Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

258 Chapter 6 Quantum Chemistry: The Strange World of Atoms
108. Iridium (element 77) is one of the metals that can be found
in the Earth’s crust not combined with other elements. It
is a brittle, lustrous metal and has a melting point over
2400
o
C.
a. Judging on the basis of iridium’s position in the periodic
table, what other elements would it most resemble?
b. Does iridium have any unpaired electrons?
c. Using the Aufbau principle, determine to what sublevel
the 25th electron was added to the configuration of
iridium.
Comprehensive Problems
109. a. The light produced in the explosion of a dramatic fire-
works display reaches you before the sound of the explo-
sion. Use another reference to determine the speed of
sound. Then determine the ratio of the speed of light to
the speed of sound.
b. What is the speed of light in miles per hour?
110. When you observe the striking colors of the fireworks at
a special occasion, you are observing emission spectra.
Describe, chemically, what has transpired in the atoms of
the elements to cause the emission of light.
111. What is the longest wavelength of the Lyman series of the
hydrogen emission spectrum?
112. Explain how both the volume and the energy of an electron
associated with an atom are quantized.
113. Using the equation that allows calculation of the radii of
energy levels for single-electron situations in hydrogen,
compare the distance between the first and second energy
levels to the distance between the third and fourth
energy levels and to the distance between the fifth and sixth
energy levels.What trend do you notice?
114. The equations used by Bohr are valid for the hydrogen
atom, but when they are applied to helium, a significant
error shows up. And when they are applied to lithium and
elements with higher atomic numbers, the error becomes so
large that the equations offer little. However, the equations
can be applied to helium and lithium ions with a fair agree-
ment with experimental values. What helium and lithium
ions would be most like the hydrogen atom?
115. When applying wave properties to electrons in atoms, we
use the expression 2πr = nλ. Explain why n can have only
integer values.
116. Define the terms discrete and continuous. List five everyday
items that have some property that is discrete and five
that have a property that is continuous on a macroscopic
scale.
117. Assign possible quantum numbers for each of these orbital
pictures. Assume that each orbital is in the lowest possible
principal shell.
a. b. c.
118. Compare the ground-state electron configurations of
potassium and argon. Explain why it is easier to remove the
outer electron of potassium than that of argon, even though
potassium has more positive charge in its nucleus.
119. A 385 nm photon of light strikes a sheet of a particular
metal and ejects an electron at a velocity of 6.1 10
5
m/s.
What is the energy, in kJ/mol, associated with this photon?
120. A 0.30 L cup of water is placed in a microwave and irradi-
ated with microwaves of 12.0 cm wavelength. The tempera-
ture of the water raises from 25°C to 80°C. How many
photons are used to heat the water?
121. a. What is the frequency and energy associated with a
photon of light from a ruby laser (see Table 6.4)?
b. What mass, in ounces, would a particle exhibiting that
wavelength have if it were traveling at half the speed of
the photon?
Thinking Beyond the Calculation
122. A photon with wavelength λ = 2165 nm strikes an excited
hydrogen atom.
a. What energy, in joules, is associated with this photon?
What energy, in joules, is associated with a mole of these
photons?
b. What is the frequency of the photon?
c. Which region of the electromagnetic spectrum contains
photons of this energy?
d. If the photon is absorbed by the hydrogen atom, to what
energy level is the electron promoted? To make the
calculation easier, assume that the electron begins in the
n = 4 energy level.
e. If the hydrogen atom relaxes back to the n = 2 energy
level from the n =7 level, what would be the wavelength
of the photon that was emitted? Which region of the
spectrum contains this photon?
f. What could you write as the electron configuration for
the hydrogen atom described in part e, after emission of
the photon?
