Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

228 Chapter 6 Quantum Chemistry: The Strange World of Atoms
Going to the three dimensions required to quantize the spatial position of
electrons in the Bohr hydrogen atom is considerably more difficult. Bohr himself
never actually assigned a wavelength to his electrons, which remained decidedly
particle-like in his own model. Bohr merely postulated that the momentum and
radius of permissible levels were quantized because they had to be if his model
were to explain the hydrogen emission spectrum. But the reason why space and
energy are quantized for the hydrogen atom arises from the wave-like nature of
the electron.
If electrons behave like waves, how do we find their wavelengths? We can do
this by using the de Broglie formula, proposed by Louis de Broglie in 1924:
λ =
h
p
=
h
mv
The equation, which shows that the wavelength, λ, is inversely proportional to
the momentum, p, through Planck’s constant h, connects wave (λ) and particle
(p = momentum) properties in a single formula. Again, note that the momen-
tum term, p, uses the velocity (v). This symbol looks similar to that for the fre-
quency (ν), but it is vastly different. The velocity has units of m/s, whereas
frequency has units of 1/s.
What is the purpose of the de Broglie equation? It illustrates the basic principle
of the wave–particle duality: Moving particles exhibit definite wave-like character-
istics. The values of wavelengths calculated by this equation are meaningful for
small, fast-moving particles such as the electron. The calculation is less meaning-
ful but still demonstrative for massive, slow-moving objects.
EXERCISE 6.5 Working with the de Broglie Equation
An electron in the ground state of the hydrogen atom has a velocity of about
2.1 × 10
6
m
.
s
−1
. What is its de Broglie wavelength?
Solution
λ =
h
p
=
h
mv
=
6.626 ×10
−34
J·s
(9.109 ×10
−31
kg) × (2.1 ×10
6
m·s
−1
)
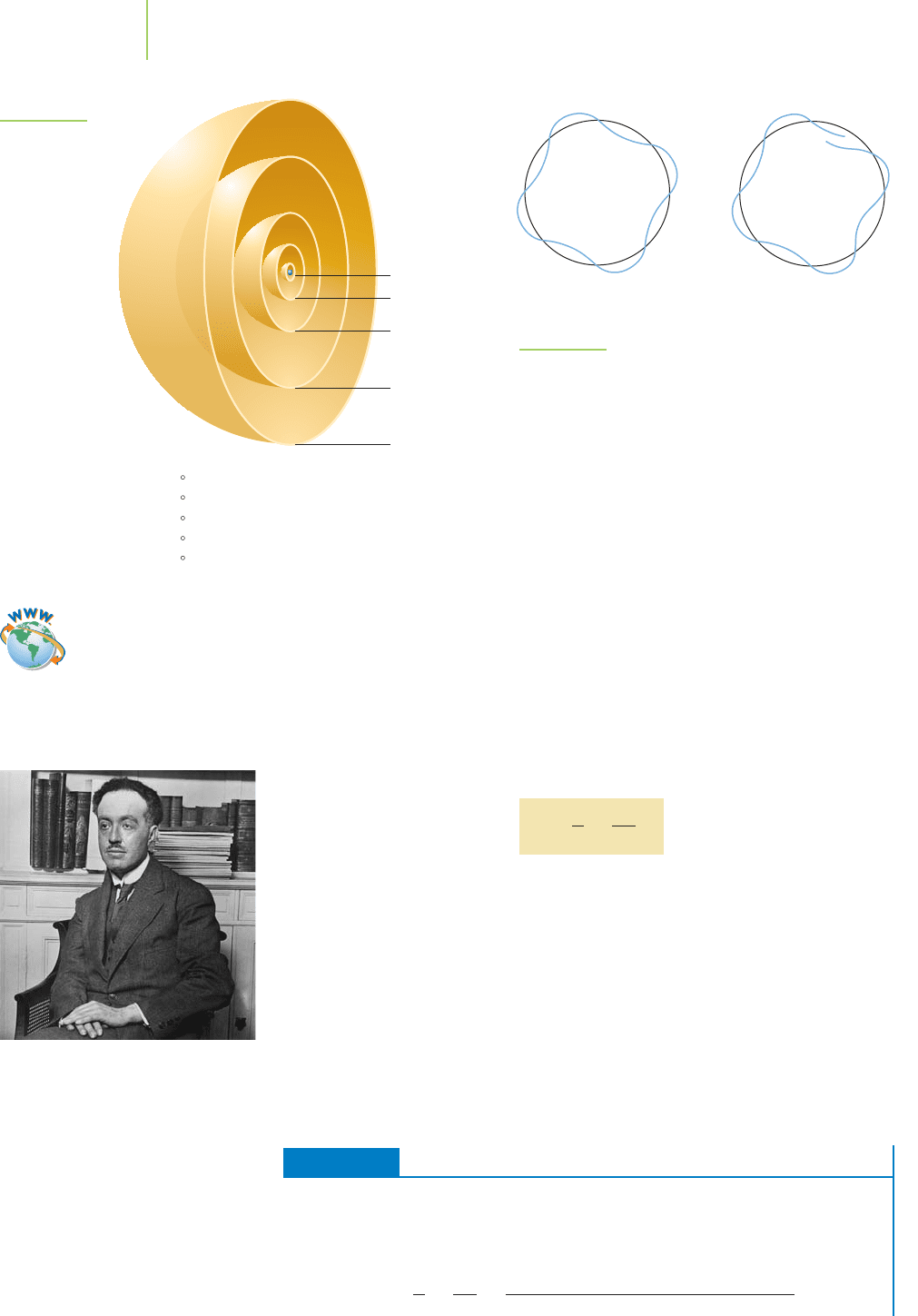
n = 1
n = 2
n = 3
n = 4
n = 5
Orbit n Distance from Nucleus
1
2
3
4
5
0.529 A
2.116 A
4.761 A
8.464 A
13.225 A
(a)
(b)
FIGURE 6.16
The requirements of a wave completing a circular path.
