Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


Further Insights
Does the answer make sense? The pressure of the gas is greater than in either of the
other balloons. At constant temperature and with the same amount of gas in each
balloon, the only way to increase the pressure of the gas is to decrease the volume of
the balloon, so that the force per unit area remains the same.
PRACTICE 10.5
How would the volume of the balloon in the previous exercise change if the pressure
were decreased to 0.975 atm?
See Problems 27, 28, 34, and 36.
Charles’s Law
As we continue to look at the gas laws, we fill a balloon at STP with equal moles
each of hydrogen and oxygen gases, so the total volume of the large Mylar balloon
is, according to Avogadro’s law, 2.0 L. That means we’ve added 1.0 L of each gas.
We then take the balloon outside into a frosty winter’s morning where the tem-
perature is −10.0
◦
F (−23.3
◦
C). We notice that the balloon seems a bit smaller, as
shown in Figure 10.15. Why?
There are several factors to consider. One of these factors involves the tem-
perature of the gases in the balloon. Temperature is a measure of the kinetic
energy of a system—the gas particles in our discussion. When the temperature
drops, the gas particles travel at lower velocities. This means that there are fewer
particle collisions with the walls of the balloon in a given time (also, each collision
will occur with a lower kinetic energy and velocity, and so each collision has less
impact). Because each gas particle collision with the balloon’s inner wall adds to
the force exerted by the gas on the balloon, a smaller number of collisions per unit
time indicates that the pressure of the gas is lowered. As a result, the balloon shrinks
until the pressure of the gas inside the balloon equals the pressure outside the bal-
loon. In this process, the pressure was the same at the beginning as at the end.
When the temperature is lowered, the volume of the balloon gets smaller. The
general result that the volume of a gas is directly proportional to the temperature (at
constant pressure and number of moles) was first reported by Jacques Alexander
Charles (Figure 10.16) in 1787 and is known as
Charles’s law.
In the early 1800s, Joseph Louis Gay-Lussac (Figure 10.17) further studied the
effect of temperature on the volume of a gas and reported that gases expand by
408 Chapter 10 The Behavior and Applications of Gases
FIGURE 10.15
The relationship between temperature
and volume of a gas (constant quan-
tity and pressure) can be illustrated
by comparing the volume of a balloon
at 0°C, on the left, with that of the
balloon on the right, at –23.3°C.
Visualization: Changes in Gas
Volume, Pressure, and
Concentration
Visualization: Charles’s Law:
A Graphical View
Visualization: Charles’s Law:
A Molecular-Level View
Video Lesson: Charles’s Law

1/273 of their volume at 0°C for each increase in temperature of 1°C. This rela-
tionship is shown graphically at standard pressure and constant amount of gas in
Figure 10.18. If we follow the line down to where the volume would become zero,
we note that the temperature would be –273.15°C. In fact, such an extension
would not be reasonable, because any gas would deviate substantially from ideal
behavior and liquefy well before that point.
In any case,
does −273.15°C seem familiar? It might. It is the temperature we
know as
absolute zero, the lowest possible temperature. This value serves as the
basis for the Kelvin temperature scale, named after William Thomson (Lord
Kelvin), who created this scale in 1848. On the Kelvin scale, a reading of zero
kelvin (0 K) is equal to −273.15°C. At this temperature, there is no kinetic energy
available, and therefore all atomic translations (movement in the x, y,or z direc-
tions) stop. Is such a temperature actually achievable? Even in the far reaches of
deep space, residual heat from the Big Bang keeps even the coldest objects at 3 K.
In the laboratory, however, scientists have been able to cool atoms down to within
a few billionths of a degree of absolute zero.
Charles’s law illustrates the relationship between volume and temperature.
