Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

118 Chapter 3 Introducing Quantitative Chemistry
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
3.1 Formula Masses
Skill Review
1. Determine the mass, in amu, for each:
a. Carbon monoxide, CO
b. Silicon dioxide (the principal component in sand), SiO
2
c. Ammonia, NH
3
d. Sodium thiosulfate (photographer’s “hypo” solution),
Na
2
S
2
O
3
e. Tristearin (a type of animal fat), C
57
H
110
O
6
2. Determine the mass, in amu, for each:
a. Water, H
2
O
b. Sodium hydroxide, NaOH
c. Fructose (“fruit sugar”)
d. Potassium dichromate,
K
2
Cr
2
O
7
e. Ammonium phosphate
(a fertilizer ingredient),
(NH
4
)
3
PO
4
Focus Your Learning
7. The price of gold fluctuates daily. However, if you paid
$10,000 for 1 kg of gold, how much would you be paying per
one atom of gold?
8. Sodium bicarbonate (NaHCO
3
) is a useful ingredient em-
ployed in baking.
a. What is the formula mass of NaHCO
3
?
b. If you paid $1.50 for 250 g of NaHCO
3
, how much would
you be paying for each formula unit?
3.2 Counting by Weighing
Skill Review
9. Convert these to grams:
a. 6.02 × 10
22
atoms of silver
b. One trillion atoms of gold
c. 2 dozen water molecules
d. One molecule of propane (C
3
H
8
)
10. Convert these to grams:
a. 3.54 × 10
21
atoms of gold
b. A gross (12 dozen) of atoms of mercury
c. 1.2 × 10
27
molecules of glucose (C
6
H
12
O
6
)
d. One molecule of carbon dioxide (CO
2
)
11. Indicate how many molecules there are in each of the
following:
a. 12.01 g of water
b. 68.3 g of sodium bicarbonate (NaHCO
3
)
c. 100 g of methane (CH
4
)
d. 2.3 mg of glucose (C
6
H
12
O
6
)
12. Indicate how many atoms there are in each of the following:
a. 100 g of silver
b. 100 g of gold
c. 12.01 g of carbon
d. 5.3 kg of water
13. a. One of the main waste products of animal metabolism is
urea. What is the mass, in amu, of one molecule of urea
(CH
4
ON
2
)?
b. What is the mass of 6.022 × 10
23
molecules of urea?
c. What would be the total mass of 6.022 ×10
22
molecules of
urea?
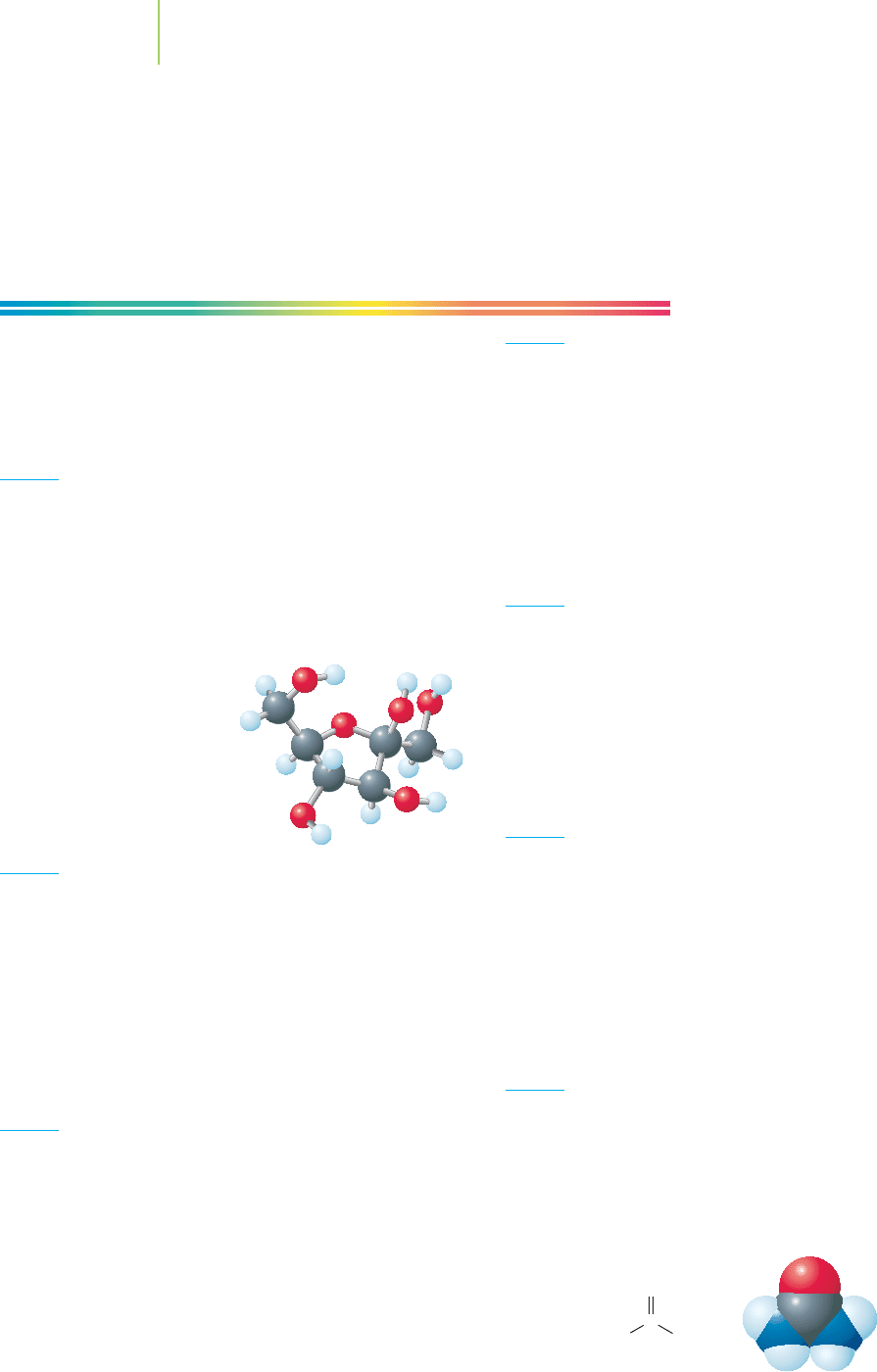
Fructose
3. Arrange these in order from lightest to heaviest on the basis
of formula masses.
C
6
H
6
H
2
OC
2
H
4
OH
CaCl
2
CO
4. Arrange these in order from heaviest to lightest on the basis
of formula masses.
