Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

We can explain the absence of noble gases by looking at their chemical
reactivity—or lack of it. Life is a very complex process of chemical change involv-
ing regulated chemical reactions. The noble gases generally do not participate in
chemical change because they are so unreactive, so we would not expect to find
them playing any part in the chemistry of life.
The fact that life is made primarily of the smaller elements, from a small sec-
tion of the periodic table, may be linked to the fact that these elements are gener-
ally more abundant on Earth, and in the universe at large, than elements with
very large atoms. As we’ll see in Chapter 21, larger atoms can be built from
smaller ones. Although there are exceptions, the larger atoms are generally less
abundant than smaller ones in nature. In any case, nearly all of the mass of a
human is made up of atoms of hydrogen (atomic number = 1), carbon (atomic
number =6), nitrogen (atomic number =7), and oxygen (atomic number = 8).
We are largely made from some of the smallest atoms of the environment.
Phosphorus (atomic number = 15) and sulfur (atomic number = 16) are also
important as part of proteins, DNA, and RNA.
Another feature of the elemental composition of human life that you may
have identified is that a relatively high number of the elements found within us
consists of transition metals, although we contain these in very small amounts.As
we mentioned earlier, the chemical versatility of transition metal ions, such as
their tendency to readily form ions of differing charges, makes them useful in
enzymes—biological catalysts of chemical reactions.
It is also notable that humans contain elements from every block of the peri-
odic table except the f-block, which comprises the lanthanides and actinides. The
elements in this block are composed of very large atoms. Many of the actinides
are unstable and therefore radioactive (see Chapter 21).
HERE’S WHAT WE KNOW SO FAR
■
The elements within a group have a common number of electrons in their
highest energy level.
■
The elements in the periodic table are arranged into three main sections: the
metals, nonmetals, and metalloids (or semimetals).
■
The structure of the periodic table includes “blocks”defined in terms of which
type of orbital is being filled. This gives us the s-block, p-block, d-block, and
f-block elements.
■
The elements that predominate in living things are carbon, hydrogen, nitro-
gen, and oxygen, with small concentrations of phosphorus, sulfur, and transi-
tion metals.
278 Chapter 7 Periodic Properties of the Elements
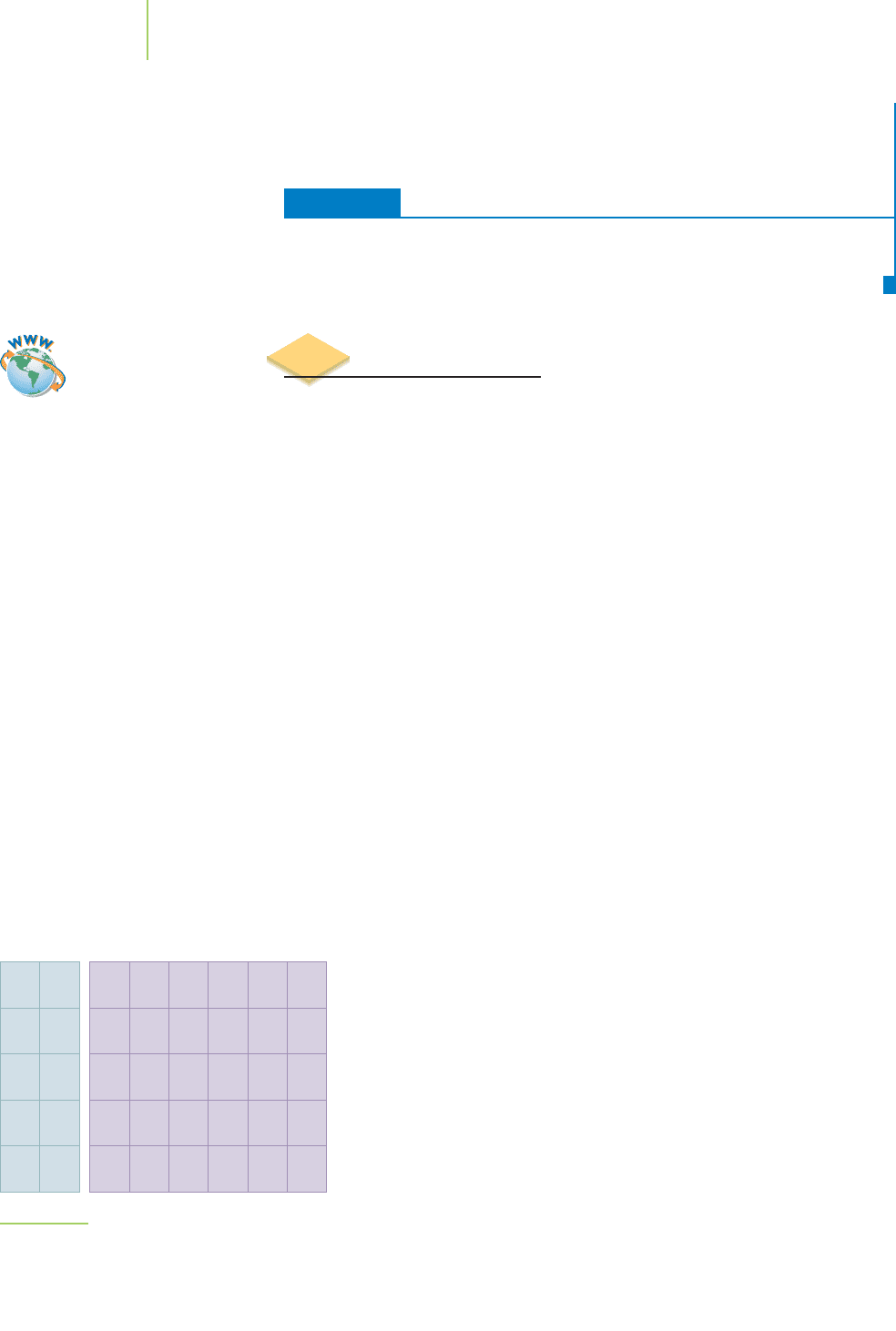
A seven-base-pair segment of double-
stranded DNA. The phosphorus atoms
are yellow.
