Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


38 Chapter 1 The World of Chemistry
electrodialysis The process of removing unwanted salts
from a solution through a series of semipermeable
membranes by applying an electrical charge. (p. 10)
element A substance that contains only one kind of
atom. All elements are listed in the periodic table of
the elements. (p. 5)
exact number A number that can be known with ab-
solute certainty. Exact numbers possess an infinite
number of significant figures. (p. 28)
extensive property A characteristic of a substance that is
dependent on the quantity of that substance. (p. 23)
heterogeneous mixture A mixture that is not uniformly
mixed, so there are different proportions of the com-
ponents in different parts of the mixture. (p. 8)
homogeneous mixture A mixture that is uniformly mixed
so that it has the same composition throughout. Also
known as a solution. (p. 8)
hypothesis A tentative explanation for an observation—
that is, a statement about what we think might be an
explanation for an observation. (p. 13)
intensive property A characteristic of a substance that is
independent of the quantity of that substance. (p. 23)
International System See SI.
kelvin (K) The SI base unit of temperature. (p. 18)
kilogram (kg) The SI base unit of mass. (p. 17)
kilogram per cubic meter (kg/m
3
) The derived SI unit
for density. (p. 22)
liter (L) A commonly used unit for volume; equal to
1dm
3
.(p. 22)
luminous intensity Brightness, expressed in the SI unit
candela (cd). (p. 20)
macroworld A term used to describe the “big world” of
everyday experience, as opposed to the “nanoworld”
of atoms, molecules, and ions, whose activities ulti-
mately determine what happens in the macroworld.
(p. 20)
mass A measure of the amount of matter in a body, de-
termined by measuring its inertia (resistance to
changes in its state of motion) and expressed in
the SI base unit kilogram. (p. 17)
matter Anything that has a mass and occupies space.
(p. 4)
meter (m) The SI base unit of length. (p. 18)
milliliter (mL) One thousandth of a liter. (p. 22)
mixture A sample containing two or more substances.
(p. 8)
mole (mol) The SI base unit of amount of substance.
One mole of entities, such as 1 mole of atoms, is ap-
proximately equal to 6.02214 × 10
23
entities. (p. 20)
molecule A particle composed of two or more atoms
bonded together. (p. 5)
nanotechnology Technology that depends on manipulat-
ing materials and their chemistry at the very small,
“nanoscale” level of individual particles or assemblies
of small numbers of particles. (p. 36)
nanoworld A term used to describe the “world of the
very small” at the level of individual atoms, mole-
cules, and ions, as opposed to the “macroworld” of
everyday experience. (p. 20)
physical change Change in the physical state of a sub-
stance, such as a change between the solid, liquid, and
gaseous states, that do not involve the formation of
different chemicals. (p. 6)
physical property A characteristic of a substance that can
be determined without changing its chemical compo-
sition. (p. 6)
precision The reproducibility of a measurement. (p. 29)
reverse osmosis The process of purifying water by pass-
ing it through a semipermeable membrane. Dissolved
solutes are unable to pass through the membrane.
(p. 10)
scientific law Concise description of the behavior of the
natural world. (p. 13)
scientific method A reliable way to find out things about
nature by making use of appropriate combinations of
these key activities: making observations, gathering
data, proposing hypotheses, performing experiments,
interpreting the results of those observations and ex-
periments, checking to ensure that the results are re-
peatable, publishing the results, establishing scientific
laws, and formulating theories. (p. 12)
second (s) The SI base unit of time. (p. 18)
SI The International System (Système International) of
agreed-upon base units, derived units, and prefixes
used to measure physical quantities. (p. 17)
significant figures Those specific numbers in a measure-
ment whose values we can trust. (p. 31)
solution A homogeneous mixture of solute and solvent.
(p. 8)
state The physical appearance of a chemical, typically
as a solid, liquid, or gas. (p. 10)
substance A type of matter that has a fixed composi-
tion. (p. 6)
theory A trusted explanation of an observation, based
on a hypothesis that has been tested in experiments.
