Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

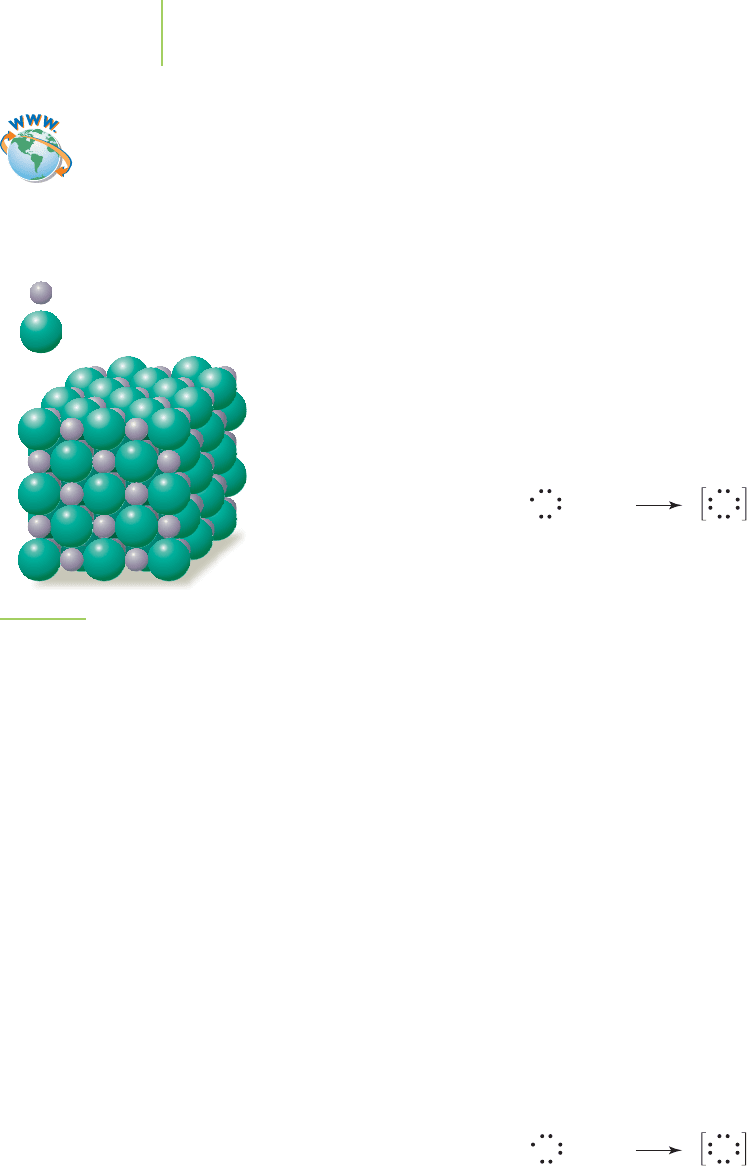
Description of Ionic Bonding
Sodium chloride is commercially mined or processed from brine (salt water, such
as from the oceans or the Great Salt Lake). In the laboratory, we can combine
sodium metal and chlorine gas in a violent reaction to make the salt. The reac-
tion, illustrated below, involves the transfer of electrons from sodium atoms to
Na(s) +
1
⁄
2Cl
2
(g) n NaCl(s) ∆H =−410 kJ
chlorine. If we look at this reaction more closely, we note that one of the atoms
(the metal) loses electrons to become a cation. In our reaction, the sodium atom
Na• n [Na]
+ e
−
loses an electron to become the sodium cation (Na
+
). The other atom (the non-
metal) gains electrons to become an anion. Addition of an electron to a chlorine
atom forms the chloride anion (Cl
−
). The charges on the sodium and chloride
ions attract each other, causing the ions to associate with each other in an
ion pair
(Na
+
Cl
−
).
But it doesn’t stop there. Other sodium cations are also attracted to the nega-
tive charge on the chloride. Other chloride anions are attracted to the positively
charged sodium. The end result is a collection of alternating sodium and chloride
ions arranged in a solid
crystalline lattice, a highly ordered, three-dimensional
arrangement of atoms, ions, or molecules (explained in detail in Chapter 13).
Within a sodium chloride crystal, each of the sodium cations has six neighboring
chloride anions, and each of the chloride anions has six neighboring sodium
cations. The forces of attraction combine to provide a neatly packed crystal of
alternating sodium and chloride ions, as shown in Figure 8.4.
Examples of Ionic Bonding
We can illustrate the formation of an ionic compound such as sodium chloride
(NaCl) through the use of Lewis dot symbols. As reflected in their positions on
the periodic table, sodium is a metal and chlorine is a nonmetal. Sodium has a
relatively low ionization energy and loses an electron to achieve the electron
configuration of a noble gas.
Na• n [Na]
+ e
−
1s
2
2s
2
2p
6
3s
1
n 1s
2
2s
2
2p
6
Chlorine, which has a very favorable electron affinity, gains an electron to achieve
a noble gas electron configuration.
1s
2
2s
2
2p
6
3s
2
3p
5
n 1s
2
2s
2
2p
6
3s
2
3p
6
In the formation of NaCl from Na metal and Cl
2
gas, the electron from the
sodium atom is added to the valence shell of the chlorine atom. We can represent
this process in equation form by showing the movement of the electron from the
sodium atom to the chlorine atom with a fishhook-shaped arrow. The result is a
sodium cation and a chloride anion that are attracted to each other. The ionic
crystal that results has the formula NaCl.
Energy must be added to the system to remove the electron from the sodium
atom. Energy, however, is released when that electron is added to the chlorine atom,
and even more energy is released when the ionic bond forms. The net result from the
ClCl e
ClCl e
308 Chapter 8 Bonding Basics
Na
+
C
l
–
FIGURE 8.4
Crystal structure of sodium chloride. In
the crystal structure of NaCl, we note
that each sodium ion is surrounded by
six chloride ions and that each chloride
ion is surrounded by six sodium ions. The
resulting crystal possesses a regular
close-packed pattern of ions (see
Chapter 14).