yyy
xx
x
zz z

259
Contents and Selected Applications
7.1 The Big Picture—Building the Periodic Table
7.2 The First Level of Structure—Metals, Nonmetals,
and Metalloids
7.3 The Next Level of Structure—Groups in the Periodic Table
Chemical Encounters: Commercial Uses of the Main-Group Elements
Chemical Encounters: The Elements of Life
7.4 The Concept of Periodicity
7.5 Atomic Size
7.6 Ionization Energies
7.7 Electron Affinity
7.8 Electronegativity
7.9 Reactivity
7.10 The Elements and the Environment
Chemical Encounters: The Elements and the Environment
Periodic
Properties of
the Elements
Our planet is composed of a wide variety
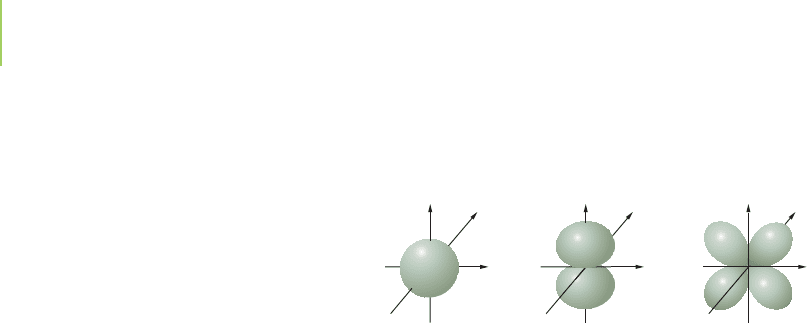
of elements.
Go to college.hmco.com/pic/kelterMEE for online learning resources.

7.1 The Big Picture—Building the
Periodic Table
The structure of the modern periodic table was initially conceived in the mid-
nineteenth century by scientists trying to make sense of the properties and
reactivities of all the elements found in the natural environment. It developed
through the recording of experimental results. That its structure is consistent with
the quantum mechanical understanding of the electronic structure of the ele-
ments that scientists arrived at in the early twentieth century, as we discussed in
Chapter 6, indicates the crucial role of electron arrangement in determining an
atom’s reactivity. It also confirms the power of the periodic table as a classification
260
We live on and within a big globe of chemi-
cals that have interacted for well over 4 billion
years to form the materially closed system that we call
the planet Earth. Our atmosphere provides the oxygen
molecules that interact with hemoglobin in our blood
and support life on this planet. The foods we eat help sus-
tain this life, and they include molecules with common names
like carbohydrates, proteins, and vitamins, as well as salts such as
sodium chloride. Our quality of life is enhanced by our clothing, which
is often made by combining molecules processed from crude petroleum
found deep beneath the seabed. The materials that we have produced for
cooking, cleaning, killing, and healing through the ages, from the Stone Age to
the Bronze Age to the Iron Age all the way through to the industrial revolution
of the late eighteenth and nineteenth centuries and on to the Information Age
that has defined the twenty-first century, have a common origin.
Throughout the ages, the stuff of life and of our way of life have been based on
the set of chemical elements listed in the periodic table.
Why is the oxygen (O
2
) that we must breathe every minute a gas, whereas
the gold we can dig up from the Earth to use as jewelry is a dense solid? Why
does this gold last for thousands of years, unchanged, while the oxygen reacts
so readily with many other elements and compounds? Why is the oxygen that
is carried around by our blood bound to the iron ions that form part of the
protein hemoglobin in our red blood cells? What makes iron so well suited to
this task? We need answers to these types of questions if we are to understand
the natural environment in which we live, its effects on us, our effects on it,
and how it is that we interact with it to acquire and process materials that are
so much a part of our day-to-day lives.
Our focus in this chapter is on the elements themselves and on how we use
the understanding of their basic structure that we developed in the last chap-
ter to gain insight into their chemical behavior. In the next two chapters, we
will look at how and why the elements interact with each other to form the
compounds that support our twenty-first-century life. As we continue our four-
chapter atomic and molecular tour, we keep in mind that the greatest single statement
of our understanding of the behavior of the elements is the periodic table into which
they have been organized. It is there that we begin our discussion.
Oxygen.
Gold.
Video Lesson: Periodic
Relationships

system that becomes more meaningful and valid with each
new chemical discovery.
The structure of the periodic table includes “blocks” de-
fined in terms of which type of orbital is being filled via the
Aufbau principle. This gives us the s-block, p-block, d-block,
and f-block, illustrated in Figure 7.1. Elements in the
s-block, such as sodium, potassium, and calcium, are natu-
rally found as positive ions, such as the Na
+
ion found in
seawater and the Ca
2+
ion that characterizes “hard” well
water. Elements in the p-block often form negative ions; ex-
amples include the Cl
−
ions in blood and the S
2−
ion that is
a part of the minerals shown in Figure 7.2, such as pyrite
(FeS
2
), called “fool’s gold” for its goldlike appearance, and
galena (PbS), mined as the main source of lead metal. Many
elements in the d-block can form positive ions with different
charges. For example, iron can form the fairly stable Fe
2+
and Fe
3+
ions and also, as part of more complex molecules
and ions, the somewhat less stable Fe
4+
,Fe
5+
, and Fe
6+
ions.
