Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


238 Chapter 6 Quantum Chemistry: The Strange World of Atoms
from the tip to the material set up an electric current (the “tunneling current”)
that increases as we bring the tip closer to the atom and decreases as we pull away.
This allows us to image the atomic positions (though only to a level of precision
limited by Heisenberg’s uncertainty principle) by scanning the tip over the mate-
rial and seeing just what kind of overlap we get. An example image is shown in
Figure 6.27. The bigger the tunneling current is, the more electrons there are in
proximity to the tip. As we pull the tip away from the surface, the tunneling cur-
rent falls off exponentially with distance. Because we assume that the atom is in
the center of the greatest electron density, we get an image of the atoms at the sur-
face of the material. The image that results from these measurements conveys
information about the positions of electrons in atoms. These are not pho-
tographs of atoms, but they are impressive testimony to our ability to detect
where individual atoms are, within the constraints of the uncertainty principle.
Even more impressive, the image that opens this chapter shows the genuine wave-
like nature of matter at the quantum scale. Look at the ripples: direct evidence
that wave–particle duality is real!
The STM is a practical application of the fact that we don’t actually have a nat-
ural end to the radial distribution function. It goes on forever and ever, just with
increasingly smaller and smaller amplitude. According to classical mechanics the
STM should not work, because electrons should not be able to “tunnel” into
the places classical mechanics says they cannot go. The STM is practical proof
that quantum mechanics is a valid description of nature.
s Orbitals
What can we say about the shapes of the orbitals within an atom? The orbital
shown in Figure 6.26 that has l = 0 is radially symmetric and has a spherical
shape. That is, as we look out from the nucleus, the probability distribution func-
tion looks the same in every direction and varies only with r, the distance from
the nucleus. All orbitals with l = 0 are called
s orbitals and can be written as s.The
principal quantum number is added to this as a prefix when we write the name of
this specific orbital. For example, when n = 1 and l = 0, we write 1s. When n = 2
and l =0, we write 2s, and so on. The 1s orbital, with n =1, has no nodes and rep-
resents the lowest energy level, or ground state, for an electron in a hydrogen
atom. The 2s orbital has one node that is concentric around the nucleus. The 3s
orbital has two radial nodes, the 4s has three, and so on, with the number of con-
centric nodes increasing along with the principal quantum number.
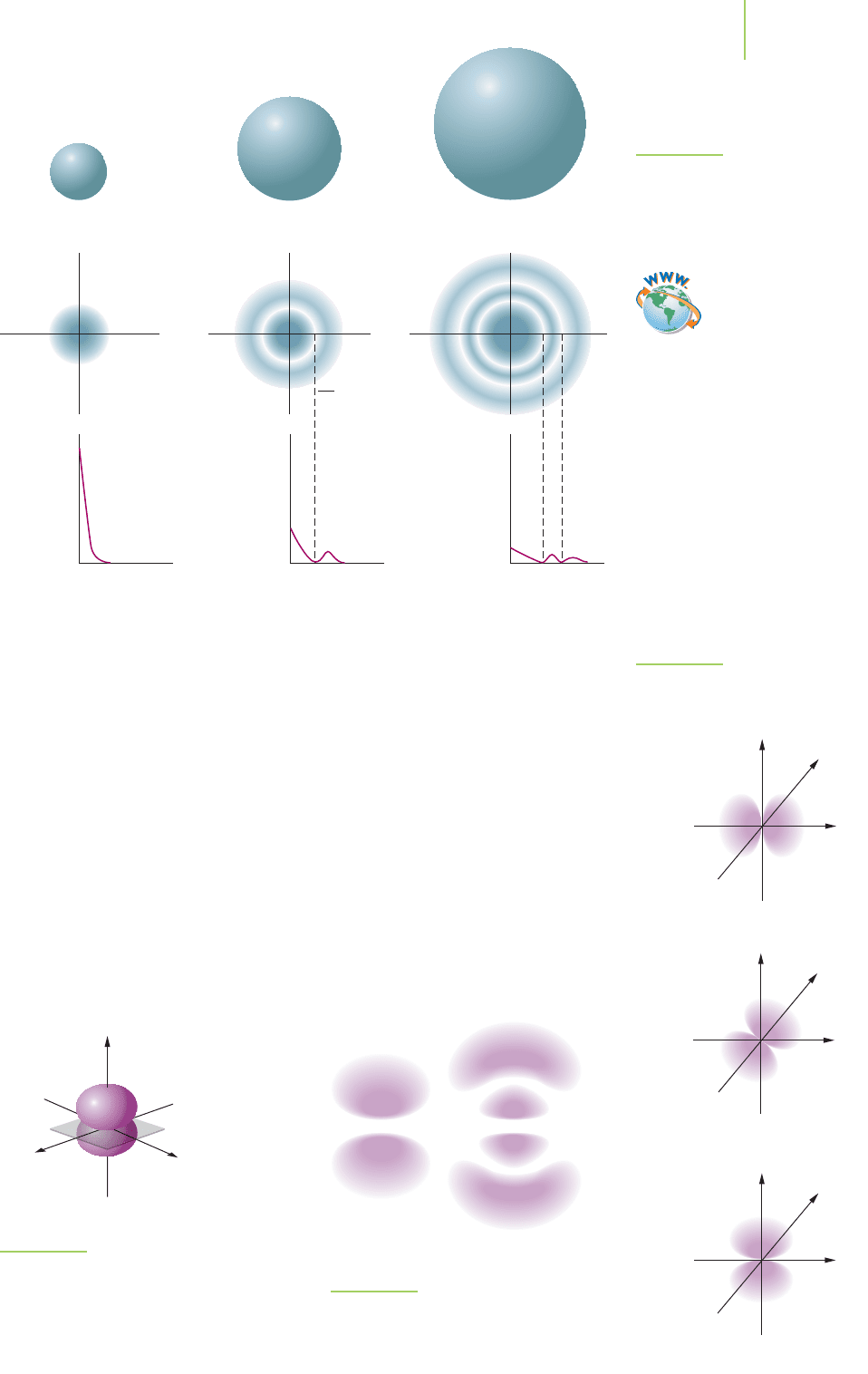
The “solid spheres”of Figure 6.28 are useful in comparing relative sizes. As we
expect, the 1s orbital is the smallest, and the size increases as n increases. This
representation does not give us any help in visualizing the nodes present; the
two-dimensional cross sections are more helpful in this respect. The last method
of representing the s orbitals takes a more probabilistic approach and uses a gray
scale to show where the probability of finding the electron is large and where it is
small. The nodes are indicated by the lightest part of the picture. All s orbitals are
spherical, with the number of radial nodes increasing from zero in the 1s orbital
to n − 1 in the ns orbital. Remember that s orbitals all have orbital angular mo-
mentum quantum numbers of l = 0 and magnetic quantum numbers of m
l
= 0.
