Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

NH
OH
OH
O
O
O
O
O
O
O
O
O
O
O
HO
528 Chapter 12 Carbon
How do drugs work? Cellular processes, such as intercellular communication,
metabolism (Chapter 14), and construction of the cell structure can be disrupted
by the binding of drug molecules to specific proteins and other biomolecules
essential to those functions. The organic chemical Taxol™ is arguably one of the
most important anticancer compounds to have been found in a plant extract over
the last 50 years. Let’s briefly trace the discovery of Taxol to show how drug
development occurs in industry.
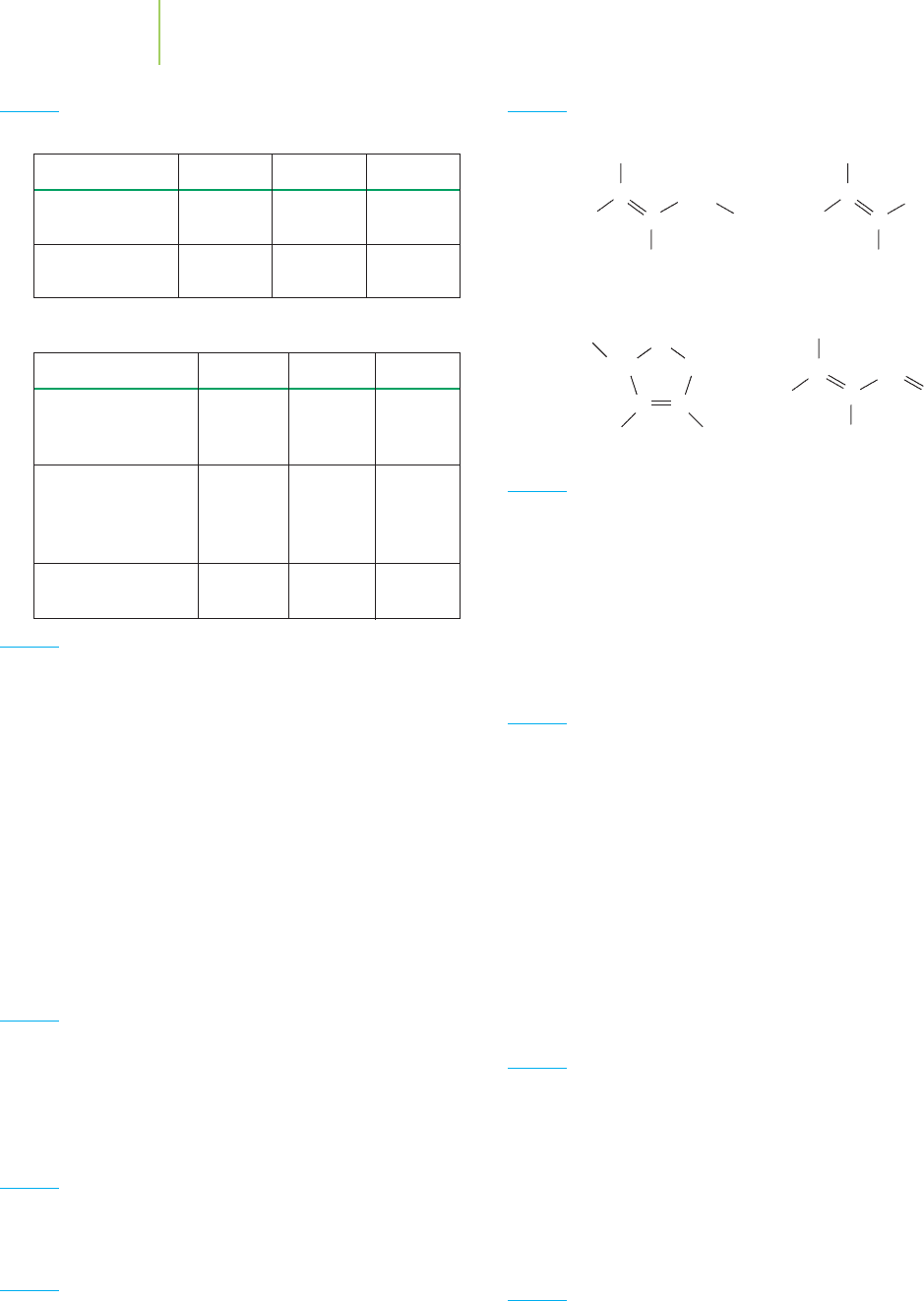
Taxol
In 1962, National Cancer Institute scientists found that extracts from the bark of
the Pacific yew tree (Taxus brevifolia) had the ability to kill some types of cancer.