Video Lesson: Ionic Bonds
Visualization: Structure of an
Ionic Solid (NaCl)
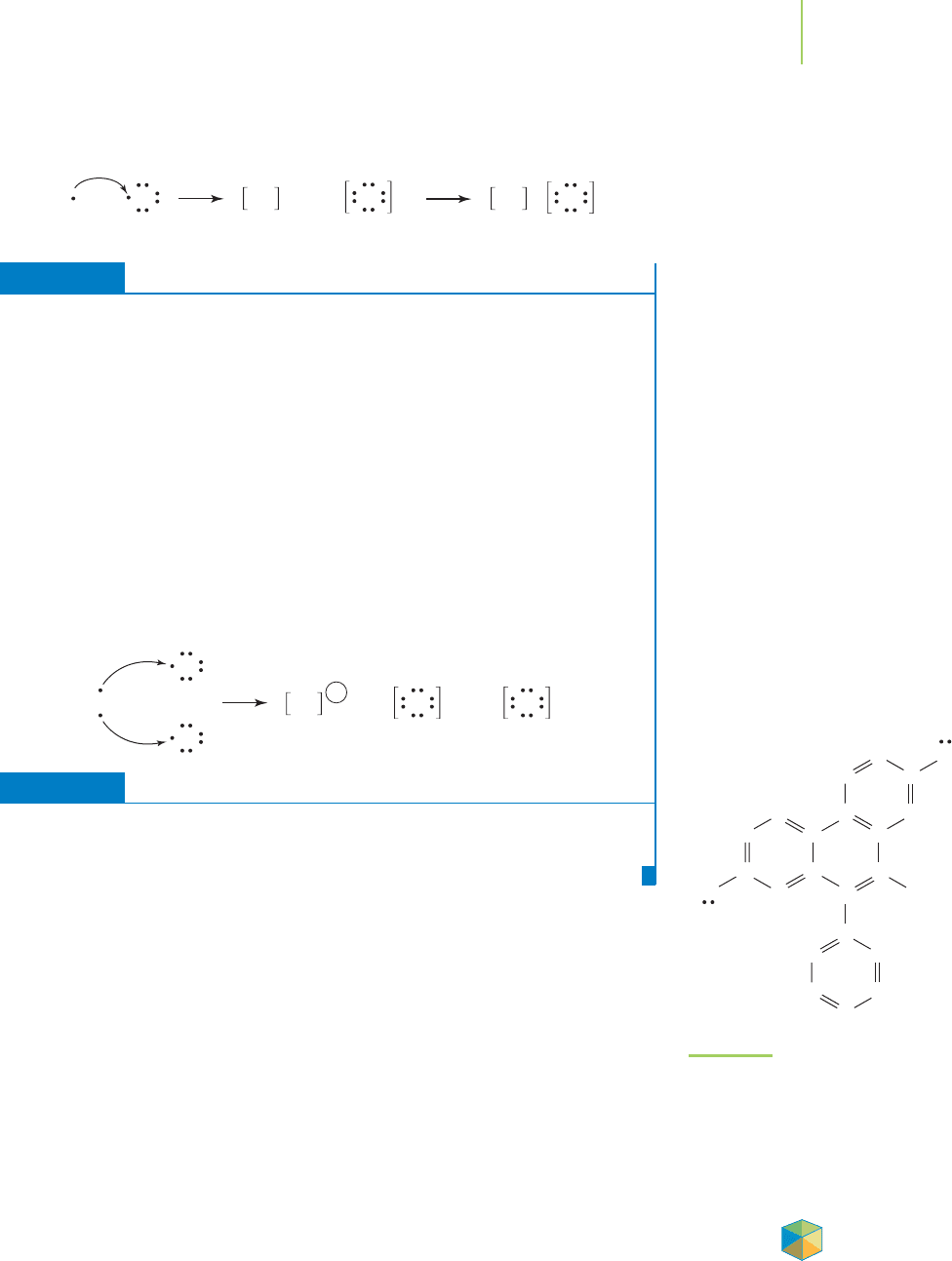
8.2 Ionic Bonding 309
formation of a crystalline lattice of sodium and chloride ions is a large release of
energy.
EXERCISE 8.2 Ionic Compound Formation
Calcium chloride, CaCl
2
, is spread on our sidewalks to melt ice. Because of its abil-
ity to absorb moisture, it is used to dry gases and prevent dust buildup in mining
and highway maintenance. Using Lewis dot symbols, show the transfer of electrons
to form CaCl
2
from calcium metal and chlorine atoms.
Solution
We start by drawing the Lewis dot symbols for the calcium and chlorine atoms.
Then we show the transfer of an electron from the less electronegative calcium to
the more electronegative chlorine atom (arrow).We show a similar transfer with the
other calcium electron to a different chlorine atom. We end with three ions (Ca
2+
and two Cl
−
) that have noble gas electron configurations. Note that the ions end
up with either a completely full valence electron configuration (and become iso-
electronic with a noble gas) or a completely empty valence shell (and become
isoelectronic with a noble gas). This is the octet rule in action.
PRACTICE 8.2
Use the Lewis dot structure model to show the formation of sodium oxide from
atomic sodium and oxygen atoms.
See Problems 21, 22, and 27–30.
Medicinal chemists, biochemists, and pharmacognocists know about the dri-
ving force for the formation of ionic bonds.Ethidiumbromide,shown in Figure8.5,
is a toxic compound that is used by researchers to stain DNA in order to make it
easier to see. This compound exhibits its effects by inserting itself into DNA
strands, causing structural changes to the DNA molecule. The negative charges
on the DNA and the positive charge of the ethidium cation help hold the com-
pound in place. These forces of attraction explain why this compound has such a
strong association with DNA. Another example of the importance of ionic bond-
ing accounts for the source of calcium in milk. Casein, one of the major proteins
found in milk, contains phosphate anions that form ionic bonds with calcium
cations.
Sizes of the Ions
Water from many wells typically contains relatively high concentrations of
calcium, often along with some magnesium and iron. When the water is heated,
dissolved calcium bicarbonate, Ca(HCO
3
)
2
, decomposes to form rock-like de-
posits of calcium carbonate, CaCO
3
, also known as boiler scale. The boiler scale
Ca(HCO
3
)
2
(aq) n CaCO
3
(s) + H
2
O(l) + CO
2
(g)
coats the inside of the pipes that carry the water in the hot-water heater and
throughout the house, so such water is called “hard.” Hard water is not hazardous
Cl
Ca Cl
Ca Cl
Cl
2
Na Cl Na
Cl
Na
Cl
NH
2
H
2
N
C
CHC
HC
CH
C
C
C
HC
CH
CH
N
C
C
C
CH
2
CH
3
Br
HC
HC
CH
CH
CH
FIGURE 8.5
Ethidium bromide. The stabilizing force
that holds the molecule in place in DNA
is the attraction between the anionic
charges on the DNA and the positive
charge on ethidium bromide.
