Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

ratio, by mass. This is called the law of definite composition. This law is true for all
compounds, irrespective of the source of the compound. We can illustrate the law
of definite composition using a compound containing the elements iron and sul-
fur, called iron(II) sulfide. If we analyze the composition of two different samples
of iron(II) sulfide, we find that their mass ratios are identical.
Result from Analysis of Two Samples of Iron(II) Sulfide
Sample A: Compound mass = 42.0 g
Sample B: Compound mass = 100.0 g
Mass Ratios
Sample A: 26.7 g Fe/15.3 g S = 1.74:1.00 = ratio of g Fe to g S
Sample B: 63.5 g Fe/36.5 g S = 1.74:1.00 = ratio of g Fe to g S
We can also think of this law in the reverse fashion. Suppose we were inter-
ested in making an 80.0-g glass of water from its elements, hydrogen and oxygen.
Given that the mass ratio of oxygen to hydrogen in water is 7.99:1.00, we would
need to chemically combine 1.00 g of hydrogen for every 7.99 g of oxygen to
make the water. In our specific example, then, we would need 8.90 g of hydrogen
and 71.1 g of oxygen to make 80.0 g of water. Water is water; it is never made up
of a different mass ratio of its components.
Preparation of Water (H
2
O)
Hydrogen 8.90 g
Oxygen 71.1 g
Water 80.0 g
Mass Ratio
Water: 71.1 g oxygen/8.90 g hydrogen = 7.99:1.00 g oxygen to g hydrogen
EXERCISE 2.1 Investigating the Law of Definite Composition
Suppose that you collected two samples of pure bottled water.
a. If you separated the water into hydrogen and oxygen and obtained the
following results, what would you report as the ratio of the mass of oxygen to
that of hydrogen in each sample?
Sample 1: 37.3 g of oxygen; 4.67 g of hydrogen
Sample 2: 69.3 g of oxygen; 8.67 g of hydrogen
b. Do these data support the law of definite composition?
c. If you obtained another sample of water and found it to contain 17.0 g of
oxygen, how many grams of hydrogen would you expect to obtain?
Solution
a. The ratios of oxygen to hydrogen in these two samples are
Sample 1:
37.3goxygen
4.67 g hydrogen
=
7.99 g oxygen
1.00 g hydrogen
Sample 2:
69.3goxygen
8.67 g hydrogen
=
7.99 g oxygen
1.00 g hydrogen
b. Yes, the ratios are the same for different masses of the same compound, so
these data support the law of definite composition.
48 Chapter 2 Atoms: A Quest for Understanding
FeS (iron pyrite).
c. Because the ratio of oxygen to hydrogen is constant in the compound called
water, we would expect to have a ratio of 7.99 g of oxygen to 1.00 g of
hydrogen. Using words, we say, “7.99 grams of oxygen is to 1.00 gram of
hydrogen as 17.0 grams of oxygen is to how many grams of hydrogen?” Using
equations, we can write
7.99 g oxygen
1.00 g hydrogen
=
17.0goxygen
?ghydrogen
Rearranging yields
17.0goxygen×1.00 g hydrogen
7.99 g oxygen
=?ghydrogen
=
2.13 g hydrogen
PRACTICE 2.1
Given the information in Exercise 2.1, determine how many grams of oxygen will
combine with 24.5 g of hydrogen to make water. How many grams of water will be
made?
See Problems 9 and 10.
The law of conservation of mass and the law of definite composition provide
two fundamental descriptions of the behavior of chemical processes. When they
were discovered, there was no satisfactory explanation for them.After all, they are
just chemical laws that describe observations of the natural world. Many scien-
tists searched for explanations of these two laws. One of these was the English
scientist John Dalton (1766–1844; see Figure 2.5).
2.2 Dalton’s Atomic Theory and Beyond
John Dalton performed experiments on compounds in which two elements
made more than one type of compound. This is not unusual. There are many ex-
amples of compounds that contain the same elements but in different ratios. For
example, we can combine oxygen and nitrogen to make many different com-
pounds, three of which are shown in Table 2.1. Note how each has a different
mass of oxygen that combines with a given mass of nitrogen.
The results demonstrate a general law formulated by Dalton: When the same
elements can produce more than one compound, the ratio of the masses of this
element that combine with a fixed mass of another element is a small whole number.
This is known as the
law of multiple proportions. Using the information in
Table 2.1, we see that in nitric oxide, 14.0 g of nitrogen combines with 16.0 g of
2.2 Dalton’s Atomic Theory and Beyond 49
FIGURE 2.5
John Dalton, a poor Quaker school-
teacher and brilliant amateur meteorolo-
gist, developed the basic points of the
atomic theory. He showed early signs of
brilliance and even began teaching others
in his small English hometown when he
was only ten years old.
Compounds Containing Nitrogen and Oxygen:
Comparing Oxygen to Oxygen
Ratio of Mass of Oxygen
Fixed Mass Mass of in Compound to Mass
Common Name of Nitrogen Oxygen of Oxygen in Nitric Oxide
Nitric oxide 14 g 16 g 1:1
Nitrogen dioxide 14 g 32 g 2:1
Nitric anhydride 14 g 40 g 5:2
TABLE 2.1
Video Lesson: Early Discoveries
and the Atom

oxygen. A different compound of these same two elements exists in which 14.0 g
of nitrogen combines with 32.0 g of oxygen. The ratio of the masses of oxygen
that combine with the same mass of nitrogen (14.0 g, in this case), is 32.0 g/16.0 g,
or 2:1, a small whole number. The same ratio in nitric anhydride is 40.0 g/16.0 g,
or 2.5:1. This is 5:2 in small whole numbers.
Why is the ratio of the components in such compounds based on small whole
numbers?