(p. 13)
uncertainty A measure of the lack of confidence in a
measured number. (p. 28)
weight A measure of the gravitational force exerted on
a body. (p. 17)
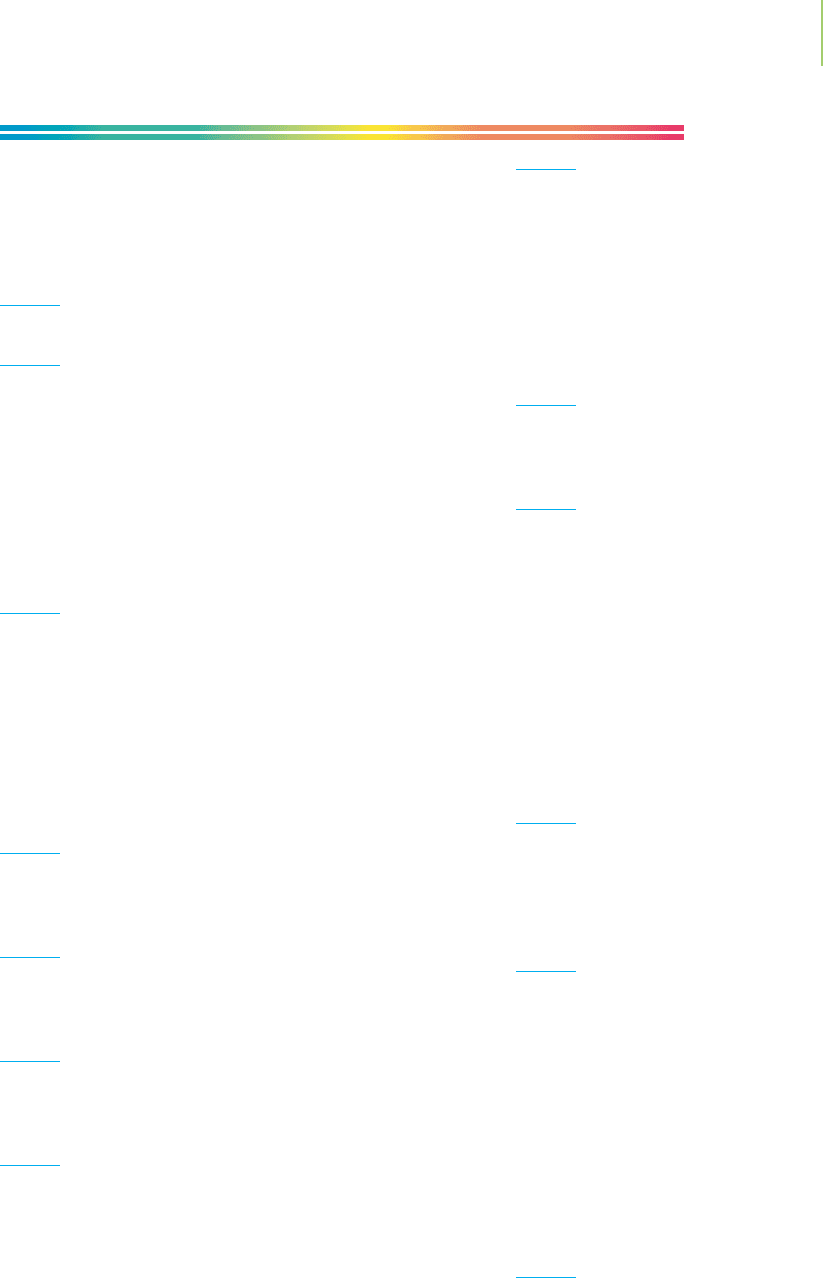
Focus Your Learning 39
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 1.1 What Do Chemists Do?
Skill Review
1. Define the term chemistry. Why is chemistry a science?
2. Explain why chemists need to be inquisitive.
3. In the section, we listed a number of things that chemists do.
Look around the room in which you are sitting. List five
additional things that you suspect are manufactured using
chemistry.
4. Look on the label of ingredients for five products in your
kitchen pantry. List some examples of substances that might
be commercially prepared. Why did you choose these? List
some substances that are added in more or less their natural
form. Why did you choose these?
Chemical Applications and Practices
5. The directions for baking a particular cake include the fol-
lowing instruction: “Carefully add 1 cup of water to the
powdered mix and stir.” Use this statement to explain why
measurements are important to the chemist.
6. A scientist has just identified a new drug for treating cancer.
Use this statement to explain why a scientist communicating
this discovery might need a chemical “shorthand.”
Section 1.2 The Chemist’s Shorthand
Skill Review
7. Define, differentiate between, and give three examples of an
element and a compound.
8. What dispute might you have with the manufacturer of a
cleanser that was labeled “chemical-free?”
9. How is it possible that oxygen, found in our atmosphere, can
be called an element and a molecule but not a compound?
10. How is it possible that gold, found as flakes in a stream, can
be called an element but not a molecule or a compound?
11. Name one characteristic that a mixture and a compound
have in common.
12. Describe a critical difference between a mixture and a
compound.
13. Which of these chemicals are elements and which are com-
pounds? For the compounds, list the elements that are pre-
sent. You may need to use the periodic table and a chemical
encyclopedia to help you answer some of these.
a. hydrogen gas d. neon
b. sodium chloride (table salt) e. copper sulfate
c. glucose f. titanium
14. Which of these chemicals are elements and which are com-
pounds? For the compounds, list the elements that are
present. You may need to use the periodic table and a chemi-
cal encyclopedia to help you answer some of these.
a. nitrogen gas d. helium gas
b. calcium chloride (sidewalk salt) e. silver metal
c. aspirin f. water
Focus Your Learning
15. Indicate whether each of these is a heterogeneous mixture or
a homogeneous mixture.
a. lake water d. soda
b. yellow notebook paper e. milk
c. marble f. dirt
16. Indicate whether each of these is a heterogeneous mixture or
a homogeneous mixture.
a. tap water d. paint thinner
b. apple juice e. spaghetti sauce
c. beach sand f. air
17. List at least two methods used to separate a homogeneous
mixture.