Application
to our health. It is just not the best thing that could happen to our house-
hold pipes. If the buildup is bad enough, the pipes can become clogged,
much as heart arteries become clogged with fatty plaques, reducing blood
flow in a human. Many homeowners and some cities “soften” water by
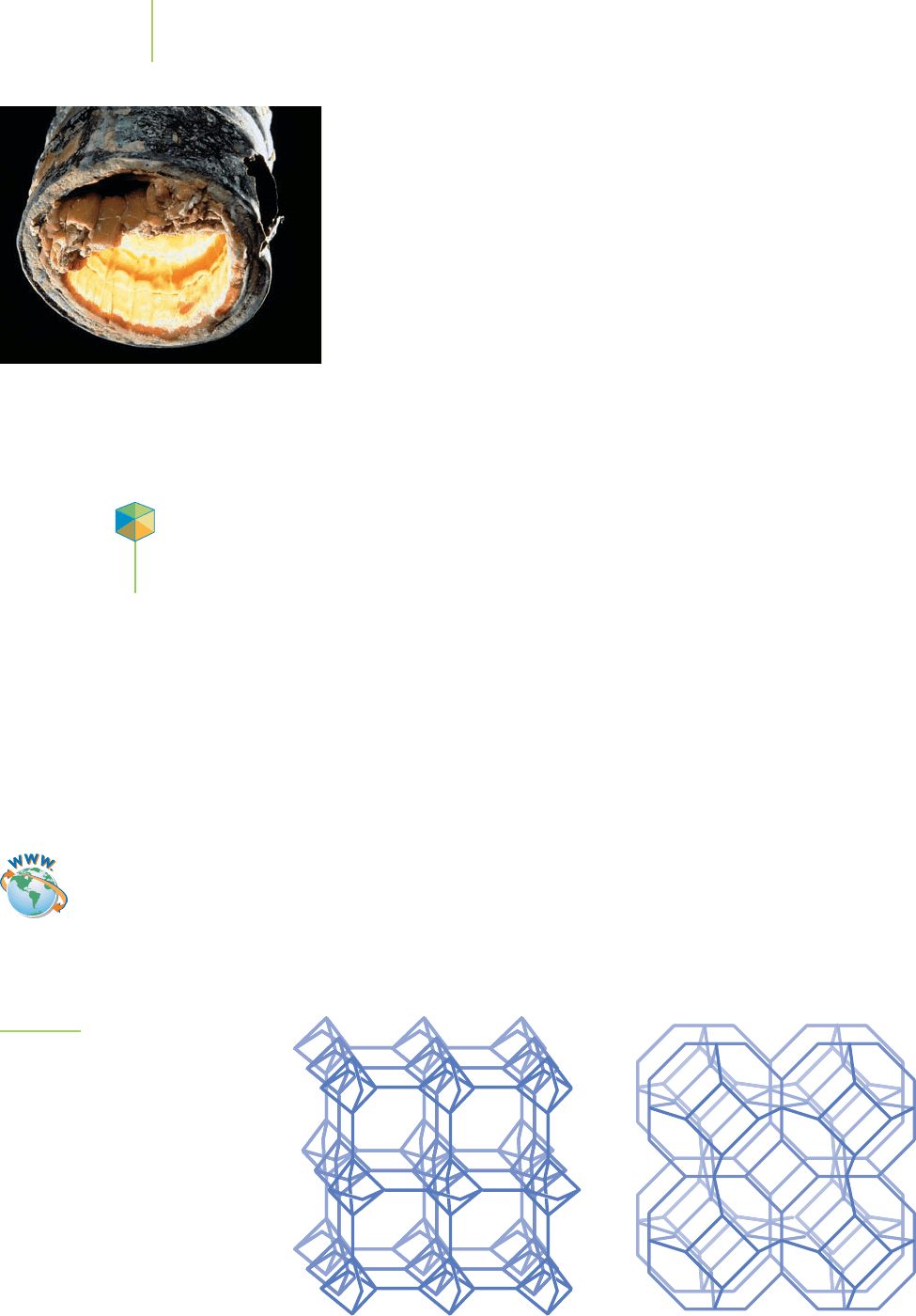
passing it through a bed of
zeolites, which exchange the calcium ions for
sodium ions that (remember our solubility rules) do not form insoluble
salts. How do these ion-exchanging zeolites work?
Zeolites like that shown in Figure 8.6 are composed of aluminum and
silicon oxide subunits containing Group IA and IIA cations. The resulting
honeycomb arrangement of subunits results in a structure full of atom-
sized holes. Ions and molecules small enough to fit through these holes can
enter the zeolite, and if they are just about the same size as the holes in the
zeolite, they get stuck inside. Interactions of the ions or molecules with the
zeolite help to hold them inside. Substances that are too small easily flow in
and out of the zeolite, whereas ions and molecules that are too big can’t
enter the zeolite in the first place. Consequently, a zeolite retains only spe-
cific sizes of ions and molecules. In exchange for the trapped ions, the zeo-
lite releases ions of the same total charge. For example, when a calcium ion is
taken up by the zeolite, it typically releases two sodium ions.
By carefully constructing the zeolite, researchers have been able to develop an
“ionic sponge” that grabs only the ions of interest to them. This is vital, in the
chemical industry, to the formation of better reaction catalysts (compounds that
significantly increase the rate of a chemical reaction without being consumed)
the filtration of polluted air, and the cleanup of hazardous wastes, among other
applications. Experiments with the zeolite known as clinoptilolite, with a pore
size of 0.4 nm, indicated that it was capable of removing radioactive cesium
(
134
Cs and
137
Cs) from cows affected by the 1986 Chernobyl (Ukraine) nuclear
accident.
