Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

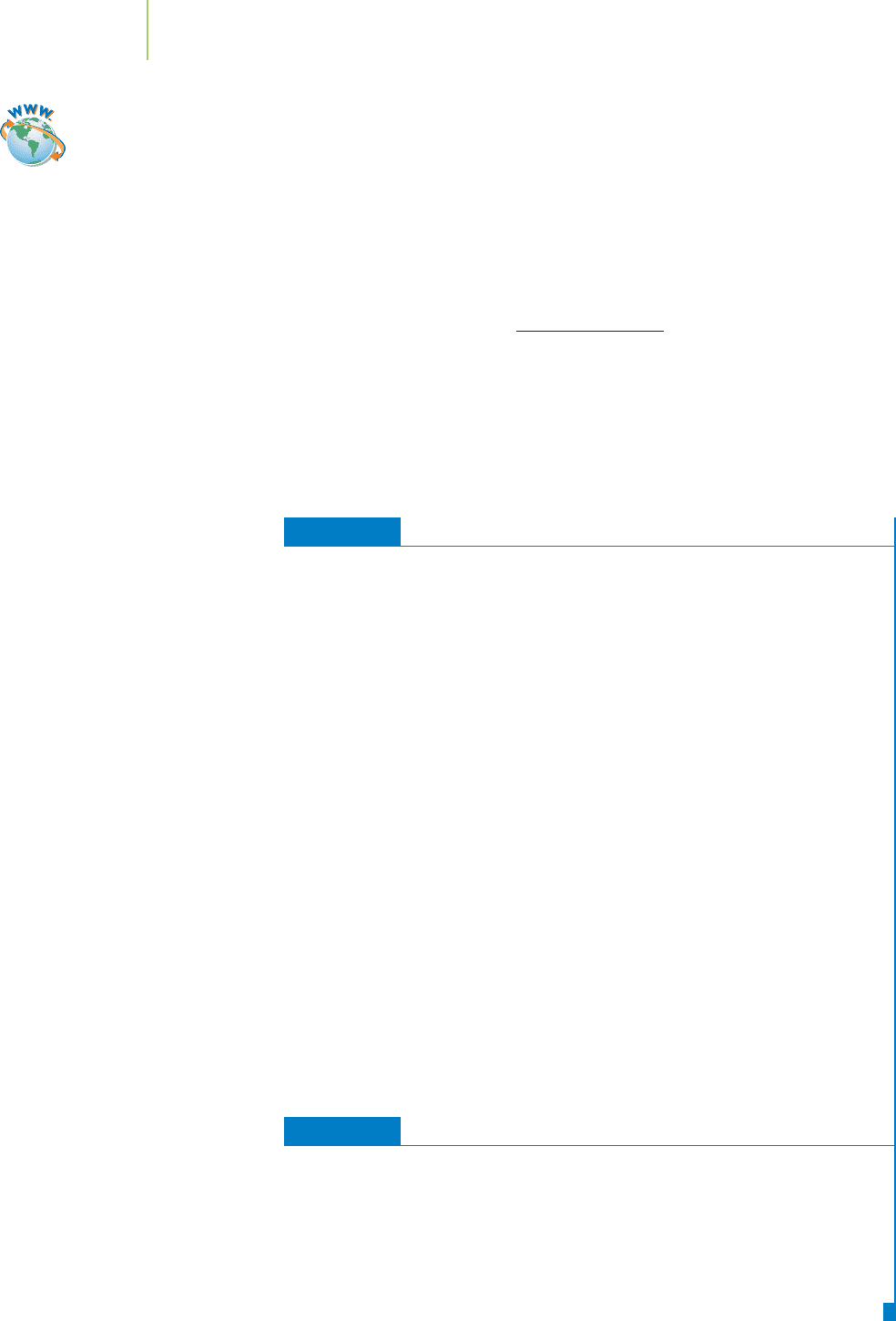
This is also the average formula mass (or formula weight) of adrenaline. Formula
mass is a more general term than molecular mass, because it also covers com-
pounds that do not exist in the form of individual molecules, such as ionic
compounds. For example, the ionic compound sodium chloride (NaCl) has an
average formula mass of 58.44 amu, found by adding the atomic mass of sodium,
22.99 amu, to the atomic mass of chlorine, 35.45 amu.
The formula mass, or molecular mass, of adrenaline is the average mass of
one molecule of adrenaline. Each molecule of the substance contains exactly the
same number of each type of atom, but different individual molecules can have
different masses, depending on which isotopes of carbon, oxygen, or hydrogen
they contain.
EXERCISE 3.1 Calculating Formula Masses
1. The formula for aspirin is C
9
H
8
O
4
. What is the formula mass of aspirin?
2. Many of us use the stimulant properties of the chemical caffeine in coffee
to remain alert, but even just the smell of coffee can seem to perk us up
in anticipation. One of the molecules that produce the aroma of coffee
is C
5
H
6
OS (2-furylmethanethiol). Calculate the formula mass of
2-furylmethanethiol.
Solution
1. Aspirin = C
9
H
8
O
4
9 atoms of carbon @ 12.01 amu = 108.09
8 atoms of hydrogen @ 1.008 amu = 8.064
4 atoms of oxygen @ 16.00 amu = 64.00
––––––––––
Formula mass = 180.154 = 180.2 amu
2. 2-Furylmethanethiol =C
5
H
6
OS
5 atoms of carbon @ 12.01 amu = 60.05
6 atoms of hydrogen @ 1.008 amu = 6.048
1 atom of oxygen @ 16.00 amu = 16.00
1 atom of sulfur @ 32.07 amu = 32.07
––––––––––
Formula mass = 114.168 = 114.17 amu
PRACTICE 3.1
Calculate the formula masses of these compounds:
a. Codeine, a painkiller with the formula C
18
H
21
NO
3
b. Magnesium sulfate, found in many medical ointments, with the formula
MgSO
4
c. Trinitrotoluene (TNT), an explosive with the formula C
7
H
5
N
3
O
6
See Problems 1–4.
NaCl
Na 22.99 amu
Cl 35.45 amu
Total 58.44 amu
88 Chapter 3 Introducing Quantitative Chemistry
Tutorial: Formula Masses
3.2 Counting by Weighing
When we do chemistry, we generally begin by measuring our chemicals using
masses. However, we need to convert these masses into some measure of the
numbers of atoms, molecules, or ions in order to relate the masses to what is ac-
tually happening in the chemical nanoworld.
Why can’t we take a sample of adren-
aline, which comes as a white powder, and use a pair of tweezers to separate out the
number of individual molecules we need?