18. Why are most heterogeneous mixtures easier to separate than
most homogeneous mixtures?
19. For each of these processes, indicate whether a chemical
change or a physical change is taking place.
a. molding melted chocolate into a bar
b. heating your home with a woodstove
c. drying your clothes in the dryer
d. snow melting in the heat of the sun
20. For each of these processes, indicate whether a chemical
change or a physical change is taking place.
a. the yellowing of an old newspaper
b. making hard-boiled eggs
c. magic ink appearing on a piece of paper
d. making dirty clothes clean in the washing machine
Chemical Applications and Practices
21. Some ancient civilizations considered air an “element.” Today
we consider air a mixture, compound (select one). What is the
basis of your choice?
22. Some ancient civilizations considered water an “element.”
Today we consider water a mixture, compound (select one).
What is the basis of your choice?
23. A “health food” store has a poster in its window that says,
“Eat natural food, not chemicals.” Briefly explain what is
wrong with this statement, and try to summarize, in a chem-
ically accurate way, what the writer of the poster was really
trying to say.
24. A box of noodles in a “health food” store indicates that the
noodles are “free of chemicals.” Briefly explain what is wrong
with this statement, and try to summarize, in a chemically
accurate way, what the writer of that phrase was really trying
to say.
Section 1.3 The Scientific Method
Skill Review
25. Suppose you sit down at your computer to draft an e-mail to
a friend. However, after you have written the e-mail, it ap-
pears that it cannot be sent from your computer. Explain how
you might use parts of the scientific method to help you solve
this problem.
26. Suppose you wanted to determine which grade, or type, of
gasoline would provide the best miles-per-gallon ratio in
your car. What would be the important variables that you

40 Chapter 1 The World of Chemistry
would control as you used the scientific method to draw your
conclusion?
27. Which aspects of the scientific method are most likely to be
subject to interpretation? Which, on the other hand, should
be least ambiguous?
28. For what reasons, apart from the sharing of information, is it
important to publish the results of scientific studies?
29. How do we distinguish a theory from a hypothesis?
30. We are familiar with how governments enact laws. How is a
scientific law formed?
Chemical Applications and Practices
31. Several studies have been done to determine the effectiveness
of various vitamins on specific health issues. Explain how it is
possible for scientific studies on the same subject to produce
conflicting results.
32. As a consumer, you encounter many claims about remedies
for various human conditions from acne to balding. What
aspects of the scientific method would you expect to see
employed before such claims are made?
33. Assume that fish are dying in a river that runs through your
town. The river begins in remote mountains, runs through
many miles of farmland, and then passes through a large in-
dustrial area before entering town. What sorts of questions
need to be should be asked in an attempt to learn why the fish
are dying? What investigations could be carried out to answer
the questions?
34. In an attempt to treat a wart on your hand, you buy a propri-
etary remedy, and two weeks later your wart has disappeared.
Does this prove that the remedy is effective? What steps
should be performed to examine the effectiveness of the rem-
edy in a proper scientific manner?
Section 1. 4 Units and Measurement
Skill Review
35. Based on the table found in this chapter, arrange these dis-
tances in order from largest to smallest:
1 millimeter 1 terameter 1 kilometer 1 nanometer
36. Based on the table found in this chapter, arrange these masses
in order from largest to smallest:
1 microgram 1 kilogram 1 milligram 1 picogram
37. Complete the conversions:
a. 100 kg to grams d. 3.20 mg to grams
b. 25.9 m to kilometers e. 9.11 nm to picometers
c. 25°C to °F f. 98.6°F to °C
38. Complete the conversions:
a. 150 mL to liters d. 2.33 L to milliliters
b. 8.42 g to milligrams e. 700 mg to grams
c. 48.5°C to °F f. –20°F to °C
39. Complete the conversions:
a. 8.7 kg to milligrams d. 3.20 dL to kiloliters
b. 25.9 dm to meters e. 9.11 s to nanoseconds
c. 190°C to °F f. 350°F to °C
40. Complete the conversions:
a. 3.99 mL to deciliters d. 14.5 L to megaliters
b. 8.42 Mg to kilograms e. 55.5 ks to gigaseconds
c. –40°C to °F f. 75°F to °C
41. A 2.00-L sample of nutrient agar would be able to be divided
into how many equal 100-cm
3
media containers for a bacter-
ial study?
42. Suppose the nutrient agar described in Problem 41 amounted
to 32 containers, each holding 50 cm
3
of media. How many
total liters of nutrient agar are in the containers?
43. Using scientific notation, express 327 kilometers in:
a. meters c. micrometers
b. millimeters d. nanometers
44. Using scientific notation, express 499 seconds in:
a. milliseconds c. deciseconds
b. microseconds d. kiloseconds
45. A 62.56-g sample of mercury was added to a graduated cylin-
der. It had a volume of 4.60 mL. What is the density of the
mercury?
46. A 22.4-g sample of a substance was added to a graduated
cylinder. It caused a 18.3-mL change in the volume of water
in the cylinder. What is the density of the substance?