NaBr KBr KMnO
4
LiOH Na
3
PO
4
Chemical Applications and Practices
5. Saccharin (C
7
H
5
NO
3
S) and aspartame (C
14
H
18
N
2
O
5
) have
both been used as sugar substitutes.
a. What is the mass, in amu, of one molecule of each sugar
substitute?
b. What is the mass ratio of aspartame to saccharin?
c. What mass, in grams, of saccharin has the same number
of molecules as 42.0 g of aspartame?
6. As you are reading this, you are breathing in some molecular
oxygen (O
2
).
a. Express the mass of an oxygen molecule in amu.
b. Convert that mass into grams per molecule of O
2
.
c. What is the mass of 6.022×10
23
molecules of O
2
?
reactants
The substances located on the left-hand side
of a chemical equation. (p. 103)
stoichiometric ratios The mole ratios relating how
compounds react and form products. (p. 108)
stoichiometry The study and use of quantitative
relationships in chemical processes. (p. 108)
theoretical yield The maximum amount of any
chemical, in grams, that could be produced in
a chemical reaction. This value can be calculated
from the equation for the reaction. (p. 115)
Urea
O
C
NH
2
H
2
N
Focus Your Learning 119
14. Ammonium nitrate is often used as a fertilizer. Express these
amounts of NH
4
NO
3
in grams.
a. one million formula units
b. ten mol
c. 0.500 kg
Chemical Applications and Practices
15. Acetaminophen, a nonaspirin pain reliever, has the formula
C
8
H
9
NO
2
. A dose of 0.500 g of acetaminophen would con-
tain how many molecules of the pain reliever?
16. a. Methane (CH
4
) is used in many laboratory burners. If
a student burned 10.0 g of
methane, how many mol-
ecules were consumed?
b. Would 10.0 g of butane
contain more or fewer
molecules?
3.3 Working with Moles
Skill Review
17. Which of these quantities of sodium chloride (NaCl) con-
tains the greatest mass?
0.100 mol 4.2 × 10
23
formula units 1.60 g
18. Which of these quantities of acetaminophen (C
8
H
9
NO
2
)
contains the greatest mass?
0.550 mol 9.1 × 10
23
molecules 80.4 g
19. Convert these to moles:
a. 65.0 g of CO
2
b. 1.5 × 10
22
atoms of neon
c. 25 g of propane (C
3
H
8
)
d. 4.5 × 10
24
molecules of ammonia (NH
3
)
20. Convert these to moles:
a. 123 g of H
2
O
b. 9.72 × 10
23
atoms of gold
c. 25.6 mg of methane (CH
4
)
d. 3.22 × 10
10
molecules of hemoglobin
(C
2954
H
4508
N
780
O
806
S
12
Fe
4
)
21. Which of these would contain the greatest number of atoms?
454 g of gold
56.0 g of O
2
245 g of graphite
22. Which of these would contain the greatest number of atoms?
100 g of tin
17.9 mg of glucose (C
6
H
12
O
6
)
2 g of water
23. Convert these to the correct number of formula units:
a. 15.0 g of CaCl
2
b. 15.0 mol of CaCl
2
c. 15.0 mL of a CaCl
2
solution that contains 0.42 mol per liter
24. Convert these to the correct number of formula units:
a. 1.0 g of K
2
CO
3
b. 15.0 mol of K
2
CO
3
c. 7.33 kg of K
2
CO
3
25. Express these quantities in grams:
a. 1500 mol of Ne
b. 12.5 mol of Na
c. 0.42 mol of N
2
26. Express these quantities in grams:
a. 0.250 mol of NaCl
b. 0.350 mol of (NH
4
)
3
PO
4
c. 1.5 × 10
21
molecules of CO
2
27. Radioactive samples of elements are used in both the treat-
ment and the detection of certain diseases. One such element
is element 43, technetium. Nuclear medicine applications of
this element include bone scans, thyroid monitoring, and
brain monitoring. If a pharmacist were preparing doses to be
given to patients that involved using 5.00 g of radioactive
technetium, how many atoms of technetium would actually
be present?
28. Two elements have been named in honor of female scientists,
curium (element 96) and meitnerium (element 109). Ele-
ment 109 is a relatively recent discovery. Often when synthe-
sizing newer elements, scientists are working with extremely
small samples, even only a few atoms. Suppose a certain
procedure produced 100 atoms of meitnerium. How many
moles is this? What would be the mass of the sample?
Chemical Applications and Practices
29. Iron is essential for the transport of oxygen in the human
body and for energy production through several biochemical
cycles.
a. What is the mass, in grams, of one atom of iron?
b. The recommended dietary allowance (RDA) for iron is
approximately 15 mg. How many atoms of iron is this?
c. Finally, express the RDA in moles of iron.
30. Howmanycaffeinemoleculesarefoundineachof thesedrinks?
a. a cup of coffee with 95 mg of caffeine
b. a cup of tea with 0.065 g of caffeine
c. a soda with 0.038 mol of caffeine
31. Cobalt is a metal required for human health.We normally in-
gest small amounts of cobalt in the food we eat. Typically, you
may have about 1.5 mg present in your body.
a. How many moles is this?
b. How many atoms of cobalt are present?
32. Male silkworms are attracted to an organic molecule, se-
creted by the females, that acts as an attractant for mating
purposes. If the mass per mole of the compound is 238 g, and
a female releases 4.20 × 10
–6
g, how many molecules are
available to be detected by nearby males?
33. Some elements exist in more than one molecular form. This
property is called allotropy. For example, phosphorus, which
is very reactive in its elemental form and is used in some
explosives and some types of matches, has three different
Butane
Caffeine
Carbon
Nitrogen
Hydrogen
Oxygen

forms. The structure of white phosphorus has interlocked
tetrahedrons. The simple formula of white phosphorus is P
4
.
a. The white streams of smoke from military bombings may
be due to the reaction of white phosphorus with oxygen.
How many grams of phosphorus would be used if an
explosive device contained 10.5 mol of P
4
?
b. The most stable of the allotropic forms of sulfur is called
orthorhombic sulfur (S
8
.) The structure resembles a
puckered crown. How many moles of S
8
are equivalent to
454 g of S
8
?