Adrenaline molecules, like all molecules,
are stunningly small—on the order of 1 nm across. This means that you would
need to line up 100 million adrenaline molecules with your tweezers to get a sam-
ple as long as your index finger (about 10 cm)! This is why we need to establish
the link between weighing things and counting them. Weighing a sample of adren-
aline gives us insight into how many molecules of adrenaline we have.
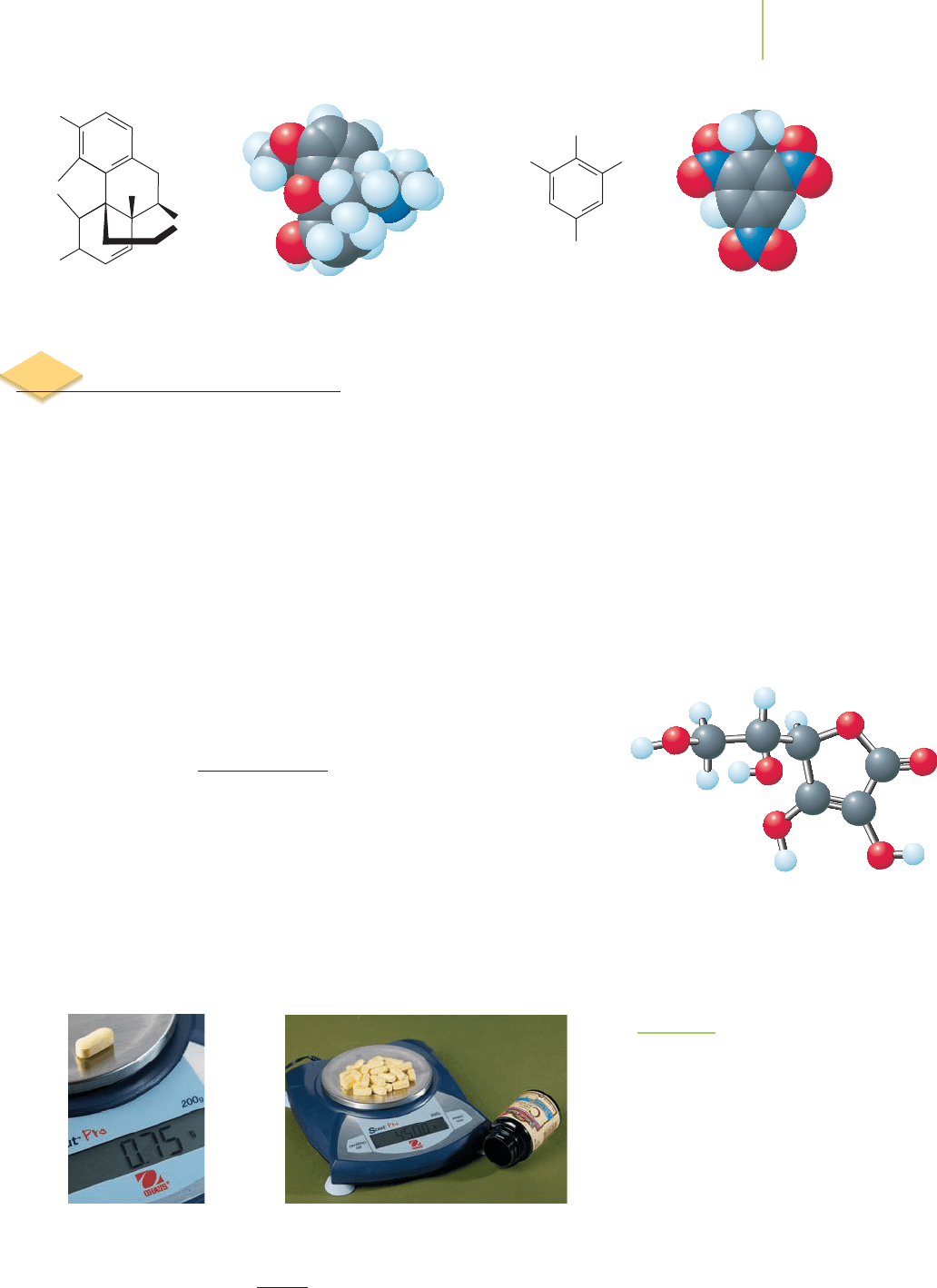
We can see how this is done on a more familiar size scale by using one tablet
of vitamin C that weighs 0.75 g. If all the tablets in a jar are placed onto a balance,
and if they weigh a total of 45.0 g, how many tablets are in the jar? As shown in
Figure 3.2, there are a total of 60 tablets in the jar.
45 g
×
1 vitamin C tablet
0.75 g
= 60 vitamin C tablets
We can do exactly the same thing with the atoms, molecules, and ionic com-
pounds, the only difference being that the numbers are most often extraordinarily
large or small.
Suppose you had just taken a pain reliever that contained aspirin, also known
as acetylsalicylic acid (see Figure 3.3). The chemical formula for aspirin is
C
9
H
8
O
4
. If the tablet you used contained 500 mg (0.500 g) of aspirin, could you
calculate how many molecules of pain reliever you had just ingested? You would
3.2 Counting by Weighing 89
O
2
NNO
2
CH
3
NO
2
H
3
CO
NCH
3
HO
Codeine TNT
H
O
Vitamin C (ascorbic acid)
One vitamin C tablet
weighs 0.75 g.
All the tablets in the
jar weigh 45 g.
45 g × = 60 tablets
1 tablet
0.75 g
FIGURE 3.2
Counting by weighing—the basic logic.