47. A 250.0-mL sugar solution had a density of 1.37 g/mL. An
additional 30.0 g of sugar was added to the solution, raising
the volume by 24.6 mL. What is the density of the resulting
sugar solution?
48. A chemist added 17.8 g of salt to 150.0 mL of a salt solution
of unknown density. The resulting solution had a final vol-
ume of 165.9 mL and a density of 1.22 g/mL. What was the
density of the original salt solution?
49. List the fundamental units that you would combine to get
these derived units (you may need to look up the meaning of
some of the terms).
a. velocity c. volume
b. acceleration d. specific heat
50. List the fundamental units that you would combine to get
these derived units (you may need to look up the meaning of
some of the terms).
a. density b. pressure c. energy d. force
Chemical Applications and Practices
51. Which of the following rulers would provide the greatest
number of significant figures in the measurement of the vol-
ume of a cardboard box?
123456789
123456789
123456789
2468

Focus Your Learning 41
61. Most samples of matter expand when heated. Assuming that
this is the case with water at room temperature, would the
density increase or decrease as the water warmed? Show the
mathematical basis of your answer.
62. Ice floats on top of water. Using the information from Prob-
lem 61, explain how this could occur and show the mathe-
matical basis of your answer.
63. In casual food preparation, one may be directed to add a
pinch or a smidgeon or “just a bit” of a particular ingredient.
If 10 pinches equaled 2 smidgeons and 10 “just a bits”
equaled 1 smidgeon, how many “just a bits” of hot sauce
should be added to a recipe that calls for 2 pinches?
64. Consider the tongue twister “Peter Piper picked a peck of
pickled peppers.” If two dozen pickled peppers are in a peck,
and Peter Piper picked 8 pecks for his girlfriend Polly, how
many peppers did Peter Piper pick?
Section 1.5 Conversions and Dimensional Analysis
Skill Review
65. Illustrate a plan of attack (in the same manner as indicated
in the text) for converting units of miles per hour (mph) to
meters per second.
66. Illustrate a plan of attack (in the same manner as indicated in
the text) for converting units of kg·m/s
2
to lb·ft/s
2
67. Osmium (element 76) is one of the most dense elements
known. The standard density is 22.6 kg/L. What is the density
in units of g/cm
3
? What would be the mass of a 0.50-L sam-
ple of osmium?
68. Mercury has a density of 13.6 g/cm
3
. What is the density in
kg/L for mercury? Calculate the weight in pounds of 3.6 L of
mercury.
69. Convert the following into the indicated units:
a. 35 mph to kph c. 733 mi/gal to km/L
b. 22.4 L/s to cm
3
/s d. 4.184 g/°C to lb/°F
70. Convert the following into the indicated units:
a. 12 doz/lb to gross/kg c. 14.4 lb/in
2
to kg/m
2
b. 16 lb/gal to g/mL d. 3.04 °C/min to °F/s
71. In the vacuum of space, light travels at a speed of 186,000
miles per second. Indicare how many miles light travels
through space in:
a. 1 minute b. 1 day c. 1 year
72. The speed of sound varies greatly depending on the medium
and conditions. If the speed of sound at sea level and zero
degrees Celsius is approximately 1100 feet per second, indi-
cate how far sound travels in:
a. 1 millisecond b. 1 dekasecond c. 1 microsecond
73. Perform these conversions:
a. 2.0 oz to pounds d. 96 in to meters
b. 4.0 qt to liters e. 13 ft to centimeters
c. 160 lb to kilograms f. 32 mi to kilometers
74. Perform these conversions:
a. 3.0 kg to pounds d. 134 oz to kilograms
b. 7400 s to days e. 75.5 cm to feet
c. 50.34 mL to gallons f. 2.88 L to quarts
52. Determine the volumes of liquid in each of these graduated
cylinders, and report your answers with the correct number
of significant figures.
53. A fast-food restaurant wants to standardize the temperature
of its coffee. It installs a coffee machine that is specified as de-
livering coffee at 75°C
± 3°C. What are the maximum and
minimum temperatures of the coffee delivered by this
machine in °F?
54. The temperature of the hot oil bath in a fast-food restaurant
determines the quality of the french fries that are produced.
The heater on the oil bath can regulate the temperature at
350°F ± 10°F. What are the maximum and minimum tem-
peratures of the french fries delivered by this machine in °C?
55. Carbon atoms have an atomic radius of approximately
77 pm. If you drew a line that was 1 cm in length using a piece
of charcoal, approximately how many atoms of carbon
would be in this line? (For the purposes of this question, as-
sume that the atoms are touching and that they form a
straight line one atom wide.)
56. The same piece of charcoal (see Problem 55) was used to
darken a box on a survey form. The box measures 5 mm on
each side. How many atoms of carbon would be in this box?
(For the purposes of this question, assume that the atoms are
touching, that they line up into a square grid, and that they
are only one layer thick on the paper.)
57. During the 1980s, several new elements were synthesized in
Germany. These elements are made of unstable atoms. Half
of the atoms in a sample of unstable atoms will decompose
over a period of time known as the element’s half-life. One of
these newly discovered elements, Meitnerium (element 109)
has a half-life of only 0.07 s.
a. What is its half-life in milliseconds?
b. What is the length of time of four of its half-lives in terms
of microseconds?