We still have to consider the Heisenberg uncertainty principle as we construct
our pictures. In order to draw sensible pictures of atomic sizes, we arbitrarily
assign the boundary of an atom to lie at some distance r from the center of the
atom, so that when we look for the electron within that value of r from the
nucleus, we find the electron a large portion (90%) of the time. We show the 1s,
2s, and 3s orbitals in Figure 6.28 at the 90% probability distribution function for
the electron and in several different formats, each of which is meant to emphasize
some aspect of the orbital shape.
An s orbital.
Visualization: 1
s
Orbital
2
1s
2
2s
2
3s
n = 3, l = 0
3s
n = 2, l = 0
2s
r
Node
rr
n = 1, l = 0
1s
(a) (b) (c)
FIGURE 6.29
The 2p
x
,2p
y
, and 2p
z
spatial component
of the hydrogen atomic orbitals at 90%
probability.
6.10 Atomic Orbitals
239
x
y
z
2p
x
x
y
z
2p
y
y
z
x
2p
z
z
y
x
p
z
FIGURE 6.30
The location of the planar node found in
the 2p
z
orbital. Although n = 2 is used in
the example, all p orbitals have a single
planar node in an analogous position.
FIGURE 6.28
The 1s,2s, and 3s spatial component of
the hydrogen atomic orbitals at 90%
probability. Note the presence of nodes
in the cutaway plots.
2p
z
3p
z
FIGURE 6.31
The 2p
z
and 3p
z
spatial component of the hy-
drogen atomic orbitals at 90% probability.
p Orbitals
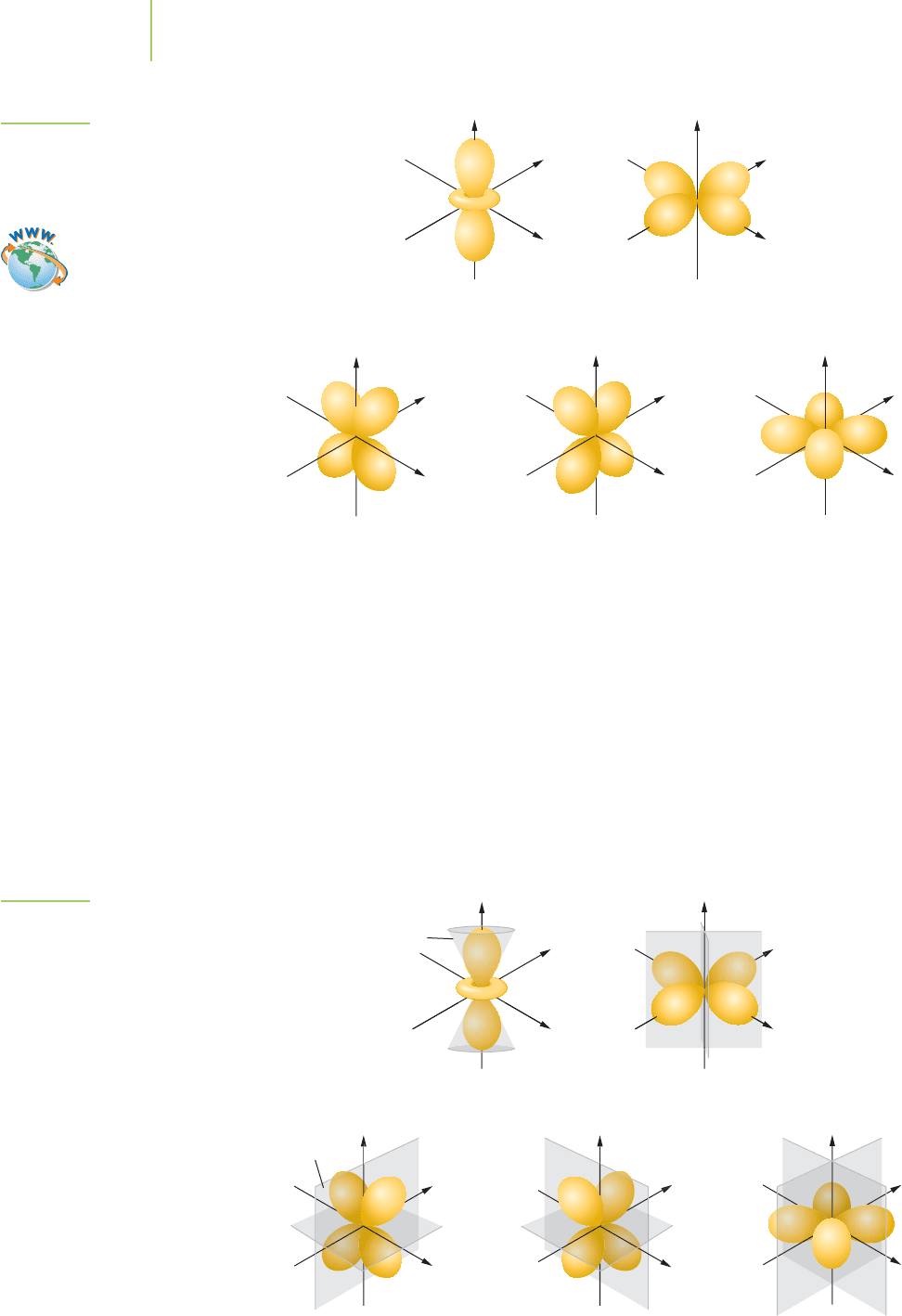
For orbitals with l = 1, which are known as p orbitals are abbreviated p, we have
a different spatial arrangement, as shown in Figure 6.29. Again we use the
convention of writing the principal quantum number as a prefix to the letter ab-
breviation for the orbital. However, because l = 1 allows for three possible m
l
values (+1, 0, and −1), there will be three degenerate orbitals that are symmetri-
cally equivalent. We can choose to orient the three equivalent orbitals along the x,
y, and z axes, and when we do so, we call them the 2p
x
,2p
y
, and 2p
z
orbitals. This
is shown for the lowest-energy 2p orbitals in Figure 6.29. The 2p orbitals have no
radial nodes, but each does have a planar node, as illustrated in Figure 6.30. For
the 2p
x
orbital, this node is the yz plane; for the 2p
y
orbital, this node is the xz
plane; and for the 2p
z
orbital, this node is the xy plane. We have set the size of the
orbital to include 90% of the probability for finding the electron within it, just as
we did with the s orbitals.
When we increase the primary quantum number to n = 3, we have three 3p
orbitals with the same kind of planar node structure (Figure 6.31), but now we also
have a radial node. The three 4p orbitals have the planar node plus two radial nodes,
the three 5p orbitals have the planar node plus three radial nodes, and so on.
Visualization: 2
p
x
Orbital
Visualization: 2
p
y
Orbital
Visualization: 2
p
z
Orbital
z
x
y
z
x
y
z
x
y
z
y
x
y
d
xy
d
x
2
–y
2
d
yz
d
xz
d
z
2
x
z
FIGURE 6.32
The 3d
xy
,3d
yz
,3d
xz
,3d
x
2
−y
2
, and 3d
z
2
spatial component
of the hydrogen atomic orbitals at 90% probability.