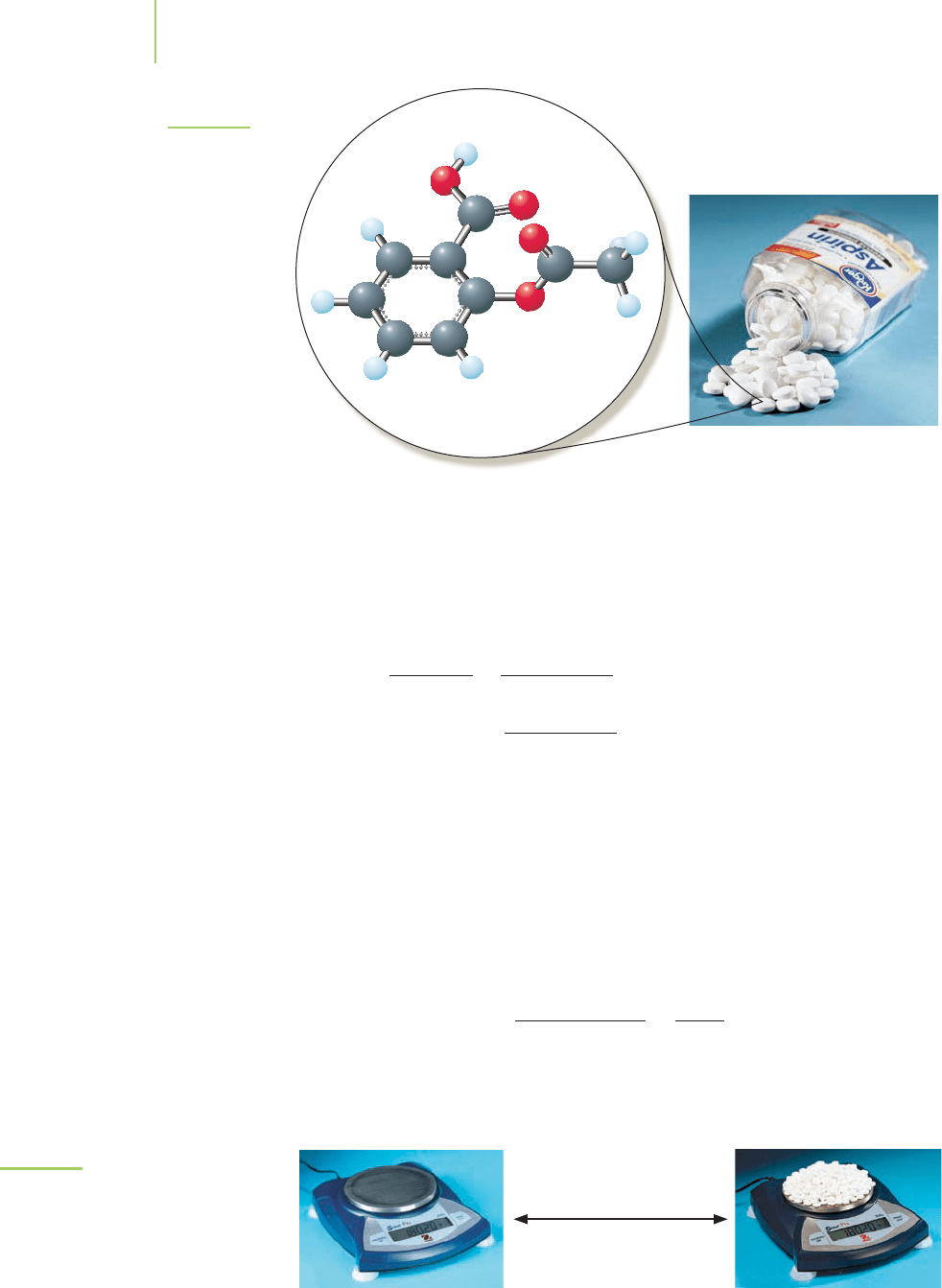
Aspirin (acetylsalicylic acid)
C
9
H
8
O
4
FIGURE 3.3
need to recall from Chapter 2 that 1 amu =1.6605 × 10
−24
g (1.6605 × 10
−27
kg).
Here is the calculation:
The average molecular mass of aspirin, calculated in Exercise 3.1, is 180.2 amu.
180.2amu
1 molecule
×
1.66 ×10
−24
g
1amu
= 2.99 × 10
−22
g per molecule
0.500 g ×
1 molecule
2.99 ×10
−22
g
= 1.67 × 10
21
molecules
This quantity is approximately 300 billion times more than the current human
population of the earth! The comparison serves as a reminder of just how tiny
molecules must be if that many are required to make up half a gram.
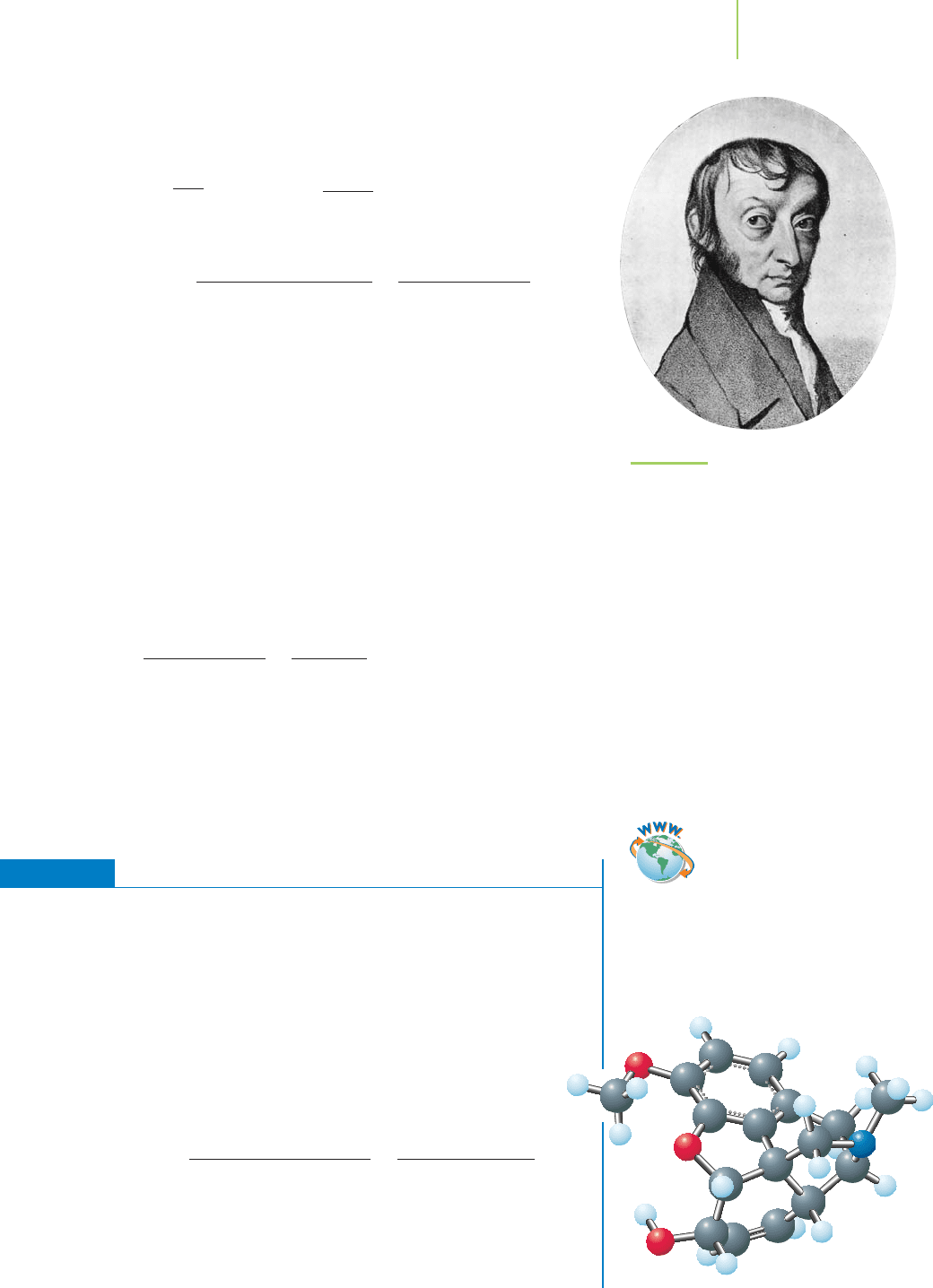
Knowing that the average molecular mass of aspirin is 180.2 amu, a chemist
at a pharmaceutical plant could weigh out a 180.2-g sample. How many mole-
cules would that sample contain? Let’s do the calculations in order to prove that
the number of molecules of aspirin (molecular mass = 180.2 amu) in a 180.2-g
sample must be the same as the number of amu’s in 1 g (see Figure 3.4). How many
amu’s are in 1 g? The mass of 1 amu = 1.6605 × 10
−24
g, so we can set up this
equation and solve it:
1amu
1.6605 ×10
−24
g
=
x amu
1g
x = 6.022 × 10
23
amu in 1 g of any substance.