The implication of these results is illustrated in Figure 2.6. Dalton won-
dered whether these results could be explained by the existence, for each element,
of some basic particle with a specific mass that would be the smallest part of every
element. Moreover, his experiments were giving results that were entirely consis-
tent with this idea. In 1803 he presented the results of his experiments before the
Manchester Literary and Philosophical Society. His ideas, which came to be
known as
Dalton’s atomic theory, are summarized in Table 2.2.
Dalton’s atomic theory was a keystone in the foundation of chemistry, but as
we shall see, not all of it is correct. However, his theory teaches us something very
important about the scientific method: Ideas don’t need to be completely correct
to be very useful and influential. Dalton was limited by the measurements he was
able to make at the turn of the nineteenth century. Our ability to know the mass
of objects is orders of magnitude better now than in Dalton’s time, and our un-
derstanding is therefore significantly better. Still, we view Dalton’s atomic theory
as a great step forward in chemistry, because it focused attention on the valid idea
that compounds are made from little bits—atoms—combining in fixed propor-
tions and that chemical reactions are rearrangements of these atoms.
The Law of Combining Volumes
One example of the refinement of ideas in the light of new information is pro-
vided by the work of the French chemist Joseph Louis Gay-Lussac (1778–1850;
see Figure 2.7). Gay-Lussac carefully measured the changes in volume when gases
reacted to produce other gases. His results are summarized in what became
known as the
law of combining volumes, which states that when gases combine, they
do so in small whole-number ratios, such as 1:1, 1:2, 1:3, and 3:2, provided that all
the gases are at the same temperature and pressure. One example is illustrated in
Figure 2.8.
50 Chapter 2 Atoms: A Quest for Understanding
Dalton’s Atomic Theory
• Every substance is made of atoms.
• Atoms are indestructible and indivisible.
• Atoms of any one element are identical.
• Atoms of different elements differ in their masses.
• Chemical changes involve rearranging the attachments between atoms.
TABLE 2.2
N: 14 g
O: 16 g
N: 14 g
O: 32 g
NO
2
NO
FIGURE 2.6
The ratio of the mass of oxygen in
NO
2
to its mass in NO is a simple
whole number.
FIGURE 2.7
Joseph Louis Gay-Lussac (1778–1850)
was the eldest of five children. His col-
leagues considered him a careful, elegant
experimentalist. He introduced the terms
pipette and burette and was the first to
isolate the element boron. He is most
noted for publishing Charles’s law (see
Chapter 10) and formulating the law of
combining volumes.
2 balloons of H
2
+
1 balloon of O
2
2 balloons of H
2
O
FIGURE 2.8
Oxygen and hydrogen combine to form water vapor. According to the law of combining volumes,
two volumes of hydrogen react with one volume of oxygen to make two volumes of water vapor.
Visualization: Oxygen,
Hydrogen, Soap Bubbles,
and Balloons
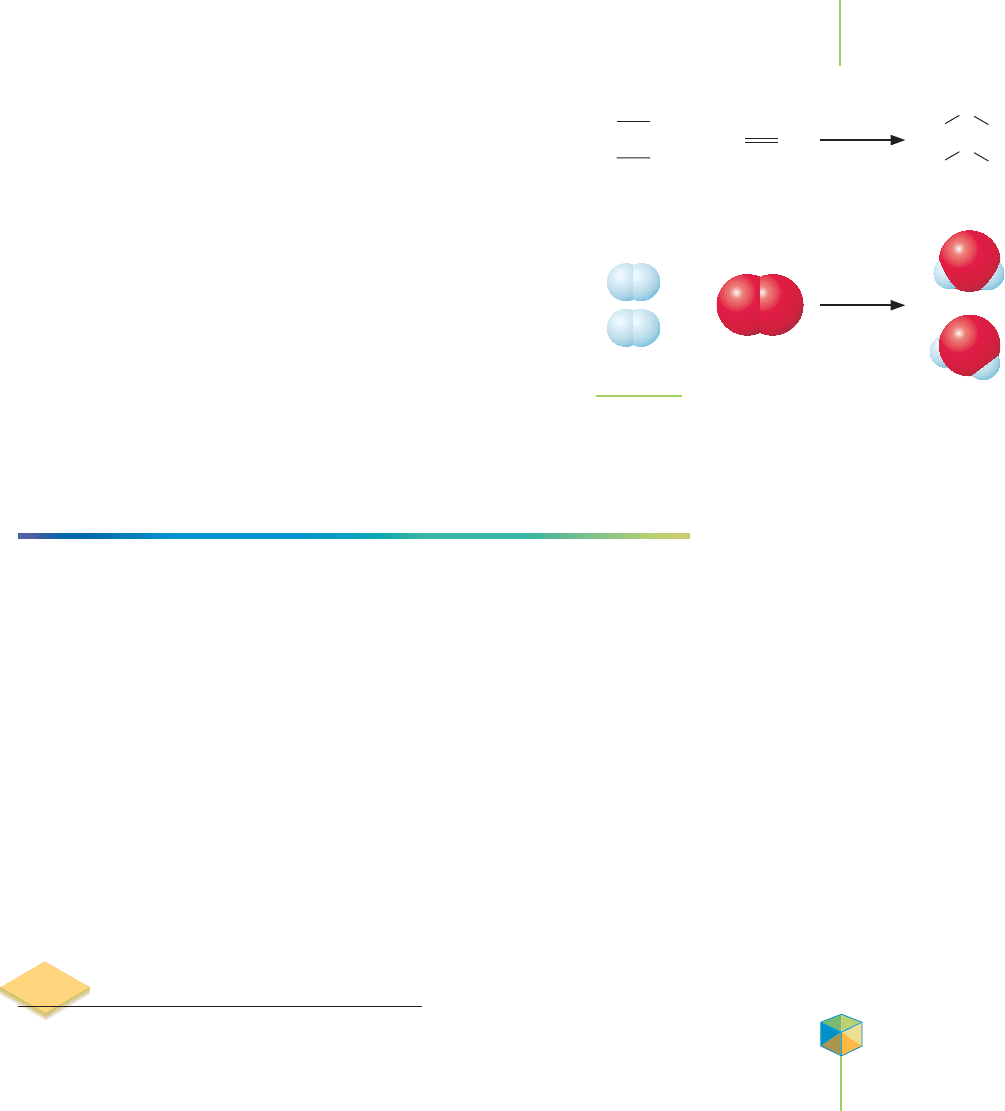
Dalton could not accept the results in that example because it
contradicted his understanding of what would have to happen at
the level of individual particles. To fit with Gay-Lussac’s law, it
seemed to Dalton that one oxygen atom would have to split in two
in order to react with two hydrogen atoms and produce two parti-
cles of water. Dalton’s atomic theory said that atoms could not be
split in two, so he presumed that either Gay-Lussac’s results or his
reasoning must be flawed. The apparent difficulty disappears,
however, when we realize that oxygen gas consists of oxygen mol-
ecules (O
2
), each composed of two oxygen atoms bonded to-
gether. We also now know that hydrogen gas is composed of H
2
molecules and that each water molecule contains two hydrogen
atoms and one oxygen atom (H
2
O). One molecule of oxygen can
combine with two molecules of hydrogen to create two molecules
of water as shown in Figure 2.9.
