Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

68 Chapter 2 Atoms: A Quest for Understanding
PRACTICE 2.4
Fill in the information missing from the following table.
See Problems 25, 26, 65, and 66.
Some substances, known as ionic compounds, are composed entirely of ions.
Calcium oxide (CaO) is one example. How do we know if the compound we are
examining is an ionic compound? Although the distinction is clear with respect
to the presence of ions in the structure, this is often not apparent at first glance
because the formula for the substance doesn’t include charges. However, the
composition of the elements that make up the compound can provide a hint.
Ionic compounds are often formed from the combination of metals and
nonmetals.
Chemists use a
chemical formula, containing element symbols and numbers,
to represent the new combination of atoms or ions known as a chemical com-
pound. A chemical formula is a kind of very precise chemist’s shorthand. It shows
the symbols for the elements found in the compound and the quantity of each of
those atoms or ions. The ratios of the atoms in a chemical formula arise from the
law of multiple proportions. For example, the formula NO tells us that each mol-
ecule of this compound contains one atom of nitrogen and one atom of oxygen.
The formula NO
2
indicates one atom of nitrogen for every two atoms of oxygen
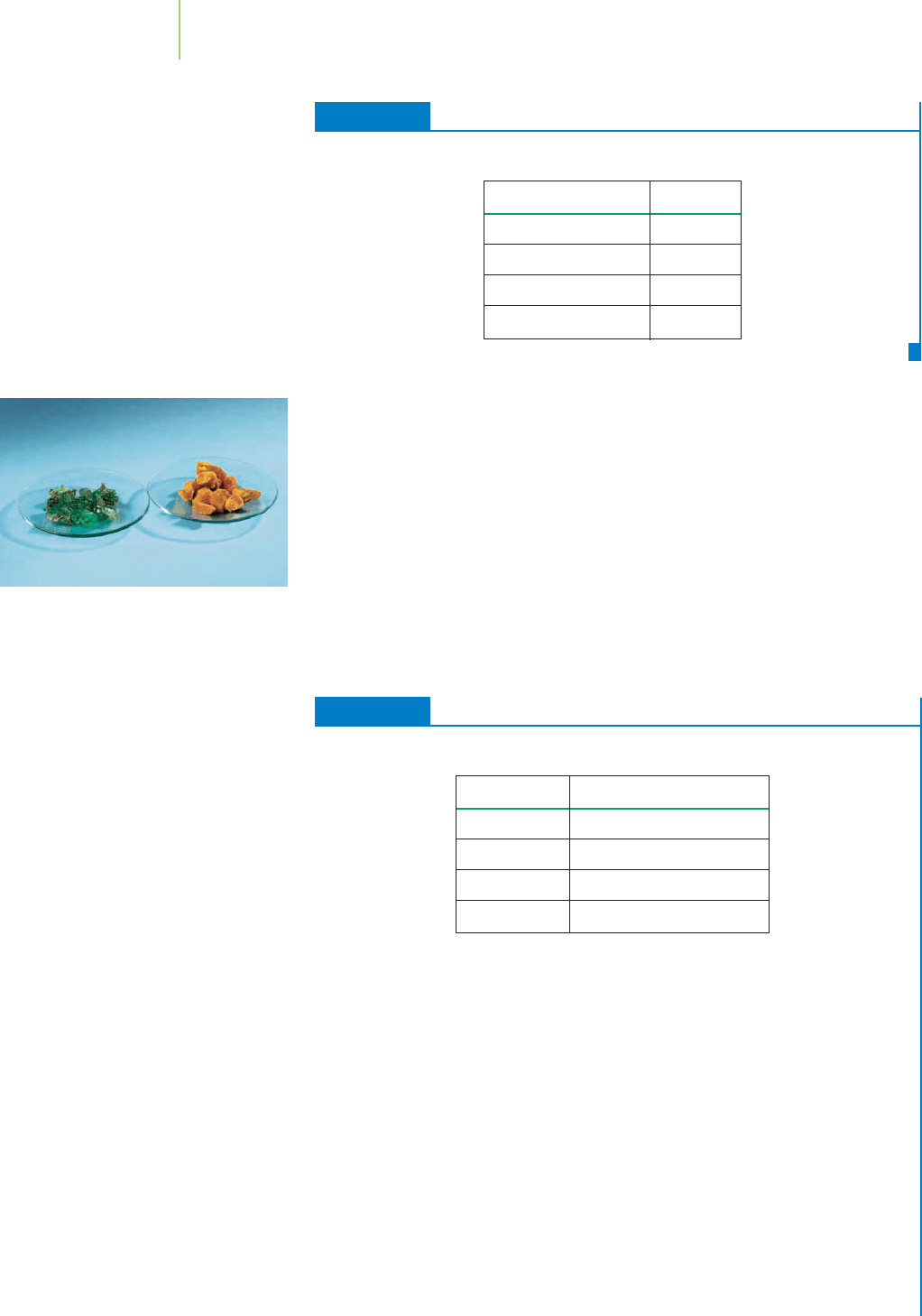
in the molecule. The formula Sb
2
S
3
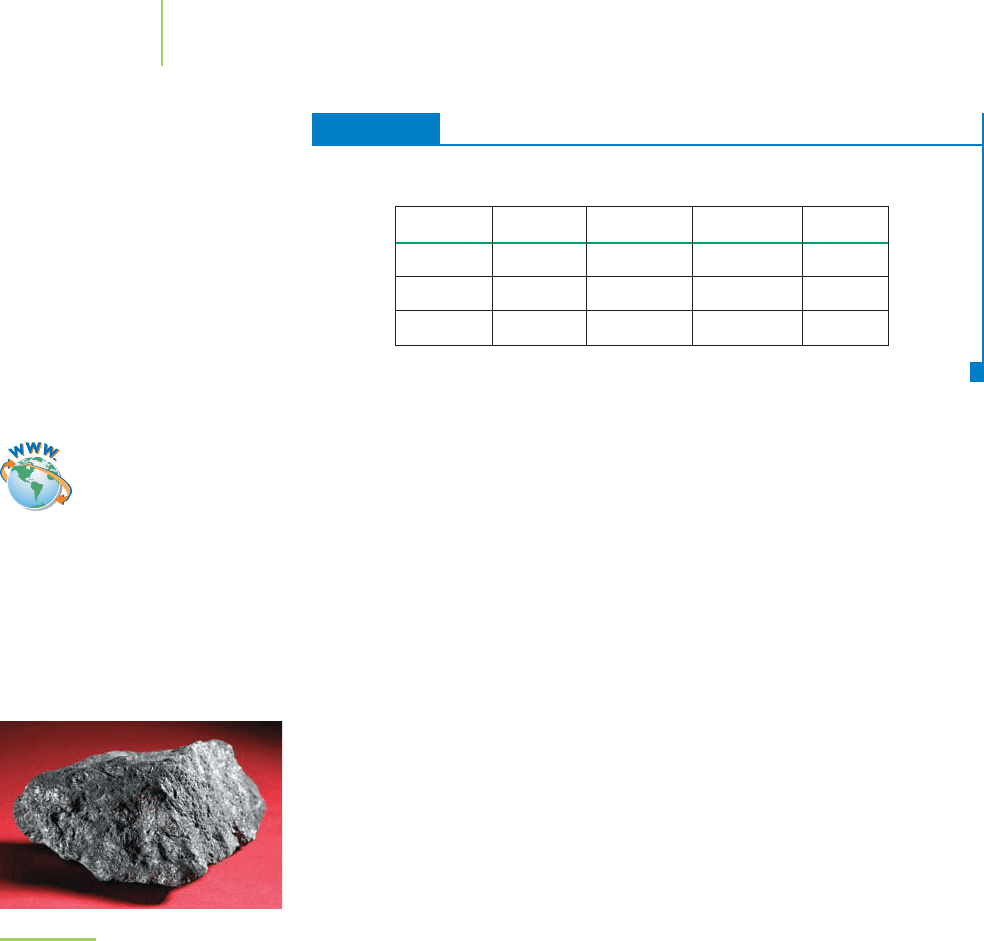
tells us that two atoms of antimony (Sb) and
three atoms of sulfur (S) make up the formula unit of the beautiful mineral stib-
nite (see Figure 2.29) used as a lubricant in antifriction alloys. Could we have de-
termined this formula without knowing more than the identity of the ions in the
compound?
