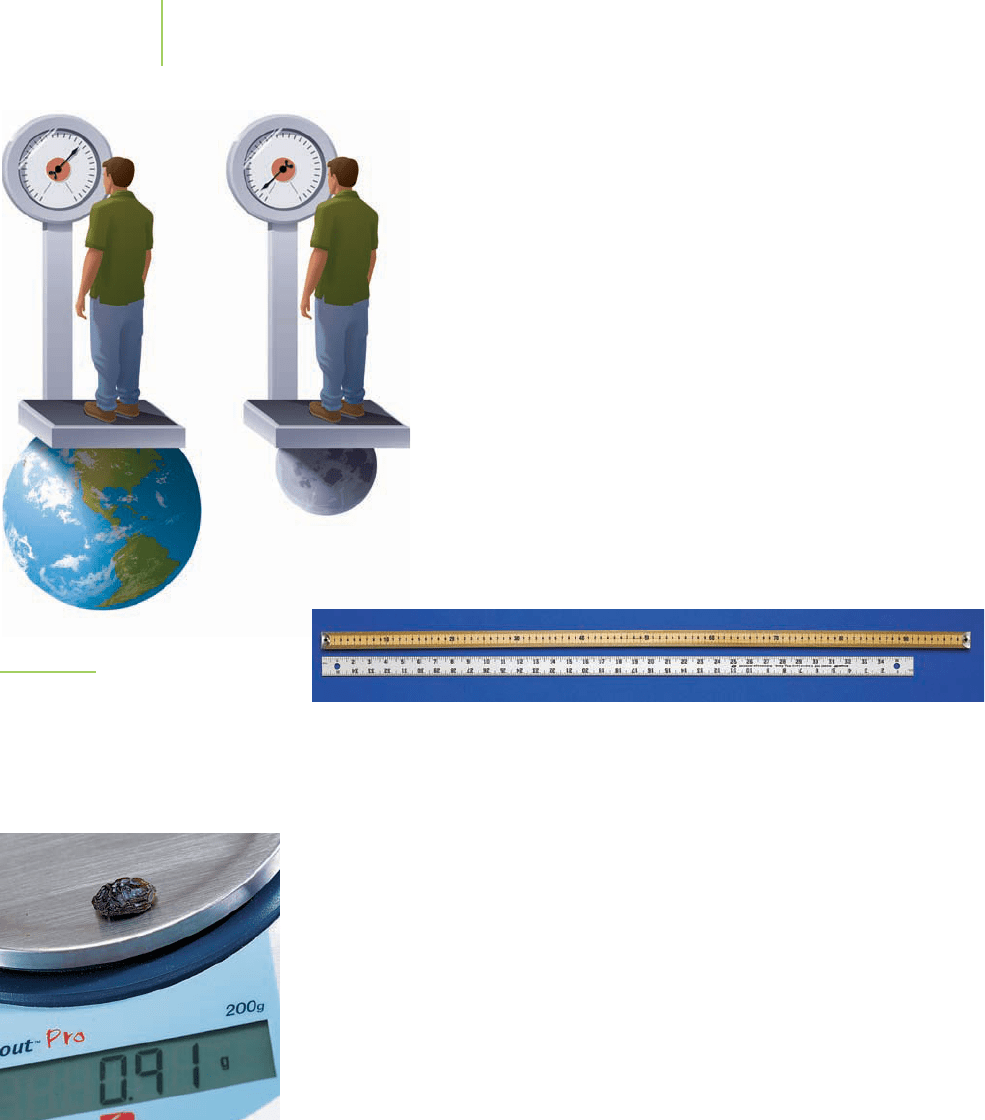
compare an object on Earth to the same object on the Moon. The
weaker gravitational force on the Moon would cause the object to
weigh less, but it would still have the same mass (Figure 1.18).
For many of our water quality measurements, we might only
need a gram of water. How much is a gram? A raisin’s mass is ap-
proximately 1 g. That is a pretty small amount, but 1000 raisins
have about the same mass as a kilogram.A kilogram weighs about
the same as 2.2 lb on Earth. However, masses with which you will
become familiar in the laboratory are much smaller than the kilo-
gram. In fact, most masses you will record in the laboratory will be
grams or milligrams. In the chemical process industries, which
produce large quantities of the chemicals used to manufacture
consumer goods, masses are often measured in tons (2000 lb) or
metric tons (1000 kg, 1 Mg, or 2200 lb).
Another important piece of information to include with our
water quality measurements would be the distance to the farthest
city that is served by the desalination plant. We would record this
distance using the SI unit of length, the
meter (m). The meter is
about 10% longer than a yard.
1 m = 1.094 yd
18 Chapter 1 The World of Chemistry
To measure long distances, we often report them in kilometers (km).
1 km = 1 × 10
3
m = 1000 m
One kilometer is equal to 1000 m, or slightly more than 0.6 mi. A runner entered
in a “5K” (5-km) road race will run 5000 m (a little more than 3 miles). In chem-
ical analysis, though, scientists measure lengths much smaller than a meter. Be-
cause the particles of chemistry are extremely tiny compared to objects in the
everyday world, their sizes are often measured in such tiny units as picometers,
nanometers, and micrometers. One nanometer is equal to 0.000000001 m, so
there are 1 billion nanometers (10
9
nm) in 1 m.
1 nm = 1 × 10
−9
m = 0.000000001 m
How long it takes to desalinate enough water for the people served by the plant
is also a key quantity. To report this value, we could use the SI unit of time, the
second (s). Luckily, the second has been used in virtually all measurement systems,
so we are pretty familiar with it. The units of hour, minute, day, and year are often
used in measurements of time. These are not SI units, but because they are so
widely used, scientists don’t often affix prefixes to the unit second to describe large
time spans. For example, instead of reporting 3600 s as 3.6 ks (which is perfectly
appropriate), the scientist would be more likely to indicate 1 hour (1 h),
3600 s = 3.6 × 10
3
s = 3.6 ks
3600 s = 1 h
Prefixes, however, are commonly used to indicate fractions of a second. One mil-
lisecond (1 ms) is one-thousandth of a second.
Temperature is a vital part of our day-to-day choices about what clothing to
wear, what liquid to drink, and a host of other decisions. The SI base unit of tem-
perature is the
kelvin (K). Notice that no “°” symbol is used with the abbreviation
K. Two other temperature units are much more common, however. The English
system uses the Fahrenheit scale, in which the unit of measure is the
degree
54
kg
9
Earth
Moon
kg
FIGURE 1.18
A person’s weight on the Moon is differ-
ent from his or her weight on Earth. The
mass of the person hasn’t changed. Only
the force of gravity is different.