Elements of the
Human Body
Abundance
Element (percent, by mass)
Oxygen 61.0
Carbon 23.8
Hydrogen 10.0
Nitrogen 2.6
Calcium 1.4
Phosphorus 1.1
Sulfur 0.2
Silicon 0.02
Potassium 0.02
Sodium 0.014
Chlorine 0.012
Fluorine 0.004
Magnesium 0.003
Iron 0.0004
Strontium 0.00005
Bromine 0.00003
Lead 0.00002
Trace amounts of Mn,
I, Cr, Mo, Se, and V
TABLE 7.13TABLE 7.13

7.4 The Concept of Periodicity 279
7.4 The Concept of Periodicity
Why is the structure of the periodic table useful to know? Once we understand the
trends as we traverse the groups and periods in the periodic table, we can make
reasonable predictions about the chemical and physical behavior of any element in
the periodic table. We will add to our understanding in subsequent chapters, even
learning how to assess the likely nuclear behavior of an element. Once we are
armed with this understanding, the periodic table serves as a most wonderful
guide to the formation and interaction of substances.
The basis of the periodic table is
periodicity, which will be revealed in many of
the properties we explore in the remainder of this chapter. When chemists talk of
“periodic properties” among the elements, they mean the way in which char-
acteristic properties recur in a periodic manner as we move through the periodic
table. For example, element number 3 in the table, lithium, is a very reactive
metal that forms an alkali on reaction with water. Element 4 (beryllium), is less
reactive, and as we move through elements 5 (boron), 6 (carbon), 7 (nitrogen), 8
(oxygen), 9 (fluorine), and 10 (neon), we find elements that become steadily less
like lithium in chemical reactivity and physical properties. Then suddenly, with
element 11 (sodium), we find another very reactive metal that forms an alkali on
reaction with water. The similarities between the reactivities of lithium and
sodium are so striking that it is clear that we are observing some significant re-
peating feature of reactivity as we move through the periodic table. If we then
move on to elements 12 through 19, we find the same thing happening again.
Elements 12 through 18 have properties less and less like those of sodium and
lithium, and then suddenly, with element 19 (potassium), the property of being
a very reactive metal that forms an alkali on reaction with water recurs. These
chemical characteristics, which we call the characteristics of the alkali metals,
recur in a periodic manner as we move through the periodic table.
That is the basic concept of chemical periodicity, and we could have chosen
various other properties to make the same point. For example, the property of
being a very unreactive gaseous element that exists as free individual atoms
occurs in elements with atomic numbers 2, 10, 18, 36, 54, and 86. As with the
alkali metals, we have a chemical property—the lack of reactivity, in this case—
that recurs in a systematic, or periodic, manner as we move through the periodic
table. The number of elements we have to pass by before a characteristic property
recurs is not constant. It is 8, 8, 18, 18, 32 in the series above (see Exercise 7.4), but
the crucial fact is that each characteristic property does recur periodically as we
move through the periodic table.
EXERCISE 7.4 Explaining the Periods Between Periodicities
The periodic property of being an “inert gas” recurs after we move on to 8, then 8,
then 18, then 18, then 32 intervening elements in the periodic table. Can you suggest
the underlying physical reason why the periodicity follows this particular pattern?
Solution
The pattern is a consequence of the way in which electrons fill up orbitals before a
particular electron arrangement associated with a particular property recurs. The
fundamental characteristic associated with being an inert gas is to have a stable octet
of eight electrons in the atom’s highest energy level, or two electrons in a completely
full energy level in the case of helium. The Aufbau principle indicates that after
helium, the stable octet will recur after the 2s and 2p orbitals have been filled, which
means eight electrons must be added before we arrive at neon, then another eight
before we arrive at argon. As we then move through Period 4, ten 3d electrons must
be added before the 4p orbitals become filled, so this time we must move through 18
FIGURE 7.7
This three-dimensional periodic table,
known as the ElemenTree, was devel-
oped by Canadian Fernando Dufour to
emphasize the periodic relationships of
the elements.