Nine years later, the compound responsible for this promising activity,
named paclitaxel or Taxol, was isolated from the yew extract, and its struc-
ture was determined to be the one shown in Figure 12.19. Taxol acts by
tight association with cellular microtubules, which are protein assemblies
essential to cell division. The idea behind using Taxol for anticancer
chemotherapy is to stop the uncontrolled cell division responsible for
tumor growth.
Given Taxol’s importance, scientists needed large quantities of this
compound for experimental treatment of cancer patients and also for basic
research. When large amounts of a natural product are required, chemists can ei-
ther purify it in bulk from its natural source or synthesize it from readily available
materials. In the case of Taxol, neither of these two avenues alone was feasible.
At the time of its discovery, the only known source of Taxol was the bark of
mature Pacific yew trees. Unfortunately, the yield of Taxol obtained from the
Pacific yew is 100 mg per kilogram of bark—a very small amount. Even worse,
bark removal results in the death of the tree. The Pacific yew tree is mainly found
in the Pacific Northwest, and large-scale harvesting of these trees for Taxol would
quickly result in extinction of the tree species. Another way to produce Taxol was
desperately needed.
A potential solution to this problem might have been to synthesize Taxol from
scratch (an approach known as
total synthesis), therefore eliminating the threat
to the Pacific yew tree. The problem is that the structure of Taxol is enormously
complex (compare the structure in Figure 12.19 to the structures of the heptane
isomers from Practice 12.1). What was needed was the development of an effi-
cient total synthesis of Taxol, and synthetic organic chemists accepted the chal-
lenge. After several years of effort, total syntheses of Taxol were accomplished. Yet
even though this was a great feat for modern organic chemistry, these total syn-
theses did little to address the supply problem. All of these total syntheses were
long, technically complex, very costly, and consequently incapable of cheaply
producing large quantities of Taxol. In summary, neither isolation from the
Pacific yew tree nor total synthesis was an environmentally or economically
viable method for the mass production of Taxol.
During the course of Taxol research, chemists found that a biosynthetic
intermediate of Taxol (10-deacetylbaccatin III, shown in Figure 12.20) could be
isolated from the needles of a relative of the Pacific yew tree, in yields of up to 1 g
per kilogram of needles. The tree first synthesizes the biosynthetic intermediate
10-deacetylbaccatin III and then converts it to Taxol. And crucially, harvesting
these needles for 10-deacetylbaccatin III is not fatal to the tree. Chemists could
then take advantage of this renewable resource for 10-deacetylbaccatin III by
finding a way to convert it into Taxol. This is one reason why the efforts to achieve
total synthesis were so important to Taxol production. All of the previous
total syntheses combined the “core”with the “side chain” to give the full structure
of Taxol. Pharmaceutical scientists adopted this same strategy by converting
10-deacetylbaccatin III into something synthetically useful and then attaching
FIGURE 12.19
The chemical structure of Taxol. Can you
identify each of the 11 chiral centers?
OH
HO
O
O
O
O
O
HO
O
HO
FIGURE 12.20
The structure of 10-deacetylbaccatin III.
12.15 Organic Chemistry and Modern Drug Discovery 529
O
OH
O
O
O
O
O
O
O
HO
HO
HO
OH
O
O
O
O
O
O
O
O
O
HO
OH
NH
10-Deacetylbaccatin III
Side chain
Core
FIGURE 12.21
The semisynthesis of Taxol.
the “side chain,” as shown in Figure 12.21. This method is called a semisynthetic
method: A portion of the structure of the desired natural product is isolated
from a natural source and then converted into the natural product via synthetic
chemistry.
In 1992, Taxol was approved for use in the United States to treat ovarian can-
cer, breast cancer, and certain types of lung cancer. Along with the drug cisplatin
(see Chapter 20), Taxol is often the first drug used in chemotherapy against can-
cer. Today, the pharmaceutical industry uses a version of the semisynthetic
method to produce Taxol.
EXERCISE 12.8 Taxol as a Drug
One problem often associated with drugs is side effects. This problem can come
from a lack of complete specificity for the target. In treating cancer, we would like to
kill the cancer cells but leave the patients’ normal cells alone. Cancer tissue grows
very rapidly, but other than that, these cells don’t have many other unusual features
compared to normal cells. This is one of the reasons why cancer is such a difficult
disease to treat. Why might Taxol have side effects?
