Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

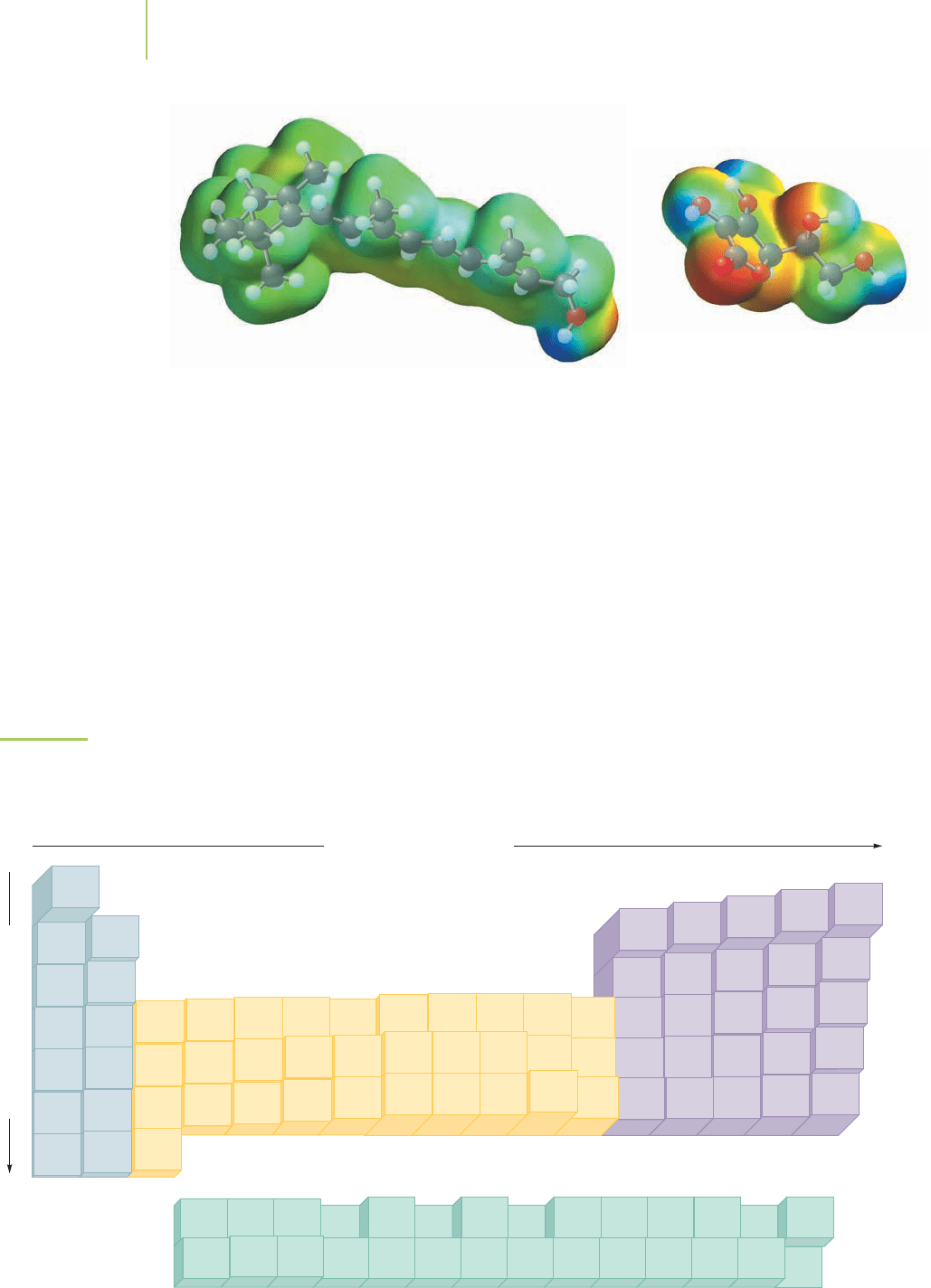
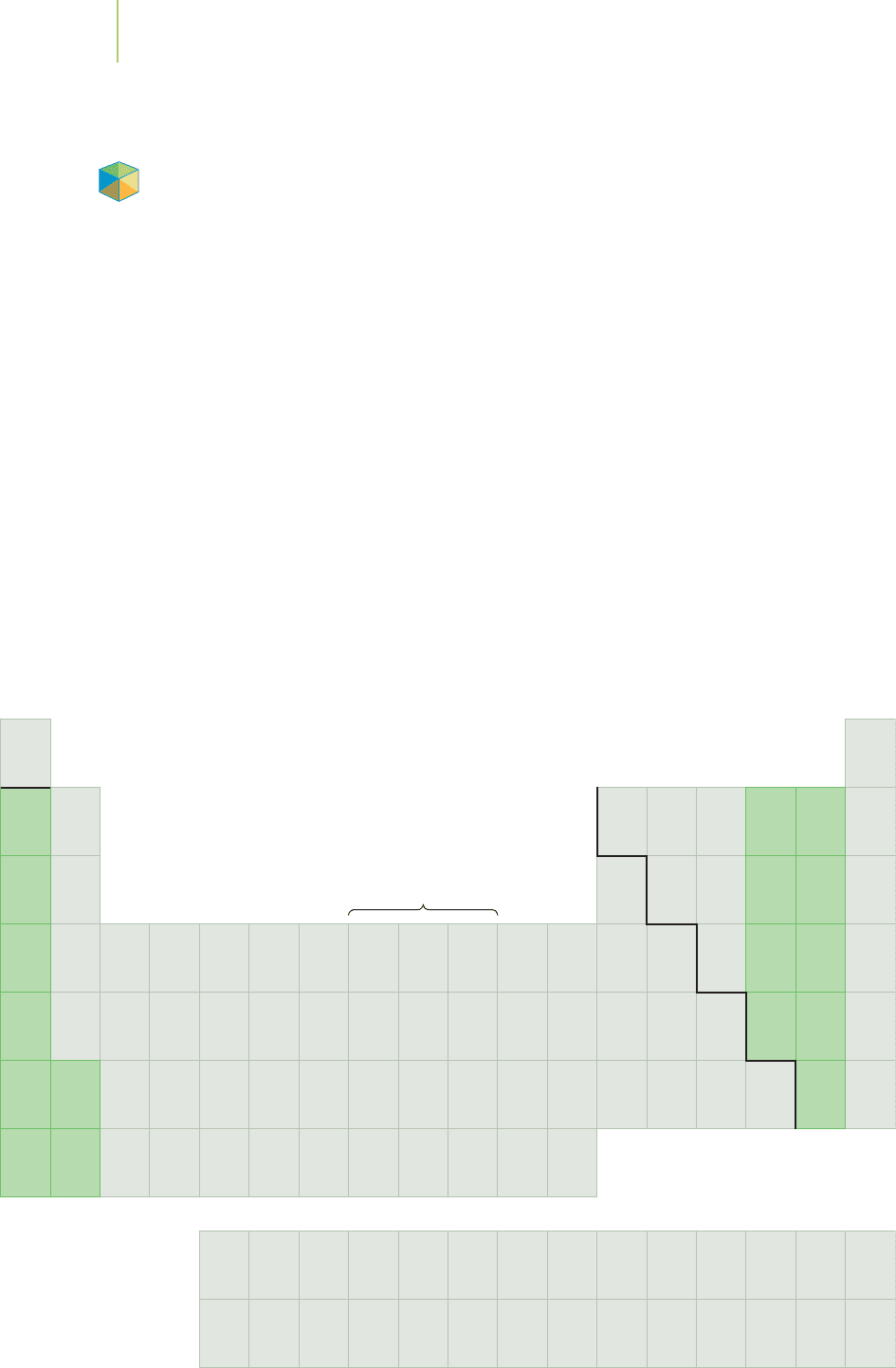
FIGURE 7.13
Electronegativity values for selected ele-
ments. Electronegativity increases as we
go up and to the right in the periodic
table.
However, our bodies require different amounts of each vitamin as part of our
daily diet. Why? Vitamin A is a fat-soluble compound that our bodies retain.
Vitamin C, on the other hand, is a water-soluble compound that is readily ex-
creted from our bodies. This means that we must consume vitamin C in larger
quantities than vitamin A and on a more regular basis. As we will discuss in the
next chapter, electronegativities are the basis for understanding the solubility of
these vitamins.
Pauling derived the electronegativity values by comparing the energies re-
quired to break various bonds, as we will discuss more fully in Chapter 8. The
values shown in Figure 7.13 reveal two general trends. The first of these trends is
that electronegativities generally increase as we move along periods all the way to
Group VIIA. The second trend indicates that electronegativities generally decrease
as we move down groups. This means that the lowest electronegativity values are
at the bottom of Group IA and that the element fluorine has the highest.
Do these
trends make sense?
The increase in electronegativity along periods occurs be-
cause the atoms are progressively smaller. In addition, atoms on the right-hand
288 Chapter 7 Periodic Properties of the Elements
Nd
1.1
H
2.1
Li
1.0
Be
1.5
Na
0.9
Mg
1.2
K
0.8
Ca
1.0
Rb
0.8
Sr
1.0
Cs