This wavelength doesn’t line up as the circular orbit is
completed (b). This doesn’t provide a suitable orbital. In (a),
the wavelength does line up and provides a useful orbital.
FIGURE 6.15
Circular cross-sections
of the atomic energy
levels of the
Bohr atom.
Louis-Victor de Broglie (1892–1987),
a French physicist and prince, published
his wave–particle duality theory in 1924.
Video Lesson: The Wave Nature
of Matter
6.7 The Heisenberg Uncertainty Principle 229
Because 1 J = 1 kg
.
m
2
.
s
−2
, substituting yields
6.626 ×10
−34
kg·m
2
·s
−2
(s)
(9.109 ×10
−31
kg) × (2.1 ×10
6
m·s
−1
)
= 3.5 ×10
−10
m = 3.5Å,or0.35 nm
PRACTICE 6.5
Calculate the de Broglie wavelength for a 1200-lb car traveling 75 mi/h.
See Problems 59 and 60.
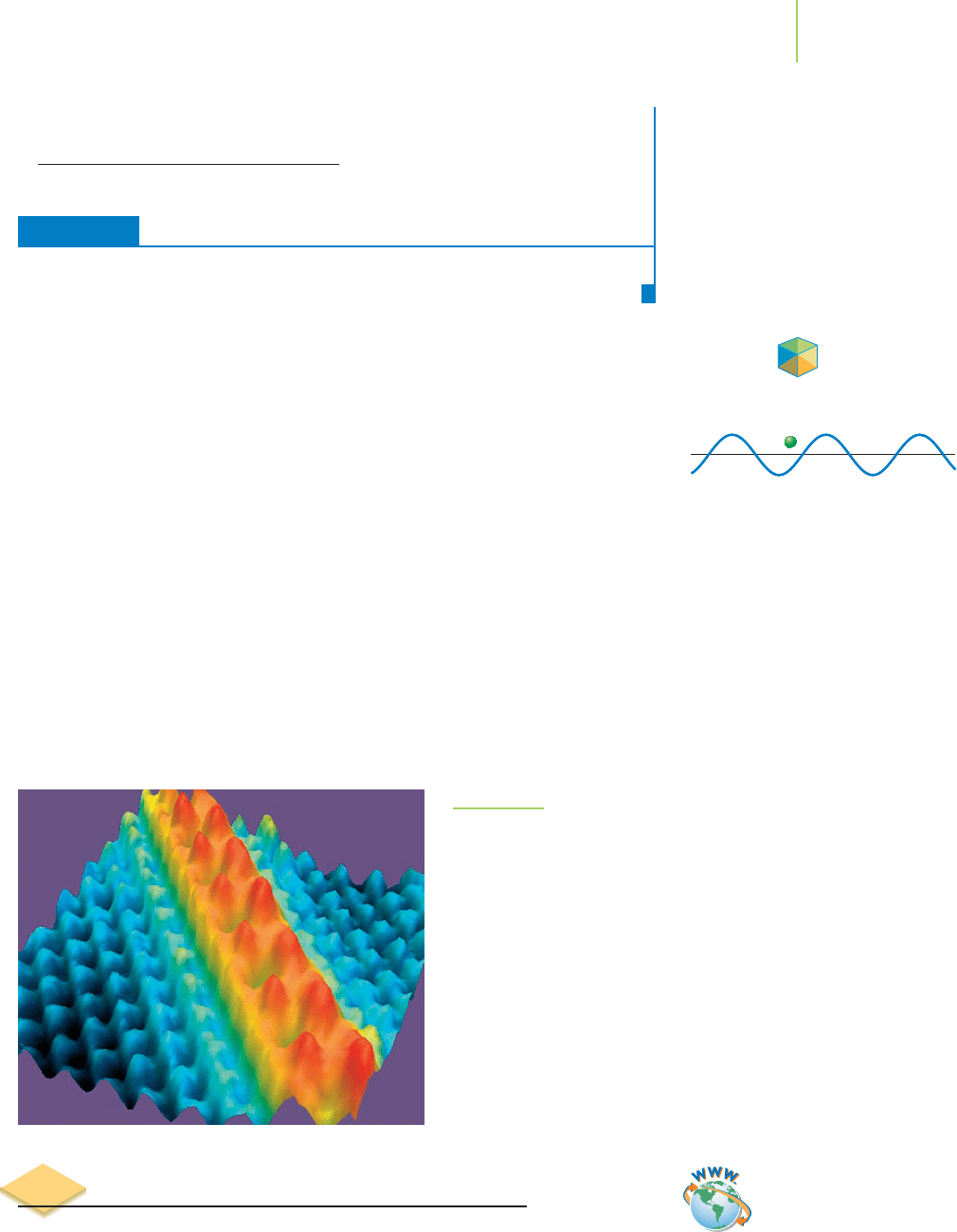
One application of the wave nature of electrons lies in their very small wave-
length. Microscopes magnify and allow us to see objects with visible light. The
limit to their magnification is the wavelengths of electromagnetic radiation (in
this case, visible light). Objects must be larger than the wavelengths of the radia-
tion in order to be seen. Using an optical microscope, we are able to see objects as
small as about 200 nm (the radius of a hydrogen atom is 25 picometers, or
0.025 nm). We are able to see objects much smaller than this if we use electrons
to image the objects. This is the principle behind the scanning electron micro-
scope (SEM). Scanning electron microscopes operate much like compound light
microscopes, but they use a beam of electrons to generate an image of the sample
rather than using light to image the sample. Because the wavelength of an elec-
tron is about 1000 times smaller than that of visible light, and given instrumental
imperfections, a scanning electron microscope can image objects or features as
small as about 3 nm. This means that the SEM can achieve a magnification of up
to 300,000-fold. Unfortunately, this is still too large to “see” individual atoms. For
that we need to use a scanning tunneling microscope (STM) (see Figure 6.17).
The STM takes advantage of another quantum effect, tunneling, which we will
discuss in Section 6.10.