First Thoughts
Cancer cells have acquired mutations in their genetic code that enable them to grow
uncontrollably. Despite this, all of the normal cellular machinery is still present in
cancer cells. First consider the mode of action of Taxol and then think about how
this might affect normal cells.
Solution
Taxol acts by binding to microtubules, which are proteins directly involved in cell
division. When Taxol binds to the microtubules, the cancer cells cannot divide to
give two daughter cells. This can stop the growth of tumors, which are masses of
cells that divide uncontrollably. Because it is nearly impossible to administer Taxol
so that only cancer cells in the patient’s body receive it, the drug is likely to interact
with normal cells as well and bind to their microtubules. As a result, these normal
cells cannot divide, which could result in the disruption of otherwise healthy tissue.
Further Insights
Taxol has revolutionized cancer chemotherapy, but it does have a few major prob-
lems. The first was mentioned before: It is in very short supply. Another problem is
its poor formulation properties. Taxol is not very soluble in water, so it is difficult to
administer effectively to patients. In addition, some cancers eventually develop drug
O
O
O
S
N
OH O
OH
FIGURE 12.22
The chemical structure of epothilone B.
530 Chapter 12 Carbon
resistance to Taxol. These cancer cells have developed proteins that dispose of Taxol
by shooting it back out from inside the cell, where it can do no good for the patient.
Fairly recently, another class of compounds called the epothilones (one is shown
in Figure 12.22) has been discovered. The epothilones are also effective against can-
cer cells, and they have the same mode of action as Taxol. Unlike Taxol, however,
epothilones are made by a bacterium that is easy to culture on a very large scale.
Therefore, there is no serious supply problem for epothilone. The structure of the
epothilones is simpler than that of Taxol, so organic chemists can more easily make
changes to improve the properties of the drug. Finally, the epothilones are more
water soluble than Taxol and also are effective against Taxol-resistant cancer cell
lines. All of these characteristics have made the epothilones very important com-
pounds in contemporary cancer research.
PRACTICE 12.8
m-Amsacrine is another anticancer drug that exhibits biological activity by binding
to DNA inside cancer cells. Once bound, it causes proteins to break the DNA mole-
cule into fragments. The result is the death of the cancer cell. Would you predict
m-amsacrine to show side effects?
See Problems 97 and 98.
EXERCISE 12.9 Functional Groups of Taxol
Taxol is a very complicated molecule, and organic chemists typically cope with
structural complexity by first looking at its most important features: the functional
groups. Look at the structure of Taxol in Figure 12.19, and point out all of the
functional groups you have learned about in this chapter.
Solution
PRACTICE 12.9
What are the functional groups in epothilone B (see Figure 12.22)?
See Problems 51–54.
OH
O
O
O
O
O
O
O
O
O
O
O
OH
OH
NH
Ester
Ester
Alkene
Ketone
Secondary
alcohol
Secondary
alcohol
Tertiary
alcohol
Ether
Ester
Ester
A
mide
Key Words 531
The Bottom Line
■
Organic chemistry is the study of carbon-containing
compounds, particularly those derived from living
things or fossil fuels. (Section opener)
■
The element carbon occurs in the form of three
allotropes: the diamond, graphite, and fullerene
forms. (Section 12.1)
■
Crude oil is our major source of carbon-containing
compounds, which are processed by the chemical
industry into a wide range of products. (Sec-
tion 12.2)
■
Hydrocarbons are compounds composed of carbon
and hydrogen only. They include the alkanes,
alkenes, alkynes, and aromatic hydrocarbons. (Sec-
tion 12.3)
■
Organic compounds can exist as structural and
geometric isomers. (Section 12.3)
■
Hydrocarbons are separated into fractions by frac-
tional distillation of crude oil. (Section 12.4)
■
Cracking and reforming are two important meth-
ods of preparing specific organic molecules. (Sec-
tion 12.5)
■
Alkanes can undergo substitution and dehydro-
genation reactions. (Section 12.6)
■
All organic compounds can be regarded as carrying
zero, one, or more distinctive functional groups on
a hydrocarbon frame. (Section 12.7)
■
Each functional group is associated with a distinc-
tive set of chemical characteristics. (Section 12.7)
■
Many modern materials are polymers composed
of thousands of organic monomer units linked
together. (Section 12.8)
■
Molecules may exhibit chirality, which may lead to
different chemical properties in two stereoisomers.