These two properties of a gas are directly proportional, so we can write
V = k
T (at constant n, P)
which can be rearranged to
V
T
= k
(at constant n, P)
where V = volume occupied by an ideal gas
T = temperature of the gas in kelvins
k
=a constant relating the two quantities (different from the prior constants)
Just as we did with the other gas laws, we can set the two ratios of volume-to-
temperature equal to each other if only the conditions of the gas have changed:
V
initial
T
initial
=
V
final
T
final
(at constant n, P)
We need to keep in mind that the relationship between volume and tempera-
ture is an approximate one (because no gas is truly “ideal”) and varies from
substance to substance.
10.4 The Gas Laws—Relating the Behavior of Gases to Key Properties 409
FIGURE 10.16
Jacques Alexander César Charles
(1746–1823), a French inventor and sci-
entist, was the first to take a voyage in a
hydrogen balloon (to a height of 550 me-
ters). He invented many scientific instru-
ments and used them in his studies, in-
cluding his confirmation of Benjamin
Franklin’s experiments with electricity.
FIGURE 10.17
Joseph Louis Gay-Lussac (1778–1850),
a French chemist and physicist, is known
for his work explaining the behavior of
gases. He and Jean-Baptiste Biot were
the first to ride in a hot-air balloon in
1804 to a height of 5 km. He was one of
the discoverers of boron in 1808, which
was later shown to be a new element.
V
0 100
–100˚–200˚ 0˚ 100˚ 200˚
200 300 400 500
Kelvin
Celcius
Temperature
Volume of gas
at constant pressure
FIGURE 10.18
In the early 1800s, Gay-Lussac found that ideal gases
expand by 1/273 of their volume for each increase of 1°C
in temperature. If we follow the line down, we intersect
the “zero volume” point at –273°C. In reality, such an
extension would not be reasonable, because any gas
would deviate substantially from ideal behavior and, in
fact, liquefy well before that point. This does, however
suggest that there is an absolute zero temperature.
Visualization: Liquid Nitrogen
and Balloons
Visualization: Collapsing Can
EXERCISE 10.6 Charles’s Law
As a demonstration of Charles’s law, a Mylar balloon is filled with 1.00 L of helium
gas at −12°C and held over a flame until the temperature of the gas within the bal-
loon reaches 150.0°C. What is the new volume of the balloon, assuming constant
pressure?
First Thoughts
What do we expect to happen to the volume of the balloon as a result of warming
the gas inside? According to our discussion about Charles’s law, it would make sense
for the volume to increase. We also note that we must change the temperatures to
the kelvin scale.
Solution
V
initial
T
initial
=
V
final
T
final
1.00 L
261 K
=
V
final
423 K
1.00 L ×423 K
261 K
= V
final
V
final
= 1.62 L
Further Insights
What would we have calculated our final volume to have been if we had kept our
temperatures in Celsius degrees?
V
initial
T
initial
=
V
final
T
final
;
1.00 L
−12
o
C
=
V
final
150.0
o
C
1.00 L ×150.0
o
C
−12
o
C
= V
final
V
final
=−13 L
This outcome of a “negative volume”sharply illustrates why the Celsius scale cannot
be used in gas law calculations.
PRACTICE 10.6
Assume the volume of the Mylar balloon described in the previous exercise is heated
until its volume reaches 2.00 L. What is the temperature of the gas within the
balloon?
See Problems 29, 30, 37, and 38.
EXERCISE 10.7 Mendeleev’s Demonstration of Nonideal Behavior
Based on his own experiments done around 1870, Dmitri Mendeleev noted that for
real gases, Charles’s law (originally known as Gay-Lussac’s Law) was not entirely cor-
rect. Mendeleev’s data showed that when several gases were heated (constant n, P)
from 0
o
C to 100
o
C, they occupied the following multiples of their original volume:
Air = 1.368
“Carbonic anhydride” (CO
2
) = 1.373
Hydrogen = 1.367
Hydrogen bromide (HBr) = 1.386
410 Chapter 10 The Behavior and Applications of Gases
If the gases were truly ideal, what fraction of their original volume should they
occupy when heated from 0
o
C to 100
o
C? Which of these gases most nearly exhibits
ideal behavior?