34. The ability of nonmetal atoms to form attachments among
themselves is called catenation. Carbon exhibits this property
more than any other element. Catenation is responsible for
the huge variety of carbon compounds.
a. Determine the number of moles in 100.0 g each of the
following carbon compounds. (In each compound, the
carbon atoms are joined to each other). C
3
H
8
(propane),
C
4
H
10
(butane), C
5
H
12
(pentane)
b. Silicon, which is found directly below carbon in the
periodic table of the elements, also has the ability to
catenate. Determine the number of grams in 0.100 mol
of each of these silicon–hydrogen compounds (called
silanes): Si
2
H
6
and Si
6
H
14
.
35. A vitamin that helps activate many enzymes in humans is
pyridoxin (also called vitamin B
6
). Its chemical formula is
C
8
H
11
NO
3
. Vitamin B
6
can be found in good supply in wheat
and legumes. If a nutritionist found 0.156 g of pyridoxine in
a food sample, how many moles would be reported? How
many molecules would be reported? How many atoms of
carbon would be contained in the vitamin sample?
36. Carbon can be found in three elemental forms: graphite, dia-
mond, and buckministerfullerene. Because all the carbon
atoms in a diamond in a ring are connected in linked tetra-
hedrons, a single diamond can be considered to be one large
molecule (often called a macromolecule). If a diamond has a
mass of 0.50 carat, how many atoms of carbon make up the
ring? (1 carat = approximately 0.20 g)
3.4 Percentages by Mass
Skill Review
37. Calculate the percent of carbon in each of these organic
compounds:
a. C
3
H
6
c. C
6
H
6
b. CH
3
OH d. C
12
H
22
O
11
38. Calculate the percent of oxygen in each of these organic
compounds:
a. CO
2
c. C
3
H
8
O
b. C
6
H
12
O
6
d. H
2
CO
3
39. Arrange these sulfur-containing compounds from the
greatest mass percent sulfur to the least.
a. H
2
Sc.Na
2
S
2
O
4
b. SO
2
d. H
2
SO
3
40. Arrange these oxygen-containing compounds from the
greatest mass percent oxygen to the least.
a. H
2
Oc.Na
2
C
2
O
4
b. CO
2
d. C
6
H
12
O
2
41. Boron forms many compounds, often with some unusual
bonding properties. In each of these, determine the mass
percent of boron: B
13
C
2
,Ti
3
B
4
,CaB
6
.
120 Chapter 3 Introducing Quantitative Chemistry
42. Determine the mass percent of sodium in each compound:
NaCl, NaHCO
3
, NaOH.
43. Which compound in each pair has the greater percent oxygen?
a. FeSO
4
or Na
2
SO
4
c. Fe(NO
3
)
3
or HNO
3
b. K
2
FeO
4
or KMnO
4
44. Which compound in each pair has the greater percent
carbon?
a. CO
2
or CO c. C
3
H
8
O or C
5
H
10
O
2
b. NaCN or KCN
Chemical Applications and Practices
45. The main component in kidney stones is CaC
2
O
4
.What is the
mass percent of each element found in CaC
2
O
4
?
46. Hydrates are compounds that contain water molecules as
part of their structure. For example, sodium carbonate dec-
ahydrate (Na
2
CO
3
.
10H
2
O), washing soda, has been used as a
grease remover. What percent of the compound is water?
(Note: The dot between Na
2
CO
3
and H
2
O indicates that
water is loosely attached within the crystal—in this case in a
1:10 ratio.)
47. Unsaturated fatty acids are responsible for the liquid nature
of many plant oils. In this case, the term unsaturated refers to
the presence of double bonds between two or more carbon
atoms in the molecule. Saturated hydrocarbons, which are
associated with animal fats, contain only single bonds be-
tween carbon atoms. Unsaturated compounds can be made
saturated by adding hydrogen to the double-bonded areas.
After such a reaction, often called hydrogenation, would the
percent by mass of carbon in the saturated compound be
increased or decreased over its percent by mass in the unsat-
urated hydrocarbon? Explain the basis for your answer.
48. The sidewalk salt that we spread on our walkways is mostly
CaCl
2
. A stable hydrate of this salt is CaCl
2
.
2H
2
O (see Prob-
lem 46). What is the mass percent of calcium in each of these
salts?
3.5 Finding the Formula
Skill Review
49. Suppose an orchestra has this composition: 24 violinists,
18 brass instrumentalists, 6 cellos, and 3 percussionists. What
is the “empirical formula” of this orchestra?
50. Suppose a lasagna is made from 2 boxes of noodles, 2 pack-
ages of ground beef, 2 tomatoes, and 4 packages of cheese.
What is the “empirical formula” of this lasagna?
51. A compound contains only carbon and hydrogen. The mass
percent of carbon in the compound is 85.7%. What is the
empirical formula of the compound?
52. A compound contains only carbon, hydrogen, and oxygen.
The mass percent of carbon is 52.1% and that of hydrogen is
13.1%. What is the empirical formula of the compound? If
the molar mass of the compound is 92, what is the molecular
formula of the compound?
Chemical Applications and Practices
53. a. Based on this mass percent composition, determine the
empirical formula of the compound: 57.1% carbon,
4.76% hydrogen, 38.1% oxygen.
Focus Your Learning 121
b. Which of these could possibly be the molar mass of the
compound: 56, 84, or 116? (Show proof for your answer.)
54. The compound found in many small portable lighters is bu-
tane. An analysis of butane yields the following mass percent
values. From these data, determine the empirical formula of
this useful fuel: 82.76% C, 17.24% H.
55. Potassium argentocyanide is a toxic compound that is used
in the important industrial process of silver plating. It has
these mass percent values: 19.6% potassium, 54.3% silver,
12.1% carbon, and 14.7% nitrogen. What is the empirical
formula of this compound?
56. In humans, ingested ethyl alcohol is enzymatically decom-
posed into acetaldehyde. The mass percent values of the
components of acetaldehyde are 54.55% C, 9.09% H, and
36.36% O. From these data, determine the empirical formula
of acetaldehyde.
57. Linoleic acid is a fatty acid that contains unsaturated carbon
bonding. Generally, this trait is found in many compounds
that compose vegetable oils. The molar mass of linoleic acid
is 280. The mass percent values of the elements in linoleic
acid are 77.1% carbon, 11.4% hydrogen, and 11.4% oxygen.
What is the molecular formula of this helpful agricultural
compound?
58. During extreme exercise, lactic acid is produced when aero-
bic metabolism is not available for glucose breakdown.
The mass percent values of the components in lactic acid are
40.0% C, 6.72% H, and 53.3% O. The average molecular
mass of lactic acid is 90.1. What are the empirical and mole-
cular formulas for this important biological compound?