Visualization: Periodic Table
Trends
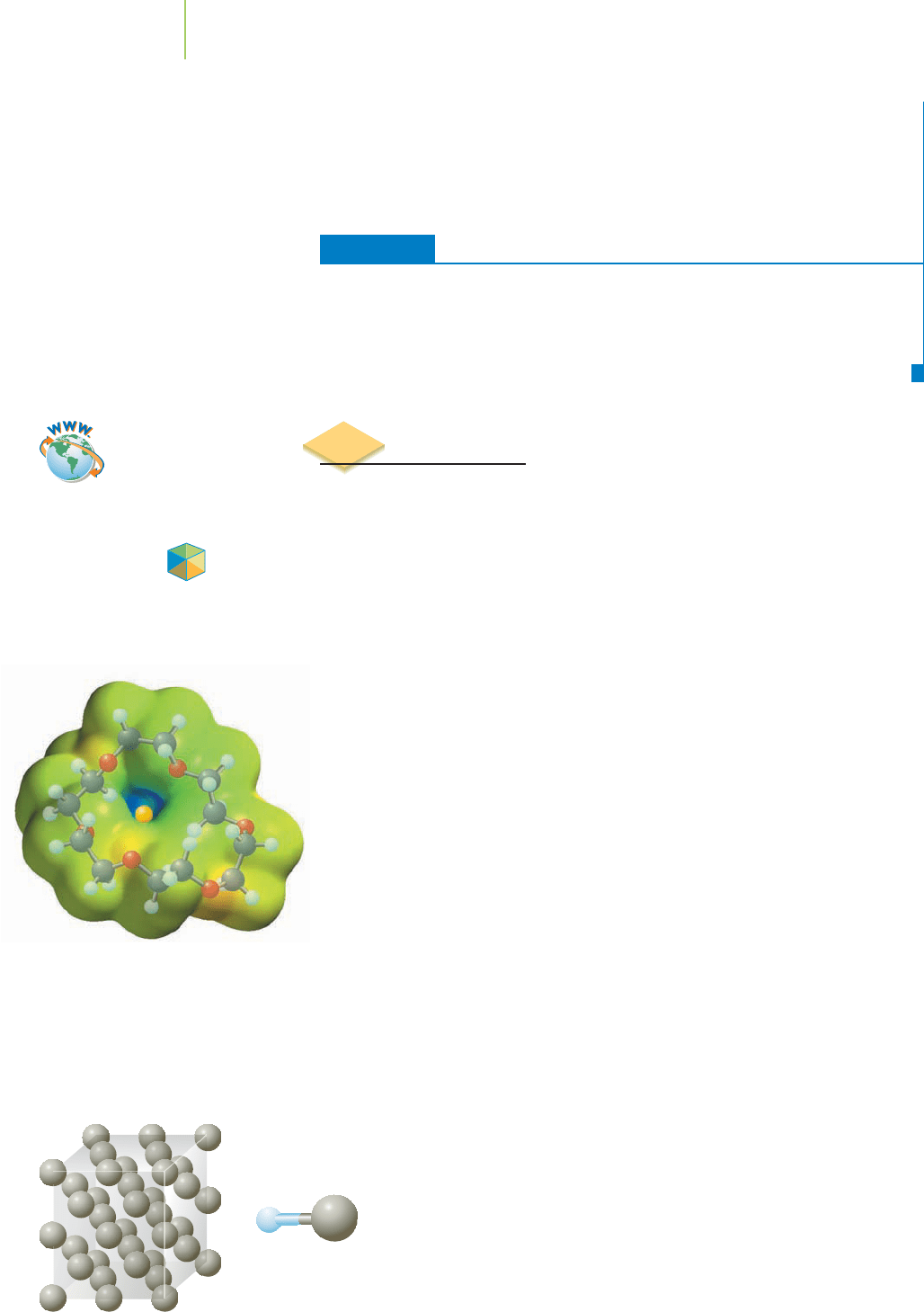
The chelating agent known as
18-crown-6 binds to sodium cations.
elements before the stable octet recurs. Similar reasoning applies to the gap between
krypton and xenon. Then the gap jumps up to 32, between xenon and radon, as a
consequence of the filling of f orbitals that must occur before the 6p orbitals of
radon can be filled. This explanation is most clearly visualized by looking at the long
version of the periodic table in Figure 7.5 or the pyramidal version in Figure 7.7.
PRACTICE 7.4
Judging on the basis of the periodicity of the periodic table, indicate what would be
the hypothetical atomic number of a metal that would be more reactive than Fr.
How many electrons would it take to fill a noble gas in the hypothetical Period 8 of
the periodic table?
See Problems 29–34.
7.5 Atomic Size
Heavy metal poisoning is unfortunately a common occurrence, particularly
among children living in homes painted with lead-based paints. In the hospital,
once the poisoning is recognized, the patient is treated with a chelating agent.
Chelating agents (see Chapter 18) specifically grab ions of a particular size and
help to remove them from the body. The size of the ion is extremely important in
how well it binds to the chelating agent. If it is too small or too large, it will not
associate with the chelating agent and be excreted from the body in that manner.
Figure 7.8 reveals the key trends and periodicities found in the size of atoms.
What do you note about the size of the atoms in the periodic table? Atomic size de-
creases as we move from left to right along any period, and it increases as we move
down any group. Figure 7.8 uses atomic radii as a measure of atomic size. The
atomic radius is defined as half the distance between the nuclei in a molecule con-
sisting of identical atoms. It is also known as the
covalent radius of an atom,
because it indicates the size of the atom when that atom is involved in covalent
bonding. Not all elements form such molecules. Some atomic radii values must
be estimated via indirect methods, such as comparing the distances between
atomic nuclei when the atoms are bound within chemical compounds instead of
in a molecule made of identical atoms. Values for the radii of metal atoms can
also be obtained by analyzing the distance between the nuclei of the atoms within
the solid structure of the metal concerned. These values are called
metallic radii.