0.7
Ba
0.9
Fr
0.7
Ra
0.9
Sc
1.3
Y
1.2
La
1.1
Ac
1.1
Ti
1.5
Zr
1.4
Hf
1.3
V
1.6
Nb
1.6
Ta
1.5
Cr
1.6
Mo
1.8
W
1.7
Mn
1.5
Tc
1.9
Re
1.9
Fe
1.8
Ru
2.2
Os
2.2
Co
1.9
Rh
2.2
Ir
2.2
Ni
1.9
Pd
2.2
Pt
2.2
Cu
1.9
Ag
1.9
Au
2.4
Zn
1.6
Cd
1.7
Hg
1.9
Ga
1.6
In
1.7
Tl
1.8
Al
1.5
B
2.0
Ge
1.8
Sn
1.8
Pb
1.9
Si
1.8
C
2.5
As
2.0
Sb
1.9
Bi
1.9
P
2.1
N
3.0
Se
2.4
Te
2.1
Po
2.0
S
2.5
O
3.5
Br
2.8
I
2.5
At
2.2
Cl
3.0
F
4.0
Increasing electronegativity
Decreasing electronegativity
Lu
1.3
Yb
Tm
1.2
Er
1.2
Ho
1.2
Dy
1.2
Eu
Sm
1.2
Tb
Gd
1.2
Pm
Pr
1.1
Ce
1.1
Lr
No
1.3
Md
1.3
Fm
1.3
Es
1.3
Cf
1.3
Bk
1.3
Cm
1.3
Am
1.3
Pu
1.3
Np
1.3
Pa
1.4
U
1.4
Th
1.3
Vitamin A and vitamin C
differ in the colors displayed
on their electrostatic poten-
tial maps. The areas of blue
and red in vitamin C’s map
indicate differences in the
electronegativity of the
atoms in this molecule.
These differences result
in the water solubility of
vitamin C.

7.9 Reactivity 289
side of each period possess an increased nuclear charge. Both of these factors
cause electrons to be attracted to the nucleus more strongly.
The decrease in electronegativity that we note as we move down groups
makes sense because the atoms are growing larger.With an extra intervening shell
of electrons at each step, the nuclear charge is being screened from the electrons
shared in a covalent bond. The effect of the increased size and electron screening
outweighs the increase in nuclear charge as we move down a group.
The trends in electronegativity, electron affinity, and ionization energy are
not without exceptions, so they are general trends rather than absolute rules.