HERE’S WHAT WE KNOW SO FAR
The information gleaned by applying the scientific method to investigate the
makeup of matter has provided us with a basic look at the nature of chemistry.
Specifically, we know that
■
Matter can be neither created nor destroyed in a chemical reaction.
■
Matter is composed of small indestructible particles called atoms.
■
Compounds are made by combining atoms in whole-number ratios.
■
The components of a compound are always present in the same ratio, by mass.
■
A chemical reaction rearranges the attachments that hold atoms together in a
compound.
These points illustrate the scientific laws identified by Gay-Lussac, Lavoisier,
Proust, and Dalton. In addition, Dalton’s atomic theory helps to solidify our un-
derstanding of these laws and of the nature of the atom. In the next sections of
this chapter, we will begin to examine the makeup of the atom.
2.3 The Structure of the Atom
Archaeology, the study of past human activities, often requires scientists to be able
to assign ages to artifacts that have been unearthed. In some cases, such relics can
be dated because they are buried underneath objects for which an age is known. In
other cases, the archaeologists must perform laboratory experiments to estimate
the age of the artifact. One exciting discovery that helped provide a wealth of
information about ancient boat construction was found buried under 6 feet of
earth in Dover, England. The “Dover Boat,” as it has become known, was discov-
ered underneath the footings of an ancient city wall (Figure 2.10).
Archaeologists knew the date that the wall was constructed from historical
records and, judging from its location, surmised that the boat was probably older
than the wall under which it was buried. To get an accurate age of the boat,
though, they needed to perform “radiocarbon dating” on small pieces of wood
from the boat.
What is the basis of this process, and what can it teach us about
atomic structure?
Some of the carbon atoms in living things emit certain particles and energy
(radioactivity) that allows the atoms to become more energetically stable (see
Chapter 21). It has been observed that the amount of radioactive carbon remains
relatively constant while the organism is alive, as life’s processes allow the
2.3 The Structure of the Atom 51
HH
H
O
H
H
O
WaterOxygenHydrogen
H
HH
OO
+
+
FIGURE 2.9
It is because the elemental forms of hydrogen and oxygen
exist as diatomic substances that the law of combining
volumes makes sense at the molecular level.
Application
C
HEMICAL ENCOUNTERS:
The Dover Boat and
Radiocarbon Dating

52 Chapter 2 Atoms: A Quest for Understanding
FIGURE 2.10
The hull of the Dover Boat is partially ex-
cavated in this figure. Note the construc-
tion of the rails running down the center
of the boat. These rails were used to
fasten the two halves of the boat
together.
exchange of atoms between, for example, a tree and its environment. Once death
occurs, however, the radioactive carbon in the tree begins to diminish. If you
can measure the amount of radioactive carbon remaining in a piece of wood,
you can estimate the year in which the tree died. The results of radiocarbon dat-
ing on the Dover Boat indicated that it was constructed around 1550
B.C.
The previous discussion implies that some carbon atoms (those that are ra-
dioactive) are different from other carbon atoms (those that are not radioactive.)
Dalton’s claim that all atoms of any one element are identical is not correct.
What
is it about the structure of the atom that gives rise to more than one type of the
same element?
A brief look back in time will help us answer this question.
Electrons, Protons, and Neutrons
In the late 1800s and early 1900s, scientists were able to make measurements that
changed our fundamental understanding of the structure of the atom. In
the 1880s, Svante Arrhenius, a Swedish researcher working at the Academy of
Sciences in Stockholm, found that some solutions contained large numbers of
“electrically charged atoms.” Arrhenius found, for example, that when copper
chloride was placed in water and the positive and negative terminals of a power
supply were immersed in the solution, copper collected at one electrode and
chlorine collected at the other. He concluded that some atoms were themselves
negatively charged and others positively charged, causing each to be attracted to
the electrode carrying the opposite charge. This work provided some of the ear-
liest evidence for the existence of what we now call ions.
In 1891, the English scientist G. Johnstone Stoney examined the physical
properties of electricity. To represent a distinct unit of electricity, he proposed
the name
electron, from the Greek word elecktron (meaning “amber”), because
rubbing a rod of amber on wool gives rise to what we now call static electricity.
Were electrons small, charged pieces of atoms? If so, then atoms could no longer
be thought of as small, indestructible or indivisible units of matter!
Many scientists noted that the discovery of the electron suggested that
Dalton’s atomic theory was inadequate. One such scientist, Joseph John (“J. J.”)
Thomson (1856–1940; see Figure 2.11), reasoned that if Dalton’s ideas about the
indestructibility of an atom were true, how could negatively charged parts of
atoms (the electrons) be released from the atom? Furthermore, if negative parti-
cles were present, wouldn’t positively charged pieces of atoms also exist? It makes
sense that an atom with negatively charged particles inside must, in order to be
Static electricity.