3.6 Chemical Equations
Skill Review
59. Provide the proper coefficients to balance each chemical
equation.
a. __ N
2
H
4
+__ N
2
O
4
→ __ N
2
+__H
2
O
b. __ Pb(C
2
H
3
O
2
)
2
+__ KI → __PbI
2
+__KC
2
H
3
O
2
c. __ PCl
5
+__ H
2
O → __ H
3
PO
4
+__ HCl
d. __Ba
3
N
2
+__ H
2
O → __ Ba(OH)
2
+__ NH
3
60. Provide the proper coefficients to balance each chemical
equation.
a. __ N
2
+__ O
2
→ __ NO
b. __ CH
4
+__ O
2
→ __ CO
2
+ __ H
2
O
c. __ H
2
SO
4
+__ KOH → __ K
2
SO
4
+__ H
2
O
d. __ CO
2
+__ H
2
O → __ C
6
H
12
O
6
+__ O
2
61. Write the proper formulas and balance the equation: Cal-
cium chloride reacts with sodium phosphate to produce
calcium phosphate and sodium chloride.
62. a. Balance this decomposition reaction:
__ (NH
4
)
2
Cr
2
O
7
→ __ Cr
2
O
3
+ __ N
2
+ __ H
2
O
b. How many atoms of nitrogen appear in the reactant side
of the balanced equation? How many nitrogen molecules
appear on the product side of the balanced equation?
63. This equation shows the proper stoichiometric ratio for all
the components but one. Fill in the correct coefficient and
compound to complete the equation.
TiCl
4
+2H
2
O → TiO
2
+__ _____
64. One method that could be used to make a chlorofluorocar-
bon (CFC) is based on this reaction:
CCl
4
(g) +SbF
3
(g) → CCl
2
F
2
(g) +SbCl
3
(s)
Freon-12
Use coefficients to balance the reaction. How many total
moles of gases are involved in the balanced equation?
Chemical Applications and Practices
65. The banned insecticide DDT could be produced using this
reaction:
__ C
6
H
5
Cl + __ C
2
HOCl
3
→ __ C
14
H
9
Cl
5
+ __ H
2
O
a. Balance the equation.
b. How many grams of DDT (C
14
H
9
Cl
5
) would be expected
from the complete reaction of 45.0 g of C
6
H
5
Cl?
66. A key ingredient for many over-the-counter antacids is alu-
minum hydroxide, Al(OH)
3
. Use the following chemical
reaction to predict the maximum number of grams of alu-
minum hydroxide that could be produced from 5.20 g of
Al
2
(SO
4
)
3
and unlimited NaOH.
Al
2
(SO
4
)
3
+ NaOH → Al(OH)
3
+ Na
2
SO
4
(not balanced)
67. The “fizz” formed when some pain relief tablets are placed in
water is caused by the following chemical reaction. The bub-
bles are produced from the release of gaseous carbon dioxide.
How many grams of NaHCO
3
would have to be present in a
tablet to react with 0.487 g of citric acid (H
3
C
6
H
5
O
7
)?
NaHCO
3
(aq) +H
3
C
6
H
5
O
7
(aq) →
CO
2
(g) +Na
3
C
6
H
5
O
7
+ H
2
O(aq)
68. If some sugar from grapes were to be fermented to make
wine, one of the primary reactions would be
___ C
6
H
12
O
6
→ __ C
2
H
6
O + __CO
2
a. Balance the equation.
b. How many grams of sugar would you need to make 100.0 g
of ethanol (C
2
H
6
O)?
3.7 Working with Equations
Skill Review
69. a. Balance this equation:
__ NH
3
+ __ O
2
→ __ NO + __H
2
O
b. Starting with 34.0 g of NH
3
and excess O
2
, what mass of
NO would be expected to be produced?
c. How much H
2
O would form?
d. If the percent yield of NO was only 35.0%, how many
grams of NO were formed?
70. a. Balance this equation:
__ Fe
2
O
3
+__ C → __ Fe +__ CO
b. If the reaction were started with 1.00 kg of Fe
2
O
3
and
0.250 kg of C, which reagent would you consider to be the
limiting component?
c. Under the conditions described in part b, what would be
the theoretical yield of Fe?
Glucose Ethanol Carbon
dioxide
+

71. a. Balance this equation:
__ Ca(OH)
2
+__ H
3
PO
4
→ __ Ca
3
(PO
4
)
2
+__ H
2
O
b. How many grams of Ca(OH)
2
are required to form
0.567 g of Ca
3
(PO
4
)
2
?
c. How many grams of H
3
PO
4
would be required for the
stoichiometry described in part b?
72. This reaction takes place when some types of matches are
struck:
__ KClO
3
+__ P
4
→ __ P
4
O
10
+__ KCl
a. Balance the equation.
b. If 52.9 g of KClO
3
, in the presence of excess P
4
,produced
25.0 g of P
4
O
10
, what would be the calculated percentage
yield of the process?
73. A fast-food restaurant serves this sandwich: 2 pieces of bread,
1 meat patty, 2 pieces of cheese, and 4 pickles. If the restau-
rant currently had on hand the following inventory, what
would be the maximum number of specialty sandwiches that
it could prepare? What ingredient would limit the sandwich
production? Inventory: 200 pieces of bread, 200 meat patties,
200 cheese slices, 200 pickles.
74. A certain laboratory set-up for general chemistry requires
four 250-mL beakers, six test tubes, and two graduated
cylinders.
a. Write an “equation” that shows the correct combination
of lab equipment to form the arrangement.
b. If a lab assistant set up 50 such arrangements, how many
test tubes would be required?
c. Would 100 beakers and 100 graduated cylinders provide
enough equipment for these 50 arrangements? Explain.
Chemical Applications and Practices
75. One method used to produce soaps includes the reaction be-
tween NaOH and animal fat. The following equation repre-
sents that process:
C
3
H
5
(C
17
H
35
CO
2
)
3
+ 3NaOH →
C
3
H
5
(OH)
3
+ 3NaC
17
H
35
CO
2
a. How many grams of soap, NaC
17
H
35
CO
2
, would theo-
retically be produced by starting with 125 g of fat,
C
3
H
5
(C
17
H
35
CO
2
)
3
?
b. If the actual amount of soap produced were 31 g, what
would be the percentage yield of the reaction?
76. The compound Na
2
S
2
O
3
is used in developing photographs.
It can be synthesized by using this chemical reaction:
Na
2
CO
3
+2Na
2
S +4SO
2
→ 3Na
2
S
2
O
3
+CO
2
a. Predict the mass of Na
2
S
2
O
3
(hypo) that could be
produced from 48.5 g of Na
2
S and unlimited quantities of
the other reactants.
b. If the procedure produced only a 78.9% reaction yield,
how many grams of hypo would you expect to obtain?