The uncertainties involved in measuring atomic radii, and the various meth-
ods that can be used, mean that the values we obtain should be regarded as ap-
proximate, and you may find slightly different values quoted in different sources.
The basic trends, however, are absolutely clear: Atomic size decreases along periods
and increases down groups. How can we explain these data? As we move down the
group, we encounter atoms with increasing numbers of occupied electron energy
levels. Each new occupied energy level, corresponding to a higher principal quan-
tum number, includes electron orbitals of greater average radius than those of the
previous level, so the atoms grow larger as we move down a group.
It might seem more difficult to explain why we find atoms of de-
creasing size as we move along any period, because the atoms actually
contain more matter as we travel from left to right, thanks to the
steady addition of protons, electrons, and neutrons. However, the
additional electrons are being added to energy levels already present
in previous elements of the period, and they are held around nuclei
whose positive charge is steadily increasing across the period. This in-
creased positive charge draws the electrons of the occupied energy
levels closer to the nucleus as we move along a period, so the atomic
radius steadily decreases.
280 Chapter 7 Periodic Properties of the Elements
Application
Atomic radii can be measured by mea-
suring the distance between the nuclei
of atoms in a metal. Measuring the dis-
tance between nuclei in a molecule
gives the covalent radius
in molecules.
Video Lesson: Periods and
Atomic Size
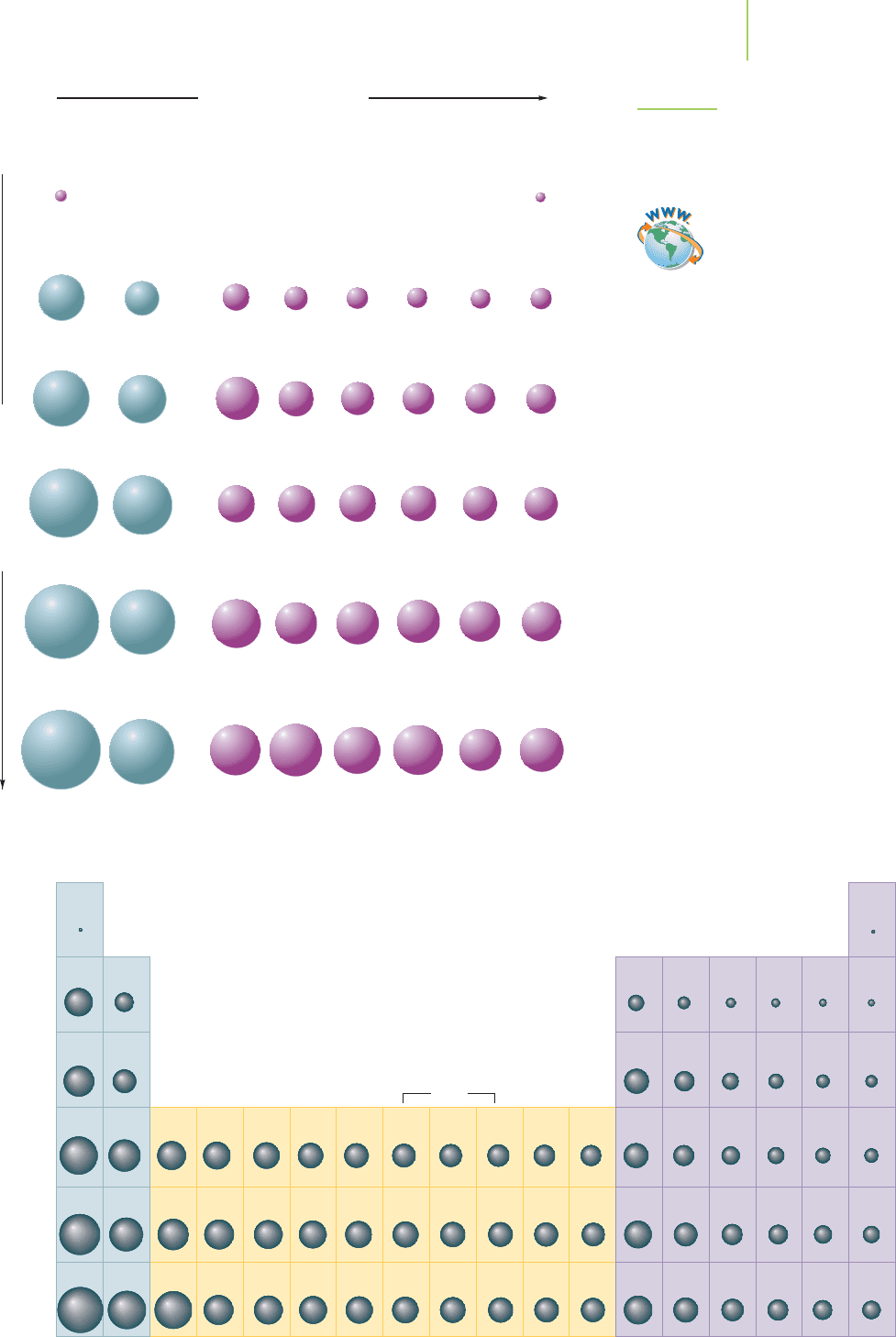
7.5 Atomic Size 281
H
37
He
32
Li
152
Be
113
B
88
C
77
N
70
O
66
F
64
Ne
69
Na
186
Mg
160
Al
143
Si
117
P
110
S
104
Cl
99
Ar
97
K
227
Ca
197
Ga
122
Ge
122
As
121
Se
117
Br
114
Kr
110
Rb
247
Sr
215
In
163
Sn
140
Sb
141
Te
143
I
133
Xe
130
Cs
265
Ba
217
Tl
170
Pb
175
Bi
155
Po
167
At
140
Rn
145
VIIIAVIIAVIAVAIVAIIIAIIAIA
Atomic radius increases
Atomic radius decreases
FIGURE 7.8
Atomic radii for selected atoms.