When we are considering ionic compounds, the great clue to the ratio is that
the ions must combine in a ratio that makes the compound electrically neutral over-
all. In sodium chloride, for example, each sodium cation has a +1 charge, and
each chloride anion has a –1 charge, so these ions will combine in a 1:1 ratio to
form the compound. Written as individual ions, they are Na
+
and Cl
−
. When we
write chemical formulas, we generally place any positive ion first, so the formula
for sodium chloride is NaCl. Note that the charges are not shown. If no number
follows the symbol of an element in a formula, it is assumed to contain just one
atom of the element, as with CaO, discussed earlier. The formula CaF
2
, for the
chemical calcium fluoride, indicates that there is one calcium ion for every two
fluoride ions.
Can we predict what the charge of an ion is likely to be in an ionic compound?
As it so often does, the periodic table gives us insight. The main-group elements
gain or lose electrons in predictable and consistent ways when they form ionic
compounds. For example, the metals in Group IIA tend to lose two electrons
when combining with nonmetals. This results in these atoms becoming cations
with a charge of +2. The metals in Group IIIA tend to lose three electrons when
forming ionic compounds, forming cations with a charge of +3. The nonmetals
of Group VA tend to gain three electrons when forming ionic compounds, to
form anions with a –3 charge. The nonmetals of Group VIA tend to gain two
electrons to acquire a charge of –2. Keep in mind that these are tendencies—
strong ones, in fact. However, as with many things in life and the chemistry that
Symbol Protons Neutrons Electrons Charge
52
24
___
6+
19 20 18
35 44 1
FIGURE 2.29
Stibnite is a mineral with the chemical
formula Sb
2
S
3
.
Visualization: Determining
Formulas for Ionic Compounds
Video Lesson: Describing
Chemical Formulas
Tutorial: Determining Formulas
for Ionic Compounds
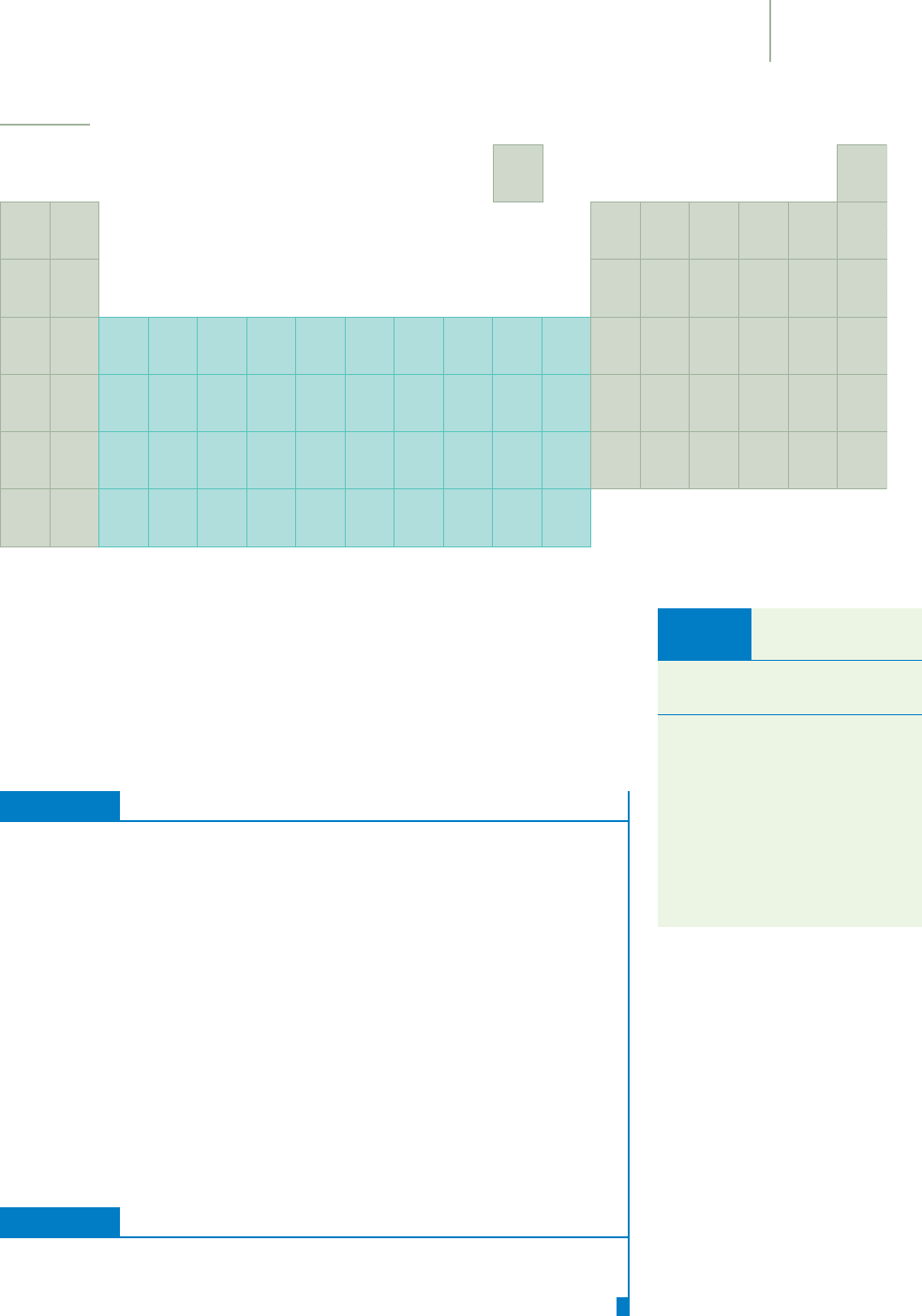
2.7 Ionic Compounds 69
is a part of it, there are exceptions, especially with main-group metals in Periods 5
and 6. For example, lead commonly exists as Pb
2+
or Pb
4+
, depending on condi-
tions. Still, it is a reasonable strategy to decide how many electrons a main-group
element is likely to gain or lose on the basis of the group to which it belongs. On
the other hand, many of the transition elements of the B groups can form ions
with different charges. A list of some common ions is given in Figure 2.30.A sum-
mary of the charges found on the typical main-group ions is shown in Table 2.6.