Application
An atom is smaller than the visible
wavelengths of light and cannot be seen
with an optical microscope.
FIGURE 6.17
Scanning tunneling microscope
image of cesium atoms lined
up in zigzag fashion on a GaAs
surface. An STM can allow us
to “see” objects at a much
higher magnification than an
optical microscope.
6.7 The Heisenberg Uncertainty Principle
How does wave–particle duality alter our ideas of how the electron is moving within
the hydrogen atom?
The Bohr model regards the electron as a particle allowed to
orbit the nucleus at only certain precisely quantized distances. However, if it is
also to behave as a wave, the electron must be spread out over all of its orbit in the
hydrogen atom. We can reconcile these two very different ideas by considering
scale. Assume that we measure the electron’s position as having a specific value of
x, y, and z in our macroscopic laboratory setting. If the electron is bound in a
Video Lesson: The Heisenberg
Uncertainty Principle

230 Chapter 6 Quantum Chemistry: The Strange World of Atoms
The position and momentum of a racing
car are known with enough precision for
the macroworld.
hydrogen atom but is somehow spread out, some uncertainty will be introduced
into our measurement of x, y, and z. But the uncertainty is very small, on the
order of 0.1 nm or so. Our macroscopic rulers are not this precise, so it looks to
us as if the electron is at a specific and absolute position in space.
If we then look at the electron on the microscopic level, we find that the best
we can do to establish its position is to say it is somewhere in the atom, within a
certain range from of the atom’s center. We see a particle’s behavior of spatially
discrete positions with continuous possible values when we are looking at things
on a macroscopic scale, and we see a wave’s behavior of being allowed only in spe-
cific regions of space but spread out to fill the “container” of the atom when we
make microscopic measurements.
This leads us to another of the “Funny things happen” phenomena that we
observe only when we are in the microscopic quantum world. On a macroscopic
level, you can measure both the position and the momentum of a particle as pre-
cisely as your instruments will allow. Quantum mechanics, however, proposes
that, regardless of the instrument used to measure them, there is an ultimate un-
certainty in both the position and the momentum of a particle, and these uncer-
tainties are connected. This is called the
Heisenberg uncertainty principle after
Werner Heisenberg, who proposed it in 1925. Mathematically, we can state the
Heisenberg uncertainty principle as
xp
x
≥
h
4π
where ∆x is the uncertainty in position, ∆p
x
is the uncertainty in momentum
along the x-direction, and h is Planck’s constant.
There is no limitation on how precisely we measure either x or p
x
. Instead, the
limitation is on the product of their uncertainties ∆x∆p
x
. In other words, as the
uncertainty in x becomes smaller, the uncertainty in p
x
gets larger. Therefore, as
∆x approaches zero and we know exactly where the particle is positioned, ∆p
x
be-
comes infinite and we do not know anything of its momentum. In essence, it can
have any value of momentum possible, including infinity. If we know the mass of
the particle, then infinite momentum implies infinite velocity. In short, limita-
tions are set on real-world behavior by the Heisenberg uncertainty principle,
which among other things limits the maximum achievable resolution of micro-
scopes, the ultimate size of computer chips, and, in the nanoworld, the behavior
of actual atoms and molecules.
6.8 More About the Photon—
the de Broglie and Heisenberg Discussions
We have focused on the electron in our previous discussion, but the principles
apply to all particles, although the effect will really be evident only on the micro-
scopic level. Any small particle such as the electron, proton, or neutron would be
a good candidate for testing the Heisenberg uncertainty principle. Bowling balls,
elephants, and cars would not. But what about phenomena that are wave-like in
our macroscopic world? What happens to waves on the quantum scale?
Electromagnetic radiation is wave-like on a macroscopic level. Light, in a
manner similar to chemical substances, can be decreased only so far. Eventually,
you will come to its smallest, indivisible unit. For light, the smallest unit is the
photon, and, like particles, photons have been shown to carry momentum,
another of our particle-like characteristics. In fact, we can calculate the momen-
tum using the de Broglie equation.
What does such a calculation tell us? Photons,
although we think of them primarily as waves, act like particles too. Again, the
calculation verifies the particle–wave duality.
6.8 More About the Photon—the de Broglie and Heisenberg Discussions 231
EXERCISE 6.6 Photons and Momentum
How much momentum is carried by a single green photon and by a mole of green
photons? Assume that green photons have wavelengths of 530 nm.
Solution
We can use the de Broglie equation to calculate the momentum of a single photon.
Because
λ =
h
p
,
p =
h
λ
=
6.626 ×10
−34
J·s
5.30 ×10
−7
m
=
6.626 ×10
−34
kg·m
2
·s
−2
× s
5.30 ×10
−7
m
= 1.25 × 10
−27
kg·m·s
−1
This is for a single photon. If we want the momentum for a mole of photons, we
must multiply this value by Avogadro’s number:
p = 1.25 × 10
−27
kg·m·s
−1
·
photon
−1
× (6.022 × 10
23
photons
.
mol
−1
)
= 7.53 × 10
−4
kg·m·s
−1
·mol
−1
Does our answer make sense? In comparison, a 68-kg (150-lb) person ambling
down the road at 2 mi/h has a momentum of 60
kg·m·s
−1
, so you can see that even
a mole of green photons has a much smaller momentum.
PRACTICE 6.6
Calculate the momentum of a mole of photons with a wavelength of 200 nm, of
700 nm, and of 1000 nm. Is there a difference? Explain.
See Problems 65–70.
A word of caution about this type of calculation: The wavelength will have the
same value, as will the frequency, whether you have one photon or a mole of pho-
tons. These properties of a photon are intrinsic properties—they do not change
with a change in amount. The total momentum and energy carried by the elec-
tromagnetic radiation, however, are extrinsic properties because both depend on
the number of photons you have.When we use the formula E = h
ν
, we always use
the energy of a single photon. Similarly, with the de Broglie equation, we always
use the momentum of a single photon.