H
Li
Na
K
Rb
Cs
Be
Mg
Ca
Sr
Ba
Sc
Y
La
Ti
Zr
Hf
V
Nb
Ta
Cr
Mo
W
Mn
Tc
Re
Fe
Ru
Os
Co
Rh
Ir
Ni
Pd
Pt
Cu
Ag
Au
Ga
In
Tl
B
Al
Ge
Sn
Pb
C
Si
As
Sb
Bi
N
P
Se
Te
Po
O
S
F
Cl
Br
I
At
Ne
Ar
Kr
Xe
Rn
He
1A
IIA
IIIB IVB VB VIB VIIB VIIIB IB IIB
IIIA IVA VA VIA VIIA
VIIIA
1
2
3
4
5
6
Period
Zn
Cd
Hg
Trend in atomic radius.
Visualization: Determining the
Atomic Radius of a Nonmetal
(Carbon)
Visualization: Determining the
Atomic Radius of a Nonmetal
(Chlorine)
Visualization: Determining the
Atomic Radius of a Metal
(Molybdenum)

1
2
3
4
5
6
IA IIA IIIA IVA VA VIA VIIA VIIIA
B
800
C
1086
N
1402
S
1005
Te
869
Se
941
O
1314
Si
780
Pb
715
Bi
703
Xe
1176
Kr
1356
Ar
1527
Ne
2088
F
1681
Cl
1255
P
1060
As
947
Rn
1042
Sb
834
Po
813
At
(926)
I
1009
Br
1143
Sn
708
Ge
761
Al
580
Ga
579
In
558
Tl
589
H
1311
Be
899
He
2377
Mg
735
Ca
590
Sr
549
Ba
503
Li
520
Na
495
K
419
Rb
409
Cs
382
FIGURE 7.9
First ionization energies for selected
elements.
7.6 Ionization Energies
Most of the elements in the periodic table are metals. When they react with other
elements to form compounds, they generally do so by losing one or more elec-
trons to form positive ions. An input of energy is required to remove one or more
electrons from an atom, and the energy involved is known as
ionization energy.
The smaller the ionization energy, the more likely it is that ionization will occur.
All elements, not just the metals, have ionization energy values. By examining the
ionization energies of the elements, we can see why metals are particularly prone
to form positive ions, and we can see how the differing reactivities of metals
matches their differing ionization energies.
The
first ionization energy (I
1
) of an element is the energy required to remove
the highest energy electron from an atom of the element in the gaseous ground
state. The first ionization energy is typically quoted per mole of atoms being ion-
ized and can be symbolized in general terms, using X to represent any element, as
X(g) → X
+
(g) + e
−
For example, the first ionization energies for sodium and chlorine are
Na(g) → Na
+
(g) + e
−
I
1
= 495 kJ/mol
Cl(g) → Cl
+
(g) + e
−
I
1
= 1255 kJ/mol
Why is the first ionization energy of sodium so much smaller than that of chlorine?
Note the resulting ion’s electron configuration. The sodium ion has a noble gas
electron configuration. The chlorine ion does not. The first ionization energies of
selected main-group elements are listed in Figure 7.9.
The
second ionization energy (I
2
), of an element is the energy required to
remove one electron from each singly charged +1 ion of an element in the
gaseous state:
X
+
(g) → X
2+
(g) + e
−
Third, fourth, and all subsequent ionization energies can be similarly defined, as
successive electrons are removed.
We can see the link between ionization energies and reactivities by examining
some of the alkali metals. Lithium, sodium, and potassium are all reactive metals
that form alkalis on reaction with water. These metals, when they react, form ions
of Li
+
,Na
+
, and K
+
, respectively. We can get an indication of how readily the for-
mation of these ions occurs by considering these ionization energies:
Li(g) → Li
+
(g) + e
−
I
1
= 520 kJ/mol
Na(g) → Na
+
(g) + e
−
I
1
= 495 kJ/mol
K(g) → K
+
(g) + e
−
I
1
= 419 kJ/mol
282 Chapter 7 Periodic Properties of the Elements
Video Lesson: Ionization Energy

EXERCISE 7.5 Implications of Ionization Energy Trends
The reactions of lithium, sodium, and potassium metals with water to form an
alkaline solution are as follows:
2Li(s) + 2H
2
O(l) → 2Li
+
(aq) + 2OH
−
(aq) + H
2
(g)
2Na(s) + 2H
2
O(l) → 2Na
+
(aq) + 2OH
−
(aq) + H
2
(g)
2K(s) + 2H
2
O(l) → 2K
+
(aq) + 2OH
−
(aq) + H
2
(g)
Given the values listed in the text for the first ionization energies of these metals,
which metal will react most vigorously if added to water? If we were to put a chunk
of cesium metal in water, how might it react?