7.1 The Big Picture—Building the Periodic Table 261
1
H
3
Li
3
Li
11
Na
19
K
37
Rb
55
Cs
87
Fr
4
Be
12
Mg
20
Ca
38
Sr
56
Ba
88
Ra
21
Sc
39
Y
57
La*
89
Ac
†
22
Ti
40
Zr
72
Hf
104
Rf
23
V
41
Nb
73
Ta
105
Db
24
Cr
42
Mo
74
W
106
Sg
25
Mn
43
Tc
75
Re
107
Bh
26
Fe
44
Ru
76
Os
108
Hs
27
Co
45
Rh
77
Ir
109
Mt
110
Uun
111
Uuu
28
Ni
46
Pd
78
Pt
29
Cu
47
Ag
79
Au
3
IIIB
4
IVB
5
VB
6
VIB
7
VIIB
11
IB
12
IIB
31
Ga
49
In
81
Tl
5
B
13
Al
32
Ge
32
Ge
50
Sn
82
Pb
6
C
14
Si
33
As
51
Sb
83
Bi
7
N
15
P
15
P
34
Se
52
Te
84
Po
8
O
16
S
9
F
17
Cl
35
Br
53
I
85
At
10
Ne
18
Ar
36
Kr
54
Xe
86
Rn
2
He
58
Ce
90
Th
59
Pr
91
Pa
60
Nd
92
U
61
Pm
93
Np
62
Sm
94
Pu
63
Eu
95
Am
64
Gd
96
Cm
65
Tb
97
Bk
66
Dy
98
Cf
67
Ho
99
Es
68
Er
100
Fm
69
Tm
101
Md
70
Yb
102
No
71
Lu
103
Lr
Transition elements
d-block
1
IA
2
IIA
13
IIIA
14
IVA
15
VA
16
VIA
17
VIIA
18
VIIIA
Metals
Metalloids
Nonmetals
*Lanthanides
†
Actinides
112
Uub
30
Zn
48
Cd
80
Hg
1
2
3
4
Period
5
6
7
9
VIIIB
810
Main-group elements
s-block
Main-group elements
p-block
Inner transition
elements
f-block
FIGURE 7.1
The main ways in which we divide the periodic table into different sections. The s-, p-, d-, and f-blocks contain elements with outer electrons in the
same type of orbital. The horizontal rows are referred to as periods. The vertical columns are called groups. The color coding indicates the metals,
metalloids, and nonmetals. Helium is misplaced in the typical periodic table; it should be located next to hydrogen in the s-block.
The careful recording of experimental results made it possible to
determine physical properties of the elements that occur in the
natural environment.

262 Chapter 7 Periodic Properties of the Elements
Helium atoms are small enough to
escape from a balloon.
We see, then, that there is some relationship between the blocks in which ele-
ments reside and their chemical and physical properties.
The horizontal “period” of the periodic table to which an element belongs in-
dicates how many energy levels contain electrons in the ground state of atoms of the
element. Elements in Period 1 have electrons in only the first energy level (princi-
pal quantum number n =1). Elements in Period 2 have electrons in the first two
energy levels (principal quantum numbers n =1 and n =2), and so on. The more
occupied energy levels an atom has, the bigger will be the atom. Helium atoms,
from Period 1, are tiny compared to radon atoms, from Period 6. The tiny size of
helium atoms explains why a party balloon filled with helium deflates in just a
few days, as the atoms escape through little pores in the material of the balloon.
Again we see that position in the periodical table is related to what elements do.
Another key link between electron arrangement and position in the periodic
table is that elements in any one main group (Groups IA through VIIIA) have the
same number of electrons in their highest energy level, so that the members of
Group VIIA all contain seven electrons in their highest energy, or valence, level.
FIGURE 7.2
Iron pyrite, FeS
2
(left), and galena PbS (right). The sulfur
in these two minerals has gained electrons and exists
as S
2−
in PbS and S
2
2
−
in FeS
2
.
Day 1 Day 8
Groups
Periods
Periods and groups in the periodic table.