Video Lesson: Understanding
Electrons

electrically neutral overall, have a balancing positive charge associated with it.
Thomson envisioned such an atom. In 1904, he wrote that “the atoms of the ele-
ments consist of a number of negatively electrified corpuscles (electrons) en-
closed in a sphere of uniform positive electrification.” His model of the atom
(shown in Figure 2.12) was known as the “plum pudding model.” This model
helped scientists understand what an atom might look like.
Radioactivity and the Structure of the Atom
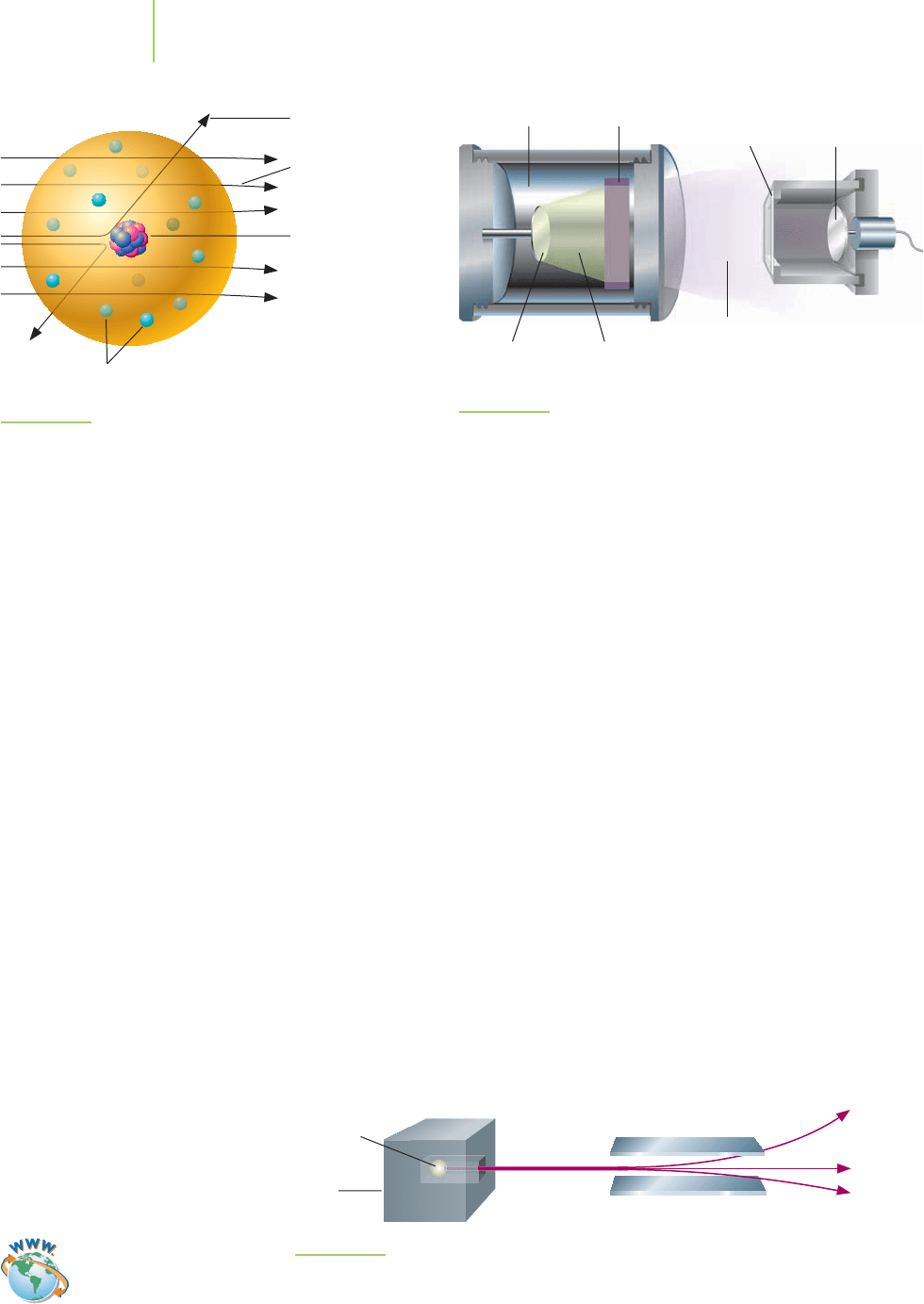
In 1909 Ernest Rutherford (1871–1937; see Figure 2.13), a New Zealand–born
physicist and former student of J. J. Thomson, and his student Ernest Marsden
(1889–1970), performed experiments in which positively charged radiation was
directed toward a thin sheet of gold foil (Figure 2.14). If Thomson’s plum pud-
ding model of the atom were correct, most of the positively charged radiation
would be expected to pass through the foil and undergo slight deflections as
its positive charge interacted with the negatively charged electrons scattered
throughout the atoms. Instead, most of the radiation went straight through the
foil without deflection, some of the radiation was scattered at very wide angles,
and—most amazingly—some was deflected nearly straight back toward its source,
as shown in Figure 2.15. In 1911 Rutherford explained this startling result. His ex-
planation led to a new model of the atom in which a small region of very
concentrated positive charge existed within a large area of mostly empty space
containing negatively charged electrons (see Figure 2.15).
Rutherford used the term
proton (from the Greek protos, meaning “first”) to
describe the nucleus of the hydrogen atom. However, his calculations of the mass
2.3 The Structure of the Atom 53
FIGURE 2.11
Joseph John (“J. J.”) Thomson
(1856–1940) provided the first model
of the structure of the atom, known
as the plum pudding model. 1906, “in
recognition of the great merits of his
theoretical and experimental investi-
gations on the conduction of electric-
ity by gases,” he was awarded the
Nobel Prize in physics.