We also need to be careful of the answer that the de Broglie equation gives us
for the mass of a photon. The mass that appears in the de Broglie formula is
known as the
rest mass, the mass the particle would have if it were not moving at
all. Photons, however, are moving at 2.998 × 10
8
m/s, and slowing them down to
zero speed is just not feasible. The rest mass you calculate for photons from the de
Broglie formula does not make any sense for a photon at the speed of light. Blame
this discrepancy on Einstein’s relativistic effect, if you like.
HERE’S WHAT WE KNOW SO FAR
■
Small particles behave both as waves and as particles.
■
Photons have both wave and particle characteristics.
■
The de Broglie equation illustrates the relationship between wave and particle
characteristics.
■
The Heisenberg uncertainty principle further verifies that small particles have
wave-like characteristics.
232 Chapter 6 Quantum Chemistry: The Strange World of Atoms
Erwin Schrödinger (1887–1961)
was awarded the 1933 Nobel Prize
in physics for his development of
the theory we use to describe the
structure of an atom.
6.9 The Mathematical Language
of Quantum Chemistry
The language of quantum chemistry is mathematics. In quantum chemistry,
knowing a few of the more important relevant mathematical concepts will pave
the way for us to explore the current understanding of atomic structure, which
has developed far beyond the Bohr model. The heart of our current understand-
ing of the atom lies within a simple-looking mathematical equation:
ˆ
H
n
= E
n
n
This is the time-independent Schrödinger equation. This equation allows us to
calculate the exact energies, E
n
, available to the electrons in an atom, if we know
the mathematical description,
n
, called the wave function, that describes the
positions and paths of the electron in its given energy level (n). Although it appears
harmless, the wave function,
n
, is a complex equation containing variables, con-
stants, and quantum numbers. This function tells us everything we can possibly
know about our system from a quantum mechanical perspective. It is called a
wave function because it is derived from equations developed to predict
the properties of waves. The
ˆ
H
in the Schrödinger equation is known as the
Hamiltonian operator after William Hamilton (1805–1865), an Irish mathemati-
cian who early on did work that hinted at wave–particle duality (Figure 6.18). In
simple terms, an operator is a mathematical term, such as +, −,
∗
, ⁄, or (in this
case)
ˆ
H
, that tells us how to proceed—what operation to do—in a mathematical
equation. The Hamiltonian is one of the more complex operators that we’ll en-
counter; it is too complex for us to carry out, in this discussion, the operation it
calls for. However, the conclusions that result from the operation are fundamental to
our proper view of atomic structure.
To illustrate the implications and utility of the Schrödinger equation, let’s ex-
amine the values of a wave function, shown in Figure 6.19, for a particle con-
strained to move in only one dimension.
FIGURE 6.18
William Hamilton (1805–1865), devel-
oper of the Hamiltonian operator.
Wave function
Energy
Probability density
8.35 10
–22
J
33.38 10
–22
J
75.11 10
–22
J
133.54 10
–22
J
208.65 10
–22
J
n = 1
n = 2
n = 3
n = 4
n = 5
FIGURE 6.19
The wave functions, energy levels, and
probability functions for a particle con-
fined to a one-dimensional box. The par-
ticle is defined by a wave function that
has nodes at each end of the wave, indi-
cating that the particle cannot be found
at the wall or outside the box.

6.9 The Mathematical Language of Quantum Chemistry 233
In this example, our particle can move between two points within that one
dimension. Think of this as a one-dimensional container or box that will confine
a particle. If we use the Schrödinger equation and solve for the energy of the par-
ticle, we find the first three wave functions shown starting from the bottom of the
box in Figure 6.19. These are called standing waves, which oscillate in time just
like the string on a violin, and we show them as a snapshot in time at their great-
est amplitude. The first level is described by half the wavelength of a sine wave.
The second level is described by a full sine wave and has a
node in the center of the
wave function—a point in the wave function where the amplitude is always zero.
The next level, represented by one and a half sine wavelengths, has two nodes that
divide the wave function into three segments. The wave function with n = 4
would have an additional node for a total of three, and the wave function n = 5
would have still another for a total of four.
Additionally, complex calculations of a particle in a one-dimensional box
result in an equation illustrating the permitted energy levels for this model:
E
n
=
n
2
h
2
8 mL
2
where n is the quantum level (n =1, 2, 3, . . .), h is Planck’s constant, m is the mass
of the particle, and L is the length of the box. Although the one-dimensional
model that gave rise to this equation is simple (it is based on a box containing a
particle), it explains the intense colors observed in leaves and flowers as well as
other materials. Certain molecules within a flower petal contain delocalized
electrons (electrons that are able to move across a molecule; we’ll have much more
to say about this in Chapter 9). The molecule itself acts as a one-dimensional
“box” because of its bonding structure. When a photon is absorbed by one of
these electrons, it becomes excited and moves to a higher energy level described by
our simple one-dimensional model. The energy differences between the lower
energy level and the excited energy level correspond to the color we observe. The
length of the bond or bonding structure defines the length of the box, L in the above
equation.
If we calculate the energy of an electron confined to a box the size of a single
covalent bond (1.5 × 10
−10
m), the wavelength of the energy needed to excite the
electron corresponds to ultraviolet light. If several atoms define the “box,” as is
seen in some types of molecules common within flower petals, then the energy
is smaller (because L is bigger), and the electrons can absorb visible light. For
example, cyanidin-based compounds, shown in Figure 6.20, are responsible for
the red color of apples, autumn leaves, roses, strawberries, and cranberry juice.
Changing the structure of the molecule changes the size of the box, which
changes the color of the photons absorbed.
Introducing Orbitals
One of the more interesting outcomes of the Schrödinger equation is that we can
use the wave function to reveal where an electron is most likely to be found within
an atom. We say“most likely”because we can never pin the electron down exactly,
as the Heisenberg uncertainty principle tells us. Instead, we can only determine
the probability of finding it at each point in space. The space that the electron is
allowed to occupy in a given energy level on an atom is called an
orbital.