The mass of a raisin is about 1 g.
A meter and a yard.
Fahrenheit (°F). The metric system uses the Celsius scale, in which the unit of
measure is the
degree Celsius (°C). All three systems can be used to accurately re-
port temperatures, but they are based on different physical phenomena. The
Fahrenheit scale was created by the German physicist Gabriel Daniel Fahrenheit
in 1714. Fahrenheit used a mercury-based thermometer to define 0°F as the cold-
est temperature he could make from a mixture of water and ammonium chlo-
ride. He called his body temperature 100°F. (Linus Pauling, a chemist whose work
we will discuss at many points later in this text, mused that Fahrenheit might have
had a slight fever.) Using his two reference points of cold and warm, he found the
boiling point of water to be 212°F.
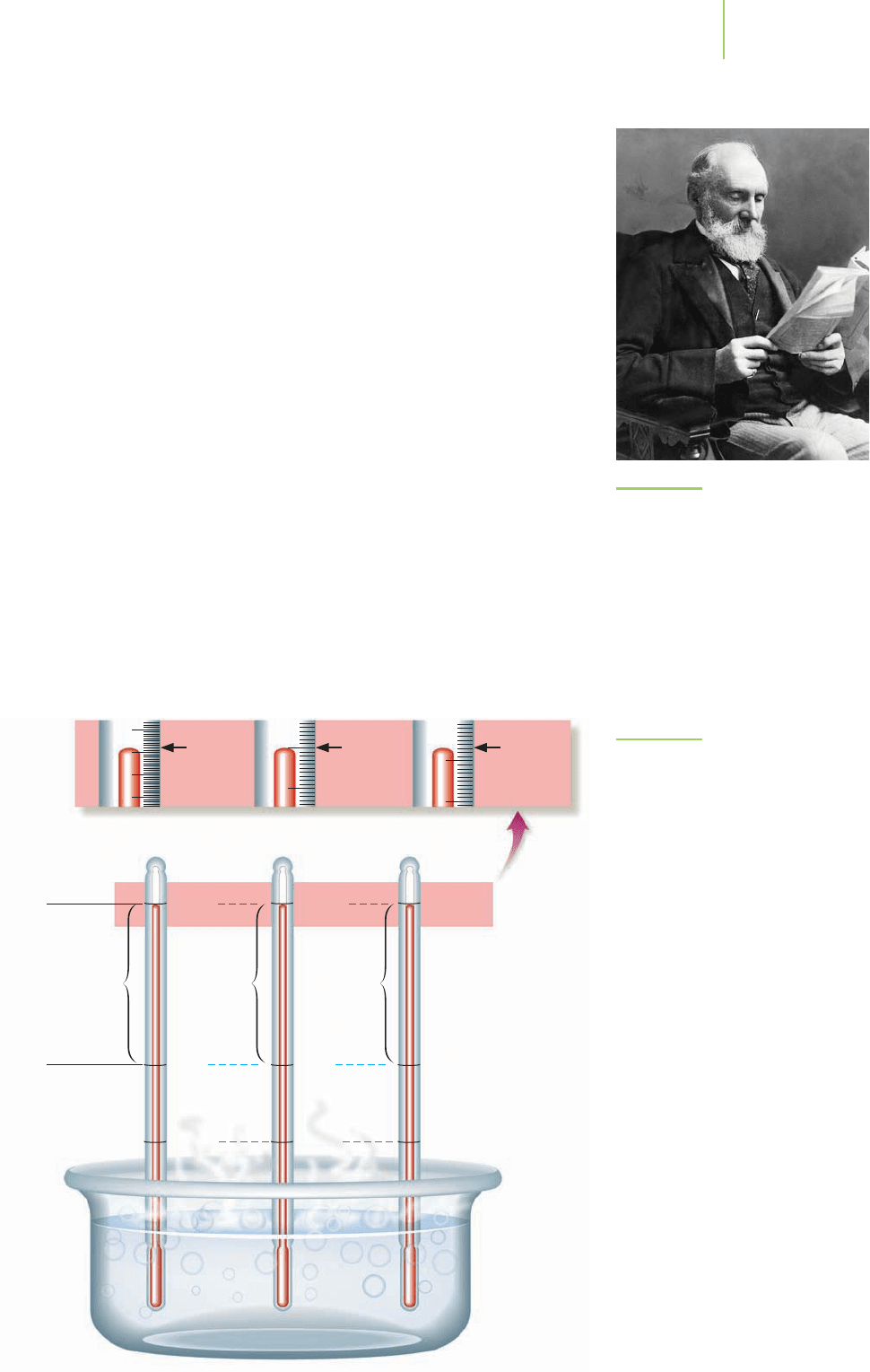
The Swedish astronomer Anders Celsius developed the Celsius scale in 1742,
based on dividing the difference in temperature from the freezing point, 0°C, to the
boilingpoint,100°C,of waterinto100individualunits(degrees).In1848,theBritish
physicistLord Kelvin (Figure 1.19) inventedthe temperaturescale named for him in
1954 in which, fortunately, one kelvin is equal in magnitude to 1 degree Celsius,
although the scales just startat different points.Although Kelvindid not recognizeit
at the time,the point we now call zero on the Kelvin scale equals –273 on the Celsius
scale,so a temperature in Celsius can be converted into Kelvin by adding 273.
T
K
= T
C
+ 273 and T
C
= T
K
− 273
More information and the exact definition of the Kelvin scale is presented later.
Converting between the Celsius and Fahrenheit scales is a little more compli-
cated. The formula shows that the temperature of an object changes by 9 Fahren-
heit degrees for every 5 Celsius degrees. This means that if you use a Fahrenheit
thermometer to measure an 18-degree drop in temperature (9
× 2), a ther-
mometer based on the Celsius scale will note a 10-degree drop (5
× 2). A
Kelvin-based thermometer would also show a 10-degree drop in temperature,
because 1 K equals 1°C (see Figure 1.20). Figure 1.20 also indicates the different
1.4 Units and Measurement 19
FIGURE 1.19
Lord Kelvin (1824–1907) was born
William Thomson in Belfast. He was
knighted in 1866 and given the title of
Baron Kelvin of Largs in 1892.