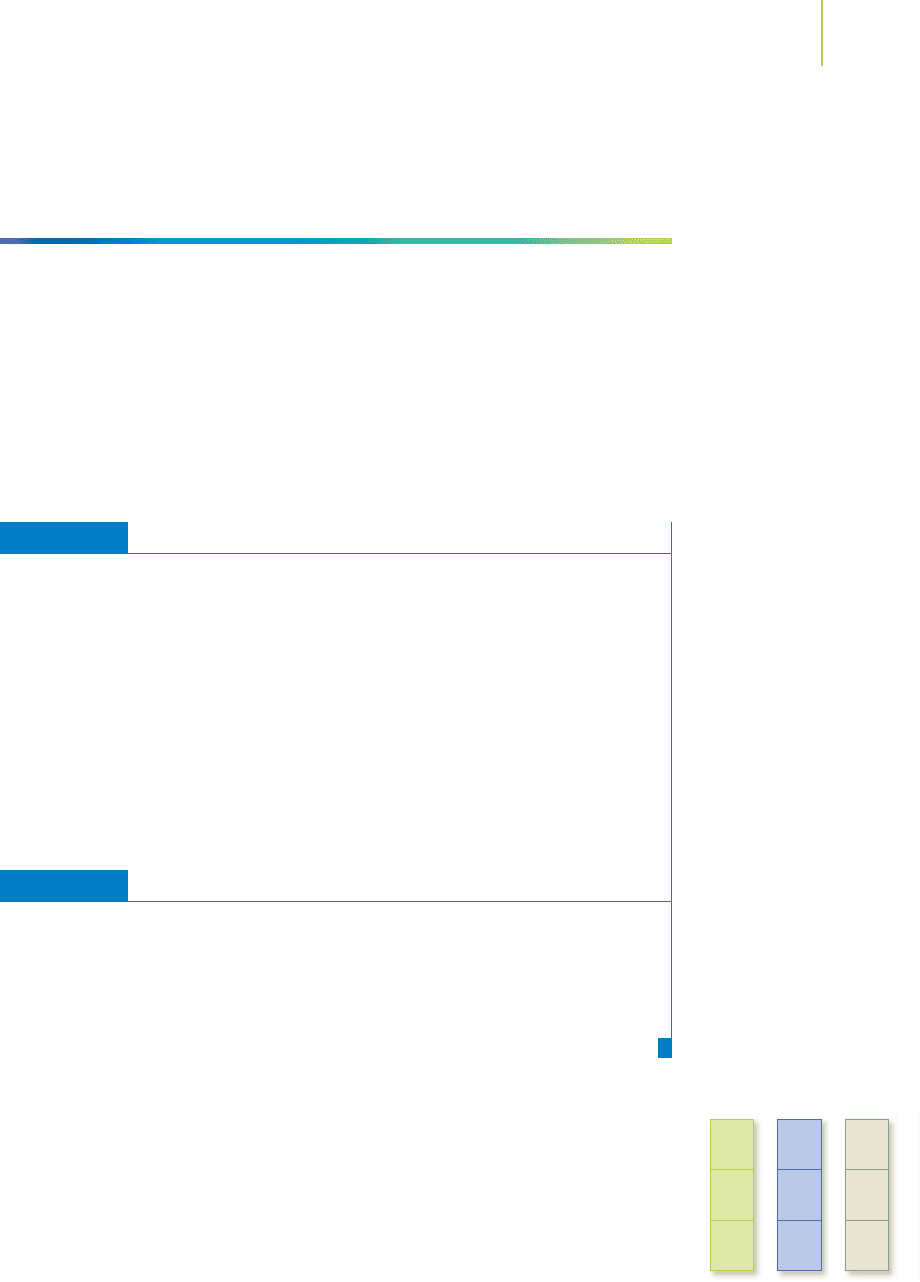
That is essentially why elements from each main group share some very signifi-
cant chemical characteristics—why helium and neon are both unreactive gases,
for example.
HERE’S WHAT WE KNOW SO FAR
■
The periodic table is organized into blocks. The s-block, p-block, d-block, and
f-block indicate the orbitals that are partially full in the highest energy level of
the element.
■
Rows in the periodic table are known as periods. The period number indicates
the highest energy level in which electrons are found in the ground state on
each element.