77. When acid reacts with a base, the typical reaction yields water
and a salt product formed from the anion of the acid and the
cation of the base. For example, hydrochloric acid added
carefully to sodium hydroxide forms water and NaCl. Use the
balanced equation shown here to calculate the maximum
yield of NaCl that can be formed when a solution containing
0.155 mol of HCl reacts with 4.55 g of NaOH.
HCl(aq)
+NaOH(s) → NaCl(aq) +H
2
O(l)
78. A “magic show” demonstration often performed to illustrate
volcanic action is to react vinegar with baking soda. The re-
122 Chapter 3 Introducing Quantitative Chemistry
lease of carbon dioxide gas that results produces a frothing
action of bubbles. How many moles of carbon dioxide could
be produced from the complete reaction of 10.0 g of baking
soda (NaHCO
3
) with excess acetic acid (HC
2
H
3
O
2
) in
vinegar?
HC
2
H
3
O
2
(aq) +NaHCO
3
(s) →
H
2
O(l) +CO
2
(g) +NaC
2
H
3
O
2
(aq)
79. Different baking powders are used in cooking to achieve
various results in food textures as carbon dioxide gas is
released. One such baking powder contains cream of tartar
(KHC
4
H
4
O
6
) and sodium hydrogen carbonate (NaHCO
3
).
Typically, starch is also added to keep moisture away from
these active ingredients.When water from the recipe contacts
these two compounds, this reaction takes place:
KHC
4
H
4
O
6
+ NaHCO
3
→ NaKC
4
H
4
O
6
+H
2
O +CO
2
What is the maximum amount of NaKC
4
H
4
O
6
(also known
as Rochelle salt) that could be formed if a baking powder
sample contained 10.0 g each of the starting ingredients?
80. Often in dying cloth, a substance must be added that helps
the dye to adhere to the cloth. Such a substance is known
as a mordant. One such compound is aluminum sulfate
(Al
2
(SO
4
)
3
.
18H
2
O). One method to obtain this useful com-
pound is as follows:
__ Al(OH)
3
+__ H
2
SO
4
+__ H
2
O → Al
2
(SO
4
)
3
.
18H
2
O
Balance the equation and determine the maximum yield that
could be obtained from reacting 25.0 g of Al(OH)
3
with 50.0 g
of H
2
SO
4
and unlimited water.
81. The following equation shows the conversion of ethanol in a
wine sample into acetic acid through the action of oxygen
from the air. This reaction drastically changes the taste of the
wine. If 100.0 mL of wine initially contained 12.0 g of ethanol
(C
2
H
5
OH), and after a period of time 4.00 g of acetic acid
(C
2
H
4
O
2
) were detected, what percent of the ethanol had
been oxidized? C
2
H
5
OH +O
2
→ C
2
H
4
O
2
+H
2
O
82. Sodium hypochlorite (NaOCl) is the active ingredient in
most household bleach products. One method of preparing
this important compound is as follows:
Cl
2
(g) + 2NaOH(aq) → NaOCl(aq) +NaCl(aq) +H
2
O(l)
a. Starting with 50.0 g of Cl
2
(g) and 50.0 g of NaOH, predict
the theoretical yield of NaOCl.
b. If the reaction, under a given set of conditions, formed
only 38.9 g of NaOCl, what would you report as the
percentage yield?
83. The following reaction can be used to represent the rusting of
iron. (Annually, about 20% of the steel manufactured is used
to replace steel corroded as a consequence of the oxidation
of iron.) Determine how many grams of oxygen and iron
reacted if 454 g of Fe
2
O
3
were formed. (First balance the
equation.)
Fe(s)
+O
2
(g) → Fe
2
O
3
(s)
84. You may have noticed an athletic trainer spraying an injured
player with a quickly evaporating liquid during a time out.
The liquid is probably chloroethane, which quickly cools
down an injured area with a slight numbing sensation.
Chloroethane can be synthesized via the following reaction.
If the reaction were begun using 85.0 g of ethene (C
2
H
4
) and
100.0 g of hydrogen chloride (HCl) but had only a 68.0%
yield, how many grams of chloroethane (C
2
H
5
Cl) would be
produced?
HCl(g)
+C
2
H
4
(g) → C
2
H
5
Cl(g)

Focus Your Learning 123
85. Adding yeast cells to glucose can cause the glucose (C
6
H
12
O
6
)
to be converted to ethanol (C
2
H
5
OH) and CO
2
. Bakers make
use of this process when the CO
2
gas causes bread to rise dur-
ing the baking process. Balance the following equation, and
determine the number of moles of CO
2
that could be pro-
duced from 25.0 g of glucose.
__ C
6
H
12
O
6
(s) → __ C
2
H
5
OH(l) +__ CO
2
(g)
86. Two students perform a chemical synthesis in their general
chemistry lab. Student A obtains a 90.0% yield in the reac-
tion. Student B obtains an 85.0% yield in the same reaction.
Can you now determine which student obtained the greater
mass of the product? Explain, or justify your answer.
Comprehensive Problems
87. Review the vitamin C controversy discussed in this chapter.
How is it possible that a compound can be both good and
bad for your health?
88. What government agency is most concerned with setting rec-
ommended amounts of vitamins and minerals?
89. Explain why it is not totally correct to use the atomic masses
given in the periodic table on the inside front cover of this
book to express the mass of one molecule of any compound.
90. How big is Avogadro’s number? If it were possible to place
circles on notebook paper to represent atoms, how many
pages would be required to complete the task? (Assume
25 lines on each side, 32 circles per line, and use of both sides
of the paper.)
91. How many grams of argon would contain the same number
of atoms as 10.0 g of neon?
92. Copper, silver, and gold are often referred to as the “coinage
metals.” If you had 454 g (approximately 1 lb) of each, which
sample would contain the greater number of atoms?
93. The human hemoglobin molecule is quite large. It is known
that each hemoglobin molecule contains four atoms of iron.
If the iron makes up only 0.373% of the total mass of the
molecule, what is the mass of 1 mol of hemoglobin?
94. In green plants, the compound chlorophyll a assists in the
production of plant products and the oxygen essential for life
on Earth. The compound contains one atom of magnesium.
The mass percent of magnesium per molecule is 2.72%.
What is the molar mass of this important compound?