180
Fahrenheit
degrees
Boiling point
of water
32°F
212°F
212°F
200°F
90°C
220°F
190°F
Freezing point
of water
Fahrenheit
100
Celsius
degrees
0°C
100°C
Celsius
273.15 K
373.15 K
Kelvin
100
kelvins
100°C
360 K
370 K
373.15 K
–
40°F
–
40°C
233.15 K
FIGURE 1.20
The Fahrenheit, Celsius, and Kelvin
temperature scales.

locations of the freezing point of water on the scales. Here are the formulas re-
quired for converting between temperatures on the Celsius and Fahrenheit scales:
T
C
= (T
F
− 32)/1.8 T
F
= 1.8(T
C
) + 32
Another property of our water sample, or of any other substance or mixture
of substances, is the number of particles of each kind we have. These particles
represent the amount of each substance in the sample. The amount of a sub-
stance is the number of entities, such as atoms, molecules, or ions (particles con-
taining an electrical charge), present in a given sample of the substance. Deter-
mining the amount of each substance in a glass of tap water is just one part of a
series of analyses that chemists do to make sure that a sample of water is whole-
some to drink. For example, the analysis for the element arsenic in drinking water
really deals with determining how many arsenic atoms are present in a known
quantity of water. This idea is explored fully in Chapter 3.
The base unit for the amount of a substance is the
mole (mol). As a preview of
Chapter 3, we will simply point out that 1 mole corresponds to a specific very
large number of entities and that this number is approximately 6.02214 × 10
23
.
Thus when we talk of 1 mole of atoms, we mean 6.02214 × 10
23
atoms; 1 mole of
molecules is 6.02214 × 10
23
molecules, and so on.
Other SI units may also be needed during our water quality measurements.
For instance, the purity of desalinated water is inversely related to its ability
to carry an electric current: The lower the electrical conductivity of the water,
the purer the water (see Figure 1.21). The SI base unit of electric current is the
ampere (A), often called the amp. This unit is used a great deal in chemistry be-
cause some chemical reactions can be used to generate an electric current (within
batteries, for example). Conversely, electricity can be used to make certain chem-
ical reactions happen. We’ll learn more about the ampere in the chapter on elec-
trochemistry (Chapter 19).
The last SI base unit is the
candela (cd). This unit describes the luminous inten-
sity
, or brightness, of something under study. For example, the Sun, which radi-
ates the light energy that powers photosynthesis, has an estimated luminous
intensity of 10
18
candelas. Light bulbs produce a vastly smaller luminous inten-
sity. We won’t deal with luminous intensity in this text.
We will refer to the world of individual atoms, molecules, and ions as the
nanoworld, in contrast to the everyday, large-scale macroworld (“big world”) with
which we are familiar. There is a huge change in the units that we use to report
quantities as we travel between the nanoworld and the macroworld. To make the
trip smoothly, we must be able to work comfortably with exponential notation
and related mathematical operations. Feel free to reinforce your understanding of
exponential notation by consulting the appendix at the back of the book.
20 Chapter 1 The World of Chemistry
FIGURE 1.21
The conductivity of water solutions
gives an indication of their purity.
One of the most important aspects of learning about chemistry is becoming
familiar with the nanoworld of chemicals and being able to visualize what
happens at that scale. The fundamental particles of chemistry are atoms
(Figure 1.22). These range in size from a diameter of approximately 75 picome-
ters (75
×10
–12
m = 7.5 ×10
–11
m) for a hydrogen atom to about 500 picometers
(5.00
× 10
–10
m) for an atom of francium. How can we get some idea of what
these numbers mean?
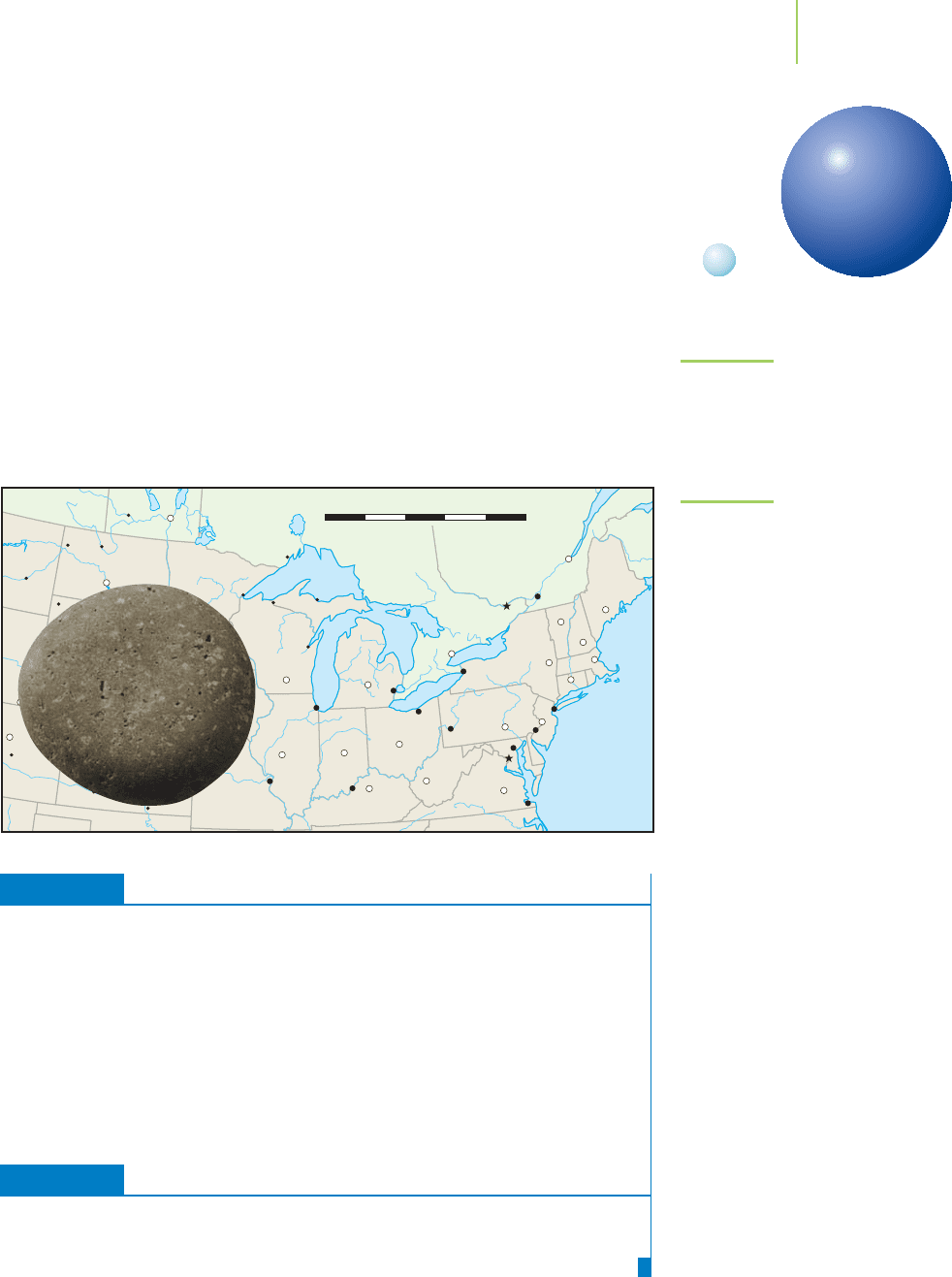
Suppose we scale things up in our minds so that atoms be-
come the size of pebbles that we can hold in our hand—some small, some larger,
but averaging around 2 cm in diameter. A real pebble scaled up in size to the same
extent would now be approximately 1000 kilometers (about 600 miles) in diam-
eter. That’s big enough to cover the entire Great Lakes (Figure 1.23). Even with
these comparisons, this extraordinary difference in scale is difficult to visualize.