By far the most common use of zeolites is as an ingredient called a “builder”
in laundry detergents for the removal of calcium ions from hard water. Size is a
critical factor in the behavior of ionic compounds. Not only does size suggest
what type of zeolite can trap a particular ion, it also plays a major role in deter-
mining the structure of the ionic crystal and the strength of the ionic bond.
What contributes to the size of an ion? We can examine the electrons in an
atom more closely to determine the answer. In our previous discussion of the
shape of the orbitals (Chapter 6), we learned that the electrons penetrate deep
into the atom. Each electron helps to balance the charge of the protons in the nu-
cleus and shields the other electrons from the nuclear charge. When an electron
310 Chapter 8 Bonding Basics
“Hard” water contains relatively high concentra-
tions of calcium, typically along with magnesium
and iron. The rock-like deposits of calcium car-
bonate that form on the inside of hot water
pipes give hard water its name.
Application
C
HEMICAL
ENCOUNTERS:
Focus on Zeolites
Natrolite Sodalite
FIGURE 8.6
Ions can enter, and they can leave: The
structure of natrolite and sodalite. These
zeolites have pores that ions of the right
size can enter.
Visualization: Ionic Radii
is removed from an atom, the amount of shielding is reduced. We say that the ef-
fective nuclear charge experienced by each electron increases. The total number
of electron−electron repulsions decreases, so the electrons are pulled closer to
the nucleus. The net effect is a reduction in the size of the electron cloud around
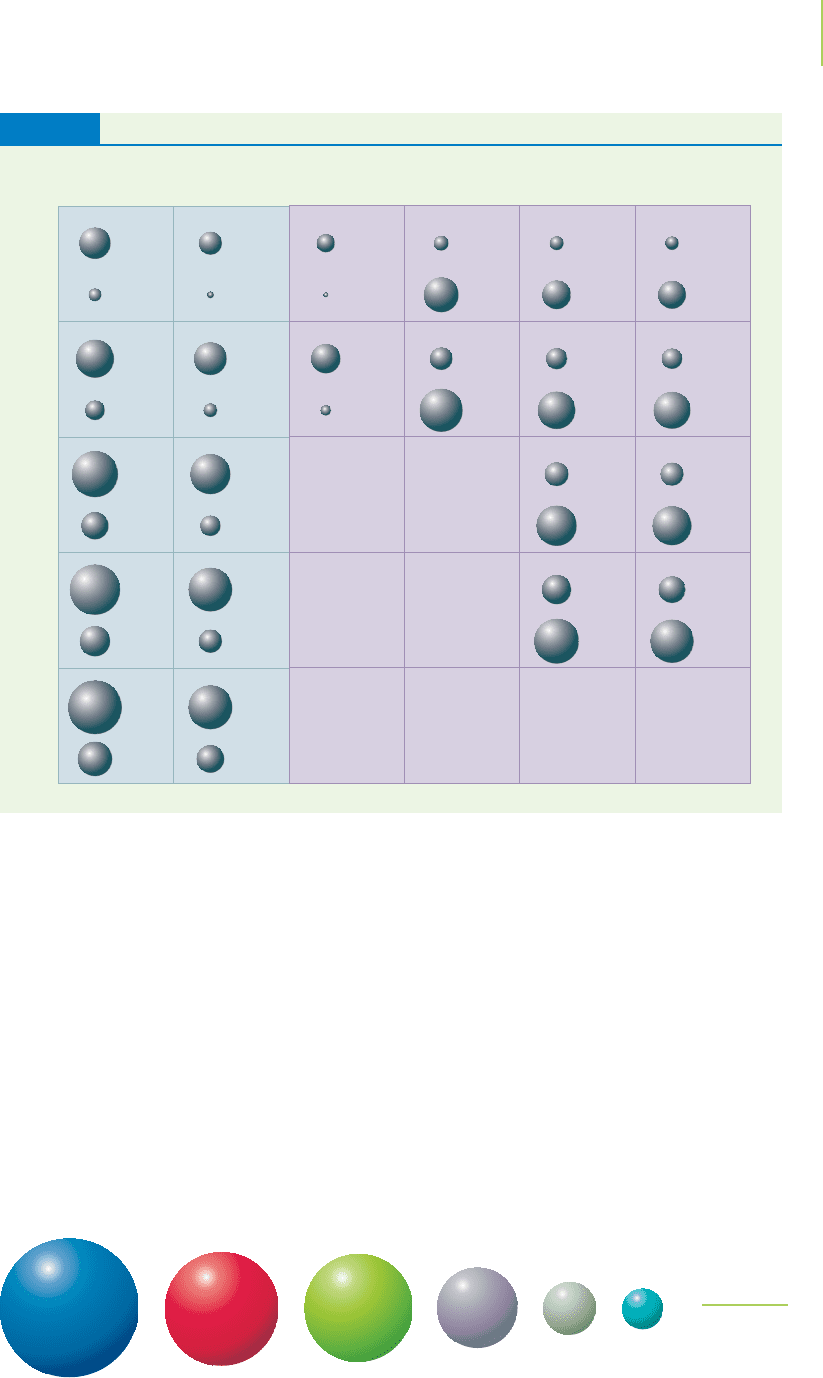
the cation. The size of the cation is less than the size of the atom (see Table 8.2).
The opposite happens when an electron is added to an atom. The extra electron
increasesthe number of electron–electronrepulsions,therebyreducingtheeffective
nuclear charge felt by each electron. This causes the electron cloud to swell in size.