What a huge number that is!
90 Chapter 3 Introducing Quantitative Chemistry
There are
6.022 × 10
23
amu in 1 g.
amu per molecule = grams per mole
6.022 × 10
23
aspirin
molecules (known as
1 mol of aspirin)
One aspirin molecule,
too small to see!
FIGURE 3.4
The link between masses in amu and
in grams.
How many molecules of aspirin are in 180.2 g of aspirin? We’ll use our di-
mensional analysis method to solve the problem. First we create a flowchart
that will help us arrive at our answer.
g aspirin
amu
grams
−−−−−→amu aspirin
molecules
amu
−−−−−→molecules aspirin
Then we perform the calculation.
180.2 g aspirin ×
1 amu aspirin
1.6605 ×10
−24
g aspirin
×
1 molecule aspirin
180.2 amu aspirin
= 6.022 × 10
23
molecules aspirin
This is the same huge number as the number of amu’s in 1 g of a substance!
By choosing to weigh out a mass in grams that has the same numerical value
as the formula mass in amu’s (180.2 grams and 180.2 amu, in the case of aspirin)
we are able to ensure that the sample contains just as many molecules as there
are amu’s in 1 g. This gives us a very useful automatic link between masses and
numbers of molecules (or atoms or ions) that works for any chemical.
This is a difficult construct, and it is entirely possible to read the preceding
discussion several times and still feel uncertain about the mass-to-amu relation-
ships. To help us reinforce the idea, let’s do the same calculation with an element,
rather than a compound. The element calcium is crucial to the manufacture
of healthy bones and teeth. The average atomic mass of the element calcium
is 40.08 amu, so we need to check how many calcium atoms are in 40.08 g of
calcium:
40.08 g ×
1amu
1.6605 ×10
−24
g
×
1atom
40.08 amu
= 6.022 × 10
23
atoms
That number 6.022 × 10
23
appears again! This is a very important and useful
number for us, because if we weigh out a mass in grams that is the same as an
element’s atomic mass or a molecule’s formula mass in amu, we will always have
6.022 × 10
23
atoms or formula units. The number 6.022 × 10
23
is known as
Avogadro’s number (N
A
) (or the Avogadro constant), after the Italian physicist
Amadeo Avogadro (1776−1856; Figure 3.5) and is abbreviated as N
A
.
EXERCISE 3.2 How Many Molecules?
Codeine, with the formula C
18
H
21
NO
3
, is often used as an analgesic (painkiller) for
intense pain. How many molecules would be in a 0.10-g sample of codeine?
First Thoughts
Our first thoughts lead us to determining the average molecular mass of codeine.
Once this mass is known, we should be able to determine the number of molecules
in that mass, using the same strategy we employed to get the number of atoms of
aspirin and the number of atoms of copper.
Solution
The molecular mass of codeine, C
18
H
21
NO
3
, is 299.4 amu.
0.10 g codeine ×
1 amu codeine
1.6605 ×10
−24
g codeine
×
1 molecule codeine
299.4 amu codeine
= 2.0 × 10
20
molecules codeine
Further Insight
How many molecules are there in 299.37 g of codeine? Again, this would give
Avogadro’s number as the answer. This number must be rather important or we
3.2 Counting by Weighing 91
FIGURE 3.5
Lorenzo Romano Amadeo Carlo Avo-
gadro, conte di Quaregna e di Cerreto
(1776–1856), was born into a family of
well-established lawyers in Italy. He, too,
prepared for a legal career, obtaining his
law degree in 1792 when he was only
16 years old. However, in 1800 he began
studying mathematics and physics pri-
vately, completing his first research pro-
ject on electricity with his brother Felice
in 1803. He was hired in 1809 at the Col-
lege of Vercelli, where he began his most
influential work in chemistry. He eventu-
ally obtained an appointment as a pro-
fessor of mathematical physics at the
University of Turin, where he worked
until he retired in 1850.
Codeine
Video Lesson: The Mole and
Avogadro’s Number

92 Chapter 3 Introducing Quantitative Chemistry
wouldn’t keep running into it! In fact, in chemistry this number is vital to many of
the calculations you will accomplish; accordingly, we give it a special name when we
do calculations (see the next section).
PRACTICE 3.2
How many atoms are there in 100.0 g of gold-197?
See Problems 9–16.
The Quantity We Call the Mole
An amount of any substance that contains 6.022 × 10
23
“elementary entities”
such as molecules, atoms, or ions is given its own name in chemistry. We call it
one
mole (often abbreviated mol) of the substance. Perhaps it seems odd to give a
special name to what is, in effect, just a number, but this is actually a very familiar
practice:
1 mole corresponds to 6.022 × 10
23
just as
1 dozen corresponds to 12
The mole is the basic counting unit used by chemists to indicate how many atoms,
molecules, or ions they are dealing with. A chemist counts moles of particles just as
a baker counts dozens of cupcakes.