Yet working with such comparisons should begin to give you some feeling for the
scale of the nanoworld of chemistry. Atoms, molecules, and ions are very small
indeed.
1.4 Units and Measurement 21
Francium
(540 pm)
Hydrogen
(75 pm)
FIGURE 1.22
The relative size of two atoms, hydrogen
(75 pm) and francium (540 pm).
U.S.A.
CANADA
Lake Erie
Lake Ontario
Colorado Springs
Fargo
Green Bay
Marquette
Minot
Norfolk
Rapid City
Rochester
Scottsbluff
St. Joseph
Wichita
Brandon
Thunder Bay
Buffalo
Dodge City
Duluth
Grand Island
Hastings
Hot Springs
Huron
Ironwood
Mankato
Miles City
Sioux City
Sioux Falls
Williston
Winfield
Cleveland
Detroit
Baltimore
Chicago
Louisville
Minneapolis
Philadelphia
St. Louis
Montreal
Buffalo
Kansas City
New York
Norfolk
Omaha
Pittsburgh
Quebec
Winnipeg
Albany
Ames
Augusta
Bismarck
Boston
Charleston
Columbus
Denver
Des Moines
Frankfort
Harrisburg
Hartford
Indianapolis
Lansing
Lincoln
Montpelier
Richmond
Springfield
St. Paul
Tr ent on
Toronto
Concord
Madison
Topeka
Washington, DC
Ottawa
L
a
k
e
M
i
c
h
i
g
a
n
L
a
k
e
S
u
p
e
r
i
o
r
L
a
k
e
H
u
r
o
n
500 Miles0
Scale
Atlantic
Ocean
FIGURE 1.23
If an atom were scaled up to the size of
a pebble on a beach, the beach pebble,
scaled up to the same extent, would be
600 miles wide.
EXERCISE 1.4 Temperature Conversions
The chemical reactions within the human body tend to proceed optimally at a tem-
perature of around 37°C. Express this temperature using the Kelvin and Fahrenheit
scales.
Solution
T
K
= T
C
+ 273 so 37°C = 37 + 273 K = 310 K
T
F
= 1.8T
C
+ 32 so 37°C = 1.8(37) + 32°F = 99°F
Normal body temperature is commonly cited as equal to 98.6
o
F, which has more
significant figures (see Section 1.6) than are justified in a comparison to 37
o
C.
PRACTICE 1.4
Water is fed into the vaporization stage of a desalination plant at 220
o
F. Express this
temperature using the Celsius and Kelvin scales.
See Problems 37c, 37f, 38c, 38f, 39c, 39f, 40c, 40f, and 53–54.
Derived Units
We often measure quantities other than the seven SI base quantities shown in
Table 1.2. Our analysis of the sample of water we obtain during our daily check of
water quality at the desalination plant or any municipal water treatment system
has several types of measurements associated with it. We’ve
recorded the mass, the time spent to purify the water in the reverse
osmosis column, the distance (using the SI unit of length) of the re-
gion served by the plant, and the temperature of the water, and now
we also note the volume and density of the sample. These last two
measures, along with many other common determinations, use
de-
rived units
, formed by the combination of SI base units. Some of the
more common derived units are shown in Table 1.4.