First Thoughts
Ionization energy is a measure of the energy required to remove the highest energy
electron from an atom. It is an endothermic process, so when comparing elements
in an otherwise equivalent reaction, we find that the lower the ionization energy, the
less energy will be required, and the more readily the reaction will occur.
Solution
The potassium metal has the lowest ionization energy of the three, so it will react
most readily with water; sodium will react less readily and lithium the least impres-
sively. Figure 7.10 shows the reactions of these metals with water. Note the flame
that potassium makes upon reaction with water. What about cesium? Its ionization
energy of 376 kJ/mol means that it will react even more vigorously than potassium.
In fact, the reaction with water is explosive.
7.6 Ionization Energies 283
The metals lithium (left), sodium
(center), and potassium (right).
FIGURE 7.10
The relative reactivities of metals can
sometimes be quite clearly visualized.
Sodium
Potassium
Calcium
Gold

Further Insights
Francium would be expected to react even more vigorously than cesium. However,
francium is exceedingly rare and has no stable isotopes. One place that prepares
samples for scientific study is the Nuclear Structure Laboratory at the State Univer-
sity of New York at Stony Brook. An isotope known as francium-210 (along with
five neutrons) is prepared by slamming oxygen-18 nuclei atoms into gold-197
nuclei using a nuclear accelerator:
197
Au +
18
O →
210
Fr + 5 n
This, and other isotopes of francium, can be produced at the rate of about 1 million
atoms per second. Keep in mind, though, that 1 million atoms are not very many
compared to the 6.02 × 10
23
atoms that make up a mole. The francium that is
collected can be studied to determine its physical and chemical properties, but the
nuclei decay so quickly that it is impractical to collect francium-210 for other uses.
PRACTICE 7.5
Which of the metals in Group IIA would you expect to react most vigorously with
water? Explain your answer in terms of the ionization energies of the elements.
See Problems 45, 46, 73, and 75.
We have just identified one of the general trends linking ionization energy
values with an element’s position in the periodic table. Ionization energies gener-
ally decrease moving down any group. We can explain this trend by noting that as
we move down a group, the valence electrons being removed are coming from
energy levels of successively higher principal quantum numbers. Therefore, as we
move down a group, the electrons being removed are screened from the nucleus
by additional inner energy levels occupied with electrons.
The other main trend in ionization energies is that they generally increase from
left to right along any period. We can rationalize this as due to the extra energy
needed to release an electron from the pull of the increasing nuclear charge found
as we move along a period.
These trends in ionization energy can be clearly seen in the laboratory if
we choose metals with significantly different reactivities (see Figure 7.10). The
trends can also be plotted graphically, as shown in Figure 7.11, in a way that
makes the periodicity in this property apparent. As we move through Figure 7.11,
we see the ionization energies rising and falling in a periodic manner.
284 Chapter 7 Periodic Properties of the Elements