EXERCISE 2.5 Determining Formulas
1. The ionic compound used in some sidewalk de-icers is made from calcium
and chlorine. What formula would represent that compound?
2. Magnesium combines with oxygen to give a bright flash of light used in some
flares and fireworks. What formula would represent the resulting compound?
Solution
1. Because Ca is in Group IIA, it will form ions with a charge of +2. Chlorine is
in Group VIIA and so will tend to form ions with a charge of –1. To form an
electrically neutral ionic compound, there must be two chloride ions for every
calcium ion. Therefore, the formula will be CaCl
2
.
2. Magnesium is found in Group IIA, so it will form cations with a charge of +2.
Oxygen is found in Group VIA and so will form anions with a charge of –2.
A one-to-one combination produces an electrically neutral compound.
Therefore, the formula will be MgO.
PRACTICE 2.5
What is likely to be the formula of a compound resulting from the atomic combi-
nation of cesium and chlorine?
See Problems 65–70.
H
+
Li
+
Na
+
K
+
Rb
+
Cs
+
Fr
+
Be
2+
Mg
2+
Ca
2+
Sr
2+
Ba
2+
Ra
2+
Sc
3+
Y
3+
Fe
2+
Fe
3+
Cu
+
Cu
2+
Ag
+
Zn
2+
Cd
2+
Al
3+
N
3–
P
3–
O
2–
S
2–
Se
2–
F
–
Cl
–
Br
–
I
–
1
IA
2
IIA
3
IIIB
4
IVB
5
VB
6
VIB
7
VIIB
8
VIIIB
9
VIIIB
10
VIIIB
11
IB
12
IIB
13
IIIA
14
IVA
15
VA
16
VIA
17
VIIA
18
VIIIA
FIGURE 2.30
Some common ions and their locations in the periodic table.
Charges on Typical
Main-Group Ions
Group Most Likely
Number Ionic Charge
IA +1
IIA +2
IIIA +3
IVA +4/−4
VA −3
VIA −2
VIIA −1
VIIIA 0
TABLE 2.6
70 Chapter 2 Atoms: A Quest for Understanding
Ionic compounds most often exist as part of large units such as the three-
dimensional crystals of NaCl that you shake on your french fries. The crystal is
composed of large numbers of sodium ions and chloride ions in equal amounts.
These ionic compounds are typically brittle solids that are difficult to melt. For
example, cesium fluoride (CsF) is an ionic compound with a melting point of
682°C. Ionic compounds vary in their solubility in water. Some, such as table salt
(NaCl), are quite soluble. Others, such as strontium fluoride (SrF
2
), are fairly in-
soluble. When soluble compounds such as NaCl are placed in water, their ions