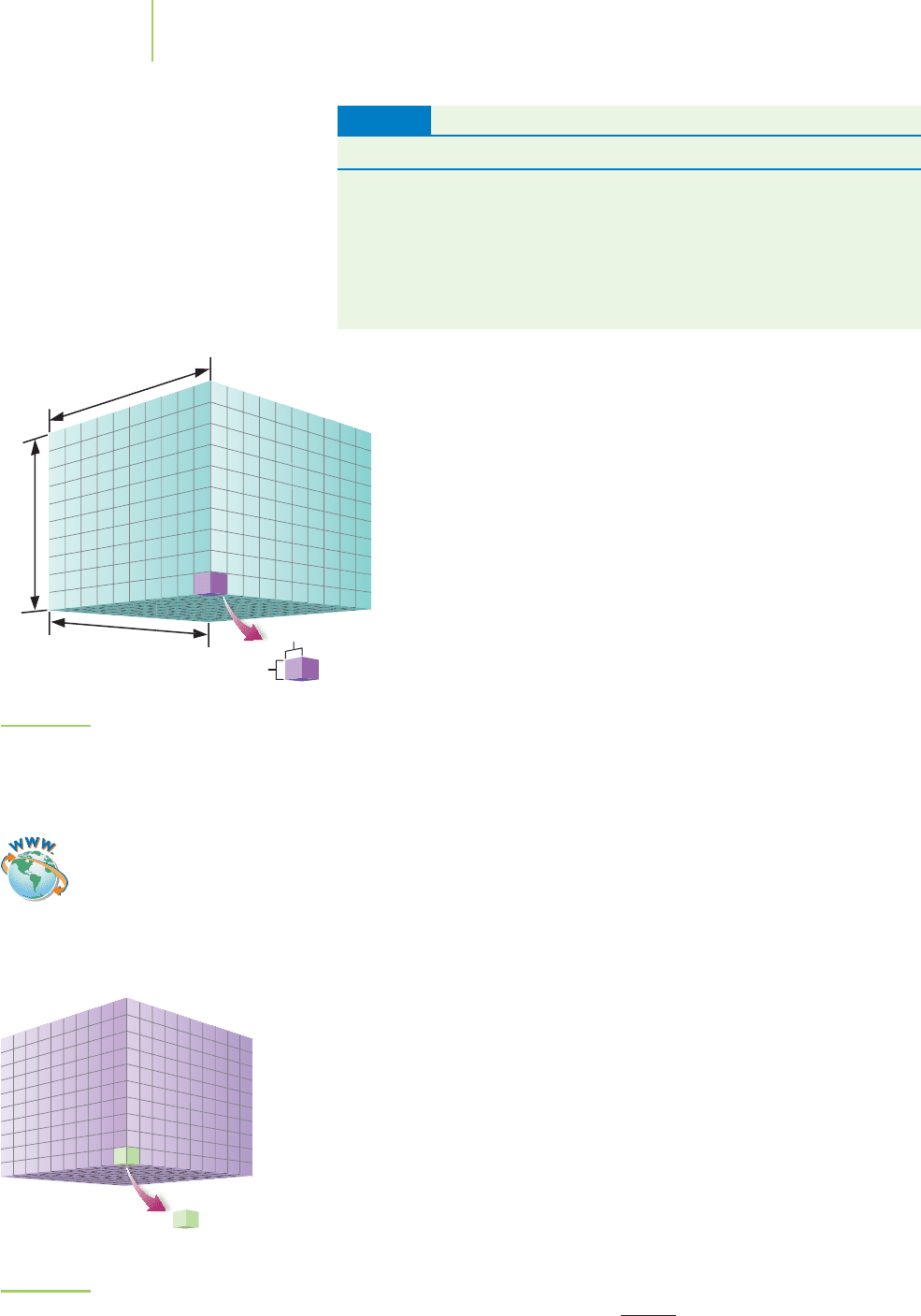
The volume of a water sample obtained from the desalination
plant is a measure the space occupied by the sample. We could mea-
sure the volume of the sample in SI base units as
cubic meters (m
3
).
Specifically, a cubic meter is the volume described by a cube of mat-
ter measuring 1 m on each side (see Figure 1.24). The SI unit for
volume, the cubic meter, is derived from the SI unit for length, the
meter.
Although the cubic meter is the SI unit for volume, this derived
unit is often much too large to reflect the sample sizes that we would
obtain. Chemists tend to measure volumes using smaller derived units, such as
the
cubic decimeter (dm
3
), which is commonly used in most parts of the world,
with the notable exception of the United States. As Figure 1.25 shows, a cubic
decimeter is the volume of a cube that measures 10 cm on each side. This means
that there are 1000 dm
3
in every cubic meter. Because we use the cubic decimeter
in most of our measurements, a different name has been given to this unit. The
cubic decimeter is also known as the
liter (L), which is the common volume mea-
surement used by scientists in the United States.
Another common derived unit for volume is the cubic centimeter (cm
3
). This
unit describes a cube measuring 1 cm on each side (see Figure 1.25). In the health
professions, one cubic centimeter is often abbreviated as 1 cc. There are 1000 cc
in 1 L, so the cubic centimeter is also known as the
milliliter (mL):1 cm
3
= 1 cc =
1 mL. Note that the unit for liters is a capital L, not a lowercase l.
1 L = 1000 mL = 1000 cc = 1 dm
3
= 0.001 m
3
Judith Fairclough-Baity, in her report on the characterization of three types of
silicone tubing, indicated the
density of the tubes she made. Density reports the
mass of a substance that is present in a given volume of the substance. During our
analysis of the quality of water from a desalination plant, we might also report the
density of the sample. The SI unit for measuring density is
kilograms per cubic
meter (kg/m
3
), although scientists often use smaller units, such as grams per cubic
centimeter (g/cm
3
). Because 1 cm
3
equals 1 mL, units of density can be reported
for liquids and many solids in grams per milliliter. The tubing with which
Fairclough-Baity worked had a density of between 1.1 and 1.2 g/mL.
Density
=
mass
volume
22 Chapter 1 The World of Chemistry
L
Liter (L)
1 dm
1 dm
1 m
Cubic meter (m
3
) = 1000 dm
3
= 1000 L
1 m
1 m
Volume: 1 dm
3
Volume: 1 cm
3
1 mL
“1 liter” = 1 L = 1 dm
3
1000 cm
3
= 1000 mL
Selected Derived Units
Physical Quantity Derived Unit Name of Derived Unit
Volume m
3
cubic meter
Density kg
.
m
–3
kilograms per cubic meter
Force kg
.
m
.
s
–2
newton (N)
Pressure kg
.
m
–1
.
s
–2
(N
.
m
–2
) pascal (Pa)
Energy kg
.
m
2
.
s
–2
(N
.
m) joule (J)
Velocity m
.
s
–1
meters per second
TABLE 1.4
FIGURE 1.24
The cubic meter, the SI unit for volume.
FIGURE 1.25
One cubic centimeter is equal to
a milliliter.