For example, if we plot the wave function of an electron around a nucleus, we
get a figure similar to that shown in Figure 6.21. We can mathematically manip-
ulate the wave function to create a new function depicted as
∗
, which looks a
lot like the square of the wave function . The great usefulness of
∗
is that it
is proportional to the probability of finding the particle at a particular point in space.
The nodes in remain at the same places in
∗
, but all values of
∗
are pos-
itive. In short, the probability function describes the spatial distribution of the elec-
tron around an atom;
∗
describes the shape of an orbital.
HO
OH
OH
OH
OH
O
Cyanidin
+
FIGURE 6.20
This molecule is responsible for many of
the colors found in plants.
The colors we see in a flower are due to
the molecules that are present in the
petals. These molecules can be thought
of as examples of the one-dimensional
particle in a box.
234 Chapter 6 Quantum Chemistry: The Strange World of Atoms
12345
0.4
0.6
0.3
0.2
0.1
0
0.5
Radial distance
Wave function value
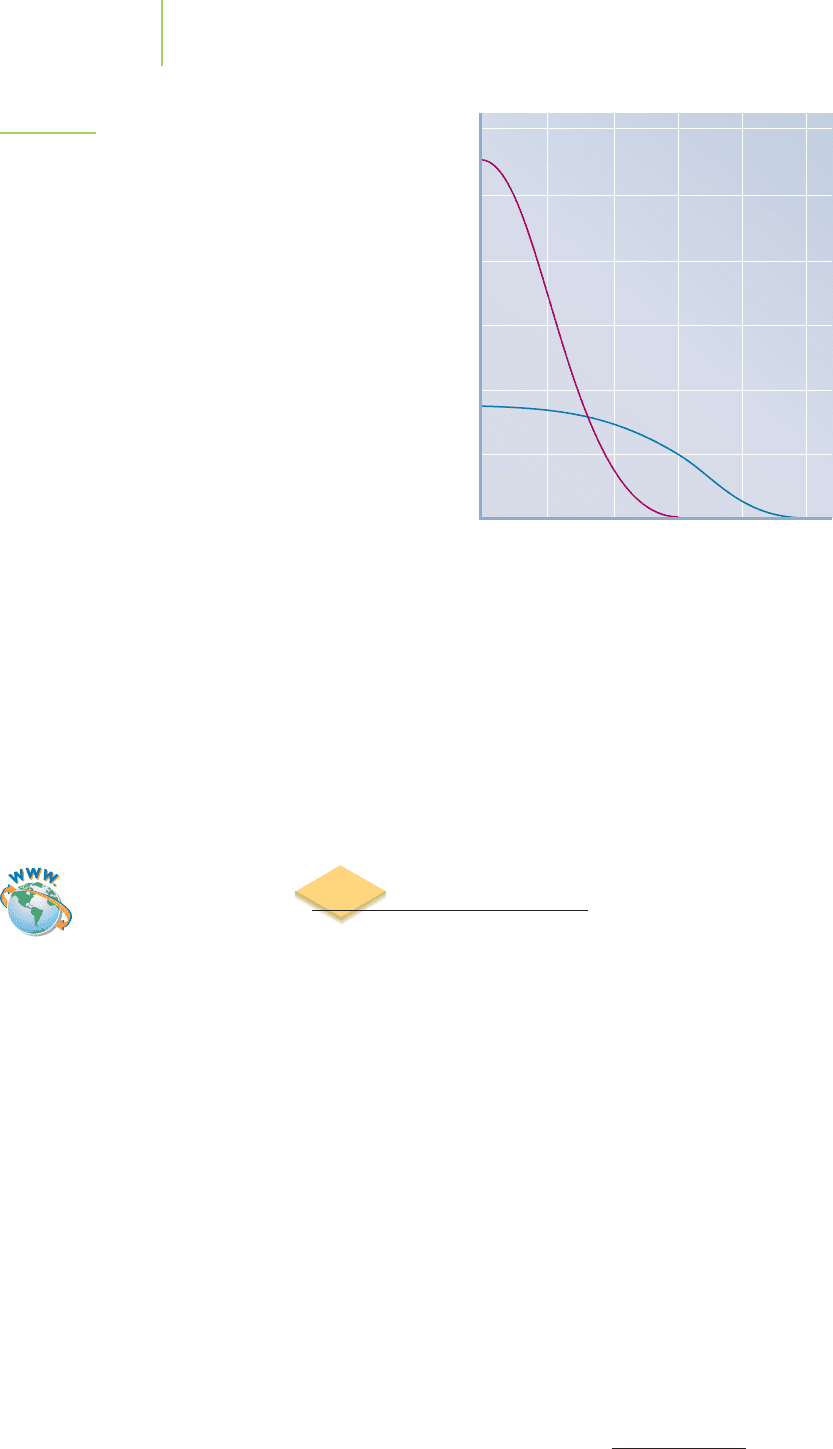
FIGURE 6.21
A plot of the wave function (blue line) represented as the
distance from the nucleus. The probability function (*;
red line) represents the most likely shape of the orbital re-
sulting from the wave function.
The idea that a single particle, like an electron, doesn’t exist at a single point
in space, but is merely more likely to be found at certain places and less likely to
be found at others, is admittedly rather odd. But it fits the ideas presented here
and years of measurements that confirm these ideas. Note that there are nodes
where we can guarantee that the particle will never be found. At these nodes, the
probability function,
∗
, is equal to zero. An electron can never be found at a
node. The electron can be found on either side of the node, but never at the node.
How does it go from one side to the other, if it is never allowed to be on the node?
It does this by behaving like a wave, which has amplitude on both sides of the
node: a clear demonstration of wave–particle duality.
6.10 Atomic Orbitals
Both the Hamiltonian operator and the wave functions for the hydrogen atom
are known, and they allow us to develop both mathematical and visual represen-
tations of the orbitals that electrons can occupy in the three-dimensional space
around the hydrogen nucleus. Specialists in quantum chemistry describe this
three-dimensional system in terms of a radial part and an angular part.