can separate and move around relatively independently. These mobile and elec-
trically charged ions allow electricity to be conducted through the resulting
solution.
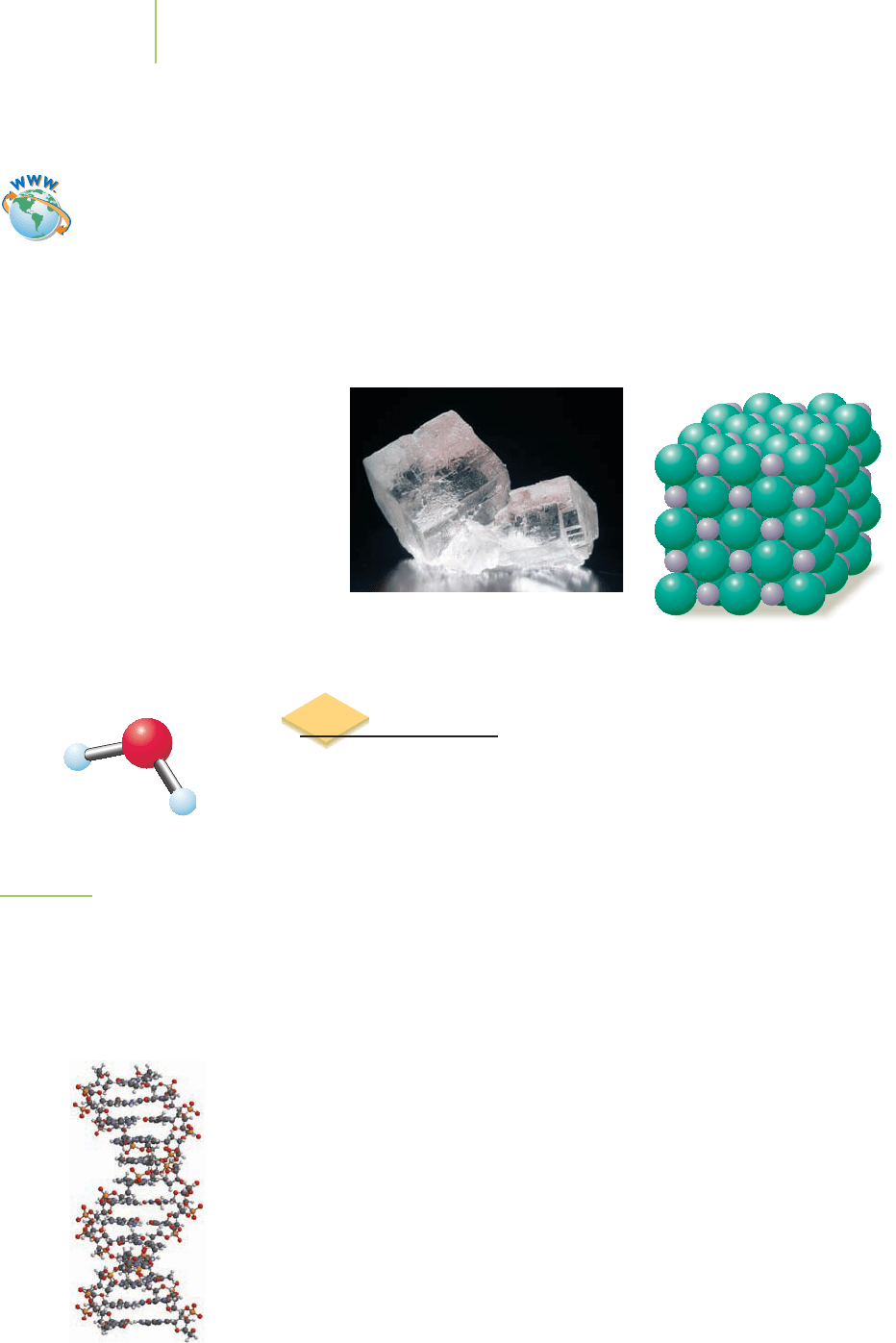
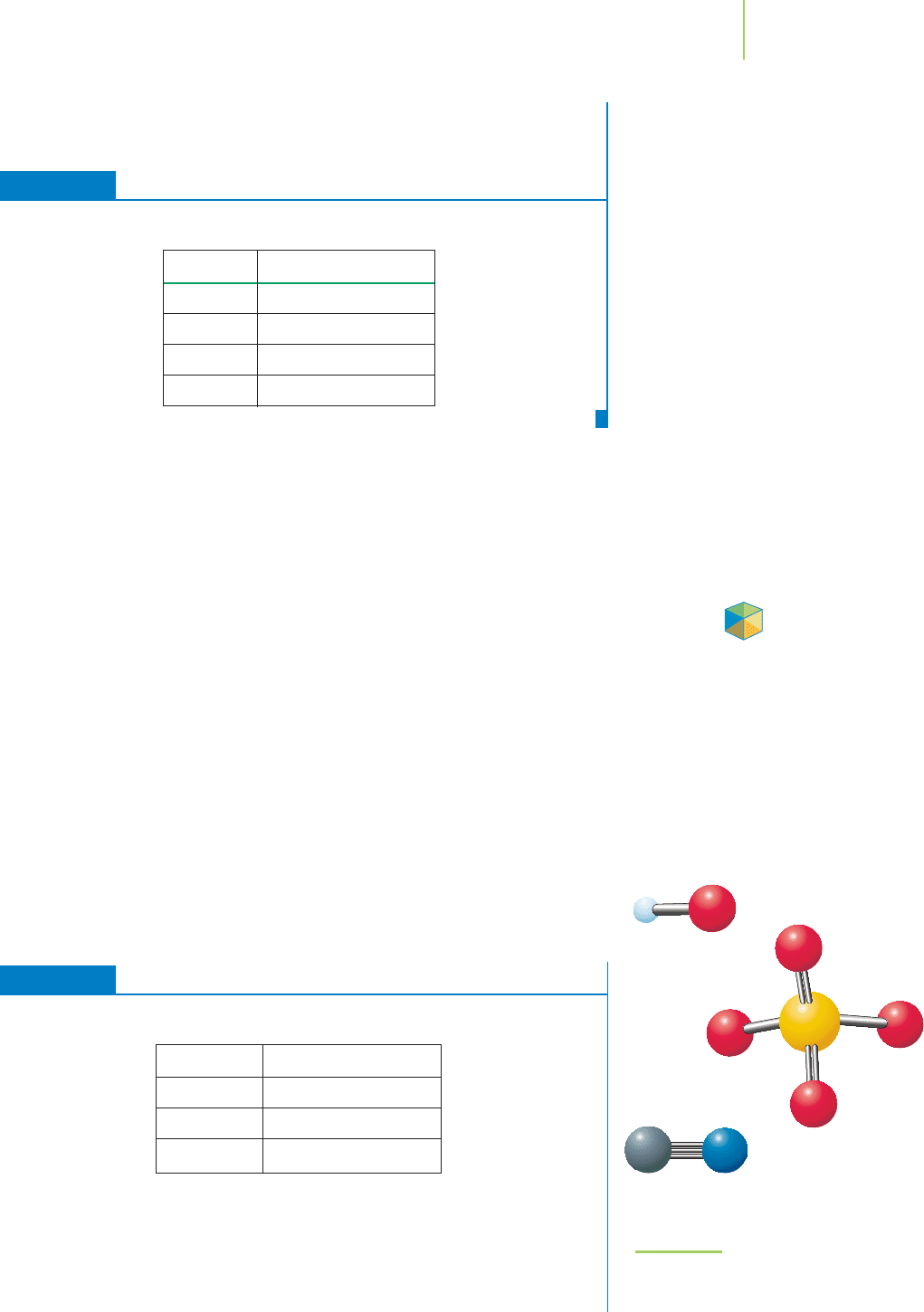
Molecular formula
H
2
O
H
H
O
FIGURE 2.31
The molecular formula for water
indicates that two hydrogen atoms are
combined with one oxygen atom.
Because water is a molecule, none of
these atoms is an ion.
Sodium chloride.
2.8 Molecules
How is carbon dioxide (CO
2
) different from sodium chloride (NaCl)—table salt?
Sodium chloride has properties that we associate with ionic compounds, such as
a high melting point and the ability to conduct electricity. Carbon dioxide is a gas
that doesn’t conduct electricity. These two substances are fundamentally different
in their physical properties, and this is an indication of an essential difference in
chemical structure. Sodium chloride is an ionic compound. Carbon dioxide is a
molecule.
Molecules are distinct substances made up of two or more atoms linked to-
gether by sharing electrons between their nuclei, rather than by the transfer of
electrons from one atom to another. We call bonds that are formed by sharing
electrons between atoms
covalent bonds. Many molecules, such as CO
2
, water
(H
2
O), and methane (CH
4
), are composed of only nonmetals that interact via co-
valent bonds. They differ from ionic compounds in that they are not made up of
ions. When we write H
2
O as the molecular formula for water, we mean that each
molecule contains just two H atoms and one O atom (Figure 2.31). This is in con-
trast to the formulas for ionic compounds, which state the ratio in which ions are
present without saying anything about the exact number of ions in any particu-
lar sample of the compound, because that number is an extensive property that
varies depending on the size of the sample.
Molecules can range in size from diatomic molecules such as N
2
and O
2
, the
main components of air, to giant molecules containing many thousands, or even
millions, of atoms. For example, the DNA that makes up the human genome and
the protein molecules that control most of the chemistry of life are giant molec-
ular compounds.