Video Lesson: CIA
Demonstration: Differences in
Density Due to Temperature
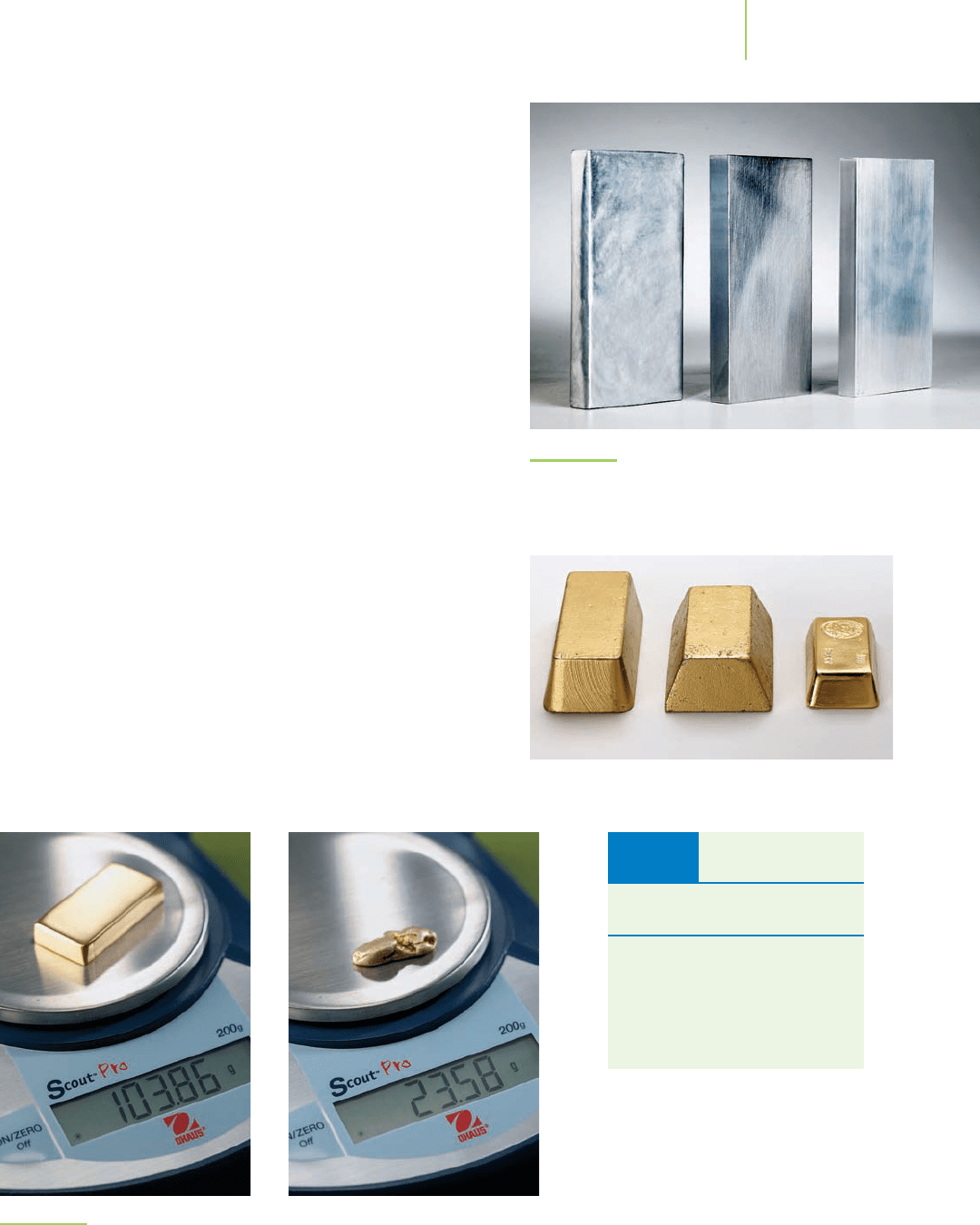
An interesting example of the use of density is in the compari-
son of three cubes of material. As shown in Figure 1.26, each block
of material looks the same. However, one is silver-tinted wax, one
is lead, and the third is aluminum.
How can we tell the wax, lead,
and aluminum apart?
There are many physical properties that differ
among the three blocks, such as color and hardness. Density is an-
other. The wax has a density of 0.95 g/cm
3
, the lead has a density of
11.4 g/cm
3
, and the aluminum has a density of 2.70 g/cm
3
. There-
fore, although the volumes of all the cubes are identical, they differ
in mass.
Extensive Versus Intensive Properties
Why does a log float on water? Mistakenly, we might say that the
log is “lighter” than the water. Why does a pebble sink to the bot-
tom of a lake? Again, we might mistakenly say that it is “heavier”
than water. The heaviness or lightness of an object is a measure of
the weight of the object. It is an
extensive property, one that is de-
pendent on how much sample you have. Length is another exten-
sive property. If the sample is a precious metal, such as gold or plat-
inum, then weight and mass are related to total cost, and all of
these are extensive properties.
On the other hand, density, the mass of the metal per cubic
centimeter (or milliliter) is an
intensive property, because it re-
mains the same irrespective of the amount of sample you have.
Gold will still cost about $400 per ounce whether if you have
1 ounce (1 oz) or 1000 oz. The cost per ounce is an intensive prop-
erty. The density of the gold, 19.3 g/mL, is the same no matter how
much gold you have. However, the cost of a chunk of gold is an ex-
tensive property because it depends on the size of the chunk. Large
chunks cost more than small chunks (Figure 1.27). Table 1.5 lists
several intensive and extensive properties.
1.4 Units and Measurement 23
FIGURE 1.26
A block of silver-colored wax, a block of lead, and a block of
aluminum. They look the same but are chemically quite different.
We can tell which is which by measuring their densities.
$212,000 $311,000 $54,000
Mass, length, and cost are extensive properties.
FIGURE 1.27
Extensive and intensive properties of gold. The weight and cost of a chunk of gold
depend on the amount (extensive properties); the color and density of the gold remain
independent of the amount (intensive properties).
Intensive and
Extensive Properties
Intensive Extensive
Properties Properties
Color Mass
Density Length
Temperature Volume
Odor Cost
Physical state Energy
TABLE 1.5

Balsa wood
Hexane
Oak wood
Chloroform
Lead
Water
10.00 mL
Experimental determination of density. A picnometer, which contains an exact volume, can
be used to measure the density of a liquid.
EXERCISE 1.5 Extensive Versus Intensive Properties
During an expedition to the top of a mountain, a camper wishes to cook some noo-
dles for the entire group. In order to heat the noodles, the camper must boil an en-
tire pot of water. Is the temperature at which water boils an intensive or an extensive
property?
First Thoughts
Initially, this problem seems confusing because we know it will take longer to bring
a pot of water to a boil than to heat a cup of water to its boiling point. Let’s consider
the question this way: Does the amount of water make a difference in how hot it
must get before it boils?
Solution
The boiling point of water is an intensive property. The amount of water does not
make a difference in how hot it must get in order to boil. In other words, the pot of
boiling water and the cup of boiling water have the same temperature.
Further Insight
Why, then, does it take longer to boil a pot of water? Answering this question reveals
another extensive property. The amount of water determines how much heat must
be used to boil the water. The amount of heat used to boil the water is an extensive
property of the water.
PRACTICE 1.5
Is the viscosity (the resistance of a liquid to flow, or the syrupiness of a liquid) of
molasses an intensive or an extensive property?
See Problem 115.