240 Chapter 6 Quantum Chemistry: The Strange World of Atoms
d Orbitals
Orbitals with l = 2 are called d orbitals (abbreviated as d) and have two nonradial
nodes. The first set of quantum numbers for which l can equal 2 occurs when
n = 3. Five d orbitals occur, because m
l
takes the values of 2, 1, 0, −1, −2 when
l = 2. For all but the m
l
= 0 d orbital, the nodes are planar and are at 90° to each
other. These orbitals have four lobes that are labeled d
xy
, d
yz
, d
xz
, and d
x
2
−
y
2
and
are oriented as shown in Figure 6.32. The remaining d orbital is labeled d
z
2
and looks a bit different but is equivalent in energy (degenerate) to the other four
d orbitals provided that it has the same principal quantum number. The d
z
2
or-
bital has two hyperbolic nodes that give the orbital a dumbbell structure with a
little toroidal “donut.” When n = 3, no radial nodes occur, as shown in Figure
6.33, but n = 4 has one radial node, n = 5 has two radial nodes, and d orbitals
with higher principal quantum numbers have n − 3 radial nodes.
z
x
y
z
y
z
x
y
z
y
x
y
Nodal
surface
Nodal
plane
d
xy
d
x
2
–y
2
d
yz
d
xz
d
z
2
x
z
x
FIGURE 6.33
The location of the two planar nodes found in the
3d
xy
,3d
yz
,3d
xz
, and 3d
x
2
−y
2
lobes and the two hy-
perbolic nodes orbitals of the 3d
z
2
lobe. Although
n = 3 is used in the example, all d orbitals have
two planar nodes in analogous positions.
Visualization: 3
d
x
2
−
y
2
Orbital
Visualization: 3
d
xy
Orbital
Visualization: 3
d
x
2
Orbital
Visualization: 3
d
yz
Orbital
Visualization: 3
d
z
2
Orbital
6.10 Atomic Orbitals 241
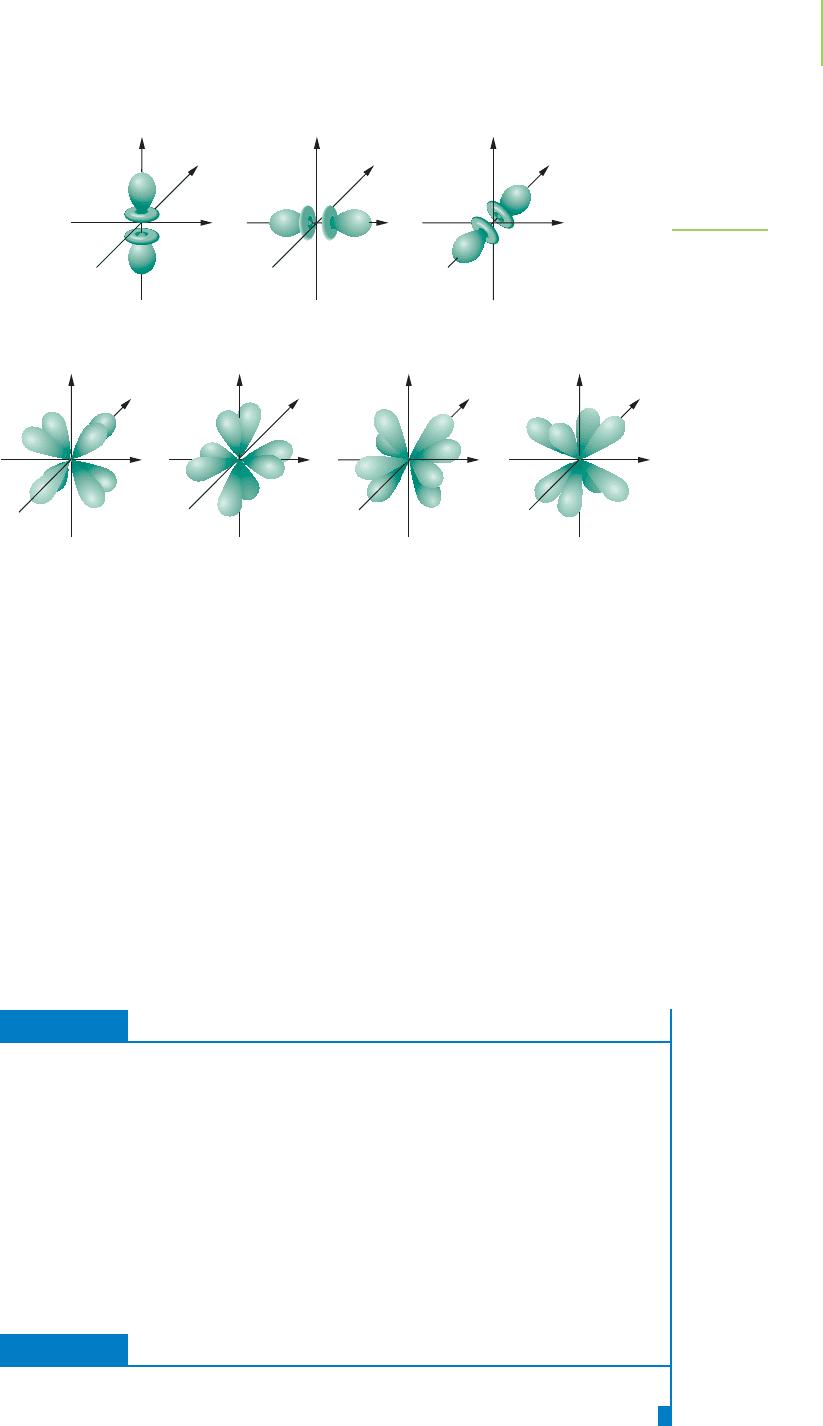
f Orbitals
Much of our use of orbitals focuses on the s, p, and d levels. The one exception is
the
f orbitals (abbreviated f ) with l = 3 and m
l
= 3, 2, 1, 0, −1, −2 , −3. These
seven orbitals will be used in our description of heavy atoms, such as those in the
lanthanide and actinide series of the periodic table. They are shown in
Figure 6.34. Note that they are multilobed and geometrically quite complicated.
At last we have arrived at our current “quantum mechanical” or “wave me-
chanical” model of atomic structure, although we can depict it only by showing
the places around the nucleus where an electron in any orbital will most probably
be found. In painting a more accurate picture of what electrons are doing, we
have had to acknowledge the uncertainty and strangeness—the funny things that
happen—down in the quantum world.
EXERCISE 6.7 Quantum Numbers
Indicate the possible values for the quantum numbers (n, l, m
l
) that would corre-
spond to the 3d orbital.
Solution
A single 3d orbital would correspond to one of these sets of quantum numbers:
n = 3, l = 2, m
l
=−2
n = 3, l = 2, m
l
=−1
n = 3, l = 2, m
l
= 0
n = 3, l = 2, m
l
=+1
n = 3, l = 2, m
l
=+2
PRACTICE 6.7
Indicate the orbital described by the quantum numbers, n = 2, l = 1, m
l
= 0.