■
Columns in the periodic table are known as groups. Elements in the same
group have the same number of electrons in their highest energy level.
■
Elements in the same group have similar properties.
EXERCISE 7.1 Identifying Elements
Give the block, the period number, and the group number for each of these
elements.
K, Sc, Al, C, Br
Solution
Potassium is an s-block metal found in Period 4 and Group IA.
Scandium is a d-block metal found in Period 4 and Group IIIB.
Aluminum is a p-block metal found in Period 3 and Group IIIA.
Carbon is a p-block nonmetal found in Period 2 and Group IVA.
Bromine is a p-block nonmetal found in Period 4 and Group VIIA.
PRACTICE 7.1
Identify each of these elements.
a. A p-block element in Period 5 and Group IVA.
b. A d-block metal from Period 6 and Group VB.
c. An s-block element in Period 1 and Group IA.
See Problems 3–6.
The Historical Development of the Periodic Table
We have just summarized the structure of the periodic table from a modern “elec-
tron arrangement” viewpoint. However, the first recorded steps in building the
table were made from the quite different viewpoint of the experimental chemist
observing what the elements actually do when they react with one another. These
first steps were taken by the German scientist Johan Dobereiner (1780–1849)
when he identified several groups (he called them triads) of elements whose
properties were very similar. Dobereiner’s “triads” included lithium, sodium,
and potassium (now found in Group IA of the modern periodic table), calcium,
strontium, and barium (now in Group IIA), and chlorine, bromine, and iodine
(Group VIIA).
In 1864, Englishman John Newlands (1837–1898) arranged some elements
in order of increasing atomic mass and found that similarities in properties
7.1 The Big Picture—Building the Periodic Table 263
20
Ca
40.078
38
Sr
87.62
56
Ba
137.327
17
Cl
35.453
35
Br
79.904
53
I
126.904
16
S
32.066
34
Se
78.96
52
Te
127.60
The three triads of elements
discovered by Dobereiner.

occurred in elements eight places apart in his scheme. Newlands’s system of ele-
ments, arranged in what he called octaves (an octave is a musical interval of eight
tones, as in the eight white keys on the piano from one C note to another) pro-
vided an early glimpse of the regular repetition, or periodicity, in properties that
gives the periodic table its name. We know today that the elements have to be
arranged in order of increasing atomic number for the periodic nature of the
periodic table to be observed.
The modern form of the table first began to take shape through the work of
German chemist Julius Lothar Meyer (1830–1895) and Russian chemist Dmitri
Mendeleev (1834–1907). They added many more elements to the table, and
Mendeleev took the crucial step of predicting that certain elements must exist to
occupy the gaps that were left in the table. In 1869, Mendeleev published the pe-
riodic table shown in Figure 7.3. Within a few years of Mendeleev’s predictions,
three elements were discovered that matched these predictions and filled some of
the gaps. For example, Mendeleev predicted the existence of the element “ekasili-
con,” which we now know as germanium; it was discovered in an ore called argy-
rodite by the German chemist Clement Winkler in 1886. Table 7.1 gives some of
germanium’s properties as predicted by Mendeleev and as later measured after
the element was discovered. The other two elements discovered were ekaboron
(scandium) and ekaaluminum (gallium).
Mendeleev’s realization that the periodic table could be used as a predictive
tool is probably the main reason why Mendeleev, rather than Meyer, is now
264 Chapter 7 Periodic Properties of the Elements
John Newlands noticed that every
eighth element had similar properties,
a principle that he called the law of
octaves. Eight consecutive white keys
on a piano comprise an octave.
Dmitri Mendeleev (1834–1907), the
youngest of the 17 children in his family,
was born in Serbia. After his father died,
his mother moved the family to St. Peters-
burg, Russia, so that Mendeleev could get
the best education possible. After 20 years
of work, he developed the basic layout of
the periodic table that we still use today.
Predicted (Ekasilicon) and Measured Properties of Germanium
Ekasilicon (Ek) Germanium (Ge)
Predicted in 1871 Discovered in 1886
Atomic mass = 72 Atomic Mass = 72.6
Density = 5.5 g/cm
3
Density = 5.47 g/cm
3
Density of EkO
2
= 4.7 g/cm
3
Density of GeO
2
= 4.70 g/cm
3
EkCl
4
will be liquid, density = 1.9 g/cm
3
GeCl
4
is a liquid, density = 1.89 g/cm
3
TABLE 7.1
FIGURE 7.3
Mendeleev’s periodic table. The atomic mass of each of the elements is recorded in the table. Dashes in
the table indicate elements that Mendeleev proposed but had not yet been discovered. The symbols at
the top of the table (R
2
O, RO, R
2
O
3
, etc.) are chemical formulas written in the style of the 1870s.