Radial
means “along the radius” and angular means “as a function of the angles that
describe the orientation of the radius.” Because the calculations of these three-
dimensional orbitals are quite complicated, we present only the results here. We
will use these results to explain, over the next several chapters (and, from time to
time, for the remainder of the textbook), how and why atoms and molecules
interact the way they do.
Quantum Numbers and s Orbitals
There are four different quantum numbers in the wave function for the electron in
the hydrogen atom, and they are given the symbols n, l, m
l
, and m
s
. The n is
known as the
principal quantum number and can be any whole number n = 1, 2,
3,...,∞. We are already familiar with the principal quantum number. This n is
what Bohr referred to as the energy levels in his atom. Based on the possible
values of n, there are an infinite number of energy levels for the hydrogen atom.
The energy of each is given by
E
n
=−
2.1786 ×10
−18
J
n
2
Video Lesson: Atomic Orbital
Size
6.10 Atomic Orbitals 235
which is just what was predicted by the Bohr model. In atoms with more than one
electron, any electrons with the same value for the principal quantum number
(n) are said to occupy the same
principal shell, but only the energy levels in the
hydrogen atom are given by this equation.
Two of the other three quantum numbers, l and m
l
, depend on the value of n.
The
angular momentum quantum number, l, can be any number from zero to n − 1
in whole-number steps:
l = 0,1,2,...,n − 1
This quantum number is considered to represent the shape of the electron orbital.
In the first energy level, n = 1, the equation used to calculate the angular mo-
mentum quantum number reveals that l =0. This implies that only one shape of
orbital is possible in the first energy level. In the second energy level, n = 2, the
value of l can be 0 or 1. This means that there are two different shapes of the
orbitals in the second energy level. When n =3, l can be 0, 1, or 2 (three different
shapes). And so on, all the way to infinity. Any electrons that share the same
values for n and l are said to occupy the same
electron subshell.
The m
l
quantum number is the magnetic quantum number (also known as the
orbital angular momentum quantum number). It is based on the value of l and
can have any value from l to −l in whole numbers.
m
l
=−l,...,−2, −1,0,1,2,...,l
This quantum number can be thought of as defining the direction in which the
individual electron orbitals are pointed. For example, a specific orbital could be
oriented along the x axis or the y axis. The value of m
l
indicates this direction.
The number of possible values for the magnetic quantum number indicates
the number of specific orbitals of a specified shape, as illustrated in Figure 6.22.
For example, in the second principal shell, when n = 2, we determined that there
are two values for l, l = 0 and l = 1. We need to consider each of these values to
generate all the possible sets of quantum numbers. When n =2 and l =0, m
l
can
only be 0 (one orbital). When n =2 and l =1, m
l
can be −1, 0, or +1. In this case,
we have three specific orbitals of the same shape oriented in three different direc-
tions. Calculating all of the possible orbitals within an energy level can be a
n
1
2
2
3
3
3
4
4
4
4
l
0
0
1
0
1
2
0
1
2
3
m
l
0
0
1, 0 –1
0
1, 0, –1
2, 1, 0, –1, –2
0
1, 0, –1
2, 1, 0, –1, –2
3, 2, 1, 0, –1, –2, –3
Subshell
notation
1s
2s
2p
3s
3p
3d
4s
4p
4d
4f
Number of
orbitals in the
subshell
1
1
3
1
3
5
1
3
5
7
Number of
electrons needed
to fill subshell
2
2
6
2
6
10
2
6
10
14
Maximum possible
number of electrons
in shell
2
8
18
32
FIGURE 6.22
Allowed values for n, l,
m
l
, and m
s
quantum num-
bers for hydrogen atomic
orbitals.
Video Lesson:
Atomic Orbital
Shapes and
Quantum
Numbers
(n, l, m
l
, m
s
)
Energy Level 1:
Energy Level 2:
Energy Level 3:
(1, 0, 0, + ); (1, 0, 0, – )
(2, 0, 0, + ); (2, 0, 0, – )
(2, 1, 1, + ); (2, 1, 0, + ); (2, 1, –1, + )
(2, 1, 1, – ); (2, 1, 0, – ); (2, 1, –1, – )
(3, 0, 0, + ); (3, 0, 0, – )
(3, 1, 1, + ); (3, 1, 0, + ); (3, 1, –1, + )
(3, 1, 1, – ); (3, 1, 0, – ); (3, 1, –1, – )
(3, 2, 2, + ); (3, 2, 1, + ); (3, 2, 0, + ); (3, 2, –1, + ); (3, 2, –2, + )
(3, 2, 2, – ); (3, 2, 1, – ); (3, 2, 0, – ); (3, 2, –1, – ); (3, 2, –2, – )
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
1
–
2
FIGURE 6.24
Systematic bookkeeping for quantum numbers.
236 Chapter 6 Quantum Chemistry: The Strange World of Atoms
daunting task because the number of orbitals gets large fairly
quickly, as shown in Figure 6.23. But as long as you are systematic
about what is essentially a bookkeeping task, confusion can be
kept to a minimum.
The final quantum number, m
s
, is known as the electron spin
quantum number
(it is also known as the spin angular momentum
quantum number). Fortunately for our bookkeeping, the only
possible values for m
s
are +
1
/
2
and −
1
/
2
. The value of m
s
can be
either +
1
/
2
or −
1
/
2
for every set of n, l, and m
l
allowed.
We can use shorthand to indicate the four quantum numbers
that describe the position of electrons in an atom using the nota-
tion (n, l, m
l
,m
s
). As shown in Figure 6.24, for n = 1 the sets of quantum num-
bers for the electrons are (1, 0, 0,
1
/
2
) and (1, 0, 0, −
1
/
2
). For n = 2 the sets are (2,
1, 1,
1
/
2
), (2, 1, 1, −
1
/
2
), (2, 1, 0,
1
/
2
), (2, 1, 0, −
1
/
2
), (2, 1, −1,
1
/
2
), (2, 1, −1, −
1
/
2
),
(2, 0, 0,
1
/
2
), and (2, 0, 0, −
1
/
2
). If you try n = 3, you should get 18 sets of quantum
numbers, and for n =4 you should get 32. Note how this follows a general trend.