Theanion,then,islargerthanthe atomfromwhich itwasmade.Theseeffects continue
as more electrons are removed from or added to an atom. All of the ions shown in
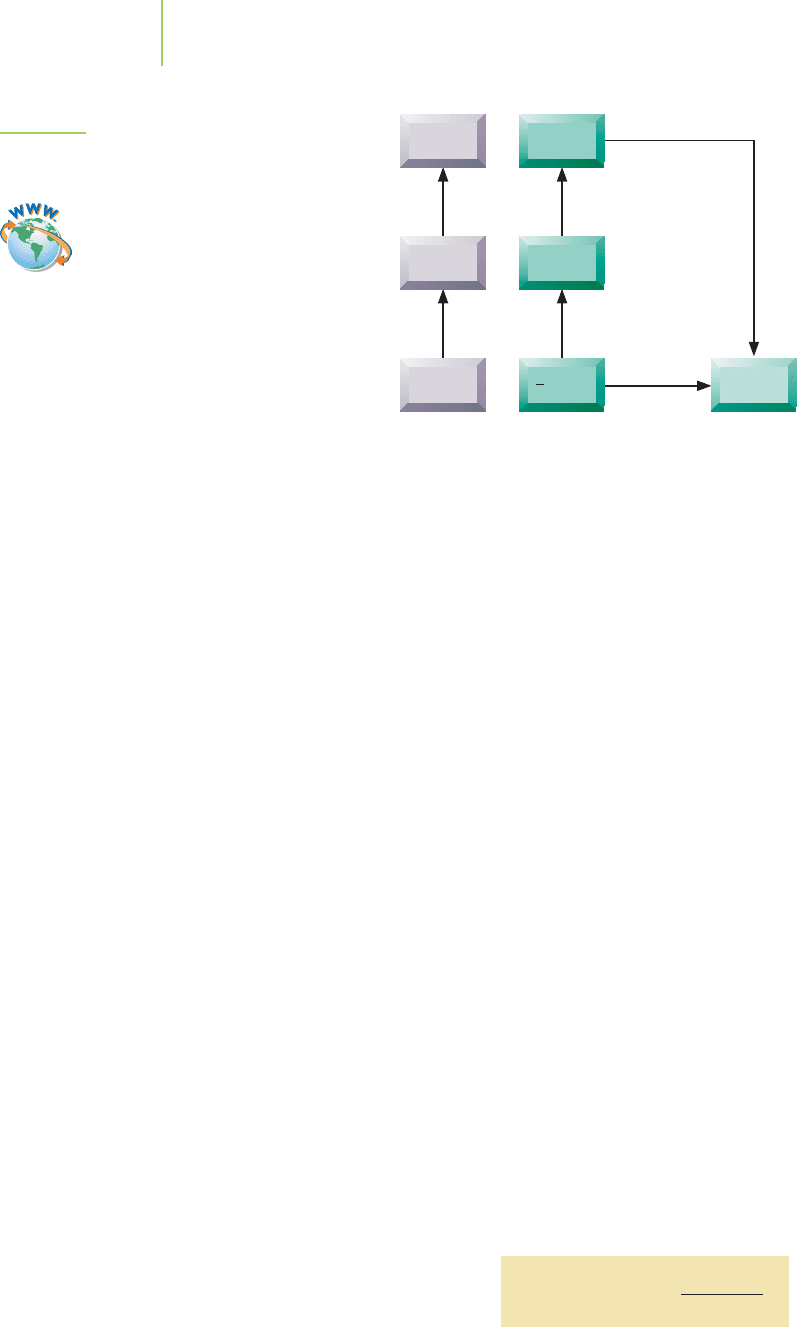
Figure 8.7 have the same electron configuration, but they differ in the charge of the
nucleus.We can see that as the nuclear charge increases,the size of the ion decreases.
We noted that the removal of the first electron greatly decreases the size of an
ion. Similar reasoning holds for the removal of subsequent electrons. In general,
8.2 Ionic Bonding 311
The Radius of Some Common lons and Atoms (in picometers)
TABLE 8.2
2+
Rb
247
5
Period
Group
Rb
+
148
Cs
265
6
Cs
+
169
K
227
4
K
+
133
Na
186
3
Na
+
99
Li
152
2
Li
+
60
IA IIA
Te
143
Se
117
S
104
S
2–
184
O
66
O
2–
140
VIA
Sr
215
Sr
2+
113
Ba
217
Ba
2+
135
Ca
197
Ca
2+
99
Te
2–
221
Se
2–
198
Mg
160
Mg
2+
65
Be
113
Be
2+
31
VA
P
110
P
3–
212
N
70
N
3–
171
IIIA
Al
143
Al
3+
50
B
88
B
3+
20
I
133
Br
114
Cl
99
Cl
–
181
F
64
F
–
133
VIIA
I
–
216
Br
–
195
N
3–
171 pm
O
2–
140 pm
F
–
133 pm
Al
3+
50 p
m
Na
+
99 pm
Mg
2+
65 pm
FIGURE 8.7
The radii of a series of isoelectronic ions.
the cation gets smaller as more electrons are removed. Removal of the first electron
from an atom greatly decreases the radius of the resulting ion. Removal of the
second and all subsequent electrons from the ion has the same effect, but in a less
pronounced way. This is due to the fact that electrons in the same subshell do not
shield each other very effectively from the nuclear charge.
As you descend within a group in the periodic table, the principal quantum
number of the valence electrons increases. The distance from the nucleus in-
creases with an increasing principal quantum number. The size of the ion
increases. This trend is shown in Table 8.2.
EXERCISE 8.3 Ions and Their Sizes
Argon is a noble gas found in the atmosphere in relatively large concentration
(0.934% by volume). It is used as an inert atmosphere in the chemical laboratory for
reactions that would react with normal atmospheres. List four ions that are isoelec-
tronic with argon, and arrange them in order of increasing size.
First Thoughts
The question is really asking us to complete two tasks. The first is to find four atoms
that can be made into ions with the same electron configuration as argon. There-
fore, we should limit our search to those atoms that have an atomic number close to
that of argon. Second, the question asks us to organize the ions we’ve created by size.
We know that cations are smaller than atoms and anions are larger.
Solution
Ca
2+
K
+
Ar Cl
−
S
2−
Further Insight
Knowing the size and electron configuration of an ion is helpful in constructing the
bigger picture for a model of a compound. The five species we’ve arranged here have
the same number of electrons around their nuclei, but they differ greatly in size.
Moreover, their having the same number of electrons doesn’t imply that these ions
have any properties in common. They are completely different in their reactivity
and in their physical properties.
PRACTICE 8.3
Arrange four ions that are isoelectronic with Ne in order of increasing size.
See Problems 23, 24, 31, and 32.
HERE’S WHAT WE KNOW SO FAR
■
The electron configuration of an ion determines the number of electrons in its
outer shell.
■
We can use the electron configuration of an ion to draw the Lewis dot symbol
for the ion.
■
Anions, atoms with extra electrons, are larger than their corresponding atoms.