Electrons
Cloud of
positive charge
FIGURE 2.12
The plum pudding model of the atom.
FIGURE 2.13
Ernest Rutherford (1871–1937) was
born in New Zealand after his family
emigrated there from Scotland in the
mid-1800s. As a student of Thomson
at Cambridge, he developed an in-
strument that could detect electro-
magnetic radiation. Later, while
he worked at McGill University
(Montréal, Canada) and at the Uni-
versity of Manchester (England), he
investigated the actions of alpha
particles. For his discoveries, he was
awarded the Nobel Prize in chem-
istry in 1908.
Radioactive
source
Screen to detect
scattered particles
Gold foil
particle beam
FIGURE 2.14
The Rutherford gold foil experiment. Positively charged radiation is directed toward a small piece
of gold foil. Although most of the radiation passes through the foil, a noticeable percentage is
scattered backward by the foil. This led Rutherford to develop a new model of the atom.
Video Lesson: Understanding
the Nucleus
Visualization: Gold Foil
Experiment
of an atom made from a nucleus of protons didn’t agree with the experimentally
determined mass of the atom. He theorized that some other kind of particle must
be in the nucleus too and suspected that it was probably electrically neutral be-
cause the positive electric charge on the nucleus already balanced the negative
charge of the electrons.
In 1932 James Chadwick (1891–1974) set up an experiment that allowed radi-
ation from beryllium atoms to strike a block of paraffin wax. This resulted in the
loss of protons from the wax. After calculating the force that would be needed to
cause the ejection of the nuclei from the sample of paraffin, Chadwick realized
that the radiation from the beryllium must be electrically neutral and have a mass
approximately equal to that of the proton. Chadwick had discovered the
neutron.
The diagram in Figure 2.16, reproduced from the original article in which he pub-
lished his results, illustrates the process that Chadwick used.
These discoveries also led to understanding of the different forms of radia-
tion. In short, some atoms are unstable and can undergo
radioactive decay
by spontaneously emitting high-energy radiation, sometimes accompanied by
fast-moving particles. Collectively, this phenomenon is known as
radioactivity.
The three most common types of radioactivity are
gamma rays (γ rays), alpha
particles
( particles) and beta particles (β particles) (Figure 2.17).
54 Chapter 2 Atoms: A Quest for Understanding
Electrons
Some α particles
are scattered.
Most α particles
pass straight
through.
Nucleus at the
center with a
positive charge
FIGURE 2.15
Some of the positively charged radiation
interacts with the positive nucleus of the
atom.
Vacuum
Radioactive
particles
Neutrons
Radioactive
source
Beryllium
Thin wax
sheet
Detector
FIGURE 2.16
Chadwick’s experiment directed neutral radiation at a block of paraffin
wax. The energy of the particles that resulted from the collision of the
radiation and the wax led to the discovery of the neutron. The radiation