95. When 1.00 g of one of the main components of gasoline was
completely combusted, it produced 3.05 g of CO
2
and 1.50 g
of H
2
O. On the basis of this information, determine the mass
percent values of carbon and hydrogen in the compound and
the empirical formula of the compound.
96. The main compound responsible for the characteristic
aroma of garlic is allicin. From the following mass percent
values, determine the empirical formula of this familiar com-
pound: 44.4% C, 6.21% H, 39.5% S, and 9.86% O.
97. When we examine balanced equations, we find that total of
the coefficients on the left-hand side of the equation arrow
does not always equal the total of the coefficients on the
right-hand side. Explain why this does not violate the law of
conservation of mass.
98. Examine the formula of salicylic acid in Section 3.6. How
many molecules of salicylic acid would be required to react
with 0.247 mL of ethanoic anhydride (d 1.080 g/mL)?
99. A sample of ore is found to contain 14.5% aluminum oxide
by mass. How many pounds of the sample would be re-
quired to make exactly 100 grams of pure aluminum?
(Assume the reaction to make aluminum metal and oxygen
gas from aluminum oxide occurs with only a 58% yield.)
100. Zinc oxideis oftenused in sunscreenstohelp protectyourskin
fromharmful UV rays.If aparticularsunbather wishes to coat
0.75 m
2
of his exposed skin with a layer of zinc oxide that is
1.0 mm thick, how many grams of zinc oxide would be
needed? (Assume the sunscreen lotion has a density of 1.10
g/mL, and that the lotion contains 18% zinc oxide by mass.)
101. Palladium(II) nitrate is a reagent that can be used to link
two smaller molecules together with a covalent bond. This
reagent can be made by treating metallic palladium with
nitric acid.
a. What is the balanced reaction for the preparation of the
reagent?
b. How many grams of palladium(II) nitrate can be made
from 10.0 grams of palladium metal and 5.6 mL of 15%
(by mass) nitric acid whose density is 1.056 g/mL?
102. A researcher claims that a 1.00 gram sample of gold(II)
chloride contains a greater mass of chloride than does a
1.00 gram sample of gold(IV) chloride.
a. Is the researcher correct? Explain your answer.
b. Which sample contains a greater mass of gold?
c. If a 2.0 cm cube of gold(II) chloride was spread so that
it was only 1/8 inches thick, what area would the
compound occupy in in
2
?
Thinking Beyond the Calculation
103. Xylene (ZIGH-leen) is an important organic molecule iso-
lated from petroleum oil. It is often used as a thinner for oil-
based paints.
a. Elemental analysis of a sample of xylene shows that the
mass percent of carbon is 90.51% and the mass percent
of hydrogen is 9.49%. What is the empirical formula of
xylene?
b. The molar mass of xylene is 106.17 g/mol. What is the
molecular formula of xylene?
c. In the laboratory setting, xylene burns in limited oxygen
environments to produce carbon monoxide and water
vapor. Write the balanced reaction for this reaction.
d. If 12.5 g of xylene were combusted using the equation
from part c, how many grams of oxygen gas would be
required to react completely with the xylene?
e. A vessel containing 45.8 mL of xylene (density =
0.8787 g/mL) and 31.0 g of O
2
produced carbon
monoxide and water. Which reagent is the limiting
reagent in this reaction? How many grams carbon
monoxide are produced in the reaction?
f. If 1.40 g of CO were actually isolated from the reaction
in part e, what would be the percentage yield of the
reaction?

Solution
Stoichiometry
and Types of
Reactions
View of a gold mine in the Black Hills of
South Dakota. The gold ore is placed in
“heap-leach pads,” where cyanide solution
is added. The solution percolates through
the pads and collects in the ponds. Gold
metal can be obtained from these ponds.
124
Contents and Selected Applications
Chemical Encounters: Gold Mining and Cyanide Leaching
4.1 Water—A Most Versatile Solvent
Chemical Encounters: Sports Drinks and Electrolyte Balance
4.2 The Concentration of Solutions
Chemical Encounters: Maximum Levels of Chemicals in Drinking Water
4.3 Stoichiometric Analysis of Solutions
4.4 Types of Chemical Reactions
4.5 Precipitation Reactions
Chemical Encounters: Focus on Lead Sulfide
4.6 Acid–Base Reactions
4.7 Oxidation–Reduction Reactions
4.8 Fresh Water—Issues of Quantitative Chemistry
Chemical Encounters: Revisiting the Maximum Levels of Chemicals in
Drinking Water
Go to college.hmco.com/pic/kelterMEE for online learning resources.

“There’s gold in them thar’ hills!” That state-
ment, and others like it, conjured up images of
great wealth in a worldwide gold rush that began in
the late 1840s, stretching from the hills of California to
Australia. Whether our excitement comes from finding a nugget
in a mountain stream, buying a necklace at the store, or just watching
a show about the bullion bars stored at Fort Knox, we are fascinated with
gold. The use of gold is not a recent phenomenon; it has been
known for a large part of recorded history. In fact, gold was first
used as coinage in 700
B.C
., and it continued to flourish in coin form
until the early 1900s.
In our modern world, gold is prized for its rarity, beauty, and high
price. In recent years, its price per troy ounce (31.1 grams, or about
1.1 “avoirdupois ounces” in the units commonly used in the United
States) has been as low as US$ 102 (1976) and as high as US$ 850
(1980). Currently it commands around US$ 500 per troy ounce.
Gold is a fine electrical conductor, is not very reactive, and is quite
malleable, making it ideal for use in electronic products, including
computers. It is also highly reflective, so it is used as a shielding coat-
ing to protect spacecraft from solar radiation (Figure 4.1).
Gold used to be found in large nuggets as relatively pure metal, and miners
would dig, sort, and harvest visible chunks of gold by almost every means possible.
These days, in both large- and small-scale operations, gold ore is mined, pulverized
into particles the size of grains of sand, and then mixed with a solution containing
cyanide ion (CN
−
).
4Au(s) + 8NaCN(aq) + O
2
(g) + 2H
2
O(l) → 4NaAu(CN)
2
(aq) + 4NaOH(aq)
The gold in the ore is dissolved by the solution, and the waste ore is discarded.
Metallic gold is then obtained by precipitation and/or electroplating (see Chapter 19)
Application
C
HEMICAL
ENCOUNTERS:
Gold Mining and
Cyanide Leaching
FIGURE 4.1
Gold has many uses in today’s so-
ciety. It blocks harmful UV rays in
an astronaut’s visor and serves as
corrosion-resistant connections in
a computer.
Gold has been used as currency for a very
long time.