To work out the mass of one mole of a chemical, you just look up its formula
mass and convert the units of that number from amu into grams. The resulting
quantity, expressed in grams per mole (
g
mol
), is known as the
molar mass—the
mass of one mole of a substance. Remember these relationships (illustrated by the
example in Figure 3.4):
■
The mass of 1 mol of any chemical is its formula mass expressed in units of
grams. This is the molar mass of the substance. The molar mass of aspirin is
180.2g
mol
.
■
1 mol corresponds to 6.022 × 10
23
entities, such as atoms, molecules, or ions.
■
6.022 ×10
23
is known as Avogadro’s number. It is often abbreviated as N
A
.
Although you should learn to think of the mole as simply equal to 6.022 × 10
23
,
it is formally defined in a more complicated way by reference to the carbon-12
isotope. This formal definition states that a mole of a substance is that amount
of the substance that has the same number of atoms as exactly 12 g of the isotope
carbon-12.
Does this make sense in terms of what we have already said about the mole? We
can check that the definition fits with the “1 mol = 6.022 ×10
23
” rule by calcu-
lating the number of particles in exactly 12 g of the isotope carbon-12. The
atomic mass of carbon-12 is exactly 12 amu.
12.00 g ×
1amu
1.6605 ×10
−24
g
×
1atom
12 amu
= 6.022 ×10
23
atoms
3.3 Working with Moles
Molecules to Moles and Back Again
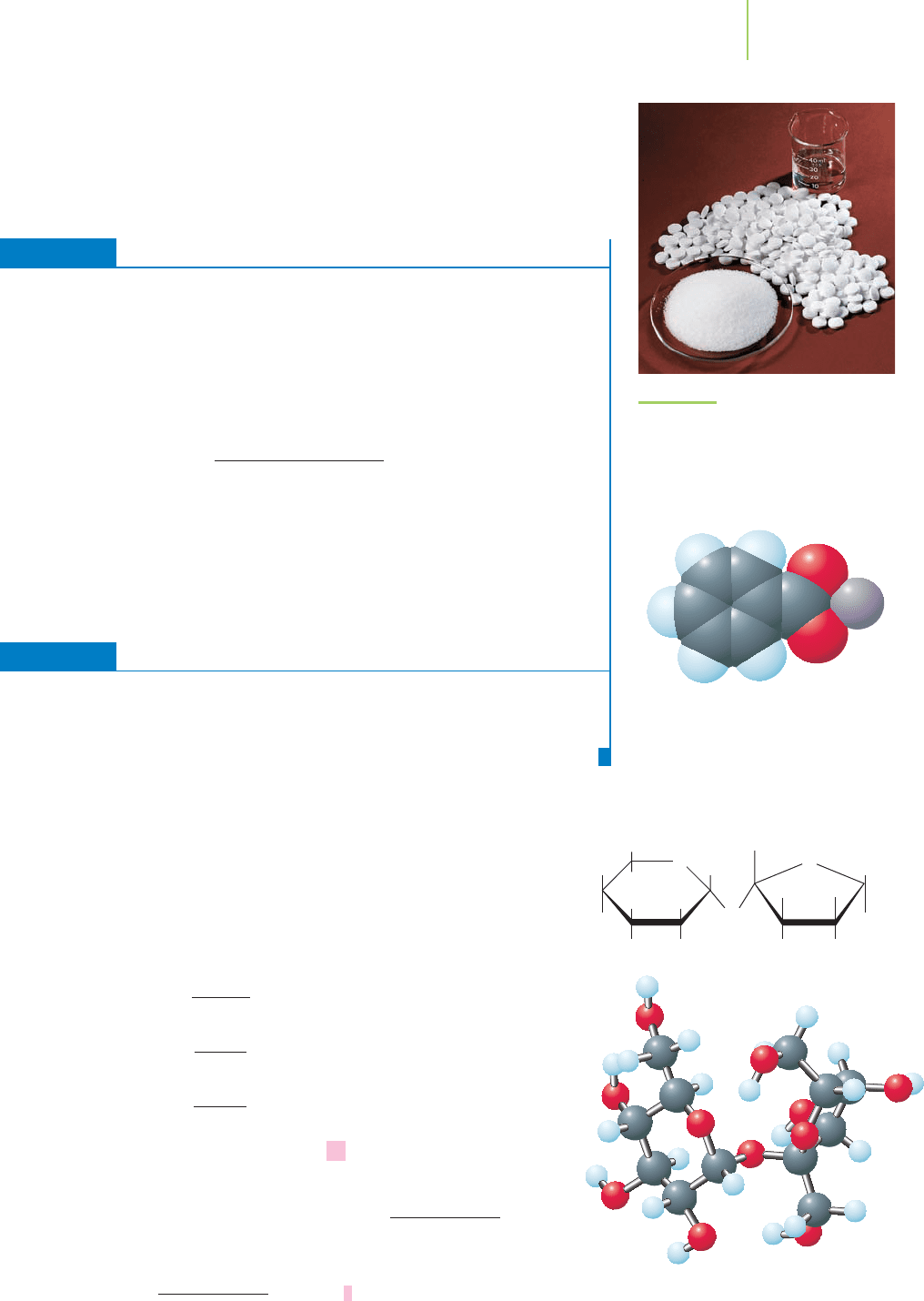
Figure 3.6 illustrates the quantities of 1 mol of water, 1 mol of sodium chloride
(“table salt”), and 1 mol of aspirin—three different chemicals of great signifi-
cance to life and medicine. Each has a different mass and occupies a different
The “dozen” is a counting unit. Its use in
counting groups is similar to a pair (2), a
ream (500), and a mole (6.022 × 10
23
).
Video Lesson: Introducing
Conversions of Masses, Moles,
and Number of Particles
volume, but each contains the same number of formula units of the compounds
concerned. The containers in the figure contain 6.022 × 10
23
molecules of water,
6.022 × 10
23
molecules of aspirin and 6.022 × 10
23
formula units of NaCl. (Note:
Because each formula unit of NaCl includes one Na
+
ion and one Cl
−
ion, our
sample contains 6.022 × 10
23
Na
+
ions and 6.022 × 10
23
Cl
−
ions!)
EXERCISE 3.3 Molecules and Moles
Really large numbers such as 4.33 × 10
24
can be quite confusing. Let’s show how
using moles can simplify our work.
a. How many moles of water are there in 4.33 × 10
24
molecules of water?
b. How many moles of codeine (C
18
H
21
NO
3
) are there in 4.33 × 10
24
molecules
of codeine?
Solution
a.
4.33 ×10
24
molecules ×
1 mol
6.022 ×10
23
molecules
= 7.19 mol
This number is much easier to work with.
b. Note that the formula of the compound doesn’t change the number of
molecules that exist in 1 mol of a substance. Therefore, the answer is the same.