58. Before filling, an empty, irregularly shaped container has a
mass of 0.1956 kg. When this container is totally filled with
water,the combined mass of the containerand wateris 305.6 g.
Whatis the mass of wateradded to the containerin kilograms?
59. A certain red tomato has a mass of 45.6 g. A green tomato
also has a mass of 45.6 g. However, when placed in water, the
green tomato floats and the red one sinks. (Try this. Depend-
ing on the degree of ripeness, it actually happens!) Which of
the two is denser? Explain how two tomatoes with the same
mass can have different densities.
60. Various plastics have identifiable density values. For example,
you may have heard of high-density polyethylene and low-
density polyethylene. If you had pieces of each that were
equal in volume, which would have the greater mass?
1
5
(
a
)
16
17
18
19
2
3
4
5
6
(b)
42 Chapter 1 The World of Chemistry
75. A typical chemistry lecture lasts for approximately 50 min-
utes. What is that time in years? . . . in centuries? ...in
decades? Report each number using a proper metric prefix
that enables you not to have any zeros in your final answer.
76. Some people live to be 100 years old. What is that time in sec-
onds? . . . in minutes? . . . in hours? Report each number using
a proper metric prefix that enables you not to have any zeros
in your final answer.
Chemical Applications and Practices
77. The mass of 6.02 ×10
23
atoms of gold is approximately 197 g.
a. What is the average mass, in grams, of just one atom of
gold?
b. Select a prefix that could more appropriately be used to
report the average mass, and express the mass in that unit.
78. The mass of 6.02 × 10
23
atoms of carbon is approximately
12 g.
a. What is the average mass, in grams, of just one atom of
carbon?
b. Select another prefix that could more appropriately be
used to report the average mass, and express the mass in
that unit.
79. Suppose a football “star” signs a contract for 200 megabucks
over a five-year period. How many dollars is the star paid per
second? ...per three-hour game?
80. Which is longer; a 100-m soccer field or a 100-yd football
field? Show the mathematical proof for your answer.
81. Air is a mixture that consists mostly of oxygen and nitrogen.
The density of air varies, depending on temperature and
pressure. However, for this problem let’s assume a reasonable
value for the density of air to be 1.20 mg/cm
3
. Suppose the
dimensions of your dorm room are approximately 12 ft ×
14 ft × 9.0 ft. What would you calculate to be the approxi-
mate mass of the air in the room?
82. Modern pewter is a mixture of tin, antimony, and copper.
Formerly, pewter also contained lead. If a 1.00-lb sample of a
particular pewter alloy contained, by mass, 95.0% tin and
3.4 % antimony, how many grams of copper must be present
in the sample?
Section 1.6 Uncertainty, Precision, Accuracy,
and Significant Figures
Skill Review
83. Some chemists are playing horseshoes. In their version of the
game, each gets four chances to throw a horseshoe to hit a
stake.What can you state about the accuracy and precision of
the chemist that threw the horseshoes to arrive at the out-
come shown here?
84. Two more chemists playing horseshoes each take a turn.
Which of the two outcomes shown here is more precise?
(a)
(b)
85. Three students weighed the same sample of copper shot
three times. Their results were as follows:
a. Calculate the average mass of the sample, as determined by
each student.
b. Which set of measurements is the most precise?
c. If the true mass of the copper shot is 15.384 g, which of the
students was most accurate?
d. What are the main sources of error that could have caused
the differences in the values?
86. The same three students measured the volume of the same
sample of water four times. Their results were as follows:
a. Calculate the average volume of the sample, as determined
by each student.
b. Which set of measurements is the most precise?
c. If the true volume of the water is 24.10 mL, which of the
students was most accurate?
d. What are the main sources of error that could have caused
the differences in the value?
87. Which of these values are quoted using exact numbers? How
many significant figures are there in each of these numbers?
a. 12.000000 g d. 12 L
b. 3125 students e. 1 g
c. 12.2 L f. 42 test tubes
88. Which of these values are quoted using exact numbers? How
many significant figures are there in each of these numbers?
a. 15 apples d. 44 mi
b. 500 people e. 70°C
c. 3.2050 in f. 10 g
Measurement Student 1 Student 2 Student 3
1 25.55 mL 23.79 mL 25.02 mL
2 24.81 mL 24.01 mL 25.10 mL
3 23.03 mL 24.32 mL 25.07 mL
4 24.28 mL 24.19 mL 25.17 mL
Weighing Student 1 Student 2 Student 3
1 17.516 g 15.414 g 13.893 g
2 17.888 g 16.413 g 13.726 g
3 19.107 g 14.408 g 13.994 g

Focus Your Learning 43
89. In each of these numbers, underline any zeros that are con-
sidered significant.
a. 0.700 cm d. 100 m
b. 0.101 kg e. 0.01010 g
c. 100.0 cm
90. In each of these numbers, underline any zeros that are con-
sidered significant.