125
126 Chapter 4 Solution Stoichiometry and Types of Reactions
4.1 Water—A Most Versatile Solvent
Thinking about the range of chemicals that must dissolve in our blood reveals
water to be an extraordinary
solvent—a compound that typically makes up the
majority of a homogeneous mixture of molecules, ions, or atoms. We find ions
such as potassium (K
+
) and chloride (Cl
−
); larger molecules including glucose
(C
6
H
12
O
6
) and, to a lesser extent, cholesterol (C
27
H
46
O); and giant protein mol-
ecules composed of thousands of atoms dissolved in our blood.Water, the solvent
that dissolves these things in blood, has historically been called the universal sol-
vent because many things dissolve in it.
A
solution is a homogeneous mixture (see Figure 4.2). Those molecules, ions,
or atoms that dissolve (or are soluble) in the solvent are known as
solutes.The
solvent is typically present in the larger amount in a solution, and the solute is
typically the minor component. Exceptions arise when large amounts of solute
can dissolve in small amounts of solvent, as with a solution of sugar in water.
Considerably more than 1000 grams of sucrose (C
12
H
22
O
11
, table sugar) can
the solution. The precipitation reaction produces gold powder, although any silver
and copper in the solution is also deposited with the gold.
Zn(s) + 2NaAu(CN)
2
(aq) → 2Au(s) + Na
2
Zn(CN)
4
(aq)
The extraction of gold using this method, known as the cyanide leaching
method, takes place in a water-based or
aqueous solution. In fact, much of the chem-
istry of life, and much of the chemistry of the environment, takes place in aqueous
solution. Each cell of the body is a watery sac full of reacting chemicals. Rivers, lakes,
and oceans contain complex solutions, each with its own special chemistry. At home
we use solutions to cook and clean. And in large-scale industrial processes, such as
gold mining, aqueous chemistry is important to the economic “bottom line.”
Working with aqueous chemistry means understanding the stoichiometry (see
Chapter 3) of the reactions that take place in the solution. For example, although
too little cyanide (CN
−
) in a given volume of water means an inefficient extraction
of gold in the mining process, too much of it, or any compound in the blood, can be
fatal. For example, long-term exposure to too much cyanide can lead to health prob-
lems in those who process the gold, including central nervous system, heart, and
thyroid gland damage, and even death. Chemical reactions that require us to look at
measures of concentration, such as how much cyanide is in a given volume of water,
deal with “solution stoichiometry,” the focus of the ideas, new terms, and techniques
we will explore in this chapter.
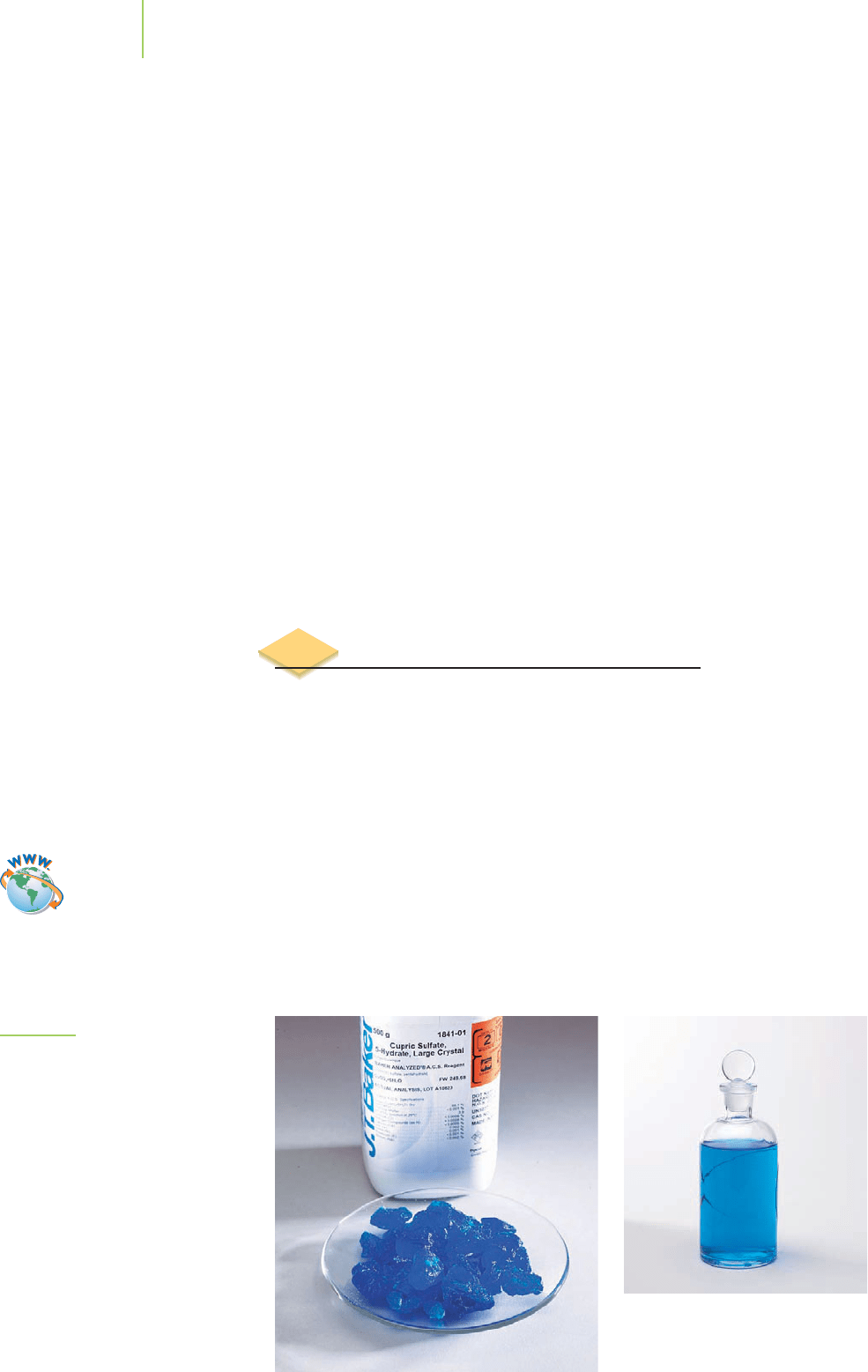
FIGURE 4.2
In solution, the particles of a solute (such
as the ions of copper sulfate, a sample of
which is shown here) form a homoge-
neous mixture with the particles of the
solvent (water molecules, in this case).
The blue color of the solute is evenly
distributed.