(Section 12.14)
■
Organic chemists can isolate and purify the active
constituents of complex mixtures in order to make
products such as pharmaceuticals. (Section 12.15)
Key Words
achiral A term used to describe molecules that have a
superimposable mirror image. (p. 5
25)
addition reaction A type of reaction in which atoms are
added to a double or triple bond. (p. 514)
addition polymer A polymer formed by the process of
addition polymerization. (p. 515)
addition polymerization A type of polymerization involv-
ing addition reactions among the monomers
involved. (p. 515)
alcohol A chemical containing the hydroxyl (—OH)
group. (p. 514)
aldehydes Compounds that carry a carbonyl functional
group (CPO) with at least one H atom bonded to
the carbonyl carbon atom. (p. 517)
aliphatic compounds Compounds in which all of the
carbon atoms are sp
3
hybridized and contain only
single bonds. (p. 496)
alkanes Saturated hydrocarbons. (p. 496)
alkenes Hydrocarbons containing at least one CPC
double bond. (p. 500)
alkyl group A hydrocarbon group, such as —CH
3
or
—C
2
H
5
, within the structure of an organic com-
pound. (p. 504)
alkyl halide A functional group in which a halogen
atom is bonded to an alkyl group. (p. 511)
alkoxy anion An anion that contains an alkyl group
attached to an oxygen anion, such as CH
3
O
−
or
CH
3
CH
2
O
−
.(p. 520)
alkynes Hydrocarbons containing a CqC bond.
(p. 501)
allotropes Different forms of the same element.
(p. 493)
amide bond A bond formed by a condensation reaction
between an amino group (—NH
2
) and a carboxylic
acid group (—COOH). (p. 523)
amine A compound containing the —NH
2
functional
group. (p. 523)
amino functional group A functional group containing
an —NH
2
attached to an sp
3
hybridized carbon
atom. (p. 523)
aromatic hydrocarbons Hydrocarbons that contain one
or more aromatic rings. (p. 504)
carbonyl functional group A functional group containing
the group CPO attached to either hydrogen atoms
(as in the aldehydes) or carbon atoms (as in the
ketones). (p. 517)
carboxyl functional group The —COOH functional
group. (p. 519)
carboxylate anion An anion resulting from the removal
of a hydrogen ion from the oxygen in a carboxylic
acid functional group. (p. 520)

carboxylic acids Organic acids that carry the carboxyl
functional group (—COOH). (p. 519)
catalytic cracking A type of cracking wherein heat and
a catalyst are used. (p. 508)
chiral A term used to denote molecules that have a
nonsuperimposable mirror image. (p. 525)
chiral center A carbon attached to four different
groups. (p. 525)
chirality The quality of being chiral—that is, of having
a nonsuperimposable mirror image. (p. 525)
cis A molecule containing groups that are fixed on the
same side of a bond that cannot rotate. (p. 503)
condensation polymerization Formation of a polymer by
condensation reactions. (p. 522)
condensation reaction A reaction in which two mole-
cules become joined in a process accompanied by
the elimination of a small molecule such as water or
HCl. (p. 520)
cracking The breaking up of hydrocarbons into smaller
hydrocarbons, using heat and in some cases steam
and/or catalysts. (p. 508)
cyclic alkanes Alkanes that possess a group of carbon
atoms joined in a ring. (p. 500)
dehydrogenation The removal of hydrogen. (p. 510)
epoxide A compound containing a triangular ring of
two carbon atoms linked by an oxygen atom, known
as the epoxide group. (p. 524)
epoxy resins Polymer resins containing the epoxide
group, in which two carbons atoms and an oxygen
atom form a cyclic ether ring. (p. 524)
esterification The process by which an ester is formed
when the —OH group of an alcohol participates in
a condensation reaction with the —COOH group of
a carboxylic acid. (p. 522)
esters Compounds formed when an alcohol and a car-
boxylic acid become bonded as a result of a conden-
sation reaction. (p. 520)
ether Any compound containing the ether functional
group (—C—O—C—). (p. 524)
fermentation The biological process that converts glu-
cose into ethanol and carbon dioxide. (p. 516)
free radicals Chemical species that carry an unpaired
electron and so are highly reactive. (p. 510)
fullerenes Forms of carbon based on the structure of
buckminsterfullerene, C
60
.(p. 494)
functional groups Groups of atoms, or arrangements of
bonds, that bestow a specific set of chemical and
physical properties on any compound that contains
them. (p. 511)