First Thoughts
Which of these gases is likely to be behave closest to ideally? Why? We note that HBr
occupies the greatest volume among the four gases, with CO
2
close behind. Why
might this be? The best way to begin is perhaps to find the increase in volume of an
ideal gas when heated from 0°C to 100°C. Let’s keep our calculations straightfor-
ward by assuming an initial volume at 0°C (273 K) of 1.000 L. Mendeleev used one
extra figure in his work, so we will do the same.
Solution
V
initial
= 1.000 L T
initial
= 273 K and V
final
= ? L T
final
= 373 K
V
initial
T
initial
=
V
final
T
final
(constant n, P)
For an ideal gas,
1.000 L
273 K
=
V
final
373 K
V
final
=
1.000 L × 373 K
273 K
= 1.366 L
Further Insights
Hydrogen, at 1.367 L, is the closest to ideal behavior. This makes sense because it is
the smallest molecule and takes up the least space. Hydrogen bromide is a much
larger molecule, requiring space for itself in order to exert the same pressure as hy-
drogen in, for example, a balloon at the higher temperature. All four gases deviate
from ideality, the more so the greater their molar mass.
PRACTICE 10.7
In addition to its having volume, give another reason why HBr gas deviates from
ideal gas behavior.
See Problem 104.
Combined Gas Equation
We can combine Avogadro’s law,
V
initial
n
initial
=
V
final
n
final
and Boyle’s law,
P
initial
V
initial
= P
final
V
final
,
and Charles’s law,
V
initial
T
initial
=
V
final
T
final
to come up with an equation called the combined gas equation. Why can we do
this? The fact that all of these relationships use a proportionality constant makes
this process easy. Our final equation takes volume, pressure, amount, and tem-
perature into account:
P
initial
V
initial
n
initial
T
initial
=
P
final
V
final
n
final
T
final
10.4 The Gas Laws—Relating the Behavior of Gases to Key Properties 411
Video Lesson: The Combined
Gas Law
Video Lesson: CIA
Demonstration: The Potato
Cannon

We can use this equation to solve for the pressure, volume, amount, or tempera-
ture of a gas, as the gas changes conditions from one state to another. If some-
thing remains constant, that condition drops out of the equation and simplifies
our calculations. Typical use of this equation implies that we use atmospheres for
the pressure, liters for the volume, moles for the amount, and kelvins for the
temperature.
EXERCISE 10.8 Combined Gas Equation
Recall our discussion of modified atmosphere packaging (MAP) in Section 10.3. If
a chicken is packaged within a nitrogen–carbon dioxide gas atmosphere at STP and
a volume of 300.0 mL of gas, what will be the volume of the gas (assuming no leak-
age through the packaging) when it is stored in a grocery freezer at a temperature of
−8.0°C and a pressure of 1.04 atm?
First Thoughts
We are given initial pressure, temperature, and volume, and we change the pressure
and temperature.We must find the final volume. What about the amount of the gas?
Apparently, it doesn’t change in the process. Therefore, we can substitute what we
know into the combined gas equation. Before doing so, we should ask ourselves,
“Would it make sense for the volume to increase or decrease as a result of these
changes?”The temperature is decreasing from 273 K to 265 K. Charles’s law suggests
that the volume would decrease as a result. The pressure is increasing from 1.00 atm
to 1.04 atm, which, via Boyle’s law, would also lead to a slight reduction in volume.
On balance, then, the volume should decrease.
Solution
P
initial
= 1.00 atm T
initial
= 273 K V
initial
= 0.3000 L
P
final
= 1.04 atm T
final
= 265 K V
final
= ?
n
initial
= n
final
P
initial
V
initial
n
initial
T
initial
=
P
final
V
final
n
final
T
final
We can rearrange variables to solve for V
final:
P
initial
V
initial
n
final
T
final
n
initial
T
initial
P
final
= V
final
Because n
initial
= n
final
, they cancel, and we have
P
initial
V
initial
T
final
T
initial
P
final
= V
final
1.00 atm ×0.3000 L ×265 K
273 K ×1.04 atm
= 0.280 L = V
final
= 280 mL
Further Insights
Does our answer make sense? We expected the volume of gas to decrease, and it
did. When the physical properties change in ways that yield opposite effects—as, for
example, when both temperature and pressure increase—we can judge what the
outcome might be before calculating by seeing which change is greater, and that
change will dominate.