■
Cations, atoms missing electrons, are smaller than their corresponding atoms.
■
Ionic compounds are the combination of anions and cations to make an elec-
trically neutral compound. The force of attraction between an anion and a
cation is the ionic bond.
312 Chapter 8 Bonding Basics
Energy of the Ionic Bond
According to the American Dental Association, the addition of fluoride to tooth-
paste and public water systems has brought about a nationwide reduction in
tooth decay. The Centers for Disease Control suggest a “safe, effective and inex-
pensive”municipal waterway fluoride concentration of between 0.7 and 1.2 parts
per million. The mineral portion of teeth is hydroxyapatite, Ca
5
(PO
4
)
3
(OH).
When you drink fluoridated water or brush your teeth with toothpaste containing
fluoride, some of the hydroxide anions in your teeth are replaced with fluoride.
Ca
5
(PO
4
)
3
(OH)
−−−→
Ca
5
(PO
4
)
3
(OH,F)
Hydroxyapatite Fluorapatite
The new mineral, called fluorapatite, is much stronger than hydroxyapatite.What
accounts for the added strength? Does the size of the fluoride and hydroxide ions
have something to do with the strength of the mineral?
The strength of the ionic bond is usually referred to as the
lattice enthalpy of
the ionic solid. The lattice enthalpy of a molecule is the amount of energy re-
quired to separate 1 mol of a solid ionic crystalline compound into its gaseous
ions (see the following equation). The lattice enthalpies of some common com-
pounds can be found in Table 8.3.
MX(s) n M(g)
+
+ X(g)
−
Although qualitative statements can be made about the relative size of the lattice
enthalpy on the basis of ionic radii, most chemists use lattice enthalpy to make
some quantitative statements about the strength of an ionic compound. Unfortu-
nately, it is quite difficult to measure lattice enthalpy accurately in an ionic crys-
talline solid. However, we can calculate the lattice enthalpy using Hess’s law (see
Chapter 5) in a process known as the
Born–Haber cycle, named after two Nobel
Prize–winning German scientists (Max Born, 1882–1970, and Fritz Haber,
1868–1934).
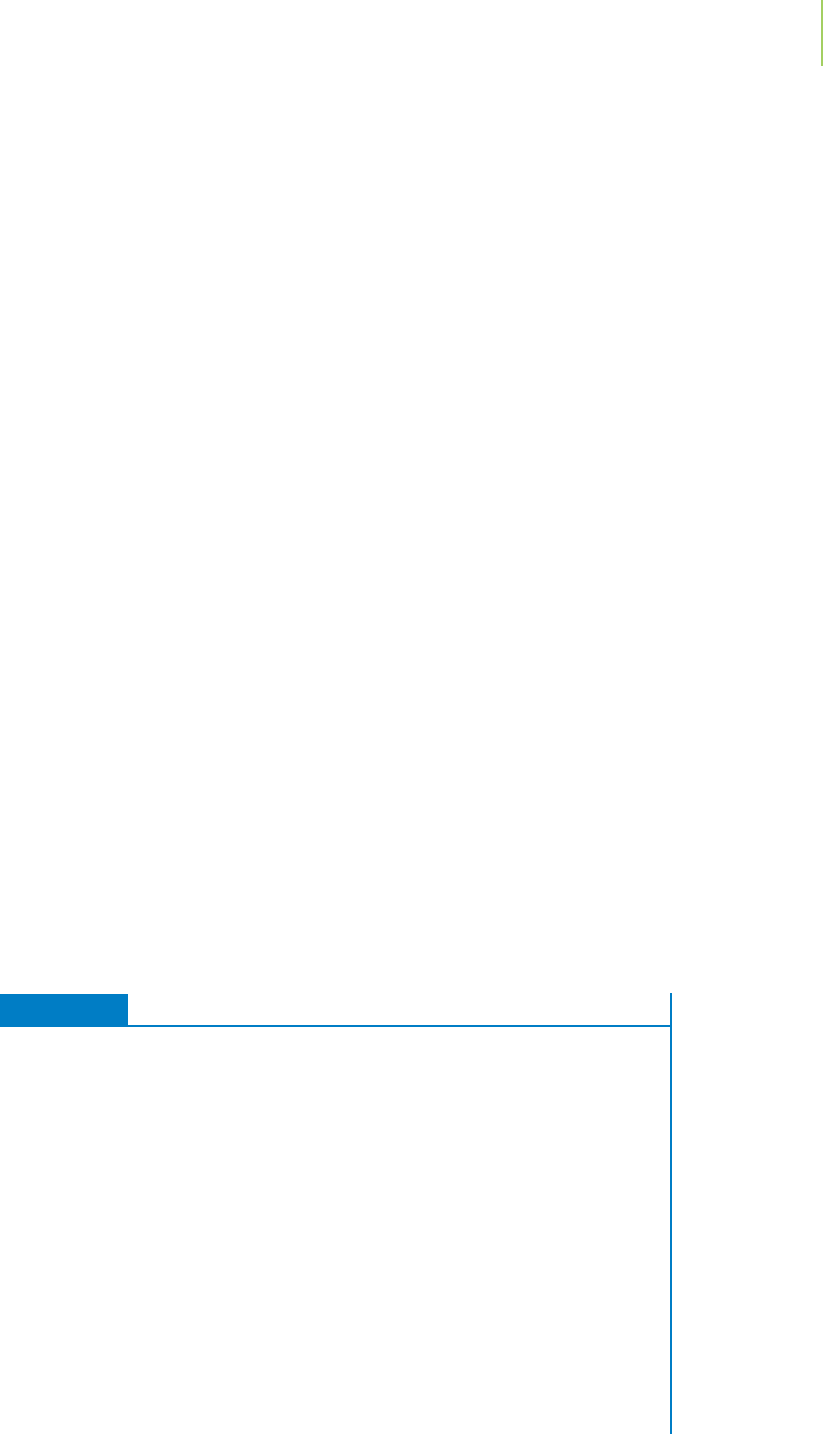
The Born–Haber cycle is a diagrammatic representation of the formation of
an ionic crystalline solid. Figure 8.8 illustrates the Born–Haber cycle for the
formation of sodium chloride from its elements in their standard states. Al-
though chemistry is a process in which all kinds of things happen concurrently
and continuously, for clarity we often break down the Born–Haber cycle into
a series of steps.