They do, however, reveal one very clear message about which atoms are most
likely to attract electrons and become negative ions, and which are most likely to
lose electrons and become positive ions. Atoms on the extreme left of the periodic
table, especially the lower extreme left, will most readily form positive ions. Atoms on
the extreme right of the periodic table, especially the upper extreme right (always
remembering that Group VIIIA is excluded), will most readily form negative ions.
EXERCISE 7.8 Trends in Electronegativity
Without examining the actual Pauling electronegativity values, predict which of
these elements in each pair is more electronegative.
Li or Be N or P S or I
Solution
Be is closer in the periodic table to fluorine. It is more electronegative.
N is only two elements away from fluorine, whereas P is three elements away.
Nitrogen is more electronegative.
S is two elements away from fluorine. It is more electronegative.
PRACTICE 7.8
Which of the following in each pair is more electronegative?
P or Na Ne or Cl N or C
See Problems 68–70.
7.9 Reactivity
The concept of reactivity is a descriptive but very useful notion in chemistry.
When we describe an element as “highly reactive,” we mean that it readily partic-
ipates in chemical reactions to form compounds. When we describe an element
as “unreactive,” we mean it does not readily participate in chemical reactions to
form compounds. As we have already discovered, most highly reactive elements
are, in their natural state, combined with other elements in the form of com-
pounds. You will never find a hunk of soft, shiny sodium in nature. Rather, you
will find it in ionic form in seawater or in salts on land. Unreactive elements can
be found in their pure form. This has great bearing on the uses we make of ele-
ments. The metals sodium and potassium, for example, would be of little use for
making automobiles or jewelry, because they react so vigorously and readily with
water. Gold, on the other hand, is an ideal metal for jewelry. Because it is so unre-
active, it can be found in its free form within the earth. The scarcity of gold makes
it unsuitable for the construction of automobiles, even though such automobiles
might last for a very long time.
We compromise when making automobiles, and build their metal compo-
nents mostly out of steel, which is largely composed of the element iron, as we
Gold in its natural state.
290 Chapter 7 Periodic Properties of the Elements
noted in the NanoWorld/MacroWorld feature in Section 7.2. Iron is not totally
unreactive; it corrodes into rust (iron oxide) when combined with the oxygen
found in air or dissolved in water. Iron lasts long enough, however, to form the
basic materials of automobiles that will last quite a few years.
Can the periodic table tell us which elements are the most reactive? The ele-
ments that react most readily generally do so by either losing or gaining electrons.
The most reactive elements will be those with the greatest tendency to form pos-
itive ions (those with the lowest ionization energies), or with the greatest ten-
dency to form negative ions (those with the largest negative electron affinities or
the largest electronegativities). These highly reactive elements are found at the
extreme left of the periodic table (Groups IA and IIA) and on the far right in
Groups VIA and VIIA. The least reactive elements of all are found in Group
VIIIA, the noble gases; and those with intermediate and low reactivities are found
between the extremes just mentioned, in the central portion of the periodic table.
In Group IB, we find gold and silver, unreactive elements we use in jewelry, and
also copper, which is sufficiently unreactive to be used in copper electric wires
and the pipes of plumbing systems.
Our discussion now enables us to find more general degrees of logic in the pe-
riodic table. We now have enough understanding to give at least rudimentary an-
swers to the questions we raised in the chapter opening about why oxygen reacts
with so many elements and why gold has a very low reactivity. These questions
can be answered by considering the location of these elements in the periodic
1
H
3
Li
11
Na
19
K
37
Rb
55
Cs
87
Fr
4
Be
12
Mg
20
Ca
38
Sr
56
Ba
88
Ra
21
Sc
39
Y
57
La*
89
Ac
†
22
Ti
40
Zr
72
Hf
104
Rf
23
V
41
Nb
73
Ta
105
Db
24
Cr
42
Mo
74
W
106
Sg
25
Mn
43
Tc
75
Re
107
Bh
26
Fe
44
Ru
76
Os
108
Hs
27
Co
45
Rh
77
Ir
109
Mt
110
Uun
111
Uuu
28
Ni
46
Pd
78
Pt
29
Cu
47
Ag
79
Au
3
IIIB
4
IVB
5
VB
6
VIB
7
VIIB
11
IB
12
IIB
31
Ga
49
In
81
Tl
5
B
13
Al
32
Ge
50
Sn
82
Pb
6
C
14
Si
33
As
51
Sb
83
Bi
7
N
15
P
34
Se
52
Te
84
Po
8
O
16
S
9
F
17
Cl
35
Br
53
I
85
At
10
Ne
18
Ar
36
Kr
54
Xe
86
Rn
2
He
58
Ce
90
Th
59
Pr
91
Pa
60
Nd
92
U
61
Pm
93
Np
62
Sm
94
Pu
63
Eu
95
Am
64
Gd
96
Cm
65
Tb
97
Bk
66
Dy
98
Cf
67
Ho
99
Es
68
Er
100
Fm
69
Tm
101
Md
70
Yb
102
No
71
Lu
103
Lr
1
IA
2
IIA
13
IIIA
14
IVA
15
VA
16
VIA
17
VIIA
18
VIIIA
*Lanthanides
†
Actinides
112
Uub
30
Zn
48
Cd
80
Hg
1
2
3
4
Period
5
6
7
9
VIIIB
810
Periodic table with the most reactive elements highlighted in green.