4.33 × 10
24
molecules of any substance is equal to 7.19 mol, whether we are
considering aspirin, adrenaline, copper, or codeine.
PRACTICE 3.3
How many moles are there in 2.88 × 10
20
formula units of sodium benzoate
(C
6
H
5
COONa), a food preservative? How many formula units are there in 1.5 mol
of sodium benzoate?
See Problems 19b, 19d, 20b, 20d, 23b, and 24b.
Grams to Moles and Back Again
Many of us sweeten our drinks by adding some “table sugar,” the com-
pound sucrose, with the formula C
12
H
22
O
11
. If you added 10.0 g of sugar
to a glass of tea, how many moles did you add? To answer this question,
we must first determine the formula mass of sucrose, which tells us the
mass of 1 mol and so gives us the mass-to-moles conversion factor that
we need. Here is the calculation:
12 mol C ×
12.01 g
1 mol C
= 144.12 g
22 mol H ×
1.008 g
1 mol
= 22.176 g
11 mol O ×
16.00 g
1 mol
= 176.0 g
Molar mass of sucrose = 342.296 = 342.3 g
This means that the mass of 1 mol of sucrose is 342.3 g of sucrose. Math-
ematically, our moles-to-mass conversion factor is
1 mol sucrose
342.3 g sucrose
.Now,
our sample of 10.0 g can be converted to moles:
10.0 g sucrose
×
1 mol sucrose
342.3 g sucrose
= 0.02921 = 0.0292 mol sucrose
3.3 Working with Moles 93
FIGURE 3.6
One mole of water (top), one mole of
sodium chloride (“salt”) (bottom),
and one mole of aspirin (center). 1 mol =
Avogadro’s number = 6.022 x 10
23
.
Sodium benzoate
OH
H
H
H
CH
2
OH CH
2
OH
CH
2
OH
OH
H
OH
HH
O
O
O
HOH
OH H
Sucrose
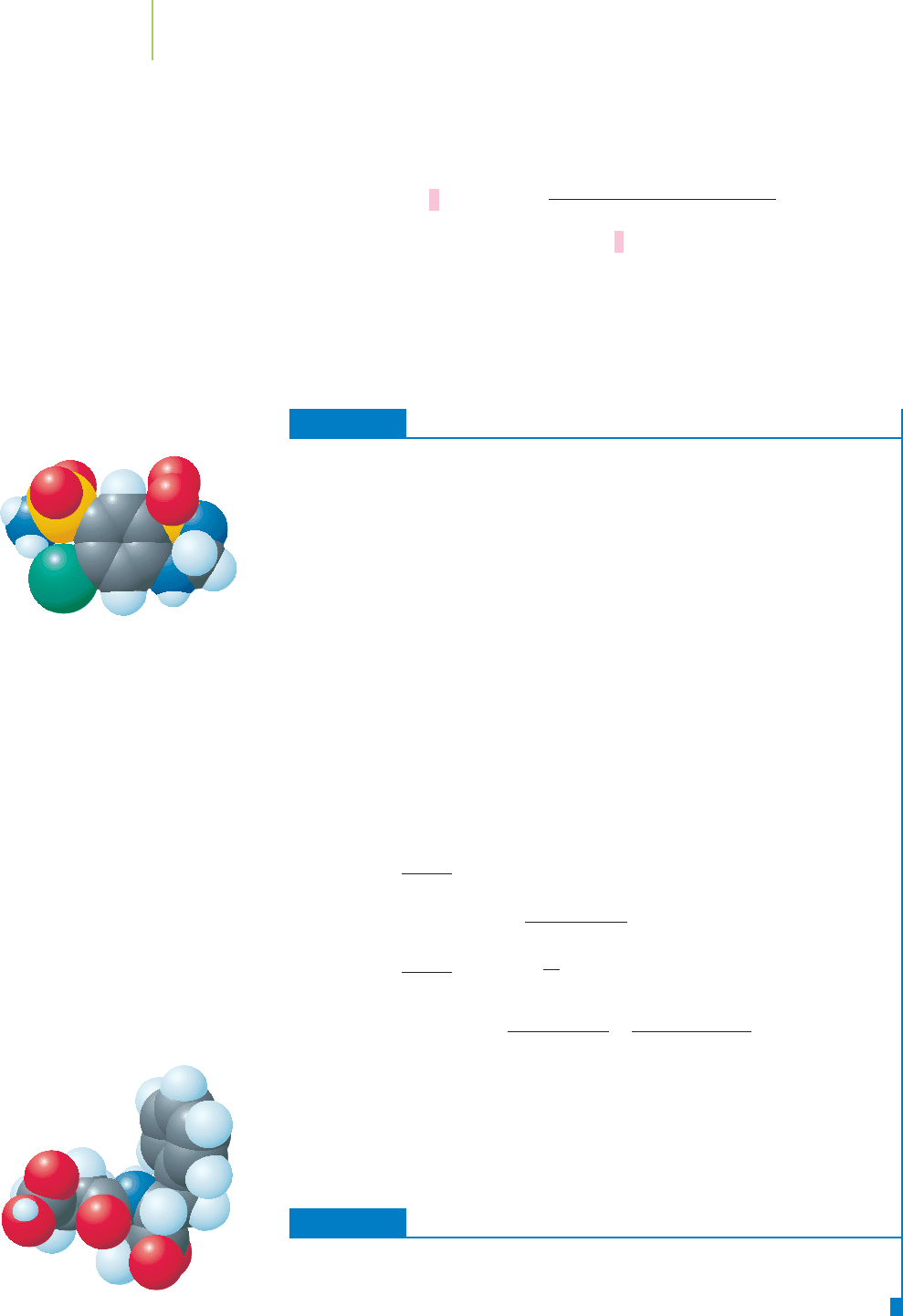
We can also determine how many molecules of sucrose have been added to our
glass of tea. We do this using Avogadro’s number, the number we call the mole,
6.022 × 10
23
.
0.02921 mol sucrose
×
6.022 ×10
23
molecules sucrose
1 mol sucrose
= 1.759 × 10
22
molecules sucrose
= 1.76 × 10
22
molecules sucrose
We would have stirred this number of sugar molecules into our tea:
17,600,000,000,000,000,000,000
EXERCISE 3.4 Calculating Masses Corresponding to Moles
Hydrochlorothiazide (HCT) is a medicine used to lower blood pressure and
deal with other illnesses related to fluid retention. Its molecular formula is
C
7
H
8
ClN
3
O
4
S
2
.
a. In a small-scale production process, a company needs 63.4 mol of the
medicine. How many grams of hydrochlorothiazide is this?
b. A common individual dosage is 3.37 × 10
−5
mol. How many milligrams are
in this individual dose?