gas chromatography (gc) Analytical technique in which
components of a mixture are separated by their dif-
ferent flow rates through a chromatography column,
driven by a carrier gas. (p. 507)
gas–liquid chromatography (glc) A form of gas chro-
matography in which the solid phase is covered with
a thin coating of liquid. (p. 507)
geometric isomers Molecules that differ in their geome-
try but have identical chemical formula and points
of attachment. See also cis and trans.(p. 502)
high-density polyethylene (HDPE) A form of polyethylene
in which the molecules are linear, with no branch
points. (p. 515)
homologous series A series of compounds sharing the
same general formula. (p. 497)
hydrocarbons Compounds composed of only hydrogen
atoms and carbon atoms. (p. 494)
hydrocracking A type of cracking that uses heat plus a
catalyst in the presence of hydrogen gas. (p. 508)
hydrogenation The addition of a molecule of hydrogen
to a compound. (p. 501)
hydroxyl group The —OH functional group. (p. 514)
ketones Compounds that have two carbon atoms
bonded to a carbonyl carbon atom. (p. 519)
knocking The rapid explosion of a gas–air mixture as it
is compressed in an automobile cylinder before the
spark plug creates the spark that is intended to cause
the mixture to explode. (p. 509)
low-density polyethylene (LDPE) A form of polyethylene
with branched-chain molecules that are shorter than
the unbranched chains found in HDPE. (p. 515)
methylene group The —CH
2
— group. (p. 497)
monomer One of the small chemical units that com-
bine with other monomers to form polymers.
(p. 515)
normal alkanes Alkanes that have no branched chains
or rings of carbon atoms. (p. 496)
octane rating A number assigned to motor fuel (gas)
on the basis of its “anti-knock” properties. (p. 509)
organic chemistry The chemistry of carbon-containing
compounds, especially those derived from living
things or fossil fuels. (p. 492)
organic compound Carbon-based compounds. (p. 492)
polyamides Polymers whose monomers are bonded
together by amide bonds. (p. 523)
polyesters Polymers in which the monomers are held
together by repeated ester linkages. (p. 522)
polyethenes Organic polymers made when huge num-
bers of ethene molecules participate in addition reac-
tions with themselves to generate long chain-like
molecules. Also known as polyethylenes. (p. 574)
polyethylenes See polyethenes. (p. 515)
polymer A chemical composed of large molecules
made by the repeated bonding together of smaller
monomer molecules. (p. 515)
primary alcohols Alcohols that have no more than one
carbon atom bonded to the carbon atom that car-
ries the hydroxyl group. (p. 517)
532 Chapter 12 Carbon
reforming A process that changes a mixture of pre-
dominantly straight-chain hydrocarbons into a mix-
ture containing more aromatic and branched-chain
hydrocarbons. (p. 508)
saturated hydrocarbons Hydrocarbons that do not con-
tain double or triple bonds. (p. 496)
secondary alcohols Alcohols that have two carbon
atoms bonded to the carbon atom that carries the
hydroxyl group. (p. 517)
steam cracking A form of cracking in which heat,
steam, and a catalyst are used. (p. 508)
stereoisomers Structural isomers that differ in their
three-dimensional arrangements of atoms, rather
than in the order in which the atoms are bonded.
(p. 525)
straight-chain alkanes Molecules that contain only
hydrogen atoms and sp
3
hybridized carbon atoms
arranged in linear fashion. (p. 497)
structural isomers Molecules with the same formula but
different structures. (p. 498)
substitution reactions Reactions in which one or more
atoms (often hydrogen atoms) are replaced by other
types of atoms (often halogens or a hydroxyl
group). (p. 510)
tertiary alcohols Alcohols that have three carbon atoms
bonded to the carbon atom that carries the hydroxyl
group. (p. 517)
thermal cracking A form of cracking that uses heat
alone. (p. 508)
total synthesis the preparation of a molecule from
readily available starting materials. Typically the
total synthesis involves many steps. (p. 528)
trans A molecule containing groups that are fixed
on opposite sides of a bond that cannot rotate.
(p. 503)
unsaturated hydrocarbons Hydrocarbons that contain
CPC or CqC bonds. (p. 501)
Focus Your Learning 533
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
12.1 Elemental Carbon
Skill Review
1. List the three allotropes for carbon, and indicate the
hybridization of the carbon atoms in each.