Application
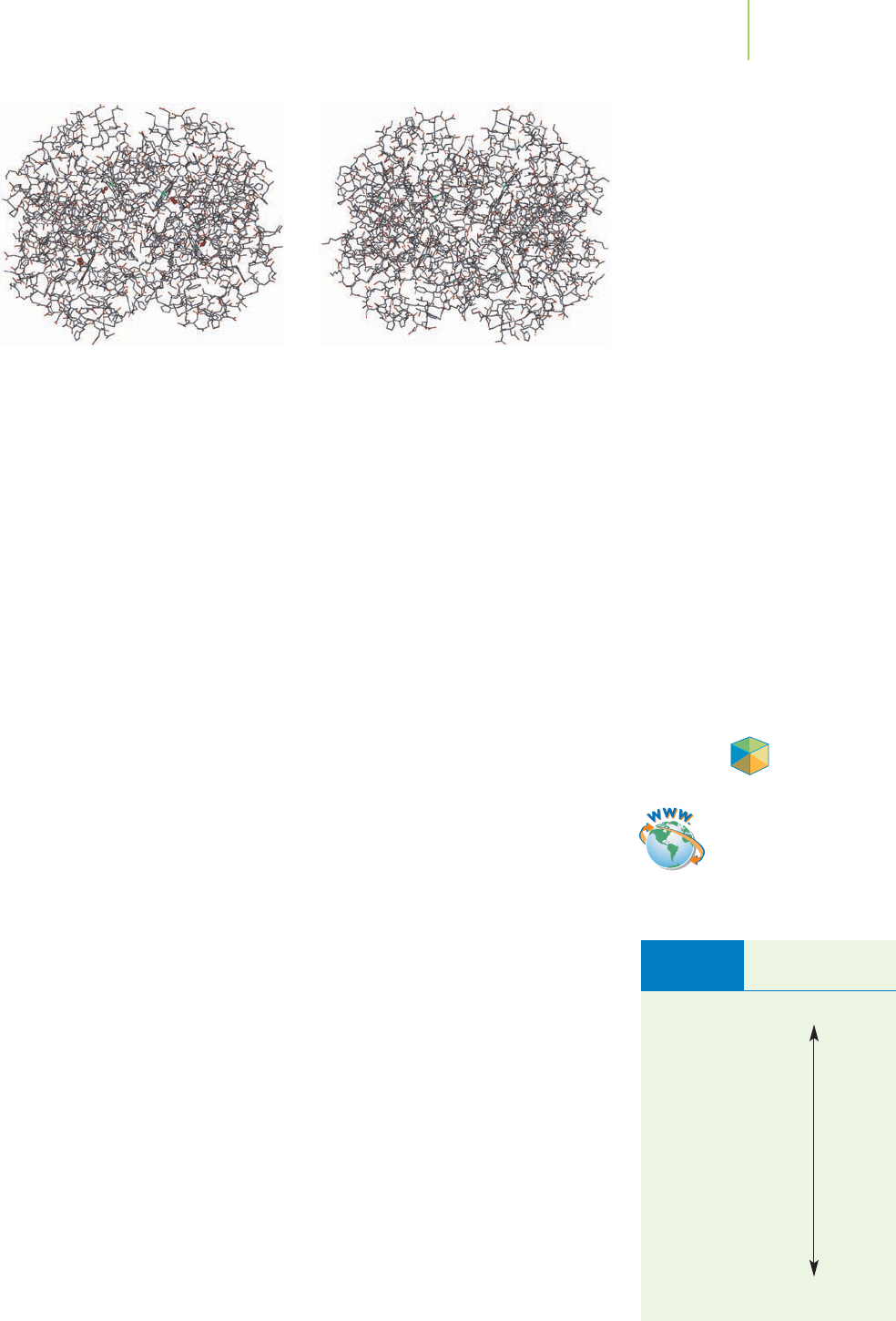
Oxyhemoglobin Hemoglobin
table and all that this means. Another question posed in our introduction was
what makes iron so well-suited to the task of carrying oxygen around the body,
bound to the iron-containing protein we call hemoglobin. The iron in hemo-
globin is in the form of Fe
2+
ions, which bind reversibly (that is, they can continue
to bind and then release, depending on conditions) to oxygen molecules (O
2
).
The binding has to be readily reversible, because hemoglobin must combine with
oxygen where oxygen is abundant, in the blood vessels of the lungs; but must re-
lease the oxygen to the tissues of the body in which oxygen levels are much lower.
A positive ion can perform the binding task by interacting with the electrons of
the oxygen molecule. In order to do that in a readily reversible way, however, the
ion and the atom from which it is derived must have neither too strong nor too
weak an attraction for electrons. A suitable ion will be from an element of inter-
mediate electronegativity, likely to be found around the middle of the periodic
table. Iron, in the middle of the periodic table, can do the job very nicely. This is
only one of several reasons why iron ions serve within hemoglobin molecules as
the oxygen carriers of life, but it is surely a significant one. Other reasons include
the availability of iron in the environment in which life originated and the suit-
ability of iron to combining with the other components of hemoglobin.
One idea that we did not discuss here but will consider in the next two chap-
ters is that judgments about the reactivity of an element cannot be made in isola-
tion. That is, atoms react with other types of atoms, and the extent to which a
reaction occurs depends on the properties of both, or even many, types of atoms.
More broadly speaking, the reaction environment must be considered in decid-
ing the nature of chemical behavior.
The Reactivity Series of Metals
The relative reactivities of some of the most abundant and most useful elements
in the environment are very significant to us. For example, in deciding which
metal is suitable for a particular industrial use, we find that the ease with which it
corrodes (oxidizes in the presence of substances in the environment, often lead-
ing to deterioration in the properties of the metal) will be very significant. The
most common form of corrosion is reaction with oxygen, and all forms of corro-
sion are chemical reactions of one kind or another. The most reactive metals will
generally corrode most quickly. Thinking about such issues led to the idea of list-
ing metals in a
reactivity series (or activity series), which ranks selected metals in
order of reactivity (see Table 7.14).
Such a list can be made by exposing metals to a range of substances, such as
hydrochloric acid, water, and air, and observing how readily they react. There are
some problems with this approach. For example, aluminum reacts more quickly
with oxygen than does iron. The aluminum soon becomes coated, however, with
a cohesive layer of aluminum oxide that protects the aluminum beneath from
further corrosion. That is why aluminum cookware is quite long-lived. Iron
7.9 Reactivity 291
The four oxygen molecules in oxyhemo-
globin can be seen near the center of
the model. Hydrogen atoms have been
omitted for clarity.