First Thoughts
We have two very different amounts to calculate. The first represents a small-scale
industrial process, the second an individual dose. But the strategy is still the same.
We can use the molar mass of the compound to go from moles to mass. The molar
mass of the compound in grams per mole will be the same as the average molecular
mass in amu.
Solution
The molar mass of hydrochlorothiazide is 297.72 g per mole. Again, we first write a
flowchart to assist us in our calculations:
a.
mol HCT
g HCT
mol HCT
−−−−−→g HCT
63.4 mol HCT
×
297.72 g HCT
1 mol HCT
= 1.89 ×10
4
g HCT
b.
mol HCT
g HCT
mol HCT
−−−−−→g HCT
mg
g
−−−−−→mg HCT
3.37 × 10
−5
mol HCT ×
297.72 g HCT
1 mol HCT
×
1 mg HCT
1 ×10
−3
g HCT
= 10.0 mg HCT
Further Insights
Do the answers make sense? We expect the industrial synthesis to result in much
more of the product than is in the individual dose, so this answer makes sense. You
may have noted from other medicines you have taken that a dose of perhaps 10 to
500 mg is standard. Therefore, our answers are reasonable.
PRACTICE 3.4
Calculate the mass in grams of 26 mol and of 0.0025 mol of each of these com-
pounds: table salt (NaCl),water (H
2
O), and the sweetener aspartame (C
14
H
18
N
2
O
5
).
See Problems 25 and 26.
94 Chapter 3 Introducing Quantitative Chemistry
HCT
Aspartame
EXERCISE 3.5 Calculating Moles Corresponding to Masses
Methylphenidate hydrochloride, C
14
H
19
NO
2
·HCl, is a medical drug used to stimu-
late the central nervous system. It is known as Ritalin, and its use in the treatment of
attention deficit hyperactivity disorder (ADHD) has been a subject of controversy
for years. A company sent a 2.00 × 10
4
-g shipment to be processed into individual
20.0-mg tablets. How many moles of Ritalin are in the shipment and in each tablet?
How many molecules of Ritalin are in each tablet? Note that the dot in the formula
is used to indicate small molecules that are chemically part of the overall formula.
Therefore, the formula methylphenidate hydrochloride could be written as
C
14
H
20
NO
2
Cl.
Solution
For the entire shipment, we calculate the chemical amount in moles as follows, after
determining that the molar mass of Ritalin is 269.76 g/mol.
2.00 × 10
4
g Ritalin ×
1 mol Ritalin
269.8 g Ritalin
= 74.1 mol Ritalin
We calculate the number of moles in each 20.0-mg tablet using the same general
strategy, being careful to add the extra conversion factor that changes milligrams to
grams before finding moles.
mg Ritalin
tablet
g
mg
−−−−−→
g Ritalin
tablet
mole
g Ritalin
−−−−−→
mol Ritalin
tablet
20.0 mg Ritalin
tablet
×
1 g Ritalin
1000 mg Ritalin
×
1 mol Ritalin
269.8 g Ritalin
= 7.413 × 10
−5
= 7.41 × 10
−5
mol Ritalin per tablet
We can convert from moles to molecules of Ritalin in a 20.0-mg tablet:
mol Ritalin
tablet
molecules
mole
−−−−−→
molecules Ritalin
tablet
7.413 ×10
−5
mol Ritalin
tablet
×
6.022 ×10
23
molecules Ritalin
1 mol Ritalin
=
4.46 ×10
19
molecules Ritalin
tablet
The individual dose has considerably fewer moles than the industrial shipment, so
the answer makes sense. The number of molecules in the individual dose is exceed-
ingly high, which is also reasonable because molecules are so small.
PRACTICE 3.5
Calculate the number of moles in 5.3 g and 0.0022 g these compounds: lactic acid,
C
3
H
6
O
3
, and sulfuric acid, used in car batteries (H
2
SO
4
).
See Problems 19a, 19c, 20a, and 20c.
As researchers have investigated the causes of heart-related health problems,
one particular molecule, cholesterol, has been found to be associated with certain
types of circulatory problems. Giving a blood sample for the analysis of your cho-
lesterol level is a routine part of a medical checkup. The situation is not as simple
as cholesterol being “bad.” Cholesterol is essential for life—without it, you would
die. Cholesterol plays a crucial role in maintaining the thin membranes that
enclose every cell of your body. It also aids in the production of hormones,
3.3 Working with Moles 95
HH
HH
H
O
H
C
C
O
H
CH
3
C
C
C
C C
C
H
H
H
H
H
H
HH
C
H
N
C
C C
C
Lactic acid Sulfuric acid
Ritalin
Application
C
HEMICAL ENCOUNTERS:
Cholesterol in the Blood
96 Chapter 3 Introducing Quantitative Chemistry
including estrogen and testosterone. So again, we find that the quantity of a
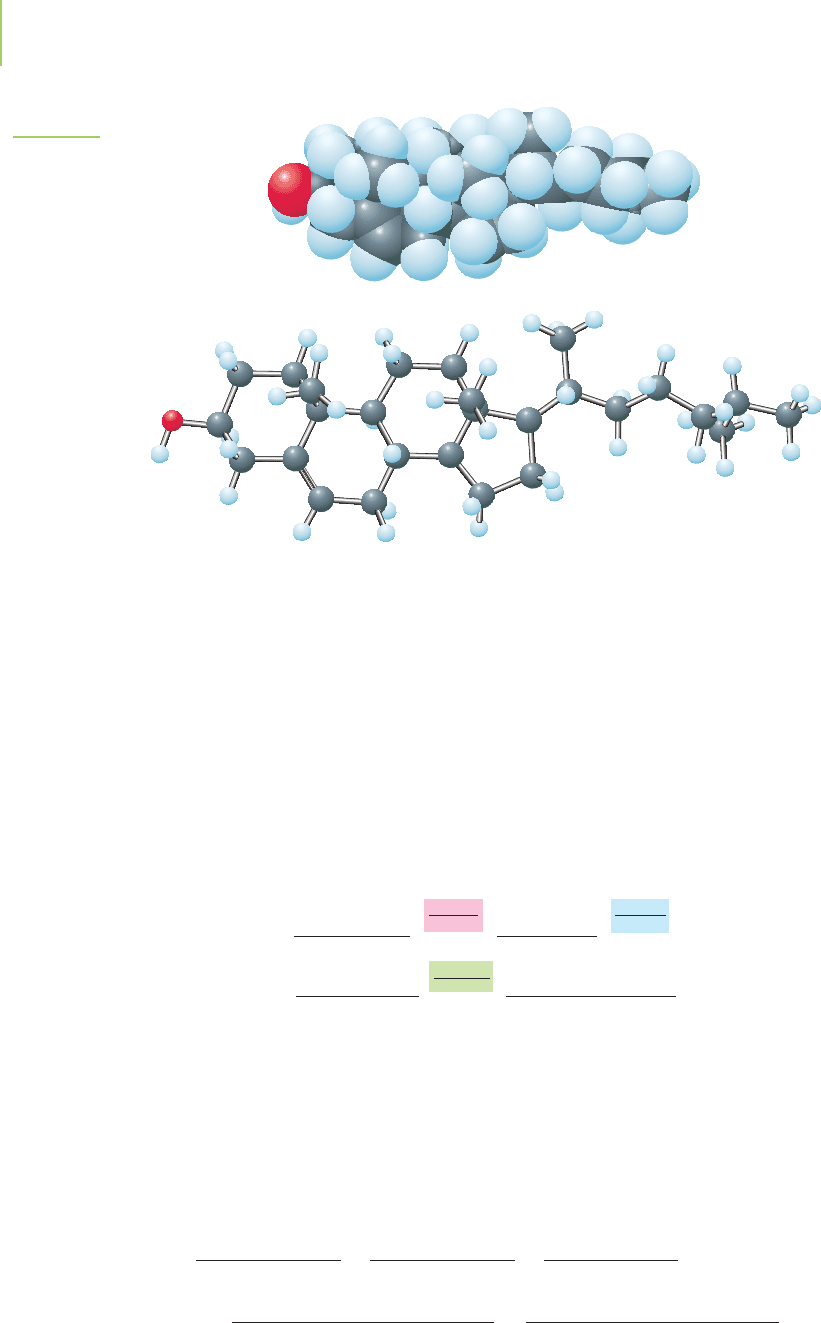
chemical is crucial. The chemical structure of cholesterol is shown in Figure 3.7.
Its formula is C