a. 1.000 cm d. 0.56 m
b. 80.2 kg e. 3000 g
c. 2104 cm
91. How many significant figures are there in each of these
values?
a. 6.07 × 10
–15
f. 1406.20
b. 0.003840 g. 0.0007
c. 17.00 h. 1600.0
d. 8 × 10
8
i. 0.0261140
e. 463.8052 j. 1.250 × 10
–3
92. How many significant figures are there in each of these
values?
a. 6.022 ×10
23
f. 250
b. 1.79 × 10
–19
g. 13.50
c. 3.00 × 10
8
h. 101.010
d. 14 i. 12.000
e. 0.0035020 j. 550.050
93. Determine the answer for each of these problems. Report
your answer using the rules for significant figures.
a. 3.44 + 6.2 f. 45.6 ÷ 2.4
b. 12.57 − 3.998 g. (754 + 0.8) ÷ 1.3
c. 2.534 + 1.23 + 2.0500 h. (49.53 × 1.20) + 12
d. 12.54 × 5.0 i. (35.865 ÷ 84.2) + 2.3890
e. 84 × 100
94. Determine the answer for each of the problems below.
Report your answer using significant figures rules.
a. 120 + 6.77 f. 140 ÷ 2.9375
b. 453 − 0.32 g. (135 + 3.2) ÷ 1.332
c. 51.8 + 7.225 + 2.01 h. (2.78 × 1.2) + 3.96
d. 2.54 × 32 i. (14.42 − 1.023) ÷ 2.3
e. 36.33 × 0.300
Chemical Applications and Practices
95. Suppose your chemistry textbook fell 10.0 cm from a back-
pack to a desk. Then it slipped off the desk and fell another
0.91 m from the desk to the top of someone’s foot. (Ouch!)
Finally, it fell the remaining 40 mm to the floor. What is the
total distance, with the correct number of significant digits,
that the textbook has fallen?
96. A piece of cheese has a mass of 250.67 g. Four people each
remove a slice of cheese from the piece. The first removes
22.5 g of cheese, the second removes 10 g, the third removes
3.557 g, and the fourth takes 80.1 g. How many grams of
cheese remain? Use appropriate significant figure rules to cal-
culate your answer.
97. Molybdenum has a melting point of over 2600
o
C. It makes
steel stronger, creates colors when added to molten glass,
and is an integral component of some biological molecules
known as enzymes. If you had a sample of the metal that had
a mass of 14.56 g and a volume of 1.43 cm
3
, what would you
calculate as the density of molybdenum? (Remember to fol-
low the rules for significant figures.)
98. A student obtains the density of water. A 243-mL sample of
water was noted to have a mass of 235.5 g. What does the
student calculate as the density of the water sample? (Re-
member to follow the rules for significant figures.)
Section 1.7 The Chemical Challenges of the Future
Skill Review
99. One of the “future frontiers” mentioned in this chapter
refers to the use of nanotechnology.
a. To what does the prefix nano- refer?
b. What would be the diameter, in centimeters, of a wheel
10 nm in diameter?
100. Ozone and other gases in the stratosphere are often mea-
sured in units known as parts per million (ppm). One ppm
ozone is equivalent to 1 g of ozone per million grams of air.
a. Determine the number of grams of ozone in 15 kg of air
containing 2.00 ppm ozone.
b. Which prefix would be best suited to identify this
quantity of ozone?
Chemical Applications and Practices
101. Use current Internet resources, or current print media, to
report one advantage and one disadvantage of genetic re-
search on corn. How long have genetically selected plants
been used in agriculture?
102. One method of cancer treatment involves chemotherapy.
What is the chemical connection to this medical term?
103. Depending on their identity and how they are used, chemi-
cals may be harmful or beneficial to our lives. Give two
specific responses to the question “What has chemistry done
for me?”
104. Ozone, like many other chemicals, may be helpful or harm-
ful. The presence of ozone at high altitudes has advantages
for us. However, at ground level it can cause several prob-
lems. Use other resources to explain how the same molecule
can have such diverse impacts.
105. What are the chief energy problems facing the United
States? How are these similar to or different from those fac-
ing other countries, both economically wealthy and poor?
106. Select one of your answers from Problem 105 and explain
how your knowledge of chemistry might play a role in solv-
ing that problem.
Comprehensive Problems
107. What aspect of daily life do you predict will be most affected
by chemistry in the generation to follow yours?
108. Name five areas of your life where chemistry plays a major
role.
109. Describe two chemical changes and two physical changes
that are important in growing food crops.
110. Someone remarks that the baking of a cake is a physical
change. Another person says that the process is a chemical
change. Who is right and why?
111. Write a sentence to describe what is happening when you
dissolve some sugar in a cup of warm water.

44 Chapter 1 The World of Chemistry
112. The height of horses is often measured in “hands.” A “hand”
is considered to be 4 inches. Furthermore, up to three-
tenths, each tenth of a hand is considered to be 1 inch. Any
value over three-tenths of a hand is rounded up to the next
hand.
a. What is the height of a horse, in inches, that stands 14.2
hands?
b. How many hands tall is a horse that stands, from the
ground to the top of the withers, 1.6 m tall?