Iso-octane, a molecular compound, is important in the definition of the oc-
tane rating used to classify different grades of automobile gas. Iso-octane consists
of molecules containing 8 carbon atoms and 18 hydrogen atoms and has the
Visualization: Comparison of a
Molecular Compound and
an Ionic Compound
Video Lesson: CIA
Demonstration: The
Conductivity of Molten Salts
Video Lesson: CIA
Demonstration Conductivity
Apparatus—Ionic versus
Covalent Bonds
Side view of DNA.
2.9 Naming Compounds 71
molecular formula C
8
H
18
. In some cases, we may be interested
only in the smallest whole-number ratio that indicates the molec-
ular formula. The simplest way to use whole numbers to express
the ratio in which carbon and hydrogen atoms are present in iso-
octane is C
4
H
9
. This is known as the empirical formula for octane.
Empirical means “derived from experiment,” and an
empirical for-
mula
is the ratio in which elements are found by experiment to be
present in compounds, regardless of the molecular structure of
the compound. This distinction is shown in Figure 2.32.
Examination of the molecular and empirical formulas for iso-
octane reveals something else about molecules. Even though mol-
ecules are electrically neutral like their ionic counterparts, they
cannot be constructed using the rules for ionic compounds. Re-
member that these are two distinctly different types of com-
pounds. The formula for many ionic compounds can be predicted from the typ-
ical charges found on the ions, especially in the A, or main-group, elements. For
molecules, on the other hand, experimentation is necessary to determine the
empirical and molecular formulas.
One of the themes of our study of chemistry is that small differences in struc-
ture can lead to big differences in chemical behavior. An important example of this
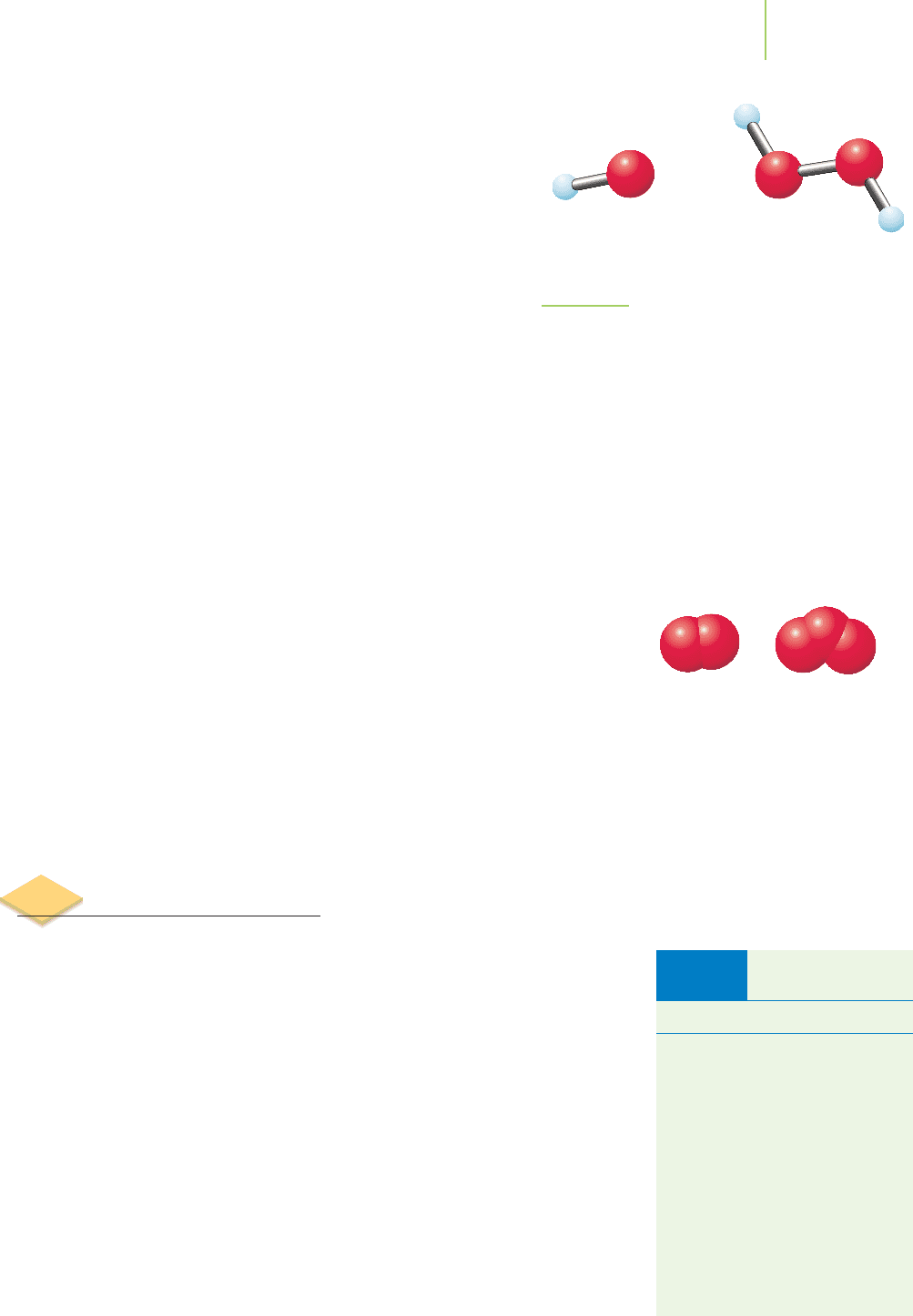
is given by two forms of oxygen, one very common, the other less so. Oxygen is
one of the
diatomic elements, in which the normal state is in the form of mole-
cules composed of two atoms bonded together. Other examples include H
2
,N
2
,
F
2
,Cl
2
,Br
2
and I
2
. The diatomic form of oxygen, O
2
, accounts for approximately
20% of the volume of air and is the form of oxygen we need to stay alive. A dif-
ferent and much less common form of oxygen, O
3
, contains three oxygen atoms
bonded together. This form of oxygen is called ozone and accounts for just a few
parts per billion of the composition of air. The extra atom of oxygen in ozone re-
sults in an
allotrope, another form of an element with different chemical and physi-
cal properties. Ozone, when it occurs in the upper atmosphere, filters out haz-
ardous ultraviolet radiation. Yet, when present at the earth’s surface, O
3
is an
irritant and poison that can cause serious damage to the tissues of the body, in-
cluding the sensitive lining of our lungs, right where O
2
is the very molecule that
is needed most!