27
H
46
O. If a blood analysis showed a cholesterol level of 195 mg
per deciliter of blood, which is equal to 1950 mg per liter, how many molecules
of cholesterol would be present per liter?
In this case you can see that the starting measurement is in milligrams, but
the question asks about the number of molecules. Remember that the “nano”
(molecules, in this case) and “macro” (moles, here) worlds are connected via
Avogadro’s number. The strategy is to express the mass as a quantity of moles of
cholesterol and then convert moles to molecules. Our dimensional analysis flow-
chart for the task looks like this:
mg cholesterol
L
g
1000 mg
−−−−−→
g cholesterol
L
mol chol
g chol
−−−−−→
mol cholesterol
L
molecules
mol
−−−−−→
molecules cholesterol
L
When we have finished our calculation, our answer should make sense. What sort
of answer should we expect?
We know that there are a great many molecules in
even the smallest sample of cholesterol-containing blood.We would therefore ex-
pect a very large number of molecules. Answers such as 10
–24
molecule/L or even
10
2
molecules/L would not make sense.
Because cholesterol has a known molecular formula, C
27
H
46
O, we can deter-
mine its molar mass as 386.6 g per mole (check that you agree). We will follow
our flowcharted strategy to get the answer.
1950 mg C
27
H
46
O
L
×
1gC
27
H
46
O
1000 mg C
27
H
46
O
×
1 mol C
27
H
46
O
386.6gC
27
H
46
O
×
6.022 ×10
23
molecules C
27
H
46
O
1 mol C
27
H
46
O
=
3.04 ×10
21
molecules C
27
H
46
O
L
Does the answer make sense? The quantity 195 mg is much less than a mole of
cholesterol (386.6 g per mole), so we would have expected an answer far less than
Avogadro’s number, but because we are counting our sample in molecules, the
Cholesterol
C
27
H
46
O
FIGURE 3.7

PRACTICE 3.6
How many molecules are there in 1.50 g of water? Would you need a bathtub, a
swimming pool, or a cup to hold 5.33 ×10
29
molecules of water?
See Problems 23a and 24a.
3.4 Percentages by Mass
Just like humans, plants undergo chemical changes that are a part of life. They
have genetic material that directs the synthesis of proteins, some of which are
part of cell structure and some of which make possible the chemical reactions
that sustain life. Nitrogen is a vital element in the genetic material and the pro-
teins in plants, so in order to grow, plants need plenty of nitrogen. For farms
throughout the world, fertilizer supplies that nitrogen in the form of manure or
industrially produced products.
Some plants need more nitrogen than others. Environmental
factors, such as temperature, rainfall, and the type of soil, also af-
fect the ability of plants to use the nitrogen in fertilizer. This
means that a farmer must apply the correct amount of nitrogen
for each particular situation. Among the most important factors
in deciding what fertilizer to use is the percent by mass,or
mass
percent
, of nitrogen in the product (see Figure 3.8). Mass percent
values are determined by dividing the mass of the component of
interest by the total mass of the entire sample. The result, multi-
plied by 100%, is the mass percent of the component in the sam-
ple. The component can be a compound (such as ammonium
nitrate, NH
4
NO
3
, which is found in many liquid and solid fertil-
izers) or it can be an ion, atom, or element.
Mass percent =
total mass component
total mass whole substance
× 100%
Which of the following two compounds would supply plants
with the more concentrated source of nitrogen: ammonium
nitrate, NH
4
NO
3
, or ammonium sulfate, (NH
4
)
2
SO
4
?
NH
4
NO
3
or (NH
4
)
2
SO
4
We can answer the question by calculating the mass percent
of nitrogen present in each compound. We need to express the
mass of nitrogen in each one as a percentage of the total mass.
number should still be quite large. Our value meets these conditions, so our
answer makes sense.
EXERCISE 3.6 Sugar Is Sweet
Sucrose (C
12
H
22
O
11
) is a wonderful molecule used to sweeten our cup of coffee. If a
packet of sucrose contains 1.50 g of sucrose, how many molecules is this?
Solution
3.4 Percentages by Mass 97
Application
C
HEMICAL ENCOUNTERS:
Nitrogen in Fertilizers
FIGURE 3.8
The mass percent of nitrogen
is often reported on the
ingredients label of a bag
of fertilizer.
1.50 g sucrose ×
1 mol sucrose
342.3 g sucrose
×
6.022 ×10
23
molecules of sucrose
1 mol sucrose
= 2.639 × 10
21
= 2.64 × 10
21
molecules of sucrose