was generated by placing radioactive polonium near a sheet of beryllium
metal. The wax, shaped into a thin sheet, was placed in front of the
detector.
Radioactive
material
Positively charged plate
Negatively charged plate
(+)
(–)
Lead
block
α particles
β particles
γ rays
FIGURE 2.17
Radiation emitted from a radioactive element placed in a lead block. Alpha particles and beta
particles are deflected by an electric field. Alpha particles are positively charged and therefore
are pulled toward a negatively charged plate. Beta particles are negatively charged and hence are
pulled toward a positively charged plate. Gamma rays are not charged and therefore are not de-
flected by the electric field.
Visualization: Nuclear Particles
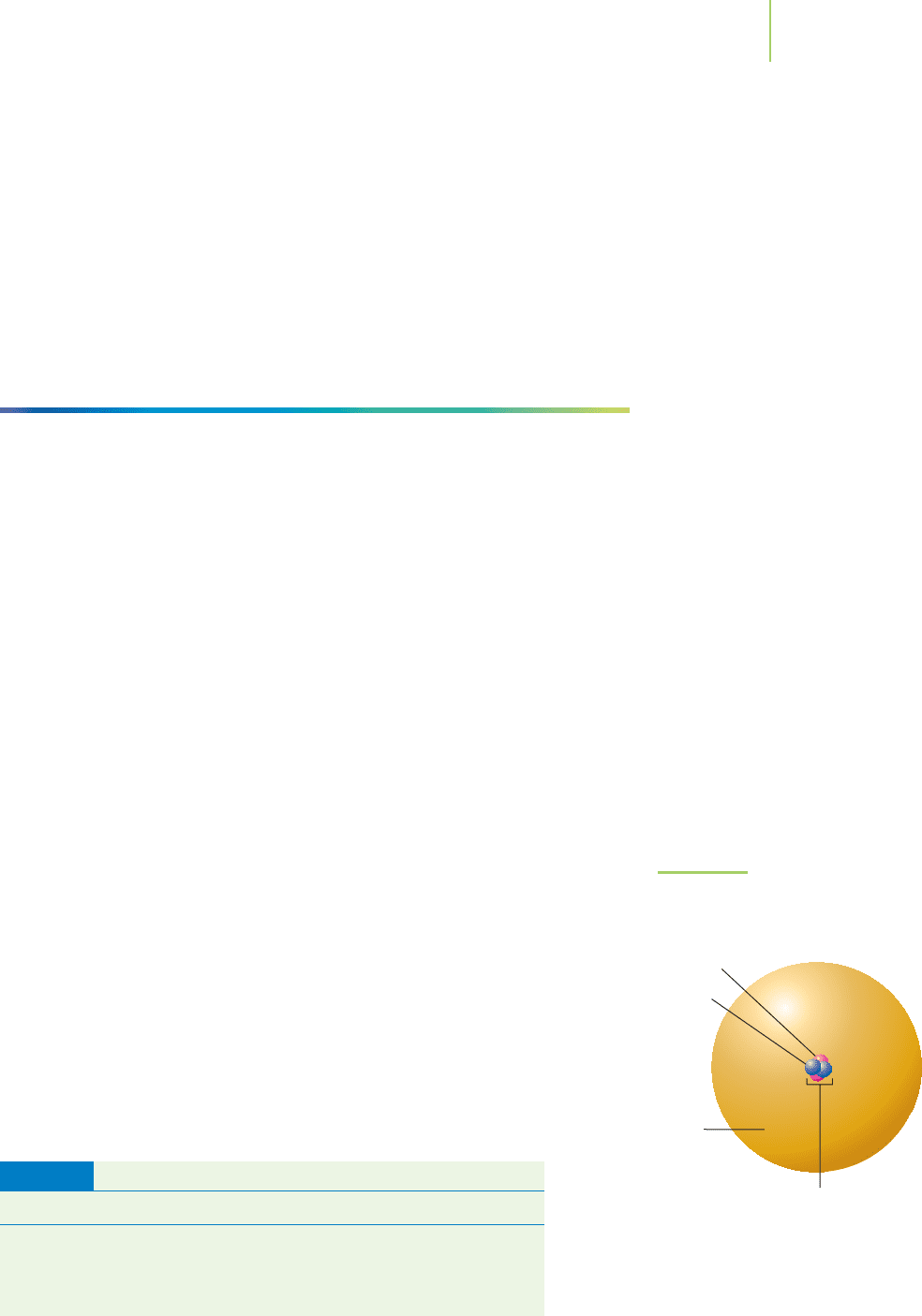
Gamma rays are a very energetic form of electromagnetic radiation, with the
same physical nature as visible light but a much higher energy. An alpha particle
is just the nucleus of the helium atom. It is composed of two protons and two
neutrons and carries a total charge of +2. Alpha particles were the type of radia-
tion directed toward the foil in Rutherford’s experiments. Beta particles are fast-
moving electrons released from the nucleus of an atom, each one carrying its
characteristic –1 charge.
The penetrating powers of radioactivity are put to many uses in modern life,
including killing cancer cells, testing the integrity of welds in metal, and looking
for hairline cracks in aircraft airframes. We will discuss the concepts and applica-
tions of radioactivity much more fully in Chapter 21.
HERE’S WHAT WE KNOW SO FAR
■
Atoms can be broken down into smaller parts. Those parts include the proton,
neutron, and electron.
■
The proton and neutron occupy the center of the atom, called the nucleus.
■
The electrons occupy the space around the nucleus.
■
Protons and electrons have opposite charges; protons are positively charged
and electrons are negatively charged.
■
The total mass of the atom is the sum of all of the particles that make up the
atom.
■
Radiation is a result of the decay of atoms. Different decay products exist, such
as the alpha particle, the beta particle, and the gamma ray.
Through the work we’ve just described, along with that done by other scien-
tists of the time, we know that the nucleus of an atom contains the protons and
neutrons. The proton has a tiny positive electrical charge (1.6 × 10
−19
coulombs)
and a similarly minute mass (1.6726 × 10
−27
kg). Neutrons do not carry a charge
and are a tiny bit more massive than the protons (1.6749 × 10
−27
kg). The elec-
trons travel around the remaining space in the atom. Although they are about
2000 times less massive (9.1094 × 10
−31
kg) than the particles in the nucleus,
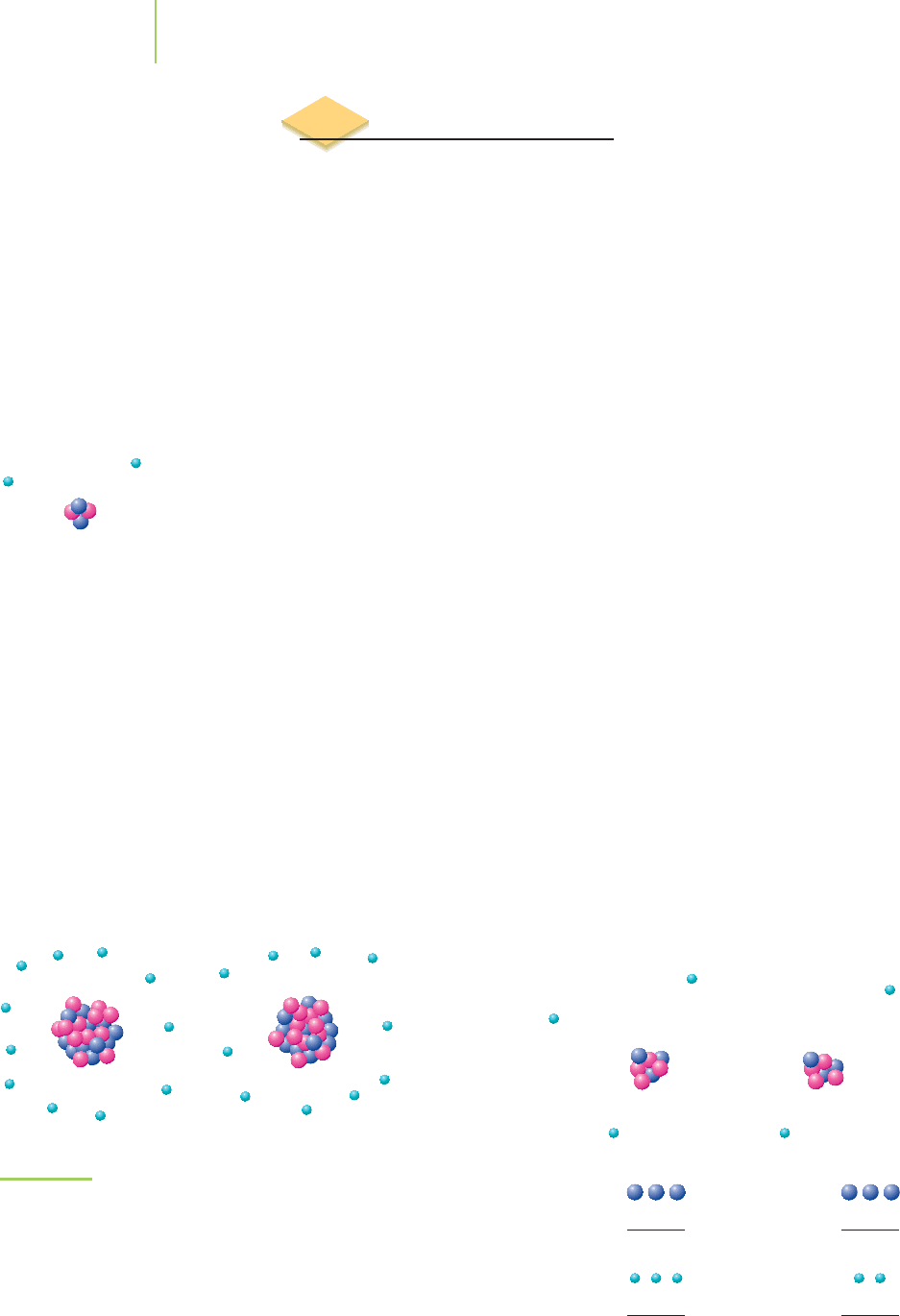
they occupy the majority of the atomic volume, as shown in Figure 2.18). The
electrons also carry a negative electrical charge, equal in magnitude to, but oppo-
site in sign from, that on the proton. To simplify counting charges, we often re-
port the charge on the proton as +1 and that on the electron as –1 (Table 2.3).