Application
A Reactivity Series
(Activity Series)
Potassium Most reactive
Sodium
Lithium
Calcium
Magnesium
Aluminum
Zinc
Iron
Tin
Lead
Copper
Mercury
Silver
Gold Least reactive
TABLE 7.14
Video Lesson: The Activity Series
of the Elements

oxide, on the other hand, does not form any protective layer but instead produces
the crumbling, weak structure we know as “rust,” which breaks away to reveal
fresh corrosion-prone metal underneath. Nevertheless, the idea of ranking metals
by reactivity has proved very useful.
An alternative, and in some ways more satisfactory, way of ranking metals by
reactivity is found in the “electrochemical series” that we will discuss in Chapter
19. This is related to the tendency of each metal to give up electrons. Remember
that when metals react, they generally do so by losing outer electrons to form
ions. The most reactive metals are the ones that lose electrons and form positive
ions most readily.
7.10 The Elements and the Environment
The elements of planet Earth are distributed throughout the environment in
places and forms that are a consequence of their physical and chemical proper-
ties, and these physical and chemical properties can be related to the elements’
positions in the periodic table. The
environment in this sense comprises the entire
world around us, including the Earth, its atmosphere, and ourselves.
Geologists describe the structure of the Earth in terms of five distinct regions:
the core, mantle, crust, hydrosphere, and atmosphere. These are shown diagram-
matically in Figure 7.14. Although nobody has traveled to the Earth’s core or has
even been able to obtain samples, we know its structure from the accumulation of
292 Chapter 7 Periodic Properties of the Elements
Rusty aluminum (left) and rusty iron (right).
FIGURE 7.14
The structure of planet Earth.
Application
C
HEMICAL
ENCOUNTERS:
The Elements and
the Environment
Core
Mantle
Crust
Hydrosphere
Atmosphere

years of indirect evidence. There is no doubt that the core is composed largely of
iron, mixed with smaller amounts of the metals nickel and cobalt and lighter ele-
ments such as carbon and sulfur. The core is largely molten, so these elements
form a molten alloy (a homogeneous mixture of metals) with some nonmetals
mixed within the alloy. The predominance of iron at the center of the Earth is a
consequence of its high density in relation to most of the other elements that
make up our planet. When the Earth formed, it went through a molten phase,
during which the densest materials were drawn toward the center. The less dense
materials rose outward, in just the same way as a mixture of cooking oil and water
will settle into layers, the dense water beneath the less dense oil.
The mantle is another region of the Earth that has never been directly sam-
pled, although a few different types of volcanic rocks are believed to have been
derived from this region. The evidence of these rocks, combined with other indi-
rect evidence, suggests that the mantle is composed largely of oxides of the
elements iron, magnesium, silicon, calcium, and aluminum. The low-density ele-
ment oxygen, which is a gas at typical atmospheric temperatures and pressures,
has been retained within the Earth’s mantle because of its chemical reactivity.
This has caused it to react with various metals to form the much more dense
compounds found in the mantle.
The Earth’s crust makes up less than 1 percent of the mass of the planet and,
as shown in Figure 7.15, it has a ratio of elements that differs greatly from that of
the Earth as a whole. However, it is the only solid part of the Earth that we can an-
alyze directly. The crust is also the part of the environment from which we draw
nearly all of our raw materials for use in industrial fabrication and as sources of
energy. The most abundant elements of the crust are listed in Table 7.15. These
elements are largely found bound within compounds, oxides being the most pre-
dominant. Oxides of silicon are among the most common components of the
crust. For example, silicon dioxide (SiO
2
) is the principal component of sand and
of the many types of rock from which sand is derived.
The hydrosphere is the name given to the waters of the Earth, comprising
oceans, seas, lakes, rivers, and underground aquifers. In addition to the hydrogen
and oxygen that make up water, the hydrosphere contains a great variety of dis-
solved substances that influence its properties and suitability for various uses.
Seawater is unsafe for humans to drink because of its high concentration of salts,
such as sodium chloride and magnesium chloride. Earth’s atmosphere contains
the low-density, gaseous materials that nevertheless are sufficiently dense to be
retained by the gravitational pull of the planet. As shown in Table 7.16, the at-
mosphere is largely composed of nitrogen and oxygen, with much smaller
amounts of argon, carbon dioxide, and other rare gases. Some of the most signif-
icant components of the environment, however, are too rare even to show up in
most tables of the atmosphere’s composition. Gases such as sulfur dioxide and
7.10 The Elements and the Environment 293
Oil
Crust Whole earth
Iron
Oxygen
Silicon
Magnesium
Calcium
Aluminum
Sodium
Potassium
Sulfur
Nickel
Other
FIGURE 7.15
Comparison of the elements in the Earth’s crust with those in the entire Earth.
Vinegar
Oil and vinegar, which is
mostly water, are immisci-
ble; they do not mix. The
denser vinegar sinks to
the bottom of this bottle.
nitrogen dioxide are extremely rare overall, but they cause problems over areas
where they are present as environmental pollutants.
Finally, it is worth pointing out that the element hydrogen (H
2
), the most
abundant element in the universe, is of such low density that any hydrogen re-
leased into the atmosphere will eventually escape into space, unless it reacts with
some other chemicals on the way. The only way for hydrogen to be retained on
our planet is within chemical compounds, such as water (H
2
O), methane (CH
4
),
or any one of millions of other compounds, including most of those found
within living things. If chemical changes had not locked up hydrogen within such
chemical compounds, we would never have evolved to ponder and study these
chemical changes.