8.2 Ionic Bonding 313
Application
C
HEMICAL
E
NCOUNTERS:
Fluoridated Water
and Tooth Decay
Lattice Enthalpies for Some Common Ionic Solids
Values in the table are in kilojoules per mole for the simple ionic compounds
(for example, the lattice energy of K
2
O is 2238 kJ/mol).
F
−
Cl
−
Br
−
I
−
OH
−
O
2−
Li
+
1030 834 788 757 1039 2799
Na
+
923 787 747 704 887 2481
K
+
821 701 682 649 789 2238
Rb
+
785 689 660 630 766 2163
Cs
+
740 659 631 604 721 ––
Mg
2+
2913 2326 2097 1944 2870 3795
Ca
2+
2609 2223 2132 1905 2506 3414
Ba
2+
2341 2033 1950 1831 2141 3029
Sc
3+
5096 4874 4711 4640 5063 13,557
Al
3+
5924 5376 5247 5070 5627 15,916
TABLE 8.3
314 Chapter 8 Bonding Basics
■
At the starting point to the cycle, we sublime solid sodium metal into gaseous
sodium metal. The enthalpy change for this process is known and is recorded
on the cycle.
■
Next, we dissociate the chlorine molecule into individual chlorine atoms,
using the equation that relates to the bond dissociation energy for chlorine.
Because we need only one chlorine atom, we take only half of the enthalpy
change for this process.
■
In the next steps, the gaseous atoms are ionized to the gaseous ions. Sodium
atoms are ionized to sodium cations with an enthalpy change that corresponds
to the first ionization enthalpy. Chlorine atoms are converted into chloride
anions with an enthalpy change that corresponds to the electron affinity for
chlorine.
■
In the final step, the sodium and chloride ions are brought together to make
the ionic crystalline solid. This enthalpy change corresponds to the negative of
the lattice enthalpy for the ionic solid.
When the heat of formation for the ionic solid is known by direct measure-
ment, the direct route from the starting point to the end of the Born–Haber cycle
(the lattice enthalpy) can be calculated by summing all of the individual enthalpy
changes. Because the individual enthalpy changes are often known (heat of for-
mation, sublimation, first ionization, bond dissociation, and electron affinity),
this provides a convenient method for determining the lattice enthalpy of the
ionic crystalline solid.
Calculation of the
lattice energy (not lattice enthalpy) of an ionic compound
can also be accomplished using
Coulomb’s law. Coulomb’s law states that the force
between two particles is proportional to the product of the charges (Q) on each
particle divided by the square of their distance of separation (d). The French
physicist Charles Augustin Coulomb (1736–1806) proved this relationship by ex-
periment. Because the force of attraction between two ions is related to the lattice
energy, a modification of Coulomb’s potential gives us the lattice energy:
Lattice energy = k
Q
+
× Q
−
d
In this equation, k is a proportionality constant, Q
+
and Q
−
are the charges on the
ions, and d is the distance between the ions. The lattice energy is related to the lat-
tice enthalpy. The difference is that the lattice energy (E
lattice
) is the amount of en-
ergy released as the solid ionic crystal is formed from its gaseous ions, whereas the
+
+
sub
H = +108 kJ
IE = +496 kJ
NaCl(s)
Na(s)
Na(g)
Na
+
(g)
diss
H = +122 kJ
EA = –349 kJ
U = –788 kJ
f
H˚
f
H˚ =
sub
H + IE +
diss
H + EA + U
f
H˚ = 108 + 496 + 122 – 349 – 788 = –411 kJ/mol
Cl(g)
Cl
–
(g)
Cl
2
(g)
2
1
FIGURE 8.8
The Born–Haber cycle for the
formation of sodium chloride.
Visualization: Born–Haber Cycle
for NaCl(
s
)
lattice enthalpy (H
lattice
) is the amount of heat at constant pressure necessary to
separate the solid ionic crystal into its gaseous ions. The good news is that these
two values are just about the same, though opposite in sign; lattice energy de-
scribes a release in energy (E
lattice
= “−”), whereas lattice enthalpy describes
energy that is absorbed (H
lattice
=“+”).
H
lattice
∼
=
−E
lattice
The magnitude of the lattice energies or lattice enthalpies increases as the
charges on the ions increase, as shown in Table 8.3. In the series ScCl
3
,CaCl
2
, and
KCl, the charges decrease on the cation: (+3), (+2), and (+1), respectively,
with a decrease in lattice enthalpies in the order ScCl
3
(4874 kJ/mol), CaCl
2
(2223 kJ/mol), KCl (701 kJ/mol). Coulomb’s law also indicates that as the
distance between the ions decreases, the lattice enthalpies increase. Evidence of
this is found by comparing LiBr (788 kJ/mol), LiCl (834 kJ/mol), and LiF
(1030 kJ/mol). The charges on the ions are the same in this series of compounds,
but the size of the anion decreases from bromide to fluoride. Because the
bromide ion (Br
−
) is larger than Cl
−
or F
−
, LiBr has the smallest lattice enthalpy.
In general, lattice enthalpies are greatest for ionic compounds that are made up of
small, highly charged particles.
Let’s revisit our section-opening question of why fluoride in our toothpaste is
important. As we noted then, the replacement of a hydroxy group in the hydrox-
yapatite, Ca
5
(PO
4
)
3
(OH), mineral that makes up our teeth gives rise to a new
mineral called fluorapatite, Ca
5
(PO
4
)
3
(OH,F). Because F
−
is smaller than OH
−
,
Coulomb’s law dictates that the force of attraction between the Ca
2+
and the F
−
should be greater than that between Ca
2+
and OH
−
in this mineral. This is the
case with, for example, nearly all binary ionic salts of fluoride compared to the
metal hydroxide, so that the lattice enthalpy of, for example, silver fluoride (AgF),
which is 953 kJ/mol, is greater than the lattice enthalpy of silver hydroxide
(AgOH), 918 kJ/mol. It is therefore reasonable to consider that the lattice en-
thalpy of fluorapatite is greater than that of hydroxyapatite. This is one of several
reasons why fluorapatite is more stable than hydroxyapatite when bathed in our
saliva—and more resistant to the formation of cavities. Fluorapatite formation is
even more compelling when fluoride is present in our mouths from municipal
water fluoridation or dental treatments; this is related to a concept called chemi-
cal equilibrium, which we will consider in Chapters 16–18.