See Problems 86 and 117.
z
x
yy y y
z
x
z
x
z
x
z
x
yyy
z
xx
f
z
3
– —
3
5
zr
2
f
xyz
f
y(x
2
– z
2
)
f
x(z
2
– y
2
)
f
z(x
2
– y
2
)
f
x
3
– —
3
5
xr
2 f
y
3
– —
3
5
yr
2
FIGURE 6.34
The 4f spatial component of the hydrogen
atomic orbitals at 90% probability.
S
(b)
SN
N
(a)
e
–
e
–
242 Chapter 6 Quantum Chemistry: The Strange World of Atoms
6.11 Electron Spin and
the Pauli Exclusion Principle
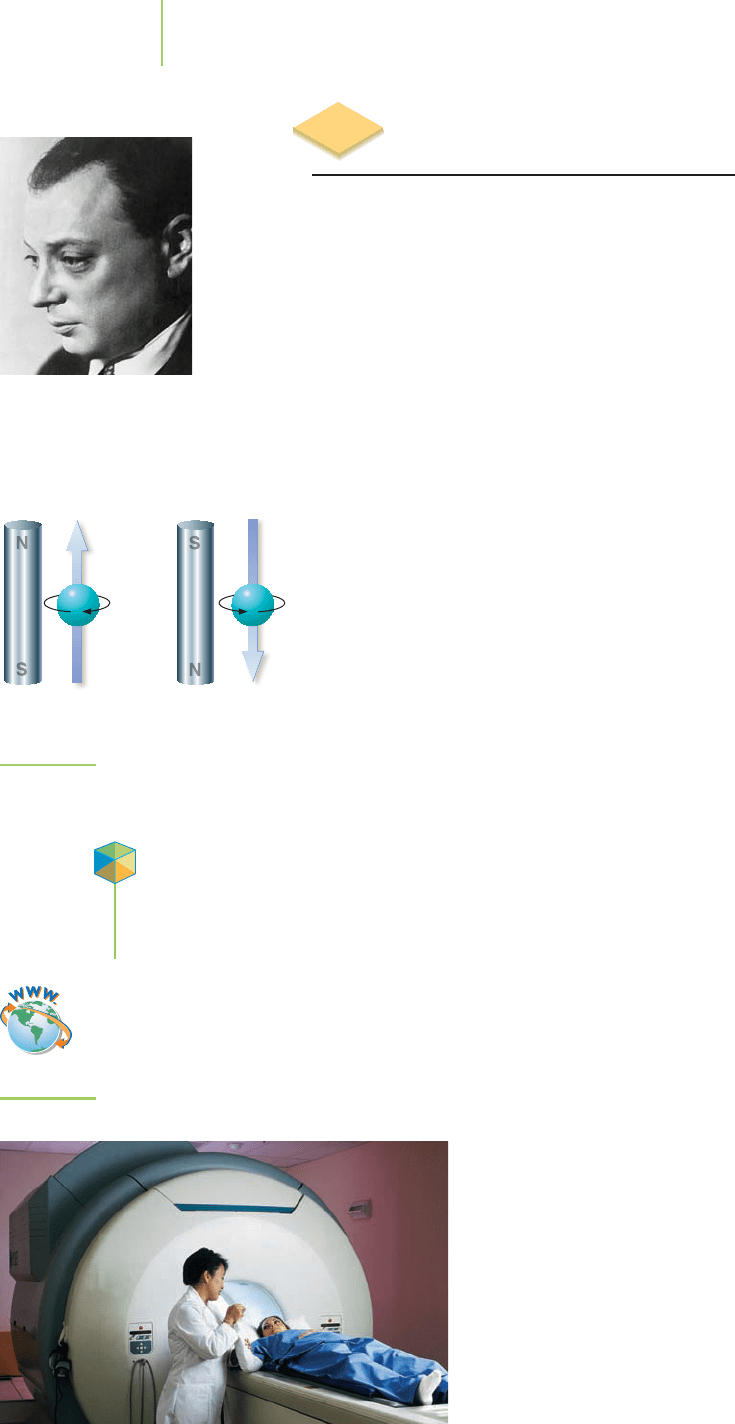
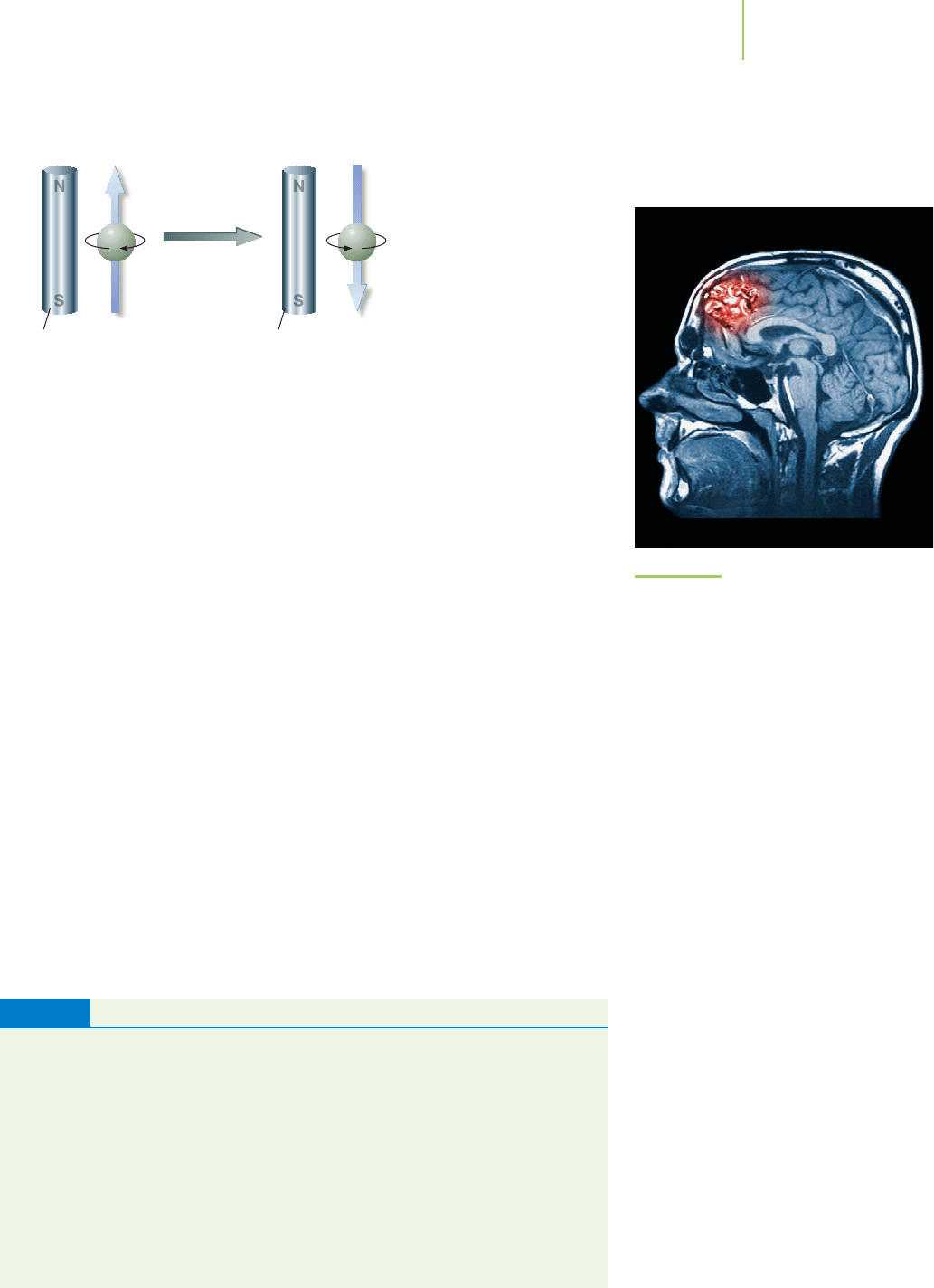
An electron behaves as though it were spinning about an axis. A “spin” in one
direction corresponds to an electron spin quantum number (m
s
) of +
1
/
2
, and a
“spin” in the opposite direction corresponds to an electron spin quantum
number (m
s
) of −
1
/
2
. These are the only two values of m
s
available; in other
words, spin is quantized into these two states, and an electron must be in one of
them. The “spin” of an electron makes the electron behave as though it were a tiny
magnet. For example, if we put hydrogen atoms into a magnetic field, electrons
with m
s
=
1
/
2
, called the “up” state, will line up parallel to the field; and those with
m
s
=−
1
/
2
, called the “down” state, will line up antiparallel to it, as shown in
Figure 6.35. We sometimes represent the up state by an arrow pointing up:
↑
.