Video Lesson: Creating the
Periodic Table
7.2 The First Level of Structure—Metals, Nonmetals, and Metalloids 265
recognized as the “father of the periodic table.” What we now appreciate as posi-
tively inspired reasoning was, at one time, dismissed as Mendeleev’s madness—a
telling illustration of how the scientific method requires time for important in-
sights to be identified among other, less fruitful ideas.
7.2 The First Level of Structure—
Metals, Nonmetals, and Metalloids
The periodic table hangs in a highly visible place on the wall of chemistry labora-
tories all over the world. It is not there for reasons of history or adornment. It
is there because it is the most important and useful work developed in the history of
science for describing what we know about the chemistry of the elements. There are
many versions of the table, and they vary in precisely what information is given
about each element. The basic structure of the table, however, in terms of the po-
sitioning of elements within vertical groups and horizontal periods, is generally
the same. A chemistry student from Russia, South Africa, Mexico, or Scotland can
look at the table and communicate its ideas with any other chemistry student in
the world. The language of chemistry, as illustrated by the periodic table, is uni-
versal. What does this basic structure tell us about the elements?
The elements in the periodic table are arranged into three main sections—the
metals, nonmetals, and metalloids (or semimetals)—as shown in Figure 7.1. The
majority of elements are metals, which possess a variety of “metallic” character-
istics, including those in this list.
Metals are generally
■
shiny in appearance
■
solids at room temperature and pressure (apart from mercury, the only metal
that is a liquid at room temperature and pressure)
■
good conductors of electricity
■
malleable—that is, able to be hammered into various shapes
■
likely to form positively charged ions when they react to form ionic
compounds
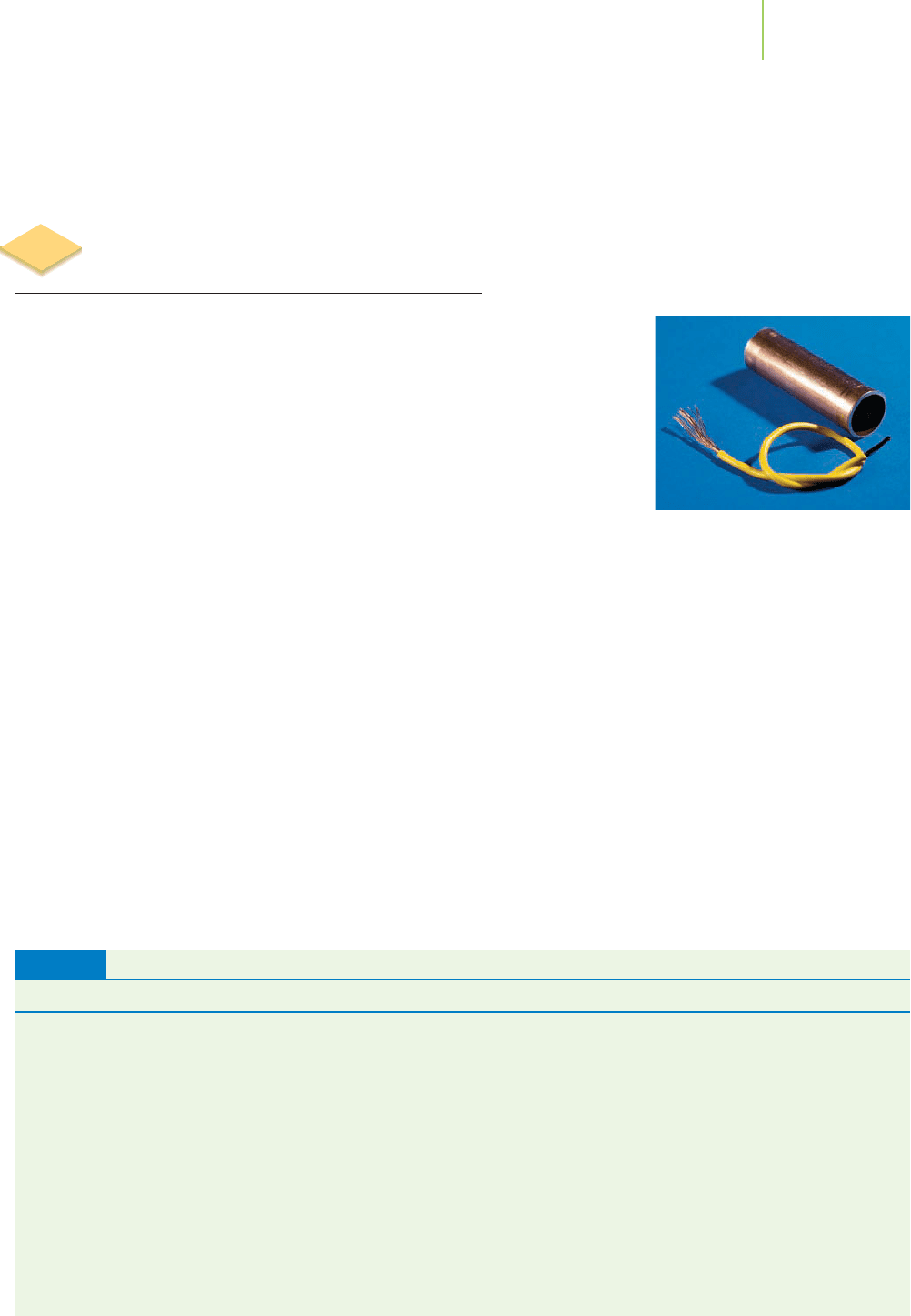
Metals, including iron, aluminum, and copper, supply us with some of our most
widely used and versatile materials for fabrication. For example, copper is used
in electric wires and in plumbing throughout your home. Iron is the basic
Copper is not very reactive—hence its
use in electrical wire and pipes.
Selected Metals, Their Sources, and Their Uses
Metal Major Sources in Nature Uses