2. Explain the differences in the properties of amorphous
(lacking a defined crystalline shape, disordered) carbon and
graphite.
Chemical Applications and Practices
3. Carbon in the diamond form and carbon in the graphite
form have widely differing uses. We pay hundreds, even
thousands, of dollars for diamond jewelry, but only pennies
for carbon in pencils. Explain, using the differences in bond-
ing, why diamonds are held together more strongly than the
carbon atoms in graphite.
4. Using hybridization, explain how carbon forms both sigma
and pi bonds in graphite, but only sigma bonds in the dia-
mond form.
5. Diamonds are typically measured in carats (1 carat is 200 mg).
If an industrial diamond contained only carbon, how many
atoms of carbon would be found in a 2.00-carat diamond?
6. Graphite can be converted to diamond with great pressure.
The type of diamond used in polishing powder can be pro-
duced with pressures on the order of 1000 MPa.
a. What is this pressure in terms of atmospheres?
b. Why is pressure a factor in conversion of the graphite form
of carbon to the diamond form?
12.2 Crude Oil—the Basic Resource
Skill Review
7. Judging on the basis of Table 12.2, what is the main difference
between the aliphatic and the aromatic compounds found in
crude oil?
8. Judging on the basis of Table 12.2, what is the difference
between the structures and formulas for compounds in the
aliphatic and naphthene classes?
Chemical Applications and Practices
9. Sulfurous compounds occur to a minor extent in crude oil,
and their presence is indicative of the source of the crude.
Explain how the decomposition of biological material could
give rise to sulfur-containing compounds.
10. Why do you think that there is such a wide diversity of com-
pounds in crude oil?
12.3 Hydrocarbons
Skill Review
11. Provide the correct name for each of these:
a. CH
3
(CH
2
)
5
CH(CH
3
)
2
c. CH
3
CH
2
CH(CH
2
CH
3
)
2
b. CH
3
CH
2
CH
2
CH(CH
3
)
2
d. CH
3
CH
2
CH
2
CH
2
CH
3
12. Provide the correct name for each of these:
a. CH
3
CH(CH
3
)
2
c. CH
3
CH
2
CH
2
C(CH
3
)
3
b. CH
3
CH
2
CH
3
d. (CH
3
)
2
CHCH
2
CH(CH
3
)
2
13. Diagram the structure of the compounds listed in Prob-
lem 11.
14. Diagram the structure of the compounds listed in Prob-
lem 12.
15. Complete this table:
16. Complete this table:
17. a. Diagram the structure of each of these:
pentane
2-methylbutane
2,2-dimethylpropane
Also give the empirical formula of each.
b. Remembering that intermolecular forces affect boiling
point, match each structure with its corresponding boil-
ing point (all are in degrees Celsius): 9.5, 36, 28.
18. a. Diagram the structure of each of these:
3-ethyl-2-pentene
cyclooctene
3-hexyne
Also give the molecular formula of each.
b. Remembering that intermolecular forces affect boiling
point, match each structure with its corresponding boil-
ing point (all are in degrees Celsius): 82, 94, 148.
19. One of the high-molecular-mass hydrocarbons that helps
form the protective waxy skin of an apple contains 28 carbon
atoms. What is the formula of this noncyclic alkane?
20. What is the formula of a saturated noncyclic hydrocarbon
containing 8 carbons? Assume that a different hydrocarbon
contains two CPC groups and has a total of 10 carbon
atoms. What is its formula?
21. Diagram five isomers of octane that all have a six-member
continuous carbon chain.
22. Diagram three isomers of octane that all have a five-member
continuous carbon chain.
23. Which, if any, of these hydrocarbons could be cycloalkanes?
a. C
2
H
6
c. C
12
H
26
b. C
6
H
12
d. C
7
H
14
24. How many hydrogen atoms are found in one molecule of
cyclodecane?
25. Identify each of these molecules as a cis or a trans isomer.
26. Identify each of these molecules as a cis or a trans isomer.
Chemical Applications and Practices
27. Ethyne (also known as acetylene) can be produced by the
reaction between calcium carbide (CaC
2
) and water. In ad-
dition to ethyne, calcium hydroxide is a product. Write and
balance the equation for the production of this important
alkyne.
28. The combustion of methane is an important reaction that
heats our homes. Incomplete combustion, however, involves
the reaction of methane with oxygen to produce carbon
monoxide and water. Write and balance the equation for the
incomplete combustion of methane.