Similarly, the down state is represented by an arrow pointing down:
↓
.
One vital consequence of this idea of electron “spin” is that it allows two elec-
trons to occupy the same atomic orbital. Wolfgang Pauli, in 1925, proposed a rule
that allows only two electrons to occupy the same atomic orbital. According to
the
Pauli exclusion principle, no two electrons can have the same set of the four
quantum numbers (n, l, m
l
, and m
s
) in a given atom. What does this tell us about
the electronic structure of the atom?
For two electrons to occupy the same orbital,
they must have the same values for the quantum numbers n, l, and m
l
. The only
way that two electrons can have these quantum numbers is if their spin quantum
numbers (m
s
) are different. In essence, one of the electrons in the orbital is in the
spin up state, and the other is in the spin down state. Because it requires four quan-
tum numbers to describe an electron on an atom, it is not possible for more than two
electrons to occupy the same orbital.
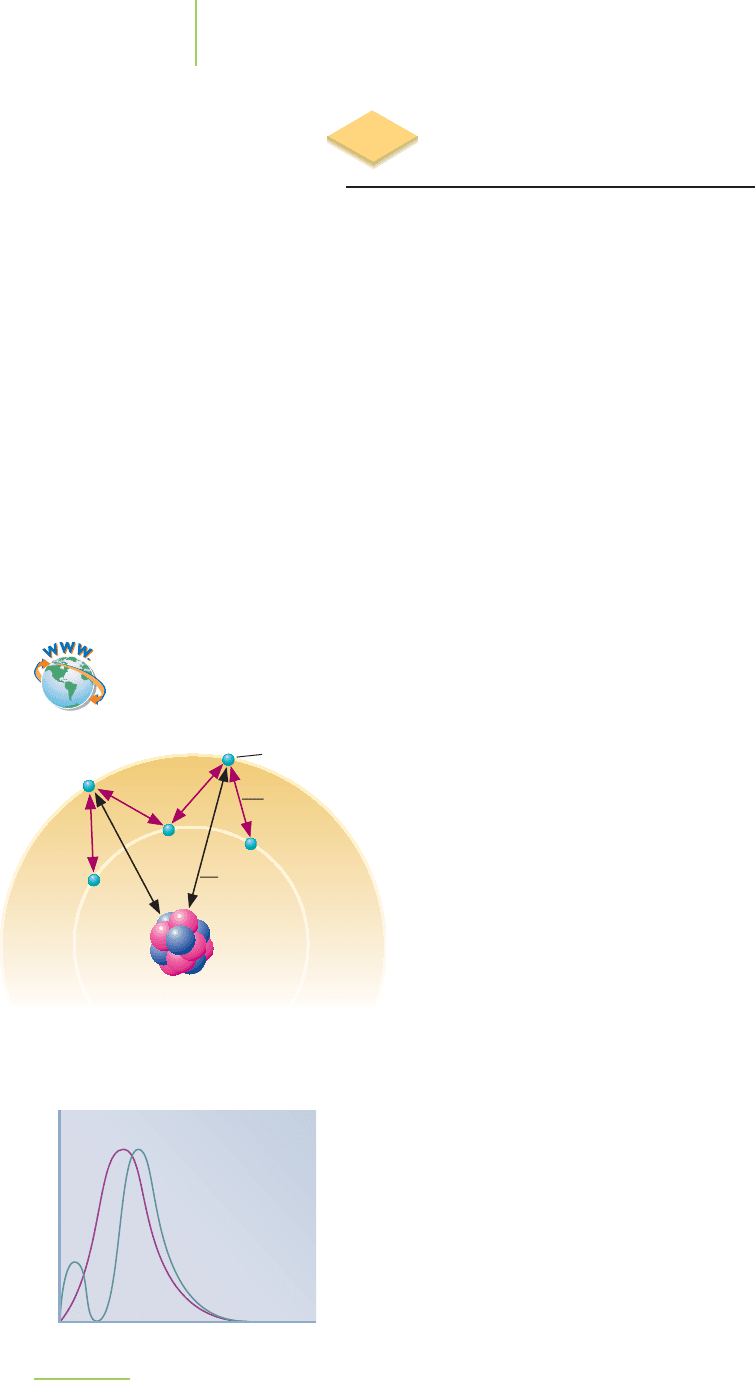
Nuclear Spin and Magnetic
Resonance Imaging
An MRI scanner consists of a large magnet with a cylindrical tube bored through
the center. An example is shown in Figure 6.36. When a person is scanned, the
body part to be imaged needs to be in the center of the magnetic field. The tech-
nique focuses on the nuclei of all of the hydrogen atoms in the body, because they
have a relatively large magnetic moment (a measure describing the magnitude
and direction of the nuclei’s magnetic field). Placing the body in the magnet
causes the hydrogen nuclei to align with the magnetic field. Just as with electrons,
there are nuclei with both up and down “spins,” so most of the nuclear “spins”
cancel each other out. But there are enough “unpaired” nuclei to result in a nice
image.
The image is created by directing radio frequency pulses toward the body part
to be examined. The radio pulses produce a miniature magnetic field aligned in a
direction perpendicular to the field of the magnet around the
body. This miniature magnetic field disrupts the alignment of
the atoms in the body part. The atoms are then said to occupy
an excited state. When the radio pulse is turned off, the atoms
relax to their original state of alignment with the magnet and
release energy. The energy is detected by the MRI and con-
verted into an image of the body part.