We can apply this to our “floating log and sinking rock” discussion by noting
that the extensive property of weight—that of the log, the rock, or the water—is
not the reason why the log floats or the rock sinks. The explanation for these
observations is related to the density—an intensive property—of the objects
compared to the density of the water. Those objects with a density greater than
the water will sink; those with a lesser density will float. Figure 1.28 illustrates this
effect for six different objects.
24 Chapter 1 The World of Chemistry
FIGURE 1.28
The densities of six different substances
are represented in this density jar.

EXERCISE 1.6 Identifying a Metal via Density
A chunk of metal has a mass of 81.76 g and
is added to a graduated cylinder (a type of
glassware that measures volume) contain-
ing 25.0 mL of water. The total volume of
the metal and water rises to 34.2 mL. What
is the identity of the metal?
First Thoughts
In this exercise, we are given only enough information to tell the metals apart by
their densities. The real question, then, is “What is the density of the unknown
metal?” The metal has a mass of 81.76 g. What volume does it occupy? The volume
of water in the graduated cylinder increases from 25.0 mL to 34.2 mL when the
metal chunk is added. The volume of the metal must then be 34.2 − 25.0 =9.2 mL.
We have mass and volume. We can now solve for density.
Solution
Density =
mass
volume
=
81.76 g
9.2mL
= 8.9g/mL = 8.9g/cm
3
The metal has the same density as nickel.
Further Insights
Knowing the density of metals is of great practical importance in manufacturing
and commerce. For example, titanium is used as a component of expensive, light-
weight bicycle frames because of its strength and low density of 4.50 g/mL. The ac-
celeration that a rider can achieve on a bicycle is another derived unit, equal to force
(itself a derived unit; see Table 1.4) per unit mass:
Acceleration =
force
mass
=
kg·m·s
−2
kg
= m·s
−2
The less mass (or weight) the bicycle has, the greater is the acceleration the rider can
achieve using a constant force.
PRACTICE 1.6
How much water would be displaced if 81.76 g of aluminum, rather than of nickel,
were added to the graduated cylinder discussed in this exercise?
See Problems 45–48 and 59–62.
1.5 Conversions and Dimensional Analysis
The SI units typically used by scientists are often quite different from some of the
units in everyday use. For instance, visitors to Canada from the United States
might wish to fill their cars with gasoline (“petrol” in Canada). Initially, they
might be surprised that the gasoline is so inexpensive. Part of that has to do with
the currency exchange rate: around $1.30 Canadian per $1.00 U.S. Another key is
that the price of Canadian petrol is reported in liters, whereas in the United
States, gasoline is sold by the gallon. Converting liters into gallons solves the dif-
ficulty caused by these differences. To do the conversion, we need an expression
that relates one unit to another. Such a
conversion factor is a mathematical ex-
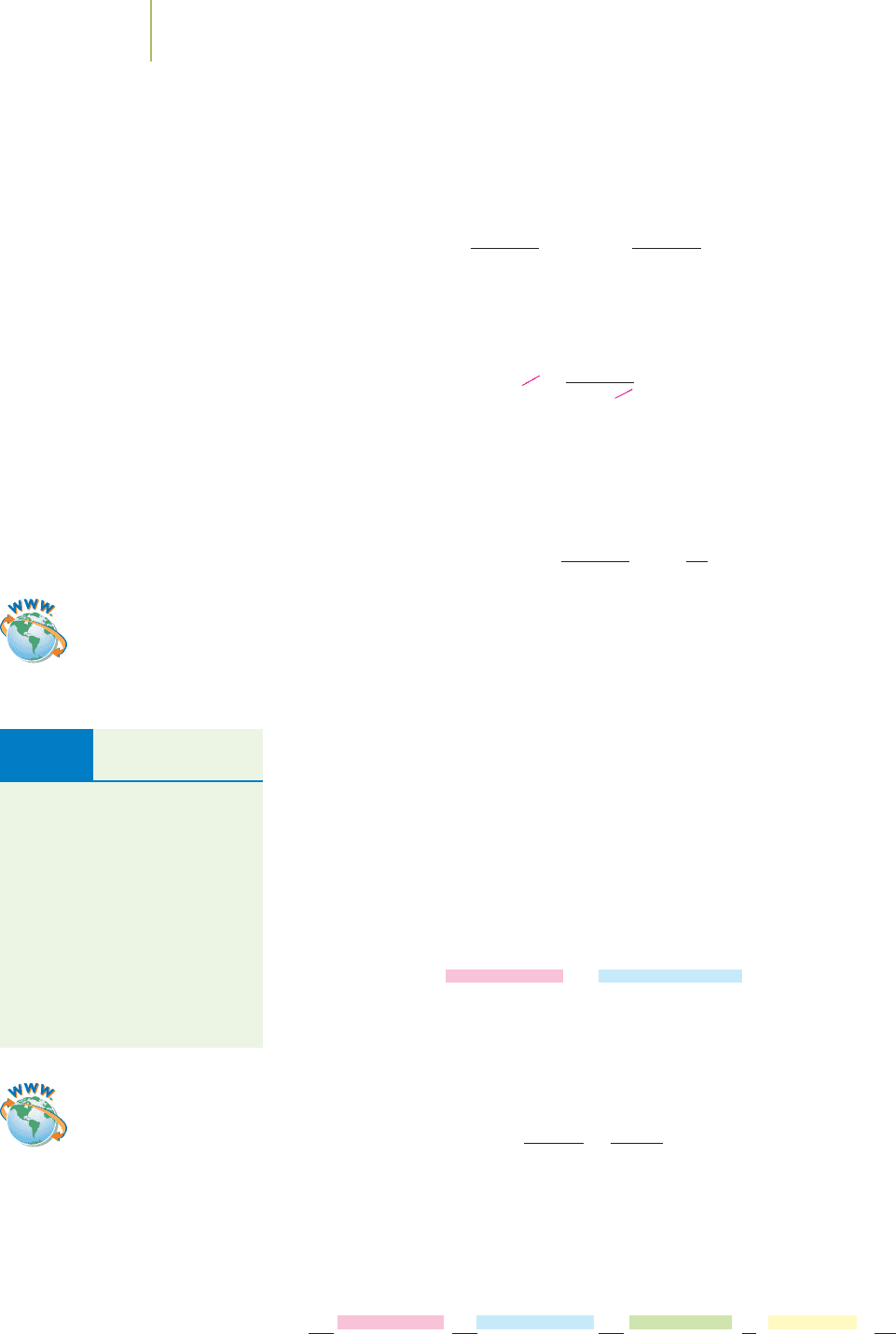
pression of the ratio of one unit to another. For example, on Earth, a 2.2046-lb
mass is equivalent to a 1-kg mass.