294 Chapter 7 Periodic Properties of the Elements
The Abundance of Elements in the Earth’s Crust
Element Abundance (percent, by mass)
Oxygen 46.60
Silicon 27.72
Aluminum 8.13
Iron 5.00
Calcium 3.63
Sodium 2.83
Potassium 2.59
Magnesium 2.09
Titanium 0.44
Hydrogen 0.14
Phosphorus 0.12
Manganese 0.10
Copper 0.0070
Gold 0.00000005
TABLE 7.15
Composition of Dry Air
Substance Volume Fraction
N
2
0.7808
O
2
0.2095
Ar 0.00934
CO
2
0.00034
Ne 1.82 × 10
−5
He 5.82 × 10
−6
CH
4
2 × 10
−6
Kr 1.1 × 10
−6
H
2
5 × 10
−7
N
2
O5× 10
−7
Xe 8.7 × 10
−8
SO
2
< 1 × 10
−6
O
3
< 1 × 10
−7
NH
3
,CO,NO,I
2
< 1 × 10
−8
TABLE 7.16
The Bottom Line
■
The structure of the periodic table was initially de-
veloped by scientists trying to make sense of the dif-
fering reactivities of all the elements found in the
natural environment. (Section 7.1)
■
The structure of the periodic table includes “blocks”
defined in terms of which type of orbital is being
filled as we imagine filling up the available orbitals
using the Aufbau principle. This gives us the s-block,
p-block, d-block, and f-block. (Section 7.1)
■
The horizontal “period” of the periodic table to
which an element belongs indicates how many
energy levels are either fully or partially occupied by
electrons in atoms of that element. (Section 7.1)
■
Elements in any one main group (Groups IA
through VIIIA) have the same number of electrons
in their highest energy level. (Section 7.1)
■
The modern form of the table first began to take
shape through the work of the German Julius
Lothar Meyer and the Russian Dmitri Mendeleev.
(Section 7.1)
■
The elements in the periodic table are arranged into
three main sections: the metals, nonmetals, and met-
alloids (or semimetals). (Section 7.2)
■
Metals and nonmetals exhibit specific types of physi-
cal properties. (Section 7.2)
■
The periodic table is divided into groups, and ele-
ments in each group are similar in electronic struc-
ture, physical properties, and chemical reactivity.
(Section 7.3)
■
Periodicity is apparent in the way important physical
and chemical characteristics recur in a periodic
manner as we move through the periodic table. (Sec-
tion 7.4)
■
Examples of characteristics that show clear periodic-
ity are atomic size (Section 7.5), ionization energy
(Section 7.6), electron affinity (Section 7.7), electro-
negativity (Section 7.8), and reactivity (Section 7.9).
■
The distribution of the elements on planet Earth is a
result of their chemical reactivities. (Section 7.10)

Key Words 295
Key Words
actinides The second set of inner transition elements.
The actinides include elements 90–103. (p. 275)
activity series A series of elements ranked in order of re-
activity. Also known as a reactivity series. (p. 291)
alkali metals The Group IA elements, excluding hydro-
gen. (p. 270)
alkaline earth metals The Group IIA elements. (p. 270)
alloy A homogeneous mixture of metals. (p. 266)
atomic radius Half the distance between the nuclei in a
molecule consisting of identical atoms. Also known
as the covalent radius. (p. 280)
catalyst A substance that greatly speeds up the rate of a
reaction without being consumed by that reaction.
(p. 272)
chalcogens The Group VIA elements. (p. 273)
covalent radius Half the distance between the nuclei in a
molecule consisting of identical atoms. Also known
as the atomic radius. (p. 280)
electron affinity The energy change associated with the
addition of an electron to an atom in the gaseous
state. (p. 286)
electronegativity The relative ability of an atom partici-
pating in a chemical bond to attract electrons to
itself. (p. 287)
environment The world around us, comprising the
Earth and its atmosphere. We ourselves are part of
the environment. (p. 292)
first ionization energy (I
1
) The energy required to remove
one electron from each atom of an element in the
gaseous state. (p. 282)
halogens The Group VIIA elements. (p. 274)
inert gases The Group VIIIA elements. Also known as
noble gases. (p. 274)
inner transition elements The elements in the f-block of
the periodic table, consisting of the lanthanides and
actinides. (p. 275)
ionization energy The energy needed to ionize an atom
(or molecule or ion) by removing an electron from it.
(p. 282)
lanthanides The first set of inner transition elements.
The lanthanides include elements 58–71. (p. 275)
main groups Groups IA through VIIIA of the periodic
table. Groups IB through VIIIB are not main groups.
(p. 269)
metallic radius Half the distance between the nuclei
of the atoms within the solid structure of a metal.
(p. 280)
metalloids A small number of elements that have char-
acteristics midway between those of the metals and
those of the nonmetals. Also known as semimetals.
(p. 268)
metals A large number of elements that share certain
typical characteristics, including shiny appearance,
malleability, and ability to conduct electricity. (p. 265)
noble gases The Group VIIIA elements. Also known as
inert gases. (p. 274)
nonmetals A collection of elements that shares certain
characteristics that are in contrast to those of the
metals, including being gases or dull, brittle solids at
room temperature. (p. 268)
octet Eight electrons in the valence shell of an atom.
(p. 275)
periodicity The recurrence of characteristic properties as
we move through a series, such as the elements of the
periodic table. (p. 279)
reactivity The ability of an element or compound to
participate in chemical reactions. Reactivity is an im-
precise but useful term, particularly when we are dis-
cussing relative reactivities under defined conditions.
(p. 289)
reactivity series A series of elements ranked in order of
reactivity. Also known as an activity series. (p. 291)
second ionization energy (I
2
) The energy required to
remove one electron from each singly charged +1 ion
of an element in the gaseous state. (p. 282)
semimetals A small number of elements that have char-
acteristics midway between those of the metals and
those of the nonmetals. Also known as metalloids.
(p. 268)
transition elements Metal elements from Groups IB
through VIIIB found in the middle of the periodic
table. (p. 275)
valence electrons The electrons that occupy the outer-
most energy level of an atom, which interact with
those of other atoms. (p. 269)

The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 7.1 The Big Picture—Building the Periodic Table
Skill Review
1. Cite two examples wherein two elements’ locations on the
current periodic table would be reversed if they were placed
in accordance with Mendeleev’s atomic mass system of
organization.