How does the computer in the MRI distinguish the struc-
tures in the body? It does so on the basis of the amount of
time it takes for the excited hydrogen nuclei to relax back to
their original alignment with the external magnetic field. The
realignment of the excited nuclei can last anywhere from a
few hundred milliseconds to a few seconds. The time it takes
Wolfgang Ernst Pauli (1900–1958) was
awarded the 1945 Nobel Prize in physics
for his discovery of what we now know
as the Pauli exclusion principle.
FIGURE 6.35
Electrons in the spin up and
spin down states.
Application
C
HEMICAL ENCOUNTERS:
Nuclear Spin and
Magnetic Resonance
Imaging
FIGURE 6.36
MRI scanner.
Video Lesson: Understanding
Electron Spin
6.11 Electron Spin and the Pauli Exclusion Principle 243
to realign is heavily dependent on the environment surrounding the hydrogen
nuclei. For example, hydrogen nuclei in water (which is the primary component
in blood and spinal fluid) relax much more rapidly than hydrogen nuclei that
make up tissues, which in turn are faster than hydrogen atoms in fats. The differ-
ence is primarily due to the fact that hydrogen atoms are attached to different
types of atoms in each of these three components of the body. It is the variation
in relaxation times, combined with the differing concentrations of hydrogen
atoms in different tissues and body fluids, that allows for the exceptional contrast
observed using this technique. Figure 6.37 shows an MRI image of a brain, where
color has been added to help distinguish the different tissues. This image can be
used to locate the position of a brain tumor and the major blood vessels leading
to it. MRI scanners are used to image many different areas of the body without
the use of invasive surgeries.
Reviewing the Significance
of Quantum Numbers
We have seen that the four quantum numbers in any electron’s wave function
specify everything we need to know about the electron’s location in the atom.
They are a bit like an electron’s address in the atom. Table 6.5 summarizes the
significance of all the quantum numbers.
S
S
External
Low Energy High Energy
+ Energy
N
N
External
Adding energy to a nucleus in a magnetic field can cause it to
change its orientation with the field. After time has passed, the
excited nucleus returns to the low energy state and emits a
photon of energy in the radio frequency range.
FIGURE 6.37
MRI brain image.
The Quantum Numbers
n = principal quantum number Indicates which of the major energy levels or
electron shells an orbital is in. Also, the larger
the value of n, the greater the total volume of the
orbital
l = angular momentum Indicates the shape of the orbital and which
quantum number quantum number type of subshell the orbital is
in, which will be an s, p, d,or f subshell
m
l
= magnetic quantum number Quantum number indicates the orientation of
the orbital in space
m
s
= electron spin quantum Indicates the orientation of the spin of the
number electron in the orbital
TABLE 6.5
244 Chapter 6 Quantum Chemistry: The Strange World of Atoms
6.12 Orbitals and Energy Levels
in Multielectron Atoms
Unfortunately, the orbitals calculated in the manner that we have discussed are
only absolutely correct for one-electron atoms and ions. You can use them for
hydrogen and its isotopes deuterium and tritium.You can also use them for He
+
,
Li
2+
, or even Hg
79+
if you adapt the equation slightly.
Problems arise when you put a second electron into an atom or ion. Because
the two electrons have the same charge, they repel each other. The interaction
between the two electrons makes it impossible to solve for their energies and
orbital wave functions exactly. Fortunately, ways exist to approximate the correct
energies and electron orbitals. The approximations also work for atoms contain-
ing three or more electrons, and the results can be quite good. How do we
describe the orbitals on an atom with more than one electron? We take the
hydrogen atomic orbitals, which are approximately correct because they describe
the effect the nucleus has on a single electron, and we change them a little to ap-
proximate the effect of other electrons. The results are useful both because they
accurately predict the physical properties of atoms, such as ionization energies
and emission spectra, and because we can use the multielectron orbitals to form
molecular orbitals, occupied by electrons in molecules (discussed in Chapter 9).
The shapes we derive for orbitals in multielectron atoms are very similar to
those in the hydrogen atom. Helium contains a 1s orbital, a 2s orbital, three 2p
orbitals, and so on, just as we found previously for the hydrogen atom. Each of
these can hold up to two electrons, one with spin up and one with spin down.
The four quantum numbers n, l, m
l
, and m
s
are called the principal, angular mo-
mentum, orbital angular momentum, and spin angular momentum
quantum numbers, just as before. The total number of nodes is n − 1,
and the number of nonradial nodes is n − l − 1, just as for the hydro-
gen atom. The kinds of the nodes and their approximate positions are
the same, and the shapes of the orbitals are similar enough that we can
use the same pictures we used above.
The one thing that will be different is the energies of the electron or-
bitals. These are so complicated that we will not be able to calculate
meaningful values for them without the aid of a computer, so we will
talk about energy only qualitatively here. One big difference in or-
bital energy levels is that whereas the energy depends only on the
principal quantum number, n, for the hydrogen atom, levels with dif-
ferent l values will now have different energies for the multielectron
atom.
This is because of something called
electron shielding (it is also known as elec-
tron screening). When a second electron is present in an atom, some of the time
this electron is between the first electron and the nucleus. Because electrons are
negatively charged, the positive nuclear charge experienced by the first electron is
smaller that it otherwise would be, and the attractive force between that electron
and the nucleus is decreased proportionately. The first electron also sometimes
spends time closer to the nucleus than its partner, and when this happens, that
second electron will be shielded too. For the two 1s electrons in helium, both are
shielded the same amount, and they remain degenerate.
When we focus on orbitals with different n and/or different l values, all elec-
trons in an atom shield all other electrons, but not necessarily to the same extent.
The 2s electrons are, on average, closer to the nucleus than are 2p electrons, as
shown in Figure 6.38, and the 2s electrons will screen the 2p electrons far better
than the 2p will shield the 2s electrons. The nuclear charge will therefore seem to
be smaller to the 2p electrons than to the 2s electrons as a result of the greater
shielding. Because the apparent nuclear charge has been decreased, the attractive
Nucleus
Electrons
Repulsion
Attraction
The outer electrons are shielded from the charge
of the nucleus by electrons closer to the nucleus.
Probability
Distance from nucleus
2s2p
FIGURE 6.38
The relative distance from the nucleus of
the electron density in the 2s and 2p
orbitals.