2.2046 lb = 1 kg
1.5 Conversions and Dimensional Analysis 25
Metal Density in g/cm
3
Aluminum 2.7
Nickel 8.9
Tin 7.3
Zinc 7.1
One kilogram weighs 2 lb, 3.30 oz
(2.20 lb).
26 Chapter 1 The World of Chemistry
Common
Conversion Factors
Length 1 mi 1.609 km
1 in 2.54 cm
12 in 1 ft
1 mi 5280 ft
Volume 1 qt 0.9464 L
1 gal 3.785 L
1 gal 4 qt
1floz 29.57 mL
Mass 2.2046 lb 1.0 kg
1 lb 453.6 g
1 oz 28.35 g
TABLE 1.6
Because these two values are equal, we can use them as a conversion factor
that can be multiplied by a number to convert its units. The operation is identical
to multiplying the original number by 1, because the numerator and denominator
are equal to each other. This equality can be written as a conversion factor in two
ways:
1kg
2.2046 lb
= 1
2.2046 lb
1kg
= 1
For instance, suppose we are interested in learning the mass (in kilograms) of
a book that weighs 3.5 lb. We can multiply the weight of the book by one of our
conversion factors.
3.5lb×
1kg
2.2046 lb
= 1.6kg
Note that we chose to multiply the weight of the book by the conversion factor so
that the unwanted units canceled. If we had multiplied by the other conversion
factor, the answer would have been different, and the units would have been
different, too! In this case, the units would not have canceled, and we would have
been left with a meaningless answer:
3.5lb×
2.2046 lb
1kg
= 7.7
lb
2
kg
This extremely important method of calculation can be used to solve a great
variety of problems in chemistry. In fact, we will use this method for many of the
calculations that we will do. It is known as
dimensional analysis (or, sometimes, as
the unit-conversion method, or the factor-label method) because canceling out the
units (or “dimensions” or “factors”) associated with each number enables us to
arrive at the proper answer. Table 1.6 lists some of the more common conversion
factors for mass, length, volume, and time. Others may be found on the inside
back cover.
Dimensional analysis can be applied to much more complex problems. For
instance, suppose we would like to know the length of our textbook in millime-
ters, given that someone has measured its length to be 10.25 inches (in). We can
perform a dimensional analysis and convert the units of inches into millimeters.
However, examination of Table 1.6 doesn’t show a direct conversion from inches
to millimeters. But the table does show a conversion factor from inches to cen-
timeters, and there is another conversion factor from centimeters to millimeters
(which we know from Section 1.4). Here’s our plan of attack:
in
inches to centimeters
−−−−−−−−−−−→cm
centimeters to millimeters
−−−−−−−−−−−−−→ mm
Let’s use this plan to construct a dimensional analysis of the units in one long
problem. Although we could do it stepwise, it is often just as easy to do a single
calculation. We’ll just have to make sure that our unwanted units cancel at each
step.
10.25 in ×
2.54 cm
1in
×
10 mm
1cm
= 260.4mm
These problems can even get longer, but they don’t necessarily have to get
harder. For example, suppose Judith Fairclough-Baity measured the density of
her tubing as 1.154 g/mL. Assume she was interested in reporting this density in
pounds per gallon. We can use conversion factors to solve this problem by con-
verting one unit at a time into the units we desire. Here’s our plan of attack:
g
mL
grams to kilograms
−−−−−−−−−→
kg
mL
kilograms to pounds
−−−−−−−−−→
lb
mL
milliliters to liters
−−−−−−−−−→
lb
L
liters to gallons
−−−−−−−−−→
lb
gal
Video Lesson: Dimensional
Analysis
Tutorial: Unit Conversions

Starting with the initial density in grams per milliliter, we set up the problem,
taking care that each unwanted unit will cancel when we’re done:
1.154 g
mL
×
1kg
1000 g
×
2.2046 lb
1kg
×
1000 mL
1L
×
3.785 L
1gal
=
9.629 lb
gal
The entire calculation converts the grams into pounds and the milliliters into
gallons.
EXERCISE 1.7 Practice with Dimensional Analysis
Manufacturing on a global scale requires working with immense quantities of raw
materials. As a close approximation of the actual amount, let’s assume that 96 bil-
lion aluminum cans are produced in a given year. If each can requires 15 g of
aluminum (Al), how many total kilograms of aluminum are used each day (assume
365 days in a year) to make aluminum cans?
First Thoughts
From the problem we can obtain these conversion factors:
365 days
1 year
9.6 ×10
10
Al cans
year
15 g Al
Al can
1kgAl
1000 g Al
If we map out our steps, there are several routes we can follow. Here is just one:
cans
year
cans to grams
−−−−−−−−−→
g
year
grams to kilograms
−−−−−−−−−→
kg
year
years to day
−−−−−−−−−→
kg
day
The key with dimensional analysis is to show how each step allows you to cancel
units so that you can end with the units you want. It doesn’t matter what order we
pick for the terms, as long as the units cancel.
Solution
9.6 ×10
10
cans
year
×
15 g Al
1 can
×
1kgAl
1000 g Al
×
1 year
365 day
=
3.9 ×10
6
kg Al
day
Further Insights
One of the most important questions we need to ask is Does our answer make
sense?
That is, did we calculate an answer that we “should be” getting? Our answer
is quite large. Should it be so, or does it make more sense to use, perhaps, 0.001 kg
of aluminum per day? If we prepare billions of cans per year, it seems reasonable
that we should use quite a bit of aluminum each day, and our answer confirms that.
This doesn’t mean that the math was necessarily correct, but the answer is at least
reasonable. Anticipating roughly what an answer should be, and then confirming
that your answer is at least nearby, is certainly part of “thinking like a chemist.”
PRACTICE 1.7
a. What is the mass in grams of 6.50 gallons of water that flow into a desalination
plant every second? Assume the density of the water in this problem is
1.00 g/mL.
b. How many gallons of gasoline are in 58.6 L of gasoline?
c. How much would 11.2 gallons of gasoline cost in Canada if the price of
gasoline in the United States is $1.34 per gallon and the exchange rate is
$1.30 CN = $1.00 U.S.? (Assume the price of gasoline is the same in
the United States and Canada.)
See Problems 65–69 and 71–80.
1.5 Conversions and Dimensional Analysis 27