Al Bauxite, clay, mica, feldspar, alumina Food wrap, kitchenware, beverage cans
Ca Lime (CaO), limestone (CaCO
3
), feldspar, apatite Manufacture of vacuum tubes, alloys, preparation of
other metals
Cu Chalcopyrite (copper iron sulfide), pure metal Coinage metal, electric wires, plumbing pipes
Fe Hematite (Fe
2
O
3
), limonite (Fe
2
O
3
·3H
2
O) Steel
Na Salt (NaCl), Borax (Na
2
B
4
O
7
·10H
2
O) Street lamps, nuclear reactor coolant
Ni Pentlandite and pyrrhotite (nickel–iron Coinage metal, stainless steel, NiCad batteries,
sulfides), garnierite (nickel–magnesium silicate) heating elements
Sn Casserite (SnO
2
) Tin cans, many alloys, including bronze
Ti Rutile (TiO
2
), ilmenite Aircraft parts, lightweight tank armor
W Wolfram ochre (WO
3
) Filaments in light bulbs
TABLE 7.2

266 Chapter 7 Periodic Properties of the Elements
constituent of a homogenous mixture of metals (known as an alloy) that make up
steel. Steel is used throughout our modern world as a structural component of
cars, trains, buildings, and bridges.
What are the industrial and commercial uses of steel? Chromium and nickel are
used to create the familiar stainless steels. These steels resist corrosion and are
commonly used in making tableware. Tellurium and selenium promote the
machinability of steel, its ability to be easily turned and shaped into bolts and
screws. Manganese makes steels that are very resistant to wear as well as to chem-
ical reaction with water. Molybdenum is used to create hard steels for use in bear-
ings. Careful addition of silicon creates electrical steels used in the generation and
transmission of electricity. The exploration of ways in which subtle changes in
the composition of steels brings about significant changes in their properties is a
continuing process. That huge research and manufacturing effort all stems from
the simple observation, hundreds of years ago, that letting hot charcoal mix in
with molten iron improved its usefulness.
EXERCISE 7.2 Heavy Metals, Both Necessary and Toxic
If you follow environmental issues, you may have heard of the term heavy metals,
which some think of as those with atomic masses greater than or equal to 63.546 g/mol.
Some of these metals are essential for life, but many of them are toxic. Some of the
heavy metals that are vital for life in low concentrations can be quite toxic in high
concentrations. Heavy metals in water and soil are a major focus of environmental
concern.