2. Some may argue that providing a list of elements in alpha-
betical order along with their properties would be the most
useful arrangement of the known elements. Explain what ad-
vantage there is to arranging them in the periodic manner
displayed in our traditional periodic table format.
3. Identify the “block” location in the periodic table for each of
these elements.
a. Sr c. S e. Se g. Sb
b. Sc d. Sn f. Sm
4. Identify the “block” location in the periodic table for each of
these elements.
a. Rb c. Ru e. Re g. Ra
b. Rn d. Rh f. Rf
5. Identify the “block” location in the periodic table for each of
these elements.
a.C c.Cu e.Cs g.Ce
b. Ca d. Cr f. Cl
6. Identify the “block” location in the periodic table for each of
these elements.
a. He c. Fe e. Xe g. Te
b. Be d. Ge f. Ne
Chemical Applications and Practices
7. The following portion of a hypothetical periodic table shows
the approximate melting points of elements that are neigh-
bors to a missing element. On the basis of the data provided,
predict the melting point of the missing element.
2610°C
3000°C ? 3180°C
8. The following portion of a hypothetical periodic table shows
the densities of elements that are neighbors to a missing ele-
ment. On the basis of the data provided, predict the density
of the missing element.
10.2 g/cm
3
16.7 g/cm
3
? 21.0 g/cm
3
9. In each of these pairs, select the larger of the two elements.
a. Mg or Ca c. Cl or Br
b. P or S d. Cs or Ba
10. In each of these pairs, select the larger of the two elements.
a. K or Ca c. Se or Br
b. O or S d. Ra or Ba
Section 7.2 The First Level of Structure—Metals,
Nonmetals, and Metalloids
Skill Review
11. Classify each of these as a metal, nonmetal, or metalloid.
a. Zn c. Ge e. Si g. Se
b. Ga d. Sn f. P h. As
12. Classify each of these as a metal, nonmetal, or metalloid.
a. Li c. Co e. C g. I
b. Ne d. Te f. Bi h. Sb
13. Most elements, at room temperature, are either solids or
gases. However, two elements, at room temperature, are clas-
sified as liquids. What are these two elements?
14. Most of the key ingredients to alloy with iron to make various
types of steel are metals. However, this is not always the case.
What are two nonmetals that can be alloyed with steel?
Chemical Applications and Practices
15. a. In the production of stainless steel, small amounts of sev-
eral elements are alloyed with iron. Two of the chief ingre-
dients are nickel and chromium. Use the chart given in
Table 7.3 to report the number of grams of each metal
found in 100.0 g of stainless steel.
b. The atoms of the added metals can become part of the
iron structure, which changes the properties of iron. How
many atoms of nickel and chromium, respectively, are pre-
sent in the 100.0 g of stainless steel?
16. Assume that the other elements (besides chromium, nickel,
and iron) found in stainless steel are insignificant. Based on
the calculations in Problem 15, how many iron atoms are
present in the sample for every atom of chromium?
17. Locate the element antimony in the periodic table. Would
this element be classified as a metal, nonmetal, or metalloid?
Antimony is used in alloys with lead or tin, is combined with
other substances to make fireproof fabrics, is mixed with
some types of glass and ceramics, and serves as the active
component of some medicines. Antimony, however, does
have some toxic properties. One way to obtain it is as follows:
Sb
2
S
3
+ Fe → FeS +Sb
Balance this equation. The annual worldwide production of
antimony is approximately 6.00
× 10
4
tons. How many
moles of antimony is this?
18. Locate the element gold in the periodic table. Would this ele-
ment be classified as a metal, nonmetal, or metalloid? Gold is
used not only in jewelry but also as a wire in some computer
parts and in other situations where a noncorrosive metal is
needed. Gold can be solubilized in water by using a mixture
of very strong acids, as follows:
Au(s) +HCl(aq) + NO
3
−
(aq) →
[AuCl
4
]
−
(aq) + H
2
O(l) + NO(g)
Balance this equation. How many grams of gold must be
solubilized to produce 1 mol of NO(g)?