PRACTICE 10.8
Assuming the same initial conditions as in Exercise 10.8, solve for the volume when
the final pressure is 0.85 atm and the final temperature is −11.0°C.
See Problems 31 and 32.
412 Chapter 10 The Behavior and Applications of Gases

10.5 The Ideal Gas Equation 413
The Gas Laws
Common Name Equation Summary
Avogadro’s law
V
n
= k (constant P, T ) Equal numbers of molecules are contained in equal
volumes of all dilute gases under the same
conditions.
Boyle’s law PV = k
(constant n, T) There is an inverse relationship between pressure
and volume of a given amount of gas at constant
temperature.
Charles’s law
V
T
= k
(constant n, P) The volume of a gas is directly proportional to the
temperature at constant pressure and moles.
Combined gas equation
PV
nT
= constant The combination of Avogadro’s, Boyle’s and
Charles’s laws relates initial and final pressure,
volume, amount, and temperature.
TABLE 10.5
To this point, we have discussed relationships among the pressure, volume,
temperature, and amount of a gas. We have seen that we can take into account
changes in any one of these and find its affect on another variable. These rela-
tionships are summarized in Table 10.5.
10.5 The Ideal Gas Equation
So far we have looked at three laws describing the behavior of gases, the laws
named in honor of Avogadro, Boyle, and Charles. The balloon that carries aloft
the ozone-detecting equipment (as was shown in Figure 10.7) bursts as a conse-
quence of the combined effects of temperature and pressure on the volume of the
gas within the balloon. In order to understand why this bursting occurs, we need
to examine how these three variables are related.
The three gas laws were summarized in the previous section as follows:
V
n
= k (constant P, T )
PV = k
(constant n, T)
V
T
= k
(constant n, P)
Setting each equation equal to V yields
V = kn (constant P, T )
V =
k
P
(constant n, T)
V = k
T (constant n, P)
We can combine all the variables on the right-hand side, because they are all pro-
portional to V via the constants k, k
and k
. To clean it up nicely, we then com-
bine all three of these constants into a single constant called R. This gives us this
equation:
V =
RnT
P
The denominator can be cleared to give the common form of the ideal gas
equation
:
PV =nRT
The name of this equation includes the term ideal because the equation assumes
ideal behavior of the gas. In fact, we know that gases will deviate from the ideal,
yet the ideal gas equation will give us meaningful approximate results unless the
gases are at very high pressures and/or low temperatures.
What is the value of R? It can be determined by measuring each of the prop-
erties (P, V, T, and n) of a gas and solving for the combined gas law shown in
Table 10.5. For example, at STP (T =273.15 K, P = 1.0 atm), 1 mol of an ideal gas
occupies 22.414 L. The value of R, then, is 0.08206 L·atm·mol
−1
·K
−1
.
PV
nT
= R =
(1.000 atm)(22.414 L)
(1.000 mol)(273.15 K)
= 0.08206
L·atm
mol·K
This is a very important number. It is known as the ideal gas constant, and it is
used quite often in chemistry. It is helpful to commit not only the number, but
also the units, to memory. Why? The units of the constant help us remember
which units to use for the other variables in the equation, and they also help us
confirm the units of our answers.
EXERCISE 10.9 Using the Ideal Gas Equation
A sample containing 0.631 mol of a gas at 14.0°C exerts a pressure of 1.10 atm. What
volume does the gas occupy?