2.9 Naming Compounds
Communication between chemists is vital to effective application of the scientific
method we discussed in Chapter 1. This communication relies on being able to
convey information in writing, over the phone, and on the Internet. Complicat-
ing matters is that, according to the United Nations, over 6000 languages are
spoken worldwide. How, then, does a chemist in Minden, Nebraska, tell a col-
league in Mexico City about a particular compound? Luckily, some compounds
have “common names” that are in widespread use across a country and around
the world. For instance, benzene (C
6
H
6
, a compound found in petroleum distil-
lates) and taxol (the anticancer compound from Chapter 1) are known by their
common names in many different countries. But this isn’t always true.As Table 2.7
illustrates for water, some compounds are so common that there is a different
name for them in every language. For other compounds, there simply isn’t a
name that is commonly known. And moreover, without a system to follow, it
would be difficult for those naming compounds to devise names that were mean-
ingful to anyone else.
A simple solution to this problem was provided by the International Union of
Pure and Applied Chemists (IUPAC), an organization formed in 1919 that con-
tinues to advise the scientific community on issues related to chemistry. IUPAC
Empirical formula
OH
Molecular formula
H
2
O
2
FIGURE 2.32
The empirical formula for hydrogen peroxide only lists the
atoms in their simplest whole-number ratios. The molecular
formula lists all of the atoms that make up the molecule.
O
3
O
2
Allotropes of oxygen.
Water in Some
Other Languages
Language Name of H
2
O
English water
Spanish agua
Hawaiian wai
Urdu pani
Swahili maji
Cebuano pagtubig
German wasser
French eau
Slovac voda
Tagalog tubig
Frisian wetter
Japanese mizu
TABLE 2.7

72 Chapter 2 Atoms: A Quest for Understanding
has a set of rules that all scientists try to follow when naming compounds. Known
as the rules of
chemical nomenclature, they establish patterns to follow in naming
existing compounds as well as compounds that have yet to be determined. The
rules for naming
binary covalent compounds (compounds composed of only two
nonmetal elements, which have atoms that interact via covalent bonds) are listed
in Table 2.8.
Consider the formula for NCl
3
. This compound is a covalent compound (it
has only covalent bonds) and is a binary compound (it has only two elements).
Using the rules in Table 2.8, we write
nitrogen
as the first step of the rules for chemical nomenclature. In the second step, we
write
nitrogen chloride
Then, to finish the name of the molecule, we add the prefixes. Note that we do
not add the prefix mono- to the first element name. If it is not stated, mono- is
assumed:
nitrogen trichloride
The following examples give you an opportunity to see these rules in practice:
N
2
O
3
dinitrogen trioxide
HCl hydrogen chloride, not monohydrogen monochloride
CO carbon monoxide; mono- is needed here
CO
2
carbon dioxide
SF
4
sulfur tetrafluoride
SF
6
sulfur hexafluoride
ClO
2
chlorine dioxide
Cl
2
O
7
dichlorine heptoxide
P
4
O
6
tetraphosphorus hexoxide
S
2
Cl
2
disulfur dichloride
EXERCISE 2.6 Naming Binary Molecular Compounds
Provide the IUPAC name for these binary molecular compounds:
PCl
5
NBr
3
AsCl
3
XeO
4
P
4
S
3
Solution
PCl
5
phosphorus pentachloride XeO
4
xenon tetroxide
NBr
3
nitrogen tribromide P
4
S
3
tetraphosphorus trisulfide
AsCl
3
arsenic trichloride
Rules for Naming Binary Covalent Compounds
The name of the compound mentions the elements in the order given in the formula.
These rules apply to binary molecular compounds—those containing two elements that
are both nonmetals.
1. Name the first element using the exact element name.
2. Name the second element by writing the stem name of the element with the suffix -ide.
3. Add prefixes as shown in the list at the right, derived from Greek, to each element
name to denote the subscript of the element in the formula. Generally, the prefix
mono- is used only on the second element in the binary compound to distinguish it
from other examples containing multiple atoms.
TABLE 2.8
Number Prefix
1mono
2di
3tri
4tetra
5 penta
6hexa
7hepta
8octa
9nona
10 deca
Video Lesson: Naming Chemical
Compounds

PRACTICE 2.6
Provide either the name or the formula for each of these binary molecular com-
pounds:
sulfur tetrafluoride carbon tetrachloride diphosphorus pentoxide
PCl
3
N
2
OOF
2
See Problems 77 and 78.
2.9 Naming Compounds 73
Rules for Naming Binary Ionic Compounds
The name of the compound has the elements in the order given in the formula.
1. Name the first element using the exact element name.
2. Name the second element by writing the stem name of the element with the suffix
-ide.
3. For metals that can have more than one stable charge, report the charge as part of
the name. Do this by writing the charge, in roman numeral form, in parentheses
immediately after the metal’s name.
TABLE 2.9
The rules for naming binary ionic compounds (compounds typically composed
of a metal and a nonmetal element, which have ions that interact via electrostatic
attractions) are similar to those for the binary molecular compounds. To sum-
marize the rules in Table 2.9, a binary ionic compound can be named by stating
the name of the atom that has become a cation (positive ion) and then adding the
name of the anion (negative ion). The anion derives its name from the parent
atom but ends in -ide. The name for a binary ionic compound does not tell us
how many of each type of ion are present. How do we tell, from the name, how
many of each type of ion are in the formula unit? Remember that ionic com-
pounds contain ions whose charges must balance to result in an electrically
neutral compound. Just use the charges to determine the number of each ion.
Sodium chloride (NaCl) offers a good example. We can tell that it is likely to
be an ionic compound because it is composed of a main-group metal (Na) and a
nonmetal (Cl). The procedure outlined in Table 2.9 indicates that we first give the
name of the metal, the element listed first in the chemical formula. Therefore, we
begin with
sodium
Then we add the name of the other element, which yields
sodium chloride
Because we know that sodium exists only as an ion with a +1 charge, we do not
need to do step 3 of the rules. According to the IUPAC rules of chemical nomen-
clature, the name for NaCl, sodium chloride, is exactly as we expected.
EXERCISE 2.7 Naming Binary Ionic Compounds
Use the rules outlined in Table 2.9 to
supply each name or formula missing
from the list on the right.
Solution
Name Formula
sodium fluoride NaF
calcium oxide CaO
aluminum sulfide Al
2
S
3
barium chloride BaCl
2
Name Formula
sodium fluoride
calcium oxide
Al
2
S
3
BaCl
2
74 Chapter 2 Atoms: A Quest for Understanding
Iron(II) chloride
FeCl
2
Iron(III) chloride
FeCl
3
PRACTICE 2.7
Supply each name or formula missing from the following list.
See Problems 79 and 80.
Let’s examine some cases where we need to use step 3 in the rules for naming
ionic compounds. One such case involves the metals found between Groups IIA
and IIIA in the periodic table. Most of these transition metals commonly exhibit
more than one positively charged state. It is therefore possible for two bottles of
iron chloride to contain two different ionic compounds. You might find FeCl
2
in
one bottle and FeCl
3
in the other. If we considered only the first two steps in
Table 2.9, we would conclude that they should both be named iron chloride.