296 Chapter 7 Periodic Properties of the Elements
Focus Your Learning
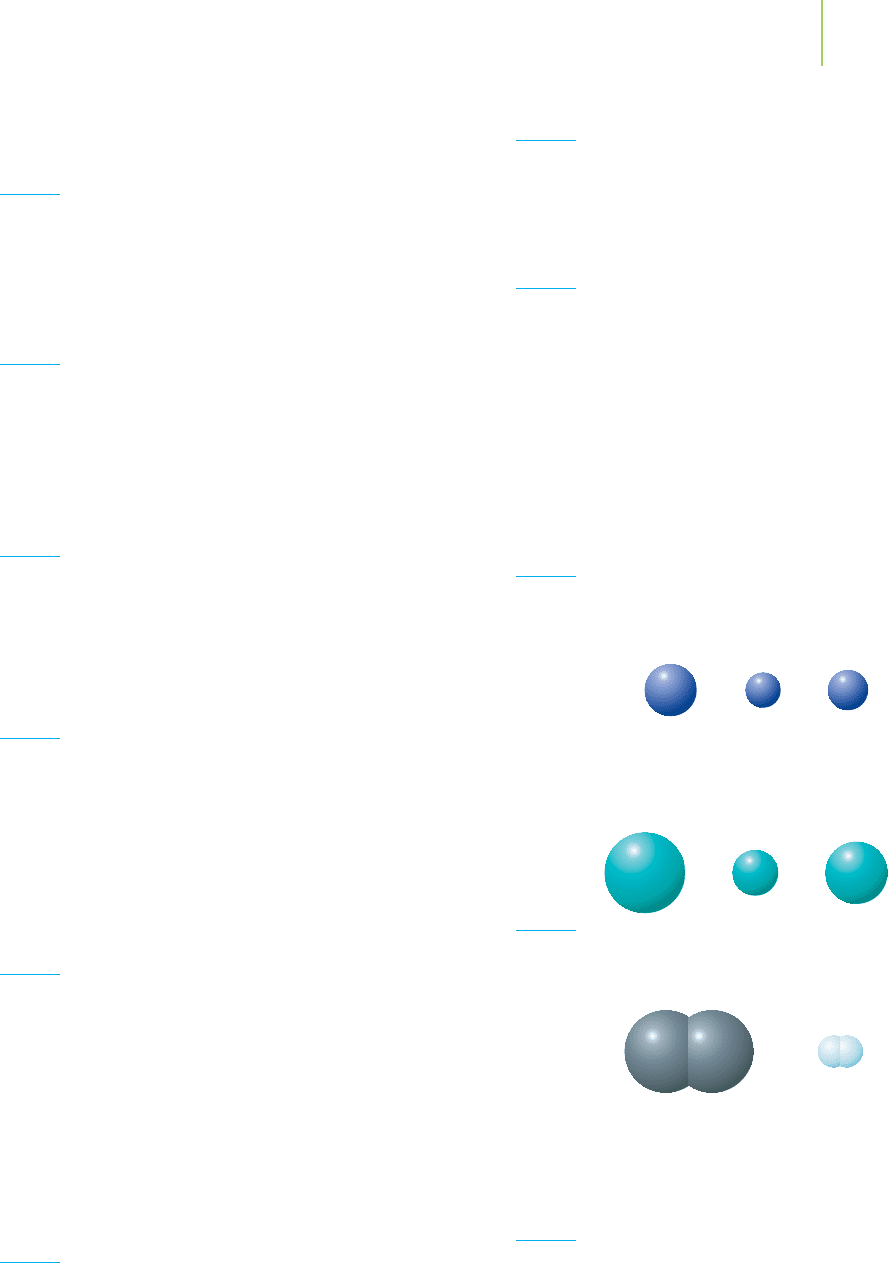
Section 7.3 The Next Level of Structure—Groups in the
Periodic Table
Skill Review
19. Using the most common oxidation number for each, write
out the formulas of the compounds that would be most likely
to form when Li,Be,B,C,N,O,and F,respectively,combine
with oxygen.
20. Write out the chemical formulas for Li, Na, K, Rb, and Cs, re-
spectively, combining with oxygen. What accounts for the
similarities in the formulas of these compounds?
21. If an element from Group VIA were going to form an ionic
compound, which type of element (metal or nonmetal)
would be most likely to form the other part of the com-
pound? Explain your answer.
22. If an element from Group IIA were going to form an ionic
compound, which type of element (metal or nonmetal)
would be most likely to form the other part of the com-
pound? Explain your answer.
23. How many unpaired electrons are found in the elements that
make up Group VIIA? How many unpaired electrons are
found in the most common ions of those elements?
24. How many unpaired electrons are found in the elements that
make up Group IIIA? How many unpaired electrons are
found in the most common ions of those elements?
Chemical Applications and Practices
25. The listing in Table 7.13 of elements in the human body is
based on mass. Considering only the first three (oxygen,
carbon, and hydrogen), indicate whether the ranking would
change if the table were based on number of atoms instead of
mass. Explain the basis of your answer.
26. The listing in Table 7.13 of elements in the human body
includes calcium. What use does our body make of this
element? In addition, calculate the quantity of calcium in a
typical human in units of milligrams per kilogram of body
weight.
27. Many dietary supplements contain a list of metals present.
This list may include zinc, iron, cobalt, and others. Name
one important role that transition metals play in human
biochemistry.
28. Iron plays several roles in humans. One of its important roles
involves the electron transport chain. Iron can easily change
oxidation states between the +2 and +3 charges. What char-
acteristic feature of iron’s electron configuration makes this
possible?
Section 7.4 The Concept of Periodicity
Skill Review
29. The noble gases, except for helium, have a stable, filled octet.
Explain why helium is still considered a noble gas even
though it does not have a stable, filled octet.
30. The alkali metals have a complete shell of a noble gas plus an
extra electron. Explain why hydrogen is often associated with
this group of the periodic table.
Chemical Applications and Practices
31. Examine the full electron configuration of calcium and
potassium. Explain why calcium is not as reactive as
potassium.
32. The same reasoning used to answer Problem 31 applies to the
relative reactivity of magnesium compared to sodium, but
why is sodium not as reactive as potassium?
33. In some of the original periodic table research, tellurium was
placed after iodine due to its slightly greater relative mass.
However, this placed tellurium in Group VIIA directly below
bromine, chlorine, and fluorine. After examining the elec-
tron configuration of tellurium and iodine explain why the
present classification is logical.
34. Nickel is placed on the periodic table immediately after
cobalt. However, its average mass is less than that of cobalt.
Explain why the placement is logical.
Section 7.5 Atomic Size
Skill Review
35. These three spheres can be used to compare the relative sizes
of sodium, magnesium, and sulfur. Which sphere would best
represent each atom?
36. These three spheres can be used to compare the relative sizes
of potassium, rubidium, and cesium. Which sphere would
best represent each atom?
37. If the bond length for a COC bond were 154 pm, what would
you determine as the radius of a carbon atom?
38. If the bond length for a HOH bond were 75 pm, what would
you determine as the approximate radius for an atom of
hydrogen?
39. If the bond length for a COCl bond were 171 pm, what
would you determine as the approximate radius for an atom
of chlorine? (Use the answer from Problem 37 to assist you in
determining the radius of a chlorine atom.)
40. If the bond length for a HOF bond were 92 pm, what would
you determine as the approximate radius for an atom of flu-
orine? (Use the answer from Problem 38 to assist you in de-
termining the radius of a fluorine atom.)
H–H bond
75 pm
C–C bond
154 pm
Focus Your Learning 297