Solution
We can use the ideal gas equation to solve for volume. By placing what we know into
the equation, we can solve for the quantity that we don’t know.
n = 0.631 mol T = 287.2 K P = 1.10 atm
PV = nRT
1.10 atm × V = 0.631 mol ×
0.08206 L·atm
mol·K
× 287.2K
V = 13.5L
PRACTICE 10.9
A 2.50-L sample of an ideal gas at 25°C exerts a pressure of 0.35 atm. How many
moles of this gas are there?
See Problems 45, 46, 51, 52, 58, and 72.
EXERCISE 10.10 The Ideal Gas Equation and Compressed Gas
How much pressure must a 48.0-L steel oxygen gas cylinder tolerate if it contains
9.22 kg of O
2
at a temperature of 25.0°C? Given the conditions, does the gas show
approximately ideal behavior?
First Thoughts
There are two differences between this exercise and Exercise 10.9. Here, we are given
mass, in kilograms, instead of the amount of moles. Second, the sample is much
larger than laboratory-sized samples. The question asks about ideal gas behavior.
The unknown here is the pressure. Gases approach ideality if the pressure is low.
High pressure creates the conditions for a great deal of interaction among gas mol-
ecules and with the container walls.
414 Chapter 10 The Behavior and Applications of Gases
Visualization: The Ideal Gas
Law,
PV
nRT
Video Lesson: The Ideal Gas
Law
Tutorial: Ideal Gas Law
Solution
We find it convenient first to convert mass into moles and then to find pressure
using the ideal gas equation. We will follow this strategy.
n
O
2
= 9.22 kg O
2
×
1000 g O
2
1kgO
2
×
1 mol O
2
32.0gO
2
= 288.1 mol O
2
PV = nRT
P ×48.0L= 288.1 mol ×
0.08206 L·atm
mol·K
× 298 K
P = 146.8atm
P = 147 atm
Further Insights
The pressure in the steel cylinder is quite high. We would therefore expect the oxy-
gen gas within the cylinder to deviate significantly from ideal behavior.
PRACTICE 10.10
A 0.250-mol sample of gas occupies 8.44 L at 28.7°C. What is the pressure of the
system?
See Problems 42, 63, and 64.
We assumed ideal behavior in the previous exercise, yet we suspect that this is
not likely for oxygen gas at a pressure of 147 atm. One of the themes in our dis-
cussion of gases has been the recognition that deviations, even small ones, from
ideality are expected. We have chosen to neglect them in our calculations, assum-
ing that the approximate answers we get are meaningful. However, as shown in