113. Numbers, and their meaning, are very important to the
practicing chemist.
a. How does the meaning of an exact number differ from
that of a number taken from a measurement?
b. Beverages are often sold in six-packs. Six six-packs would
have how many individual containers?
c. Those containers hold a total of 7200 mL of the beverage.
Which of the value(s) represented in this problem are
exact numbers?
114. What are the two most common sources of uncertainty in
measurements? Of the two, which typically produces uncer-
tainty in a consistent direction?
115. Describe two intrinsic and two extrinsic properties of
seawater.
116. A chemist and her family go on vacation and ride the train
to the mountains. They start their journey from Chicago
and travel 1223 miles to Aspen, Colorado. The trip starts at
1:50 p.m. and the train arrives in Aspen at 1:53 p.m. the next
day. How fast, in kilometers per hour, does the train average
during the trip?
117. During a typical cross-country train trip, top speeds of
80.0 miles an hour are reached. What is that speed in
kilometers per second?
118. A student notices that the contents of a 12 fl oz can of diet
soda weigh 340 g.
a. What is the density, in grams per milliliter, of the soda?
b. Will this can of soda float on water or sink?
119. A dairy wishes to deliver its milk in large trucks to reduce
the cost of transportation. Assuming that the delivery truck
has a weight of 6500 pounds, how many gallons of milk
(d 1.106 g/mL) could be placed into the truck so that it
could still make it over the small country bridge rated to
hold a maximum of 10.0 tons?
Thinking Beyond the Calculation
120. In a recent study of an allegedly pre-Columbian (before
Columbus) map of the North American continent, one re-
searcher claimed that the map was a forgery because the
black ink had a yellow tinge (older ink would sometimes do
this) that could have been made by first laying down the
yellow line and then copying a thinner black line over it.
However, another researcher analyzed the difference
between the boundaries of the black and yellow lines and
claimed, after many measurements, that the differences
were consistently so small that it could not have been done
freehand.
a. Was the second researcher using precision or accuracy to
make his claim? Explain.
b. Describe how the second researcher used the scientific
method to study the map.
c. If the black lines had an average width of 1.45 mm.
What would that width be in inches? ...in meters? ...in
yards?
121. Biological evolution is a topic that often sparks debate.
Some say it is one of several theories, and others say it is a
fact. The field of “chemical evolution,” in which chemists
study how the elements and simple compounds (such as hy-
drogen, methane, and ammonia) combined to form the
molecules of life (including proteins and DNA) has been ac-
tive for nearly a century. Look up the pioneering work of
Miller and Urey using Internet or print resources. Do their
experiments convince you that chemical evolution is possi-
ble? What sorts of experiments would you design to deter-
mine whether chemical evolution occurs? If so, would you
call it a theory or a fact? Why?
122. There has long been a debate about the relationship be-
tween the mathematical preparation of students and their
success in first-year chemistry. The debate goes beyond
standardized test scores to social issues. If you were going to
study the relationship between math preparation and suc-
cess in first-year chemistry, how would you approach the
problem? Although you would need to consider all aspects
of the scientific method in your study design, we are espe-
cially interested in three parts. What are the main questions
you would ask? What experiments would you design to an-
swer your questions? And how would you factor other, non-
quantitative data into your results?

45
Atoms:
A Quest for
Understanding
Fossils can provide evidence that life
existed millions of years ago. New
technologies have been developed
that can “see” evidence that life on
Earth is more than 3.5 billion years
old.
45
Contents and Selected Applications
Chemical Encounters: How Old Is Life?
2.1 Early Attempts to Explain Matter
2.2 Dalton’s Atomic Theory and Beyond
2.3 The Structure of the Atom
Chemical Encounters: The Dover Boat and Radiocarbon Dating
2.4 Atoms and Isotopes
2.5 Atomic Mass
2.6 The Periodic Table
2.7 Ionic Compounds
2.8 Molecules
2.9 Naming Compounds
2.10 Naming Acids
Go to college.hmco.com/pic/kelterMEE for online learning resources.

46
How old is life? Scientists have been debating
that issue for quite some time. Xenophanes of
Colophon, an ancient Greek philosopher who died
about 490
B.C., examined fossils of marine life to generate a
hypothesis—a possible explanation based on observations—
about the history of Earth. Since then, scientists have been using
fossils to date life on this planet. Until recently, however, virtually all
fossils of creatures that lived before about 2.5 billion years ago had been
nearly impossible to detect.
In the early 1990s, high-powered microscopes were used
to detect and identify fossils in rocks that were 3.5 billion
years old. Apparently, life had existed on Earth earlier than
anyone had yet imagined. In 1996 a new technique was
developed to examine rocks for the biosignatures of life.
Biosignatures—traces of life left behind when a creature
dies—were identified using this technique in rocks 3.9 bil-
lion years old. Faint traces of life have also been suggested in
a Martian meteorite found in Antarctica (see Figure 2.1)
and also in rocks found in Greenland and Australia. Is this
evidence clear proof that life on Earth began over 3.5 billion
years ago? Some scientists believe it is, but the debate rages
on. Questions are raised. Experiments are designed and
done. As we discussed in the last chapter, this is the nature
of vibrant science.