EXERCISE 8.4 Predicting Lattice Enthalpies
Use the relationship of ionic sizes to predict whether calcium fluoride (found in
toothpaste) or calcium chloride (sidewalk salt) has the greater lattice enthalpy. Also
predict whether aluminum chloride or sodium chloride has the greater lattice
enthalpy.
First Thoughts
Comparing the lattice enthalpies of two ionic compounds can be accomplished by
realizing that the magnitude of the lattice enthalpy is inversely proportional to the
distance between the individual ions and directly proportional to the size of the nu-
clear charge on the ions. The distance between the ions is directly related to the
individual ionic radii.
Solution
Calcium fluoride (CaF
2
) and calcium chloride (CaCl
2
) differ in the size of the anion
bound to the calcium cation. From the discussion of atomic size, we noted that
fluorine is smaller than chlorine. Moreover, the radius for both anions also follows
this trend; fluoride is a smaller anion than chloride. Coulomb’s law says that CaF
2
8.2 Ionic Bonding 315

has a larger, more positive lattice enthalpy. And indeed it does (2609 kJ/mol versus
2223 kJ/mol).
Aluminum chloride (AlCl
3
) and sodium chloride (NaCl) differ in the charge
of the cation. The larger charge on the aluminum cation (+3) indicates that alu-
minum chloride should have the larger lattice enthalpy. It does (5376 kJ/mol versus
787 kJ/mol).
Further Insights
Which has a greater influence on the lattice enthalpy, ionic radius or ionic charge?
The examples we’ve examined suggest that the ionic charge has a much greater
effect. We can reason, and rightly so, that the electrostatic force of attraction be-
tween a cation and an anion is a very powerful force. This powerful force assists
some proteins as they fold into a biologically active molecule.
PRACTICE 8.4
Which has the greater lattice enthalpy, FeCl
3
or FeCl
2
?
See Problems 25, 26, 33, 34, 100, 101, and 103.
8.3 Covalent Bonding
In addition to NaCl (table salt), sucrose (C
12
H
22
O
11
, common sugar) is another
compound we see at the dinner table (Figure 8.9). What is the difference between
table salt and sugar? Placed side by side, they appear relatively similar to the
naked eye. Both are colorless crystalline compounds. When added to water, they
both dissolve. However, as we saw in Chapter 4, solutions of these two com-
pounds act differently. Table salt, a strong electrolyte, dissociates into sodium
cations and chloride anions when dissolved in water. Sucrose, a nonelectrolyte,
doesn’t dissociate when it dissolves. This is why electric current passed through
the salt solution and lit the bulb, while the bulb over the sugar solution remained
unlit. Sucrose is an example of a compound containing only covalent bonds. The
atoms that make up sugar are firmly held together; bonds between these atoms
don’t dissociate upon addition to water. Because of this, an aqueous solution of
sucrose doesn’t conduct electricity. These bonds differ from the bonds in sodium
chloride, an ionic compound that dissociates in water, because the electrons in
sucrose are shared.
Knowing the type of the bonds that make up a molecule is fundamental to
those who do chemistry for a living. For example, pharmacognocists understand
that whereas many ionic compounds are readily soluble in water and can be
316 Chapter 8 Bonding Basics
FIGURE 8.9
Common table sugar. Sugar can be
crystallized in large chunks that look
much like the crystals of table salt.
Cough syrups often contain ethanol
(listed on the ingredient label as alcohol)
to increase the solubility of the active
compounds in the medicine that would
otherwise not be sufficiently soluble in
water.

administered to patients in aqueous solutions, many covalent compounds have
limited solubility in water. Drugs that are insoluble in water must be adminis-
tered in other solvents, which explains why cough syrups often contain ethanol.
In this section, we will examine the covalent bond up close, draw pictures of mol-
ecules that utilize this bonding scheme, and calculate the forces that hold these
bonds in place.
Description of Covalent Bonding
Opposite to the ionic end of the bonding spectrum is the covalent bond. In the
ionic bond, valence electrons gather on one of the atoms in the bond, leaving the
other atom with a deficiency of electrons. In the covalent bond, the atoms share
valence electrons to differing degrees. This occurs because the electronegativity of
the atoms on either end of the covalent bond are similar in magnitude, so the
atoms do not form ions. However, in order to participate in a bond and at the
same time obtain a noble gas electron configuration, the atoms in the covalent
bond must share their electrons. Examples of compounds that contain covalent
bonds include H
2
,CH
4
,CO
2
, and H
2
O (Figure 8.10).
Because of the similarities in electronegativity, the majority of covalent bonds
exist between nonmetals. There are exceptions, however, such as the covalent
bonds between lead and each of the four of the carbon atoms in tetraethyl lead,
Pb(C
2
H
5
)
4
, a compound that used to be added to gasoline to improve engine
performance. Why is this an exception? The four lead–carbon covalent bonds in
the compound exist as a consequence of the relatively small difference in the
electronegativity between the lead and carbon atoms. Because the emissions-
reducing catalytic converters required on all cars and light trucks in the
United States since 1981 are destroyed by lead, and because the combustion of the
lead-containing gasoline releases the metal into the environment, this type of
automobile fuel was banned in the United States in 1986. All of the automotive
gasoline sold today in the United States is unleaded gasoline. And as a result, the
amount of lead pollution of the environment has decreased. Table 8.4 lists the
47 countries that have completely eliminated the use of leaded gasoline in motor
vehicles. A World Bank Regional Conference on “The Phase-out of Leaded Gaso-
line in Sub-Saharan Africa,” held in Senegal in 2001, set as a goal the elimination
of leaded gasoline from that entire continent by 2005. Recent United Nations and
World Bank data show that this goal has largely been achieved.
8.3 Covalent Bonding 317
FIGURE 8.10
Examples of compounds
that contain covalent
bonds include CO
2
,H
2
O,
H
2
, and CH
4
.
Application
C
HEMICAL ENCOUNTERS:
Tetraethyl Lead in
Gasoline