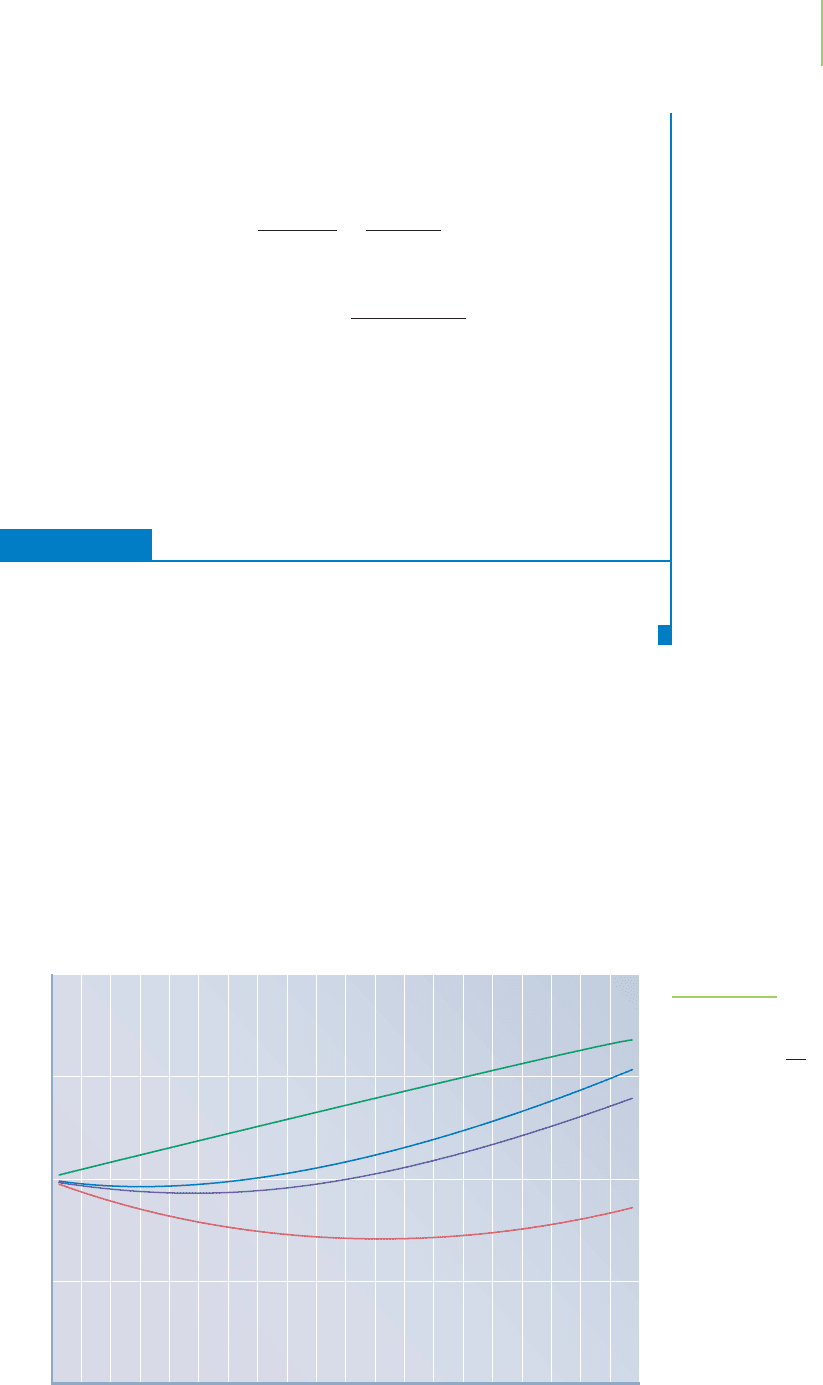
Figure 10.19, the ratio PV/nRT, is not constant, and the deviation becomes more
severe at high pressures. Is there a way to correct for relatively high pressure or
low temperatures, such as we had in Exercise 10.10 (compressed O
2
)?
10.5 The Ideal Gas Equation 415
0.8
1.0
0.9
1.1
1.2
Air
Helium
Nitrogen
Oxygen
100 500 900 1300 1700 2100
Pressure (torr)
PV/nRT
2500 2900 3300 3700 4100
FIGURE 10.19
“Ideal” is just that—a standard of
perfection that no gas can ever meet. If
PV = nRT, then = 1.00. Plotting actual
values shows that all gases deviate from
this equation, especially as the pressure
increases. Moreover, the deviation is differ-
ent for each gas.
PV
nRT
Accounting for the Behavior of Real Gases
Volume Correction
Real gas molecules occupy discrete volumes, and larger atoms and molecules take
up more space than smaller ones. Therefore, the available volume of the con-
tainer is not as large as if it were empty or if we assumed that the gas particles had
no volume. The more moles of gas in a given space, the less free volume com-
pared to ideal conditions, as shown in Figure 10.20. To take this into account, we
can express the available volume as
V
available
=V
container
− nb
in which b is a constant roughly related to the size of the molecules.
Pressure Correction
The molecules in a real gas interact with each other. Larger atoms and molecules,
and those with dipole moments, exhibit stronger interactions than smaller ones.
When particles interact with each other, they do not collide as often with the walls
of the container, and their force per unit area—their pressure—is lower (see Fig-
ure 10.20). The reduction in observed pressure can be given by
P
actual
= P
ideal
−
a
n
V
2
in which a is a constant with a magnitude that is loosely indicative of the
intermolecular forces that are possible in molecules. Note that the values of a in
Table 10.6 are large in the relatively large molecules that have more polarizable
electrons than the smaller molecules and atoms. The constant, a, is multiplied by
n
V
2
. As the volume, V, gets larger, the correction goes down, because the mole-
cules are farther apart. Similarly, when the number of moles, n, is low, the correc-
tion term is reduced.