This model of the structure of the atom is sufficiently useful for us to under-
stand the main differences among atoms and the most fundamental principles of
chemical change. However, we must realize that it is just a model. Chemists use
various models, which represent important aspects of reality in useful ways, but
none of their models is likely to be entirely correct. Yet each model has a place in
the toolbox of the chemist. We can use this model of the atom whenever it is most
useful; and we can use more sophisticated models, about which we’ll learn later,
whenever those would be most useful. What model we use depends on what
questions we are trying to answer.
2.3 The Structure of the Atom 55
Proton
Neutron
Nucleus
Space
occupied
by electrons
FIGURE 2.18
The atom is mostly empty space. The
nucleus constitutes about 1/10,000 the
diameter of the atom.
Subatomic Particles
Particle Mass amu* Charge
Electron 9.1094 × 10
−31
kg 5.486 × 10
−4
−1
Proton 1.6726 × 10
−27
kg 1.0073 +1
Neutron 1.6749 × 10
−27
kg 1.0087 0
*The atomic mass unit (amu) is an arbitrary unit used to set the masses reported in the
periodic table. We will discuss it in the next section.
TABLE 2.3
2.4 Atoms and Isotopes
The periodic table of the elements is arguably the single most important practical
outcome in the history of chemistry. Because it is an essential tool for practicing
chemists as well as chemistry students, it is located on the inside front cover of
this book for your easy reference.The periodic table lists the atoms of just over
90 elements that are known to occur naturally, as well as a smaller number of
heavy elements that have been synthesized.
One of the great beauties of the periodic table is that all of the atoms of the el-
ements in the table are constructed of the same building blocks: protons and neu-
trons surrounded by a sea of electrons. Are the numbers and types of particles
important in constructing an atom? The short answer is a definite “yes.” The
numbers of protons, neutrons, and electrons define each individual atom. The
identity of the atom is determined by the quantity of protons in the nucleus,
which is known as the
atomic number (Z). For instance, all hydrogen atoms have
one proton (Z = 1), all helium atoms have two protons (Z = 2), and all iron
atoms have twenty-six protons (Z = 26). Just as your university identification
number uniquely identifies you at your school, the atomic number unequivocally
identifies a specific element in chemistry. Potassium has an atomic number of 19,
and any element with nineteen protons must be potassium.
The number of electrons in an electrically neutral atom is equal to the number
of protons and, therefore, to the atomic number. However, when combined with
atoms of other elements, an atom can gain or lose electrons to form a charged
entity we call an
ion. Although the number of electrons that an atom contains
changes, the atomic number—the number of protons it contains—remains the
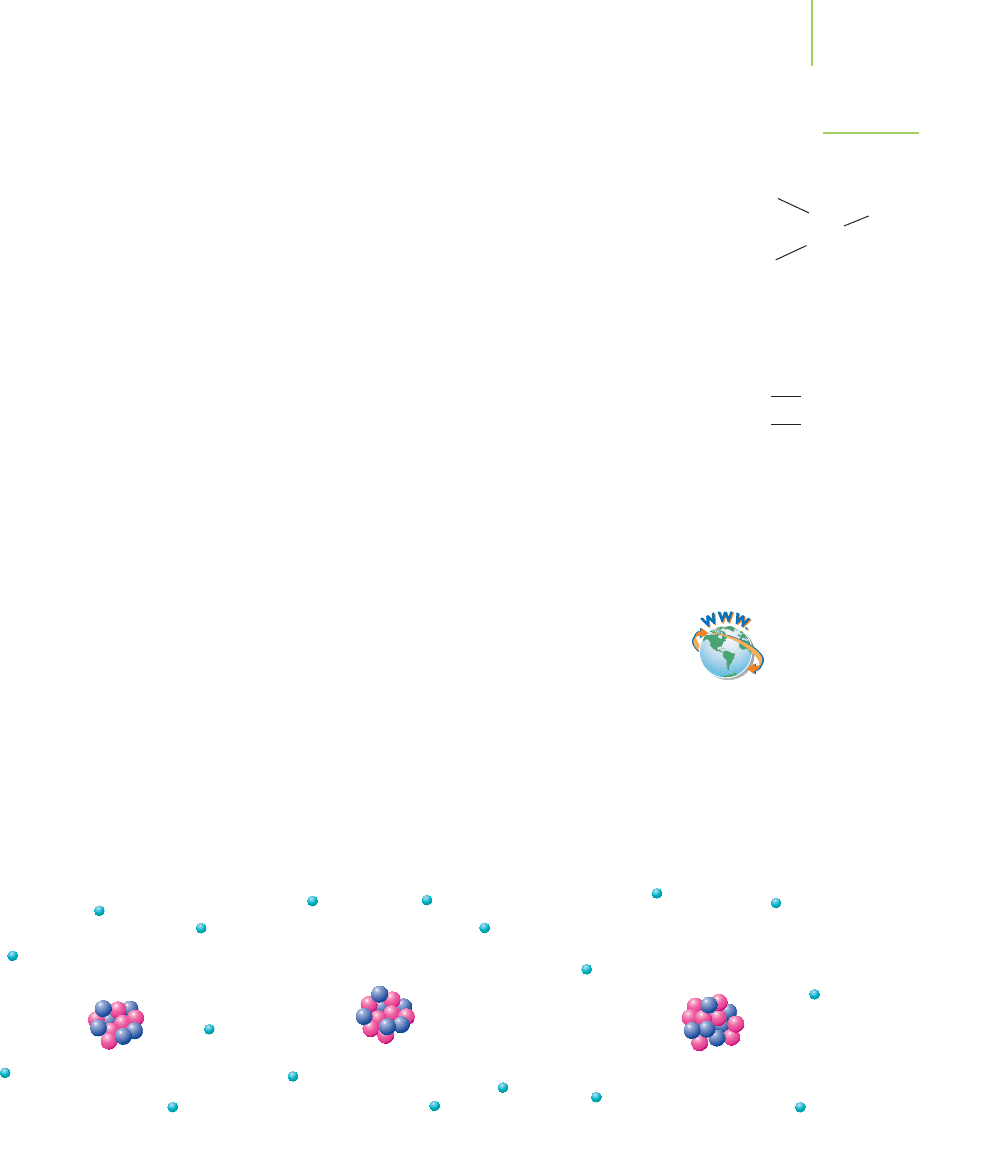
same. For example, the sodium ion shown in Figure 2.19 is still sodium, even
though its electron count is no longer the same as that of a neutral sodium atom.
The key question is
How many protons does the atom contain? The number of pro-
tons always identifies the element.
A positive charge indicates that there are more protons than electrons on the
ion. We typically refer to this type of ion as a
cation (pronounced CAT-ion). Con-
versely, a negative charge indicates that there are more electrons than protons.
This type of ion is known as an
anion (pronounced AN-ion). If we know the