However, the first bottle contains an iron ion with a +2 charge. (Remember, it
needs to be Fe
2+
in order to balance the charges of the two Cl
−
atoms.) The sec-
ond bottle contains Fe
3+
for similar reasons. To name these two compounds in
such a way as to distinguish between them, we follow step 3 in Table 2.9 and add
the charge on the iron to the name of the compound. Thus FeCl
2
becomes
iron(II) chloride, and the other bottle contains iron(III) chloride.
EXERCISE 2.8 Naming Additional Compounds
Use Table 2.9 to supply each name or formula missing from the following list.
First Thoughts
To supply these names, we need to be familiar with the rules outlined in Table 2.9.
More important, we will have to consult Figure 2.30 to determine the charges on
each ion. For those with more than one charge, we must follow step 3 in Table 2.9.
Solution
Formula Name
BaCl
2
barium chloride
TiCl
2
titanium(II) chloride
CuO copper(II) oxide
MnO
2
manganese(IV) oxide
Further Insight
Writing the names of compounds requires, first of all, that we understand the na-
ture of the atoms involved in the formulas. Often, it is easy to forget that ionic com-
pounds and molecular compounds are named using different rules. We wouldn’t
want to accidentally name CuO as copper monoxide. This implies that the formula
Formula Name
BaCl
2
TiCl
2
copper(II) oxide
manganese(IV) oxide
Name Formula
magnesium chloride
lithium fluoride
NaBr
Li
2
O
2.9 Naming Compounds 75
represents a molecule. And, as we saw earlier, molecules and ionic compounds have
markedly different properties.
PRACTICE 2.8
Use Table 2.9 to supply each name or formula missing from the list.
See Problems 83, 84, 89, and 90.
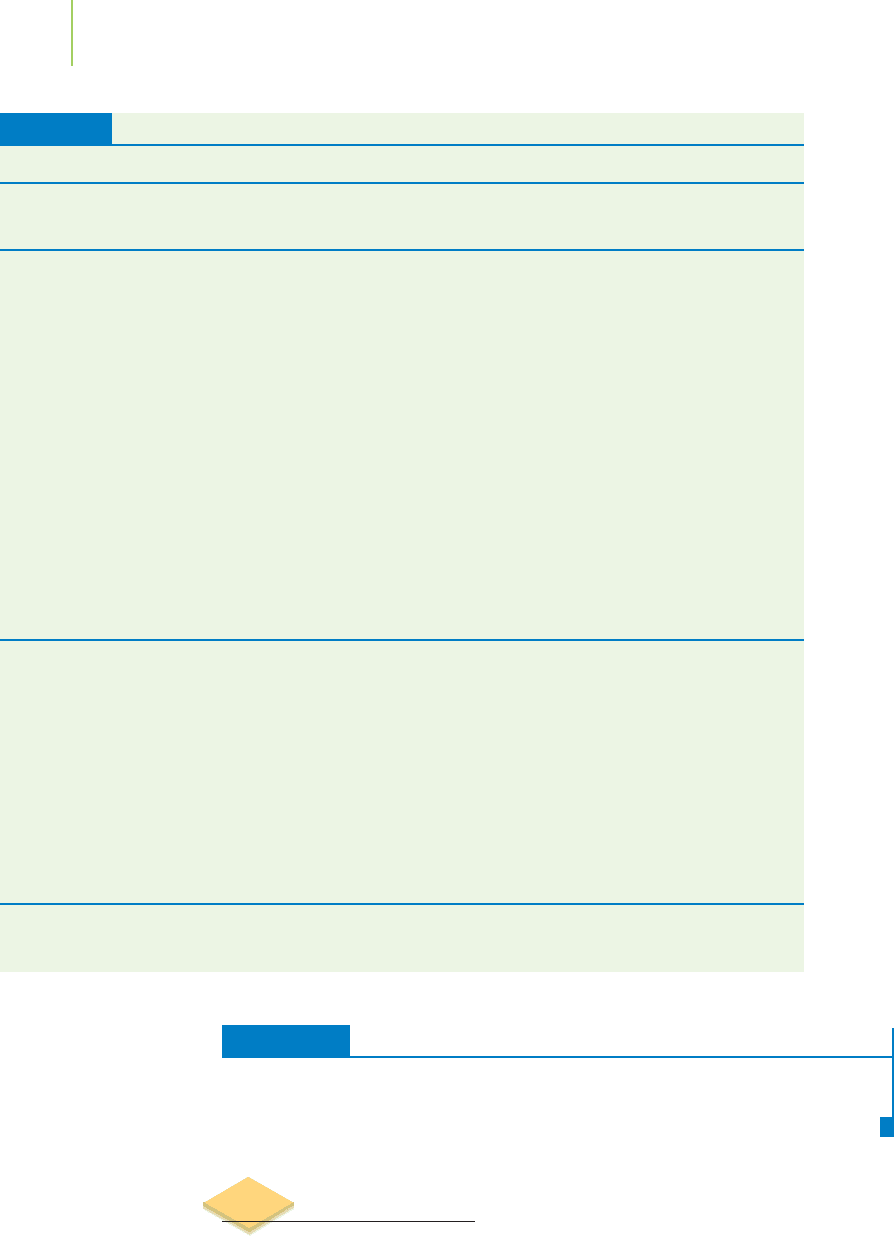
Polyatomic Ions
Many compounds are made up of more than two elements. Some of these are still
classified as ionic compounds, because they involve the association of ions. In
some cases, the ion itself contains two or more atoms. The entire ion that results
can carry a positive or negative charge and behave just like a monatomic ion. Ions
such as this are known as
polyatomic ions or molecular ions. Figure 2.33 shows the
structure of some examples. Table 2.10 lists many others.
Polyatomic ions are everywhere. For example, the famous White Cliffs of
Dover on the southern end of England are made of chalk, the same stuff we use
to write on chalkboards. Chalk has the formula CaCO
3
and is made up of two
ions, Ca
2+
and CO
3
2−
.
Using Table 2.10, we can arrive at the name calcium carbonate for this com-
pound. The CO
3
2−
ion in the compound is the polyatomic “carbonate” ion with
a charge of −2. The calcium and carbonate ions combine to form the electrically
neutral ionic compound calcium carbonate, but there are covalent bonds within
the structure of the carbonate ions.
One common garden fertilizer has the formula (NH
4
)
3
PO
4
. The ammonium
ion, NH
4
+
, has a +1 charge, and the phosphate ion, PO
4
3−
, has a −3 charge.
Three ammonium ions will be present for each phosphate ion. We must use
parentheses in the formula to indicate that our subscript 3 applies to the entire
ammonium ion, not just to the hydrogen atoms within it. What is the name of
this compound? It is known as ammonium phosphate.