The ideal gas equation assumes the use of P
ideal
. Therefore, in our modified
model, we can substitute for P
ideal
as follows:
P
ideal
= P
actual
+ a
n
2
V
2
This model of the pressure and volume deviations was put into equation form in
1873 by J. D. van der Waals. The equation he produced is
P +a
n
2
V
2
(V − nb) = nRT
hh
Pressure correction Volume correction
416 Chapter 10 The Behavior and Applications of Gases
FIGURE 10.20
As more and more molecules of a
gas are added to a given volume, the
amount of free space within the volume
decreases and the gas deviates greatly
from “ideal.” The van der Waals equa-
tion takes this into account, as we saw
in the text.
van der Waals Constants for Some Gases
Gas Formula a (atm L
2
·mol
−2
) b (L·mol
−1
)
Acetylene C
2
H
2
4.39 0.0514
Ammonia NH
3
4.17 0.0371
Argon Ar 1.35 0.0322
Carbon dioxide CO
2
3.59 0.0427
Chlorine Cl
2
6.49 0.0562
Ethane C
2
H
6
5.49 0.0638
Helium He 0.034 0.0237
Hydrogen H
2
0.244 0.0266
Methane CH
4
2.25 0.0428
Nitrogen N
2
1.39 0.0391
Oxygen O
2
1.36 0.0318
Sulfur dioxide SO
2
6.71 0.0564
Source: Maron and Prutton, Principles of Physical Chemistry. New York: MacMillan, 1959, p. 33.
TABLE 10.6
Video Lesson: Comparing Real
and Ideal Gases
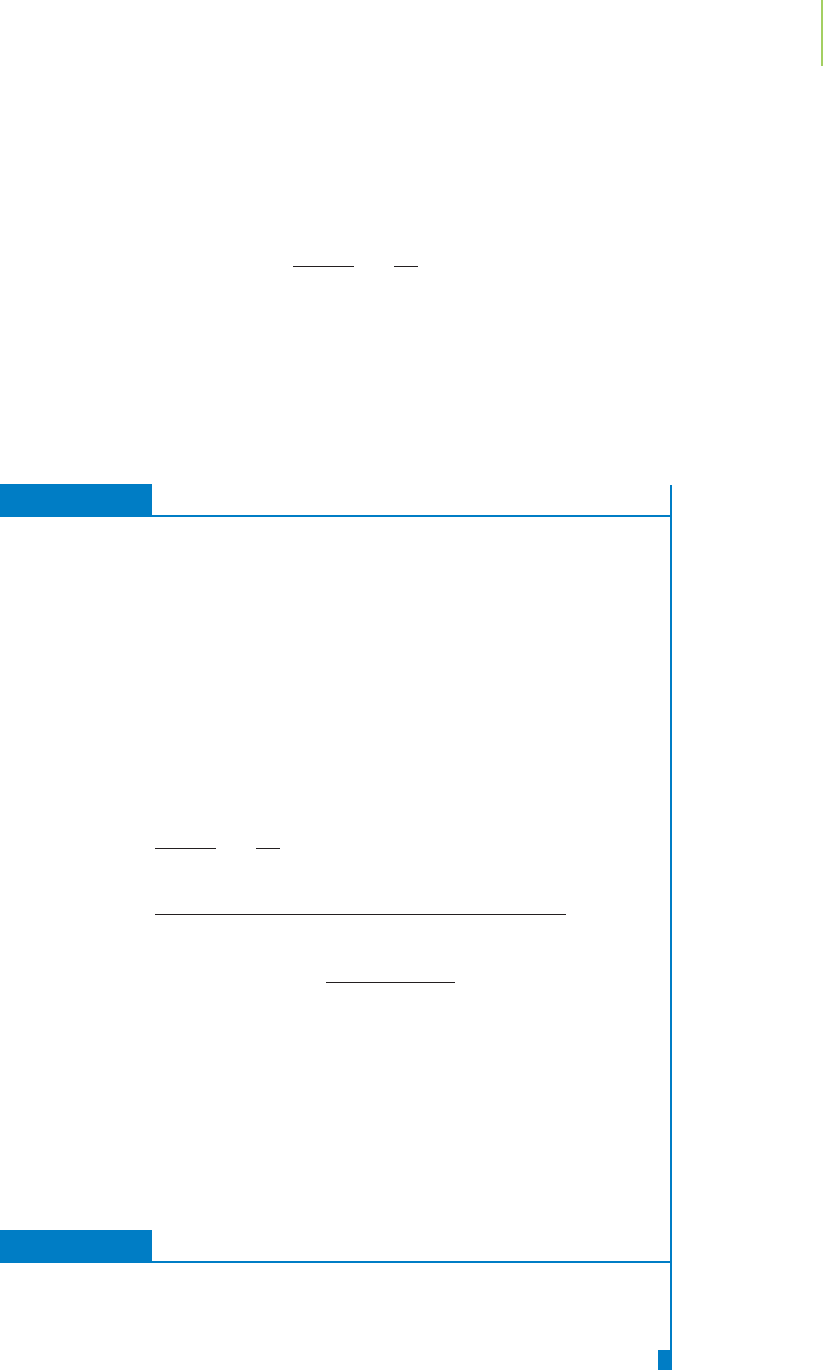
As we noted previously, the constant b is a correction indicating that real mole-
cules do have a finite volume and are not as far apart as we claim in ideal behav-
ior. The more moles of gas, n, the greater will be the correction to volume,
V − nb. The values for b are only roughly proportional to molecular size.
We can rearrange the
van der Waals equation to solve for pressure:
P =
nRT
V −nb
− a
n
2
V
2
The van der Waals equation is the most often cited mathematical model for
use with real gases because it works fairly well at normal pressures and tempera-
tures. However, it doesn’t fit the experimental data well at very high pressures and