What is the new technique that can be used to find the
biosignatures of life? All organisms, whether they are mul-
ticellular or single-celled, leave traces where they have
lived. In some cases, these traces can be seen as fossils. In
other cases, particularly the more ancient ones, the biosignature is simply a slight ex-
cess of one kind of a type of matter known as the atom. All organisms are made up of
characteristic concentrations of specific types of atoms. By measuring larger-than-
normal concentrations of these atoms, scientists can claim to have identified a biosig-
nature of life. The particular biosignature that led to the discovery of life in the
3.9-billion-year-old rock is a larger-than-normal concentration of carbon-12.
What is carbon-12? It is a specific set of particles that make up one type of carbon
atom. Although several different sets of particles can give rise to a carbon atom, only
one of these produces the carbon-12 isotope. In this chapter, we’ll learn about the par-
ticles that make up the building blocks of matter called atoms. We’ll uncover the basic
structure of an atom, and we’ll learn how atoms are arranged in the most important
organizational chart used in chemistry: the periodic table of the elements.
2.1 Early Attempts to Explain Matter
As early as 600
B.C., philosophers developed a view that the world was made of a few basic
“elements”—earth, air, water, and fire. The Greek philosopher Democritus (Figure 2.2) wondered
how he could be sitting in one part of his house and detect that bread was baking elsewhere in
the house. Could it be that small particles of the bread were breaking away from the loaf and
Application
C
HEMICAL ENCOUNTERS:
How Old Is Life?
FIGURE 2.1
An inside look at a meteorite, known simply as ALH84001,
that is presumed to have come from the surface of Mars. In
1996, scientist discovered what appeared to be microfossils
inside this meteorite. The meteorite is estimated to be
about 4.5 billion years old.

traveling through the air and into his nose? Noticing that wet clothing gradually
got drier and lighter led to the explanation that small, invisible pieces of water
were gradually leaving the clothing. What was the smallest possible piece of this
matter? In Democritus’ culture, these pieces of matter were thought to be indi-
visible, unable to be broken down further, or uncuttable. In their language, the
particles of the basic elements were atomos. This is the origin of the modern word
atom.
The lack of certain instruments that we use routinely today made it impossi-
ble, in Democritus’ time, to perform the experiments that have led to our current
understanding of matter. For instance, the invention of the balance for measur-
ing mass was one of the most significant experimental advances in the history of
science. In fact, two important chemical laws were discovered with the use of the
balance. These laws helped to shape our current understanding of the ideas put
forth in Democritus’ era.
The Law of Conservation of Mass
The French scientist Antoine Lavoisier (1743–1794) used the most precise
balances of his time, shown in Figure 2.3, to examine a variety of chemical
processes (chemical reactions). From his repeated measurements, Lavoisier con-
cluded that the total mass of the
reactants (what is consumed in a reaction) did
not differ from the total mass of the
products (what is produced in the reaction).
In other words, matter is neither created nor destroyed in a chemical reaction.We
call this conclusion the
law of conservation of mass.
Strictly speaking, this law is not precisely true because of the tiny
changes in mass resulting from something we’ll discuss in Section 21.5: the
mass-energy equivalence discovered by Albert Einstein. However, the law of
conservation of mass is accurate enough to indicate a fundamental truth
about chemical reactions: The atoms with which you start are the same as the
ones with which you end. When atoms participate in chemical reactions,
they are not destroyed or replaced by newly made atoms but, rather, are
rearranged. DNA, the genetic code in our cells, is composed of atoms of
carbon, hydrogen, oxygen, nitrogen, and phosphorus. Arranged in one way,
these atoms are the genes that lie at the heart of the chemistry of life.
Arranged in other ways, they are a mixture of water, carbon dioxide gas,
and a fertilizer called ammonium phosphate. Arranged in still other ways,
they form parts of biosignatures from billions of years ago.
The Law of Definite Composition
Another French scientist, Joseph Louis Proust (1754–1826; see Figure 2.4),
studied the composition of various minerals. In 1799 he published his work
about a compound that contained copper, carbon, oxygen, and hydrogen.
His results indicated that every time he determined the composition of his
mineral, the components of the compound were always present in the same
2.1 Early Attempts to Explain Matter 47
FIGURE 2.2
The Greek philosopher Democritus
(460
B.C.–370 B.C.). His early deductions
about the composition of the basic
components of nature led him to believe
in unseen and uncuttable particles, called
atomos.
FIGURE 2.3
Using balances such as these, Lavoisier and
others were able to reach conclusions about the
nature of chemical reactions.
FIGURE 2.4
Joseph Proust (1754–1826) was born in Angers,
France. He was a professor at different institutions in
Spain. In 1797 he published his understanding of the
Law of Definite Proportions. After returning to France
in 1808, he spent considerable time investigating
foodstuffs and discovered the molecule leucine, one
of the building blocks of proteins.