29. Cyclohexane and benzene both have a ring structure
containing six carbon atoms. Compare the two structures in
terms of:
a. The number of hydrogen atoms in each
b. Carbon orbital hybridization
c. The angles between carbon atoms
d. The number of double bonds
e. Resonance structures
30. Four alkene isomers that contain two CPC can be drawn for
C
5
H
8
. Draw these four isomers and identify the one that is
stabilized most by resonance structures.
12.4 Separating the Hydrocarbons
by Fractional Distillation
Skill Review
31. Mixtures of hydrocarbons, and of other types of compounds,
can be separated into their individual components by gas
chromatography. Briefly explain the main steps by which this
technique makes possible the identification of compounds in
a mixture.
32. For specific uses, hydrocarbon fractions obtained from crude
oil may be further modified. The two major industrial
processes that provide useful hydrocarbon products are
cracking and reforming. Compare and contrast these two
important techniques.
33. What property of hydrocarbons serves as the basis for a sep-
aration, during fractional distillation, of the components in
crude oil? What is the relationship between this property and
the structure of the components?
C
H
H
C
CH
3
CH
2
CH
H
CH
3
H
C
CH
2
CH
2
C
CH
C
Br
H
CH
CH
3
C
H
H
CCH
3
CH
3
CH
2
534 Chapter 12 Carbon
Alkane Alkene Alkyne
Type of C to C
bond
Type of
hybridization
Alkane Alkene Alkyne
Angles between
bonds to other
atoms
If made of only
two carbons, will
have __ total
hydrogen atoms
Saturated or
unsaturated?
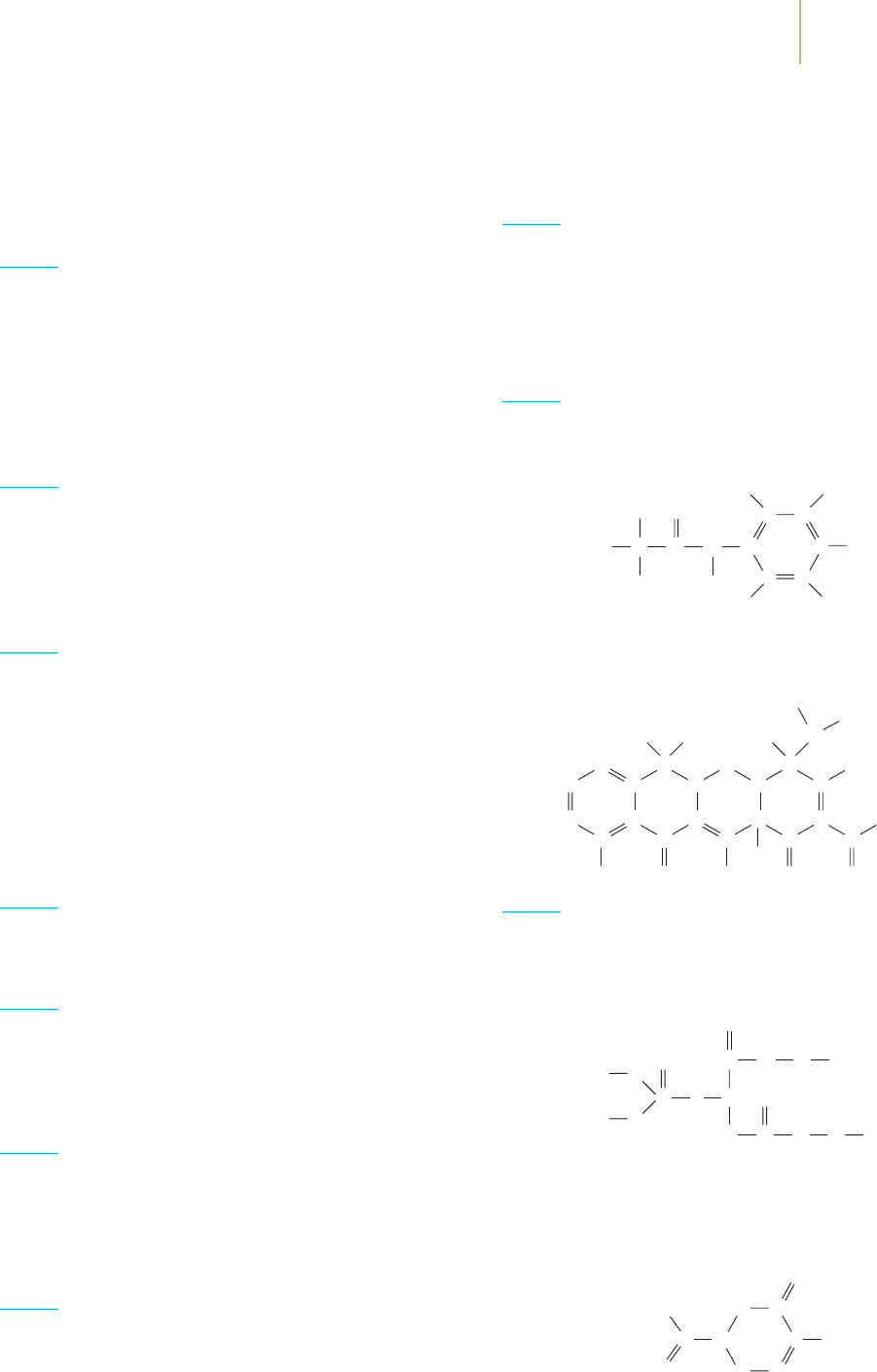
34. Although gas chromatography is often used to resolve mix-
tures of hydrocarbons, it cannot be relied on in every case.