Video Lesson: Electron Shielding
6.13 Electron Configurations and the Aufbau Principle 245
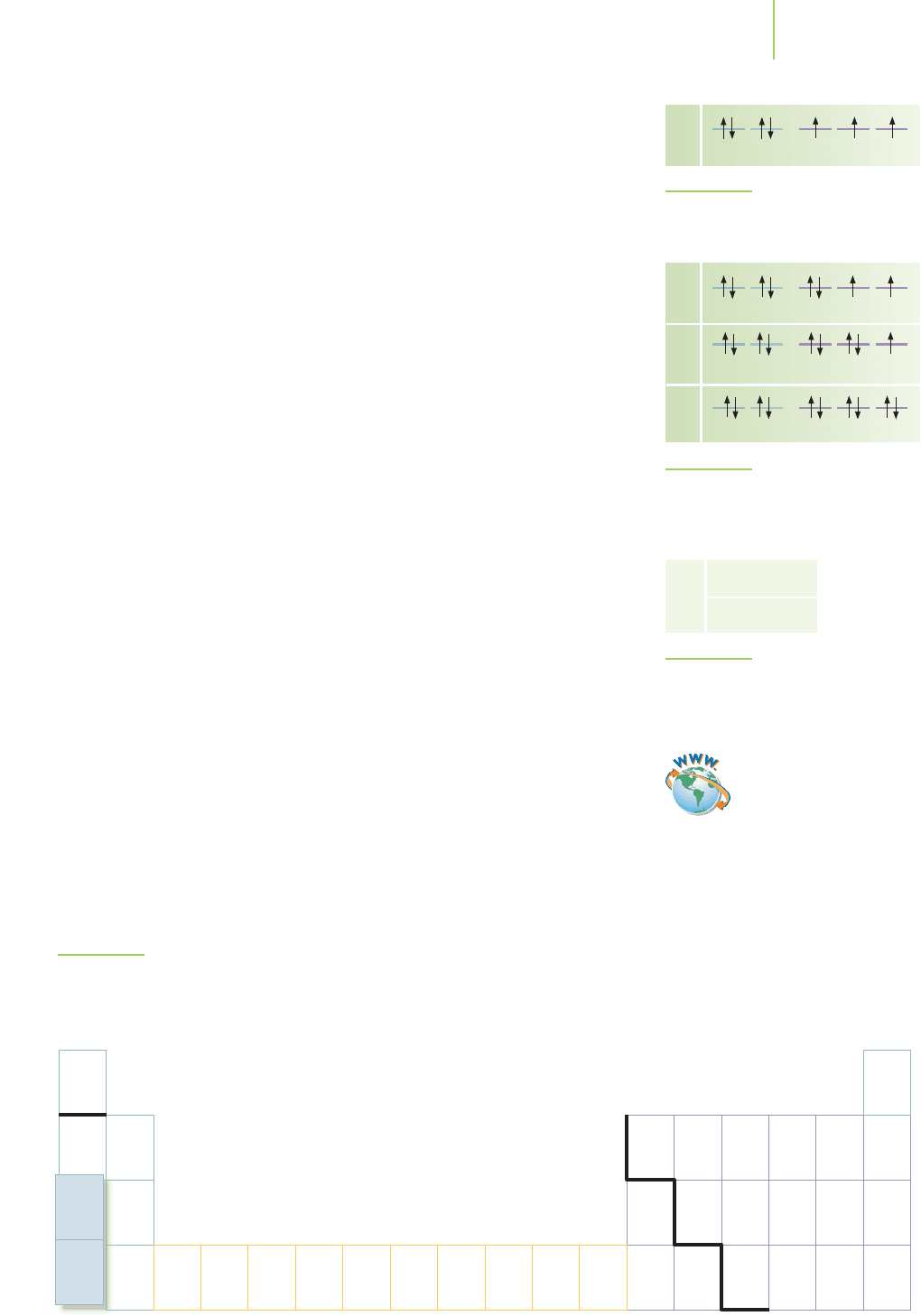
1s
1s
1s
2s
2s
2s
2p
2p
2p
2p
2p
2p
2p
3s
2s
2s
2s
0
Energy (kJ mol
–1
)
480
960
1440
1920
2400
2880
3360
3840
H He Li Be B C N O F Ne Na
FIGURE 6.39
Relative energy levels for multielectron atoms. The relative placement of the energies of the orbitals is only approxi-
mate and varies with Z, the nuclear charge, which increases to the right across the periodic table.
forces between the nucleus and the electron will also decrease. The bottom line is
that the 2p electrons will no longer be as low in energy as the 2s electrons. This is
shown schematically in Figure 6.39. The 2p
x
,2p
y
, and 2p
z
orbitals will still be de-
generate, however, because they are all at the same average distance from the nu-
cleus and will all experience the same shielding from other electrons in the atom.
For n =3, the next principal quantum number up in energy, the 3s, will be lower
in energy than the 3p, which will be lower than the 3d. For the next set of orbitals,
in which n = 4, the 4s will be below the 4p, which will be below the 4d, which
will be below the 4f.
6.13 Electron Configurations
and the Aufbau Principle
Now that we have a complete set of orbitals and a general scheme for ranking
their energies, we can list all the orbitals occupied by the electrons of any atom
in the periodic table. The complete list of filled orbitals is called the
electron
configuration
of an atom. We are often interested in the lowest energy configura-
tion, which represents the ground state of the atom. This can be generated by
adding one electron at a time to an unoccupied atomic orbital, starting with the
lowest energy orbital and working our way up in energies until all electrons in the
atom have been assigned an orbital. This method of filling hydrogen atomic or-
bitals to get multielectron configurations operates in accordance with the
Aufbau
principle
, from the German word for “building up.” The Aufbau principle says
that as protons are added to the nucleus, electrons are successively added to
orbitals of increasing energy, beginning with the lowest-energy orbitals. In
essence, we build up the electron configuration one electron at a time until all
electrons are accommodated. To convey the maximum information in the mini-
mum space, we use a chemical shorthand to indicate which orbitals are occupied.
In its ground state, the hydrogen atom will have its lone electron in the 1s orbital,
H 1s
1
He 1s
2
Visualization: Orbital Energies
Tutorial: Aufbau Principle

FIGURE 6.40
The electron configuration of the first
three elements of the second period.
FIGURE 6.41
T
hree possible electron configurations
for carbon.
246 Chapter 6 Quantum Chemistry: The Strange World of Atoms
this as 1s
1
. Moving on to helium, the next element in the periodic table, we place
the first electron in the 1s orbital, but because we can have both up and down
spins associated with the spatial part of the orbital, the second electron will go
into a 1s orbital as well. Rather than writing out the spin state explicitly, we write
the electron configuration as 1s
2
and just have to remember that the electrons in
this configuration have opposite spins.
The next element in the sequence has three electrons. This element must be
lithium, which the periodic table places in the second row, below hydrogen and
helium. Lithium has been placed here because we have used up all the orbitals
with n = 1 and now must start another quantum level, or shell, by placing the
third electron in the 2s orbital. The ground-state electronic configuration for
lithium is therefore 1s
2
2s
1
. The other elements in the same row as lithium con-
tinue to fill the second quantum shell:
Li: 1s
2
2s
1
N: 1s
2
2s
2
2p
3
Be: 1s
2
2s
2
O: 1s
2
2s
2
2p
4
B: 1s
2
2s
2
2p
1
F: 1s
2
2s
2
2p
5
C: 1s
2
2s
2
2p
2
Ne: 1s
2
2s
2
2p
6
In the above examples, note that the p orbitals fill after a total of six electrons be-
cause 2p
x
,2p
y
, and 2p
z
can each accommodate two electrons, one with spin up
and one with spin down. Remember that the p orbitals are referred to as occupy-
ing their own subshell.
When we get to the end of the row, we will have completely filled another
shell. The next row must start filling the 3s orbitals because there is no more room
for electrons in the n = 2 shell. Now you can begin to understand how the struc-
ture of the periodic table shows a splendid consistency, whether we are classifying
elements in terms of physical and chemical properties, as did Mendeleev and Meyer,
or by the quantum mechanical solution to the Schrödinger equation in the order of
increasing electron energy, as did the quantum chemists and physicists.