charge and the atomic number of an ion, we can determine the number of elec-
trons that make up the ion.
56 Chapter 2 Atoms: A Quest for Understanding
2 electrons
2 protons
2 neutrons
He
Protons
Li
++
+3
+
Electrons
Net charge = +3 – 3 = 0
––
–3
–
Protons
Li
+
++
+3
+
Electrons
Net charge = +3 – 2 = +1
––
–2
Na Na
+
FIGURE 2.19
The sodium atom and the sodium ion. Both of these combina-
tions of electrons, protons, and neutrons are sodium, despite the
difference in the number of electrons.
We call the sum of the number of protons and neutrons the mass number (A)
of the atom. The number of neutrons (n) in a particular atom can be calculated
by subtracting the atomic number from the mass number:
A − Z = n
These basic components of the atoms can be written using the chemist’s
shorthand of
nuclide notation, as shown in Figure 2.20. Nuclide notation lists the
symbol for the element, accompanied by the atomic number and the mass num-
ber of the atom.
Rather than requiring that we write numbers for all of the particles that make
up an atom or ion, nuclide notation provides a quick way to convey the quantity of
subatomic particles to other scientists. An alternative way to quickly write the
number of particles is to write the name of the element, followed by the mass num-
ber of the element. For example, when we wrote “carbon-12” earlier in this chap-
ter, we were indicating an atom containing 6 protons, 6 neutrons, and 6 electrons.
Isotopes
Let’s reexamine the radiocarbon dating of the Dover Boat. From that discussion
we learned that there must be at least two different types of carbon atoms. The
discovery of a biosignature in a rock formation is related to this fact as well. What
is different about the carbon atoms?
All atoms of an element have the same number of protons, but the number of
neutrons that make up the nucleus of an atom can differ. Atoms of the same ele-
ment must have the same atomic number (the same number of protons); other-
wise, they would be different elements. However, if they differ in the number of
neutrons they contain, they are known as
isotopes. There are actually three com-
mon isotopes of carbon, the most abundant (98.93%) of which is carbon-12.
Carbon-13 is another nonradioactive isotope of the element carbon; it makes up
only a small amount (1.07%) of the carbon atoms in the universe. The carbon-14
isotope (2 × 10
−10
% of all carbon atoms) is radioactive and is the element used
to determine the age of ancient artifacts.
2.4 Atoms and Isotopes 57
3
7
A
A – Z = number of neutrons
7 – 3 = 4 neutrons
Z
Li
Z
E
A
Mass
number
Atomic
number
Element
symbol
FIGURE 2.20
Nuclide notation.
The number of neutrons in a particular
atom can be determined by subtracting
the number of protons (Z) from the
atomic mass number (A).
6
C
12
6
C
13
6
C
14
Isotopes of carbon.
Many examples of isotopes exist among the elements of the periodic table. In
fact, it is rare for an element not to have isotopes. Some of the isotopes of hydro-
gen, carbon, and oxygen are shown in Table 2.4 in nuclide notation. Also included
in the table is their relative natural abundance. For example, 99.985% of all hy-
drogen atoms are hydrogen-1, 0.015% are deuterium (the same single proton as
hydrogen, but with one neutron), and only 10
−18
% of them are tritium (one pro-
ton and two neutrons).
Many nuclei that have either too many or too few neutrons to be energetically
stable tend to give off energy and particles to become stable. This is the reason for
the radioactivity of carbon-14.
Video Lesson: Modern Atomic
Structure
Tutorial: Isotopes