The number of possible quantum numbers in each energy level is 2n
2
.
How do these values help us model the electronic structure of atoms? Although
the specific set of four quantum numbers represents a convenient label for a spe-
cific hydrogen atomic wave function, quantum numbers are much more than
that. The principal quantum number, n, gives the energy of the system and sets
the values of the l and m
l
quantum numbers. Together, the n, l, and m
l
tell us
about the spatial distribution of the electron and define the orbital shape and
size. For example, as shown in Figure 6.25, the radial component of the orbitals
with n = 1, 2, and 3 illustrates the difference in size of the atomic orbitals.
Orbitals that have equal energies are said to be
degenerate orbitals, and the
number of orbitals having the same energy is called the degeneracy. In the
hydrogen atom, orbitals with the same value of n and l have the same specific
amount of energy, and these orbitals are therefore degenerate. Consider the
orbitals within the second energy level (n =2). When n =2, l can be either 0 or 1.
When l = 0, the value of m
l
can be only 0. There is only one orbital, so it can’t be
degenerate. However, when l =1, m
l
can be −1, 0, or +1. In this case, three orbitals
with the same value for l are possible. These three orbitals have the same amount
of energy and are degenerate. The third energy level contains two sets of degener-
ate orbitals. One set contains three degenerate orbitals (l =1, m
l
=−1, 0, +1); the
other set contains five degenerate orbitals (l =2, m
l
=−2, −1, 0, +1, +2).
n
3
0
1
2
0
–1, 0, +1
–2, –1, 0, +1, +2
l
m
l
FIGURE 6.23
Outline of n, l, and m
l
values.
Nodes
Node
3s2s1s
(b)
3s2s1s
(a)
FIGURE 6.25
(a) The radial component of the probabil-
ity for the hydrogen atomic orbitals with
n =1, 2, and 3. (b) The spatial compo-
nent of those orbitals at 90% probability.
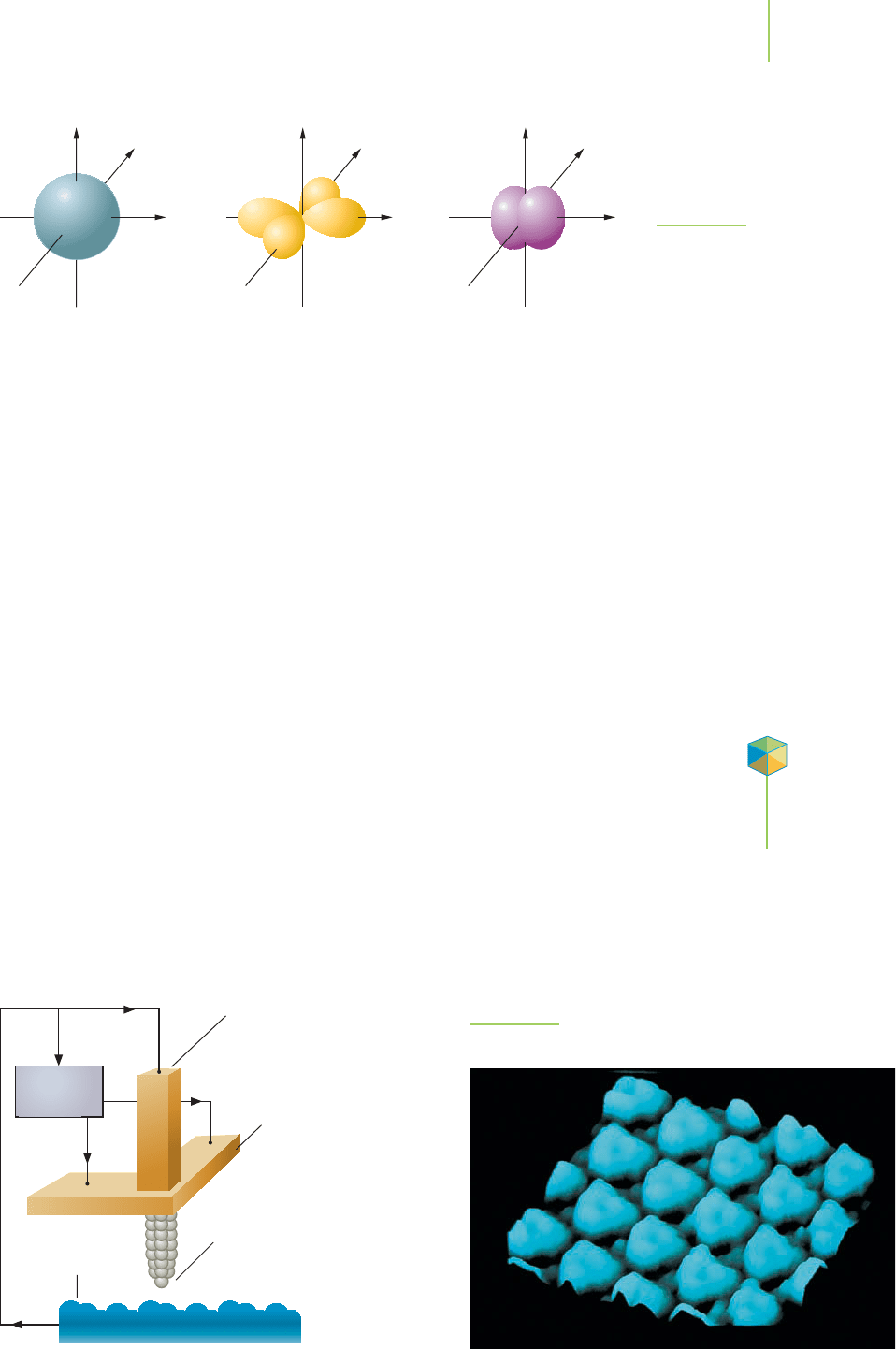
FIGURE 6.27
An STM tip scans over an object to produce an image.