low temperatures. Therefore, as pressure and temperature change, different val-
ues of a and b must be chosen. Other mathematical models that fit the data even
better (and are correspondingly more complex) have been derived.
EXERCISE 10.11 The van der Waals Equation
We now have the tools to better answer Exercise 10.10. Assuming the same volume,
temperature, and number of moles of O
2
gas in the cylinder, what is a more accu-
rate estimate of the pressure of the gas?
First Thoughts
We expect the actual pressure exerted by the oxygen gas to be less than the pressure
exerted by an ideal gas because the gas molecules are interacting more with each
other and colliding (and, therefore, interacting—the a term) less often with the
walls of the container. On the other hand, the nb correction term would make the
pressure greater by making the actual open volume less. The a-term correction is
much more significant, so we expect the pressure to be lower than the ideal value.
Solution
P =
nRT
V −nb
− a
n
2
V
2
P =
288.1 mol O
2
× 0.08206 L·atm·mol
−1
·K
−1
× 298 K
48.0L−(288.1 mol O
2
× 0.0318 L·mol
−1
)
− 1.36 L
2
·atm·mol
−2
(288.1 mol O
2
)
2
(48.0L)
2
= 181.4 atm − 49.0 atm = 132 atm
Further Insights
We expected a lower pressure, and the van der Waals equation confirmed this. The
answer of 132 atm therefore makes sense. Keep in mind that this equation is an
empirical model and fits many, but not all, situations well. It does, however, come a lot
closer than the ideal gas equation to assessing the behavior of real gases correctly.
PRACTICE 10.11
What is the pressure of 1.00 mol of O
2
gas in a 1.00-L balloon at 25°C? Calculate the
pressure using the ideal gas law and the van der Waals equation. Is there a differ-
ence? Then repeat the same calculations for ammonia.
See Problems 102 and 103.
10.5 The Ideal Gas Equation 417