1. According to the definition given above, what is the lightest of the heavy
metals?
2. Using the periodic table, can you identify some other heavy metals that are
commonly discussed as environmental contaminants?
3. Chromium is included in some lists of heavy metals. What does that indicate
about the definition of the term heavy metals?
4. Can you suggest why heavy metals can be particularly persistent environ-
mental contaminants, posing problems that are difficult to correct?
Solution
1. According to the definition supplied, copper is the lightest of the heavy
metals.
2. The metallic elements heavier than copper in the periodic table include many
that have received publicity as heavy metal contaminants of the environment.
Examples which feature prominently in news reports on this issue are cad-
mium, mercury, and lead.
3. Chromium has a lower atomic mass than copper, so its inclusion in lists of
heavy metals suggests that there is no universally accepted definition of the
term heavy metal. This is in fact the case. It is a term used rather loosely to
describe metallic elements of relatively high atomic mass that are also toxic.
4. Heavy metals are particularly persistent environmental contaminants because,
being elements, they cannot be degraded into simpler, less toxic components.
This is in contrast to toxic compounds, some of which are readily degraded
into less harmful compounds or their component elements.
PRACTICE 7.2
Which of these elements are metals? Which of these are heavy metals?
Li, Si, Ni, Ce, Ge, Al, Po, Se, Rb, Cu
See Problems 11 and 12.
Stainless steel is commonly used as flat-
ware in kitchens.

7.2 The First Level of Structure—Metals, Nonmetals, and Metalloids 267
Steel, shown in Figure 7.4, is one of our
most versatile construction materials,
with 1.05 billion metric tons (2.42 trillion pounds)
manufactured in 2004. What exactly is steel?
The most basic definition tells us that steel is a mix-
ture of iron and small amounts of carbon, suggesting
that it is a metal with a little nonmetal blended into it.
Hundreds of years ago, it was discovered that the metal
element iron could be changed into a tougher and more
resilient material by allowing carbon from wood fires
to become mixed in with it. Since then, we have discov-
ered that adding various other elements in differing pro-
portions can be used to vary the properties of steel in a
great many ways. In fact, nowadays, we can no longer
talk of steel as though it were a single thing. There are
actually over 3500 different grades of steel, each with a
particular mixture of elements added to the iron that
forms the basis of them all. Looking at the differences
among these grades reveals how small changes in the
atoms present in the steel can have very significant ef-
fects on its properties.
There are three main types of steel, known as carbon-
steel, low-alloy steel, and high-alloy steel. Carbon-steels
have as little as 0.1% and sometimes more than 2%
carbon added to their iron, but only very small amounts
of other elements. Over 90% of the world’s steel is carbon-
steel, which is further categorized as high-carbon,
medium-carbon, low-carbon, ultra-low carbon, and so
on, depending on exactly how much carbon it contains.
Alloy steels have homogeneous mixtures of metals, called
alloys, added to them. The metals most commonly added
to alloy steels are manganese, aluminum, copper, nickel,
NanoWorld / MacroWorld
Big effects of the very small: The diversity of steels
FIGURE 7.4
Steel, such as that being poured here,
has a variety of uses in the manufac-
ture of building materials, office equip-
ment, and automobile parts.
chromium, cobalt, molybdenum, vanadium, tung-
sten, titanium, niobium, zirconium, and tellurium.
Steels also often contain some added nonmetals, such
as silicon, selenium, nitrogen, and sulfur. The compo-
sitions of three standard steels are shown in Table 7.3.
The enhanced strength of the iron–carbon alloy was used initially to create
swords and armor.
The Compositions of Selected Steels
The elements alloyed with iron to make selected
steels. The numbers are reported as the mass percent
of the total composition.
Tool Basic Electric Stainless
Steel Steel Steel
C 0.864 0.215 0.225
Mn 0.341 0.393 0.544
P 0.012 0.016 0.030
Si 0.185 0.211 1.00
Cu 0.088 0.211 0.226
Ni 0.230 0.248 8.76
Cr 4.38 0.017 16.7
V 1.83 0.003 0.176
Mo 4.90 0.038 0.24
W 6.28 — —
Sn 0.029 — —
Al — 0.002 —
Co — — 0.127
TABLE 7.3