6.10 Atomic Orbitals
237
Quantum chemists have been able to show us pictures that describe what the
quantum numbers tell us. For example, Figure 6.26 also shows us shapes of many
of the orbitals that we have described with quantum numbers. Remember that at
the atomic level, we can no longer speak in terms of absolute locations but can
only give the probability of the quantum particle being in a given location. The
pictures therefore can show us only the most probable place to find the electron
in the orbital. This means that if you were in Champaign, Illinois, studying a hy-
drogen atom in an atom trap, and you asked a friend to see whether he could find
your atom’s electron in Perth, Scotland, there would be a finite probability (albeit
outrageously small) that he would be able to find it there. We do not have instru-
ments that are sensitive enough to measure the tiny probability of an electron
existing in Scotland when its atom’s nucleus is in Illinois. To be honest, we would
be hard pressed to measure the probability even a few millionths of a millimeter
from the atom.
The Scanning Tunneling Microscope
The scanning tunneling microscope (STM) allows us to draw a picture that rep-
resents the surface of a material. This microscope is so powerful that it can even
“see” single molecules and atoms. The STM basically uses an atomic-sized needle
that runs across the surface of a material. As the tip moves, it runs up and over
molecules or atoms, and changes in the position of the tip of the needle are
recorded on a graph. It works because if we bring the tip of the needle close
enough to the sample material, the wave function of the atoms in the tip will
overlap with those of the sample. This allows electrons to move from the tip into
the material by a process called quantum tunneling. The electrons that “tunnel”
Application
C
HEMICAL ENCOUNTERS:
The Scanning
Tunneling
Microscope
Computer
Sample
surface
1 atom wide
tungsten probe
Rods move probe
across sample
Probe moves up and down
to maintain same distance
from sample
z
x
y
z
x
yy
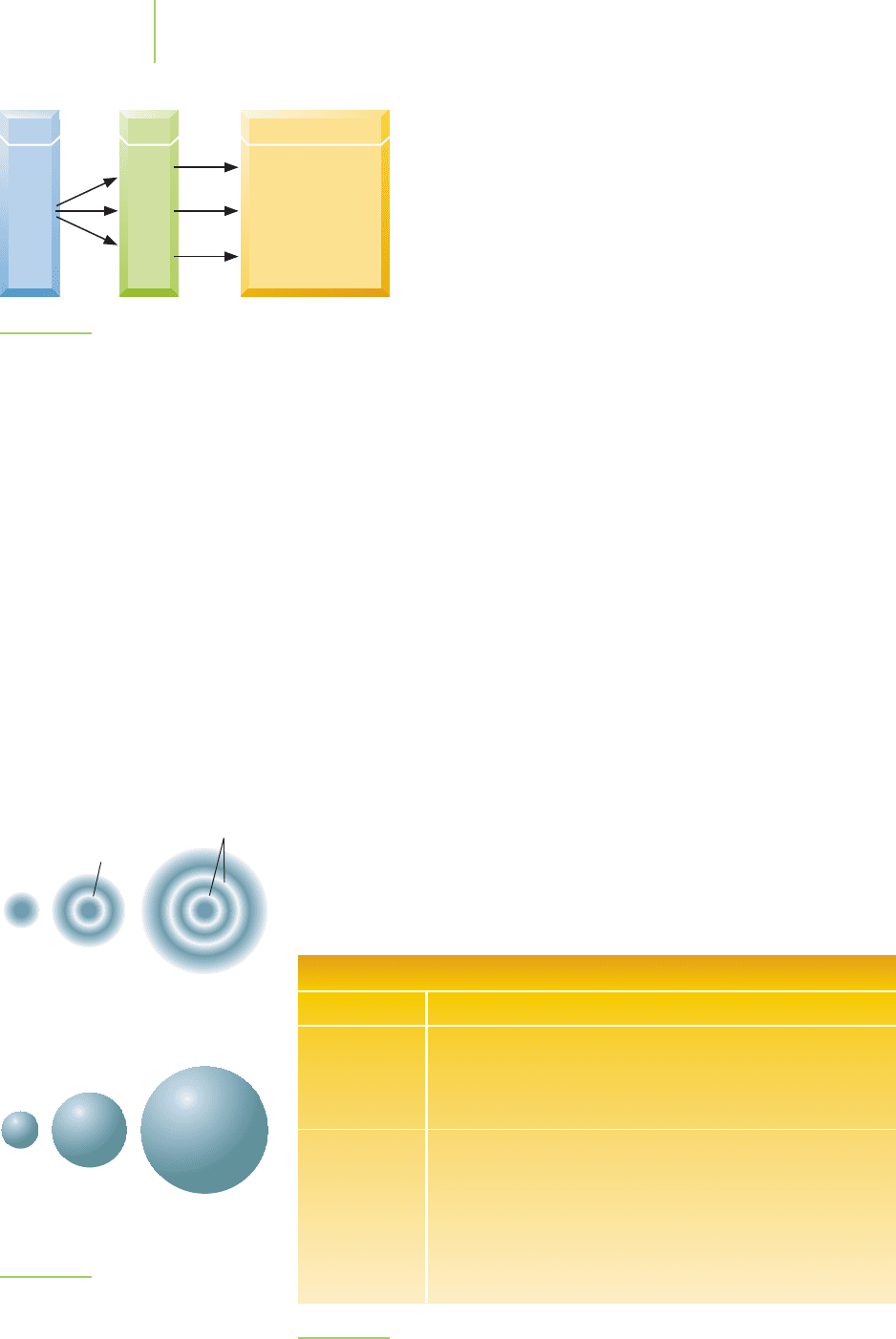
2s orbital 3d
x
2
–y
2 orbital 2p
x
orbital
x
z
FIGURE 6.26
Some atomic orbitals.