Li
Na
K
Rb
Cs
He
Ne
Ar
Kr
Xe
Rn
10
0
H
B
Be
C
O
N
F
Mg
Al
Cl
S
P
Zn
As
Br
Cd
Tl
18 36 54 86
500
1000
1500
2000
2500
Period
2
Period
3
Period
4
Period
5
Period
6
Ionization energy (kJ/mol)
Atomic number
FIGURE 7.11
The values of first ionization energies
for the elements in the first six periods.
H
Li
Na
K
Rb
Cs
Be
Mg
Ca
Sr
Ba
Sc
Y
La
Ti
Zr
Hf
V
Nb
Ta
Cr
Mo
W
Mn
Tc
Re
Fe
Ru
Os
Co
Rh
Ir
Ni
Pd
Pt
Cu
Ag
Au
Ga
In
Tl
B
Al
Ge
Sn
Pb
C
Si
As
Sb
Bi
N
P
Se
Te
Po
O
S
F
Cl
Br
I
At
Ne
Ar
Kr
Xe
Rn
He
Zn
Cd
Hg
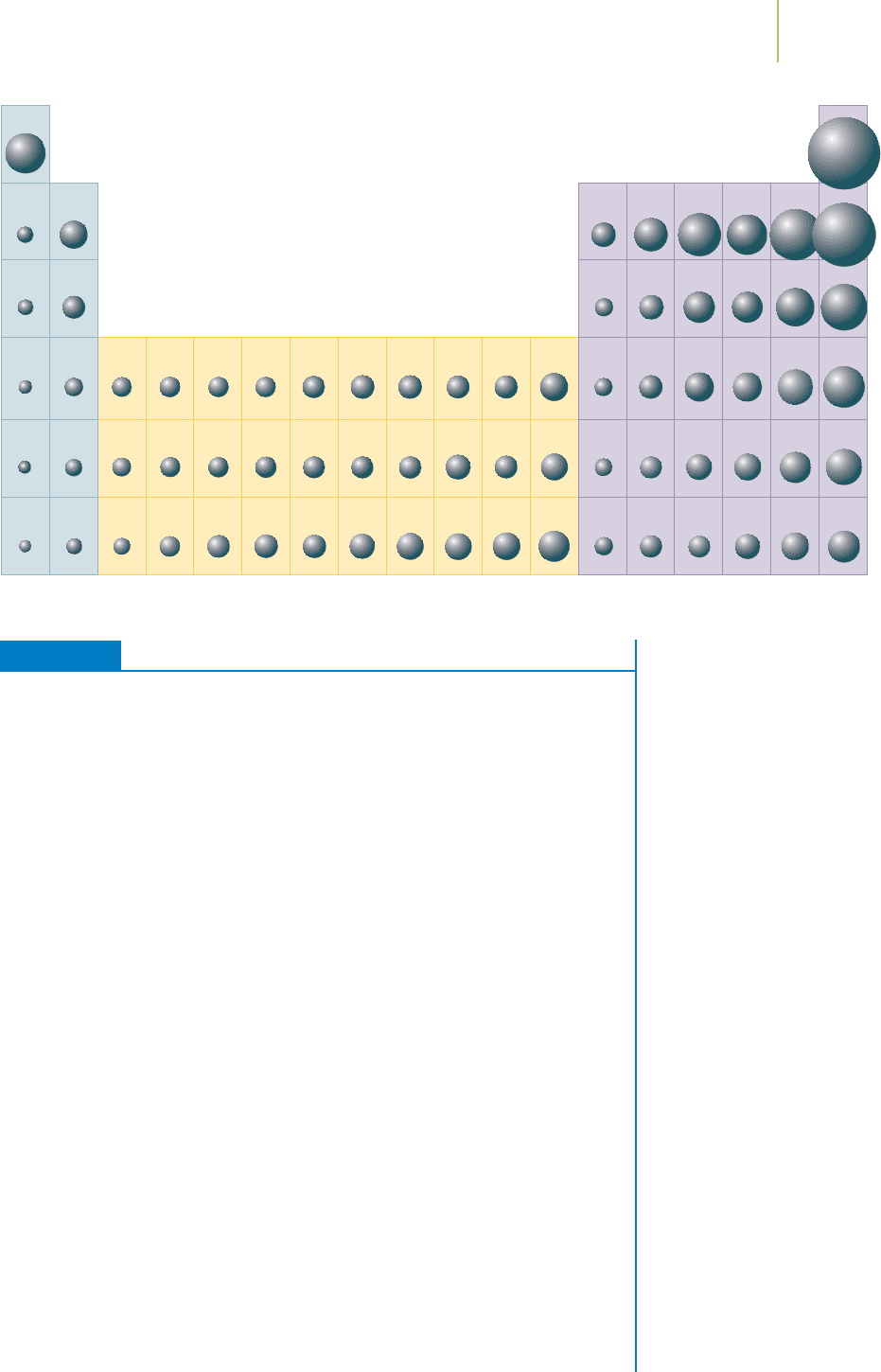
7.6 Ionization Energies 285
The trend in first ionization energy. The size of the sphere
for each atom indicates the relative magnitude of the first
ionization energy.
EXERCISE 7.6 Differences in Ionization Energies
Here are the first three ionization energies of sodium and magnesium, in kilojoules
per mole:
I
1
I
2
I
3
Sodium 495 4560 6920
Magnesium 735 1445 7730
There is a very large difference between the first and second ionization energies of
sodium. With magnesium, however, the very large difference is seen between the
second and third ionization energies. Why do the large differences in ionization
energies occur where they do?
First Thoughts
What does the ionization energy tell us? It is the energy required to remove an
electron from the atom. The key question, then, is “What is it about the electron
configuration of each element that results in such a sudden increase in ionization
energy?”
Solution
Sodium atoms have one outer-shell electron ([Ne]3s
1
), whereas magnesium atoms
have two ([Ne]3s
2
). Once sodium has lost one electron, the next electron must come
from a deeper electron shell, closer to the nucleus, so a great deal more energy is re-
quired to remove that second electron. In the case of magnesium, the transition to
removing electrons from an inner shell does not occur until the two outer electrons
have been removed, so the big jump in ionization energy is seen between the second
and third ionization energies, rather than between the first and second.
Further Insights
Iron is an example of a transition element that has several possible oxidation states,
including Fe
2+
and Fe
3+
. A most wonderful and trustworthy website to retrieve ion-
ization energy data (and so much more!) is the WebElements site at http://www
.webelements.com. Look up the ionization energy for the ions of iron at that site.
Where are the sudden increases in ionization energy, and how do they compare to
those found in a nonmetal such as phosphorus? Can you explain the differences be-
tween these two elements?
PRACTICE 7.6
Predict, and draw a graph of, the ionization energy versus electron ionized for
sulfur. Compare your graph to the measured values at the WebElements site.
See Problems 47, 48, 53, 54, 96, and 99.
7.7 Electron Affinity
Our look at ionization energies concerned the energy required to generate
positive ions (cations) from neutral atoms. The other side of the “ionization coin”
is the generation of negative ions (anions) from neutral atoms. A negatively
charged ion forms when an atom gains electrons, and the energy changes associ-
ated with electron gain are called electron affinities. The
electron affinity is the
energy change associated with the addition of an electron to an atom in the
gaseous state, and as usual, we quote the values in kilojoules per mole of atoms.