In order to proceed to the next row, or period, of the periodic table, we
still need to know a few more things about how electrons fill the orbitals. Be-
cause of their negative charge, electrons tend to remain as far apart as possi-
ble in a manner that minimizes repulsive interactions among them. When
filling the 2p orbitals for boron through neon, we could keep the electrons far-
ther apart on the average by placing them into different 2p orbitals for as long
as possible. We could also minimize the repulsive electron–electron interac-
tions by keeping the spins of the electrons parallel—that is, with their re-
spective spin axes pointing along the same direction. To illustrate this, we will
fill the electrons from the second period of the chart one more time. The first
three, shown in Figure 6.40, are unambiguous.
For boron, the 2p
x
orbital choice is arbitrary. Either the 2p
y
or the 2p
z
orbital would have been equally valid. To extend the series to carbon, we now
have to decide whether we will place the two 2p electrons in the same orbital
or in different orbitals. Three unique choices are possible, as shown in
Figure 6.41, all of which represent possible electronic states for the carbon
atom.
The ground state for the carbon atom is state 1, which has the two 2p elec-
trons in different orbitals with the spins aligned in the same direction. It is the
lowest energy state for carbon, and it is therefore the state in which you will
find a carbon atom unless you supply it with energy—by irradiating it with
electromagnetic radiation, for example.
To understand why we need to keep spins aligned the same for partially
filled levels, remember that no two electrons in an atom can have the same
four quantum numbers n, l, m
l
, and m
s
(Pauli exclusion principle). Giving the
two 2p electrons the same spin gives them the same value of m
s
and requires
that they be placed in different 2p orbitals. This keeps the two electrons
H 1s
1
Ne 1s
2
2s
2
2p
x
2
2p
y
2
2p
z
2
=1s
2
2s
2
2p
6
1s 2s 2p
x
2p
y
2p
z
1s 2s 2p
x
2p
y
2p
z
Li
Be
1s 2s 2p
x
2p
y
2p
z
B
1s 2s 2p
x
2p
y
2p
z
1s 2s 2p
x
2p
y
2p
z
State 1:
State 2:
1s 2s 2p
x
2p
y
2p
z
State 3:
Video Lesson: Electron
Configurations through Neon
6.13 Electron Configurations and the Aufbau Principle 247
spatially separated and therefore minimizes the repulsion between the two. Over-
all, this behavior is summarized by what is known as
Hund’s rule. Proposed by the
German physicist Friedrich Hund in 1925, this rule states that when orbitals of
equal energy are available, the lowest energy configuration for an atom has the
maximum number of unpaired electrons with parallel spins.
If we continue adding the electrons to the hydrogen-like atomic orbitals, we
will have the maximum number of unpaired 2p electrons for nitrogen, as shown
in Figure 6.42.
We must again begin pairing them to continue adding electrons to the or-
bitals, as shown in Figure 6.43. By the time we get to neon, the entire second shell
is filled.
To proceed to sodium, we need to add the next electron to the 3s level. Even
with our shorthand notation, the electron configurations are beginning to get
unwieldy. However, we can say that all atoms in the third row of the periodic table
must have filled the n = 1 and n = 2 shells before electrons can be added to the
n =3 level. This allows us to write the electron configuration in one of two ways,
as given in the example for sodium as shown in Figure 6.44. The first notation
shows all of the electrons explicitly, and the second uses [Ne] to take the place of
1s
2
2s
2
2p
6
. We can say that the configuration of sodium is 3s
1
with a neon core.
The electrons listed after the
core electrons ([Ne]) are called valence electrons and
will be very important in establishing the chemical reactivity of the element.
Similarly, the ground-state electron configuration for potassium could be
written either explicitly as 1s
2
2s
2
2p
6
3s
2
3p
6
4s
1
or as [Ar]4s
1
. The latter case indi-
cates only the electrons in the outermost shell of the configuration and places the
less reactive, more strongly bound filled-shell electrons into an argon core. This
does not imply that the nucleus of potassium has been replaced with argon.
Argon has one fewer proton and, for the most abundant of their isotopes, two
more neutrons than does potassium. The [Ar] core notation signifies that the
atomic configuration has the same hydrogen-like orbitals occupied with elec-
trons plus all the others noted after it.
You might have noticed that the argon ground-state electron configuration
has been given as 1s
2
2s
2
2p
6
3s
2
3p
6
but that when we added one more electron to
the set to represent the occupied orbitals of potassium, the next element in the
periodic table, we placed it into a 4s level. This makes sense from the position that
potassium occupies in the periodic table: right under sodium, as shown in
Figure 6.45. And rubidium, which is directly under potassium, has the electron
configuration of [Kr]5s
1
. In fact, all the elements in the first column of the
1s 2s 2p
x
2p
y
2p
z
N
FIGURE 6.42
The electron configuration for nitrogen.
1s 2s 2p
x
2p
y
2p
z
1s 2s 2p
x
2p
y
2p
z
O
F
1s 2s 2p
x
2p
y
2p
z
Ne
FIGURE 6.43
The electron configuration for the last
three elements of the second period.
Na
1s
2
2s
2
2p
6
3s
1
[Ne]3s
1
FIGURE 6.44
The electron configuration
for sodium can be
written two ways.
11
Na
3s
1
19
K
4s
1
1
H
1s
1
3
Li
2s
1
4
Be
2s
2
12
Mg
3s
2
20
Ca
4s
2
21
Sc
4s
2
3d
1
22
Ti
4s
2
3d
2
23
V
4s
2
3d
3
24
Cr
4s
1
3d
5
25
Mn
4s
2
3d
5
26
Fe
4s
2
3d
6
27
Co
4s
2
3d
7
28
Ni
4s
2
3d
8
29
Cu
4s
1
3d
10
31
Ga
4s
2
4p
1
5
B
2s
2
2p
1
13
Al
3s
2
3p
1
32
Ge
4s
2
4p
2
6
C
2s
2
2p
2
14
Si
3s
2
3p
2
33
As
4s
2
4p
3
7
N
2s
2
2p
3
15
P
3s
2
3p
3
34
Se
4s
2
4p
4
8
O
2s
2
2p
4
16
S
3s
2
3p
4
9
F
2s
2
2p
5
17
Cl
3s
2
3p
5
35
Br
4s
2
4p
5
10
Ne
2s
2
2p
6
18
Ar
3s
2
3p
6
36
Kr
4s
2
4p
6
2
He
1s
2
IA
ns
1
IIA
ns
2
IIIA
ns
2
np
1
IVA
ns
2
np
2
VA
ns
2
np
3
VIA
ns
2
np
4
VIIA
ns
2
np
5
VIIIA
ns
2
np
6
1
2
3
4
30
Zn
4s
2
3d
10
1
13 14 15 16 17
8 9 10 11 1234567
18
2
FIGURE 6.45
Position of potassium and sodium on the periodic table.
Video Lesson: Electron
Configurations Beyond Neon