EXERCISE 2.9 Naming with Polyatomic Ions
Provide each name or formula missing from the table.
Solution
Formula Name
CaC
2
O
4
calcium oxalate
Mg(NO
3
)
2
magnesium nitrate
CuSO
4
copper(II) sulfate
Al(OH)
3
aluminum hydroxide
CaC
2
O
4
Mg(NO
3
)
2
copper(II) sulfate
aluminum hydroxide
Formula Name
CuCl
2
CrO
3
nickel(II) oxide
palladium(IV) sulfide
Sulfate
SO
4
2–
FIGURE 2.33
Three polyatomic ions. These ions are
made up of chemically bonded atoms.
As a whole, they act as ions.
Application
Cyanide
CN
–
Hydroxide
OH
–
76 Chapter 2 Atoms: A Quest for Understanding
Selected Polyatomic Ions
Formula Name Charge for Ion
Cation with a NH
4
+
ammonium +1
+1 Charge
Anions with a CH
3
COO
−
acetate −1
−1 Charge
ClO
−
hypochlorite −1
ClO
2
−
chlorite −1
ClO
3
−
chlorate −1
ClO
4
−
perchlorate −1
CN
−
cyanide −1
HCO
3
−
hydrogen carbonate (bicarbonate) −1
H
2
PO
4
−
dihydrogen phosphate −1
HSO
4
−
hydrogen sulfate(bisulfate) −1
HSO
3
−
hydrogen sulfite (bisulfite) −1
MnO
4
−
permanganate −1
NO
2
−
nitrite −1
NO
3
−
nitrate −1
OH
−
hydroxide −1
Anions with a CO
3
2−
carbonate −2
−2 Charge
C
2
O
4
2−
oxalate −2
CrO
4
2−
chromate −2
Cr
2
O
7
2−
dichromate −2
HPO
4
2−
monohydrogen phosphate −2
SO
3
2−
sulfite −2
SO
4
2−
sulfate −2
O
2
2−
peroxide −2
S
2
O
3
2−
thiosulfate −2
Anions with a PO
4
3−
phosphate −3
−3 Charge
TABLE 2.10
PRACTICE 2.9
What is the name of the compound with the formula KMnO
4
? What is the formula
for ammonium dichromate?
See Problems 81, 82, and 85–88.
2.10 Naming Acids
Some compounds, known as acids, are often written with a hydrogen at the start
of their formula. These compounds have special names when they are in aqueous
solutions. For those that contain only hydrogen and a halogen, the name is
hydro____ic acid, where the blank is replaced by the root name of the halogen.
For instance, aqueous HCl is known as hydrochloric acid. The names of other
acids come from the name of the polyatomic ion, by replacing the -ate ending of
the ion with -ic acid or the -ite ending with -ous acid. For example, HNO
3
is nitric
acid and HClO is known as hypochlorous acid.
Key Words 77
The Bottom Line
■
All matter is composed of atoms. (Section 2.1)
■
The law of conservation of mass states that the mass
of the chemicals at the start of a reaction is equal to
the mass of the chemicals at the end of the reaction.
(Section 2.1)
■
The law of definite composition states that any par-
ticular chemical is always composed of its compo-
nents in a fixed ratio, by mass. (Section 2.1)
■
The law of multiple proportions states that when
the same elements can produce more than one com-
pound, the ratio of the masses of the element that
combine with a fixed mass of another element corre-
sponds to a small whole number. (Section 2.2)
■
Dalton’s atomic theory stated that every substance is
made of atoms; atoms are indestructible; atoms of
any one element are identical; atoms of different ele-
ments differ in their masses; and chemical changes
involve rearranging the attachments between atoms.
(Section 2.2)
■
The law of combining volumes states that when gases
combine, they do so in small whole-number ratios,
provided that all the gases are at the same tempera-
ture and pressure. (Section 2.2)
■
Atoms are composed of electrons, protons, and neu-
trons. The most common isotope of the hydrogen
atom is the only exception. It contains only electrons
and protons. (Section 2.3)
■
The modern model of the atom indicates a tiny, but
dense, positively charged nucleus surrounded by a
diffuse electron cloud. (Section 2.3)
■
Nuclei can contain both positively charged protons
and neutral neutrons. Isotopes of the same element
differ in their number of neutrons. All atoms of
the same element have the same atomic number
(the same number of protons). (Section 2.4)
■
Atoms are electrically neutral and contain equal
numbers of protons and electrons. Ions are charged
particles and are formed from atoms or groups of
atoms transferring electrons to other atoms or
groups of atoms. Cations are positively charged ions,
and anions are negatively charged ions. (Section 2.4)
■
The atomic mass of an element is the weighted aver-
age of all the isotopes of that element. (Section 2.5)
■
The periodic table of the elements lists all the known
elements, arranged into periods and groups in a
manner that reflects the chemical characteristics that
particular elements share. (Section 2.6)
■
A chemical formula makes use of the atomic sym-
bols and subscripts, as needed, to represent the
atoms in a chemical compound. (Section 2.7)
■
Molecules are distinct substances made up of two
or more atoms linked together by sharing electrons
between their nuclei, rather than by the transfer of
electrons from one atom to another. We call bonds
that are formed by the sharing of electrons between
atoms covalent bonds. (Section 2.8)
■
There are systematic rules for naming compounds
and listing their formulas. (Section 2.9)
Key Words
alkali metals The highly reactive metals found in Group
IA of the periodic table, which form alkalis upon re-
action with water. (p. 66)
alkaline earth metals The metals found in Group IIA of
the periodic table. (p. 66)
allotropes Forms of an element that have very different
chemical and physical properties, such as the al-
lotropes O
2
and O
3
.(p. 71)
alpha particle A fast-moving nucleus of helium (two
protons and two neutrons) emitted during the decay
of a radioactive element. (p. 54)
anion Ions with a negative charge. (p. 56)
atomic number (Z) The number of protons in the nucleus
of an atom. (p. 56)
atomic mass unit The arbitrary unit of mass for ele-
ments based on the isotope carbon-12. One atom of
carbon-12 is defined to have a mass of exactly
12.0000 amu. (p. 60)
beta particle A fast-moving electron emitted during the
decay of a radioactive element. The beta particle orig-
inates from the nucleus of the decaying element.
(p. 54)
binary covalent compounds Compounds composed of
only two nonmetal elements. (p. 72)
binary ionic compounds Compounds typically composed
of a metal and nonmetal element, which have ions
that interact via electrostatic attractions. (p. 73)
cations Ions with a positive charge. (p. 56)
chalcogens The elements found in Group VIA of the
periodic table of elements. (p. 66)