For example,
F(g) + e
−
→ F
−
(g) −328 kJ/mol
Cl(g) + e
−
→ Cl
−
(g) −349 kJ/mol
Br(g) + e
−
→ Br
−
(g) −325 kJ/mol
I(g) + e
−
→ I
−
(g) −295 kJ/mol
At(g) + e
−
→ At
−
(g) −270 kJ/mol
The electron affinity values for Group VIIA reveal one of the general trends in
electron affinity and also one of the complications. The values indicate to us that
electron affinities generally become less negative (or more positive), correspond-
ing to less energy being released, as we move down a group.
Does this trend make
sense?
Because added electrons are farther from the nuclei of larger atoms, they
interact less with the positive charge of the nucleus. The trend makes sense. There
are many exceptions, however, such as more energy released when a chloride ion
forms, in the above list, than upon formation of the fluoride anion.Why does flu-
orine not fit the trend? In searching for a possible explanation, we should note
that fluorine is the smallest of this group of atoms, so the electrons in
the outer p orbitals, to which the extra electron is being added, will be
much closer together than in the other atoms of this group. The larger
electron–electron repulsions between these closely spaced electrons,
compared to those of other halogen atoms, will be associated with
increased potential energy.
Figure 7.12 shows many more electron affinity values. It allows us
to confirm the general trend just identified within groups, in addition
to revealing exceptions. Figure 7.12 also confirms that elements that
form stable negative ions have large negative electron affinity values,
whereas those that form stable positive ions have much lower negative
electron affinity values, or even positive values.
Note, as well, that the values in Figure 7.12 indicate that electron
affinity values generally get more negative as we move from the left to
the right along a period in the periodic table.
Does this trend make
sense?
We know from our understanding of the trend associated with
atomic radii that atoms get smaller as we move to the right along the
period table. For the same reasons we discovered as we move down a
group, the electron affinity generally tends to get more negative as we
move to the right along a period.
286 Chapter 7 Periodic Properties of the Elements
17
VIIA
1
IA
13
IIIA
15
VA
2
IIA
14
IVA
16
VIA
18
VIIIA
Li
–60
B
–27
N
~0
F
–328
Na
–53
K
–48
Rb
–47
Cs
–46
Cl
–349
Br
–325
I
–295
At
–270
Be
>0
C
–154
O
–141
Ne
>0
S
–200
Se
–195
Te
–190
Po
–183
FIGURE 7.12
Electron affinity values, in kilojoules per mole
(kJ/mol), for selected elements. Electron affinity
values generally decrease as we go up and to the
right in the periodic table. More negative values
indicate a greater affinity for electrons.
Video Lesson: Electron Affinity
7.8 Electronegativity 287
EXERCISE 7.7 Trends
Many of the periodic properties identified in the periodic table are related to each
other. Is there a relationship between electron affinity and the number of valence
electrons?
First Thoughts
We should first construct a crude periodic table with arrows pointing to the most
negative electron affinity. Superimposing the number of valence electrons on this
drawing will reveal any relationship.
Solution
As the number of valence electrons gets larger, the atomic radius gets smaller across
a period. As the radius gets smaller, the electron affinity becomes more negative.
Therefore, as the number of valence electrons increases, the electron affinity be-
comes more negative. This trend, however, is valid only within a given period in the
periodic table, because the electron affinity changes and the number of valence elec-
trons remain constant as we move down a group.
Further Insights
Periodic trends are abundant in the periodic table. Size, electron affinity, reactivity,
and ionization potential are all related to position on the periodic table. This table
has trends in many other properties, such as the formation of acids or bases upon
reaction of elemental oxides with water, the tendency to form chlorides, the ten-
dency to behave like metals, and many more.
PRACTICE 7.7
Is there a relationship between the trends observed in ionization energy and elec-
tron affinity? Explain.
See Problems 57–62, 74, 76, 79, and 80.
7.8 Electronegativity
In our discussion about electron affinities, we are actually considering a rather
contrived situation, because most elements rarely exist in the form of free
gaseous atoms. A more meaningful characteristic of elements, one that is related
to their interaction with additional electrons and therefore is more firmly rooted
in real chemical behavior, is their electronegativity. The
electronegativity of an
atom is a measure of the ability of the atom in a molecule to attract shared elec-
trons to itself. Atoms in molecules share electrons to create covalent bonds. How-
ever, if the atoms sharing electrons are different, they will contain nuclei with
different charges and different electron arrangements. The result is that the elec-
trons are not shared equally. Electronegativity values have been calculated for
every atom in the periodic table. However, we generally do not quote electroneg-
ativity values for the noble gases (Group VIIIA) because, as a consequence of
their exceptional stability, they do not readily form bonds.
Electronegativity values for the elements in the periodic table are one of the most
powerful quantities chemists have that explain and predict the behavior of molecules
and ions. Their importance cannot be overstated. In 1932, Linus Pauling defined
the concept in terms of bond energies. The Pauling scale of electronegativities
and the variations of it are the bases of much of our discussion about bonding
in subsequent chapters. To illustrate the utility of electronegativity values, let’s
consider two essential vitamins that we must regularly consume: vitamin A and
vitamin C. Deficiencies in these vitamins are responsible for serious maladies.
Application
Video Lesson: An Introduction
to Electronegativity