Video Lesson: Properties of
Solutions
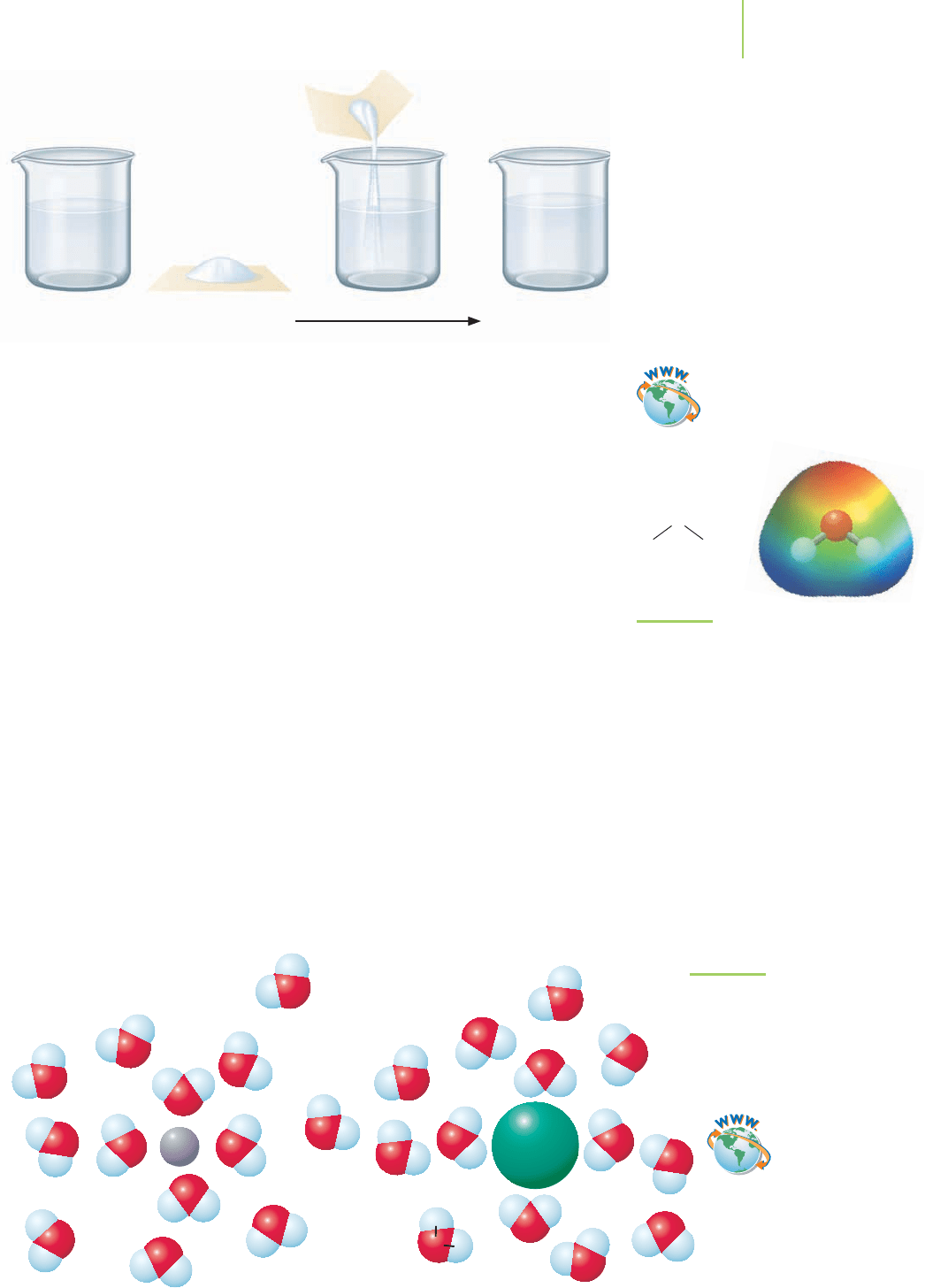
dissolve in 1000 grams of water, but we still refer to the water as the solvent because
it retains its phase. As we work with solutions here and throughout our study of
chemistry, we tend to be keenly interested in the behavior and impact of the vari-
ous solutes, such as cadmium or nitrate ions, dissolved in the water, the solvent.
Solutes dissolve in solvents because of the energetically favorable interactions
between the two components of the solution. Water is a particularly versatile sol-
vent because its structure permits it to interact with a wide variety of other chem-
icals. The resulting mixture is more energetically stable than if the compounds
remained separate (such as layers of oil and water.)
Why is this so? A closer look at
the structure of water will help us answer this question.
Water is a molecule that contains an oxygen atom rich in electrons. This oxy-
gen atom, therefore, possesses a partial negative charge. The hydrogen atoms at-
tached to the oxygen atom have a lower concentration of electrons, which results
in a partial positive charge. This is illustrated by the computer-drawn electrosta-
tic potential map shown in Figure 4.3. When an ionic compound dissolves in
water, it dissociates into its composite ions and interacts with the partial charges
on the water molecule.
As an example, let’s explore the dissolution of table salt (sodium chloride,
NaCl) in water. The solid salt is composed of equal numbers of sodium ions
(Na
+
) and chloride ions (Cl
−
). Figure 4.4 shows that as the solid salt dissolves, the
opposite charges of the ions and the water molecules attract. The water molecules
cluster around the ions in a way that allows the ions to separate and mix with
the water. The salt dissolves. The cage of water molecules around the ions is
known as the
hydration sphere of the ions, and we say that the Na
+
and Cl
−
ions
become hydrated. The ions are not static, nor are they held in place by the water
molecules. Instead, the ions separate and move about independently, though
4.1 Water—A Most Versatile Solvent 127
Water Sucrose Sugar water+
Sucrose is very soluble in water.
–
+
+
O
HH
FIGURE 4.3
The electrons in the water molecule are dis-
tributed toward the oxygen atom. The oxy-
gen end of water is electron-rich (
) and
the hydrogen end of water is electron-poor
(
). This can be represented with a colored
map of electrostatic potential. Red areas in-
dicate high electron density, and blue areas
represent low electron density. The colors in
between (ranging from yellow to green to
sky blue) indicate varying degrees of elec-
tron density.
Na
+
Cl
–
H
H
O
FIGURE 4.4
When an ionic compound (such as
NaCl) dissolves, the uneven charge
distribution in water molecules
facilitates the process by interact-
ing with the positive and negative
charges on the ions.
Video Lesson: Factors
Determining Solubility
Visualization:
Dissolution of a Solid
in a Liquid