What is a chief limitation of GC when applied to hydro-
carbon separations?
12.5 Processing Hydrocarbons
Skill Review
35. The octane rating system has been used as a standard to rate
the burning efficiency of other fuels. What is the formula of
octane? Use the formula to write and balance the reaction for
the combustion of octane.
36. The actual isomer of octane used in the rating system is
“isooctane.” The descriptive name for the compound is 2,2,4-
trimethypentane. Diagram the structure of this compound
and write a balanced reaction for the combustion of isooctane.
Chemical Applications and Practices
37. Another compound, besides isooctane, used in fuel studies
was found to have the following percentage composition:
84.0% carbon and 16.0% hydrogen. The molar mass of the
alkane is 100.0. From these data, what would you calculate as
the molecular formula of the compound?
38. Calculate the mass percent carbon and the mass percent
hydrogen of isooctane.
39. Currently, many automobiles run on fuel with a minimum
octane rating of 87. To what mixture of isooctane and hep-
tane does this fuel correspond?
40. Some additives to gasoline have a much higher octane rating.
For instance, a particular alcohol has an octane rating of 116.
What quantity of this alcohol would need to be added to
crude gasoline (octane rating 55) to give a product with an
octane rating of 92?
12.6 Typical Reactions of the Alkanes
Skill Review
41. Using methane and bromine, show the balanced equation to
form dibromomethane.
42. Using butane as a reactant, show the balanced equation that
produces 2-butene.
43. Provide the structural diagram and the name for all of the
products that would result from the substitution reaction
between Cl
2
and ethane.
44. Provide the structural diagram and the name for all of the
products that would result from the dehydrogenation of
butane.
45. What are the typical products that result from the combus-
tion of an alkane?
46. A reaction produces a molecule with a CPC bond. If the
product was made from an alkane, what would be the classi-
fication of this reaction?
Chemical Applications and Practices
47. The combustion of propane to form carbon dioxide and
water can produce approximately 2200 kJ per mole. If a
burning propane torch used 42.0 g of propane, how many
kilojoules would be released?
48. Why do arctic hikers use propane as fuel for their cook stoves
instead of pentane?
12.7 The Functional Group Concept
Skill Review
49. Provide a suitable chemical formula and an example struc-
ture of a molecule for each of these functional groups.
a. alcohol b. aldehyde c. alkene d. ketone
50. Provide a suitable chemical formula and an example struc-
ture of a molecule for each of these functional groups.
a. carboxylic acid b. alkyne c. ether d. cyclic alkane
Chemical Applications and Practices
51. The structure of the common analgesic acetaminophen fol-
lows. Identify any and all functional groups shown in the
structure.
52. The structure for tetracycline follows. Identify any and all
functional groups shown in the structure.
53. The partially complete structure (showing all atoms except
hydrogen) for the pesticide malathion follows. Reproduce
the structure and add the missing hydrogen atoms to the
molecule.
54. The partially complete structure (showing only carbons and
oxygens) for carvone follows. Reproduce the structure
and add the missing hydrogen atoms to the molecule.
CC
C
C
CC
CC
C
O
C
S
OC
OC
P
SC
O
C
C
O C C
C O C C
O
HC
HC
C
H
C
C
C
OH O O
C
H
2
C
C
C
OH
OH
C
HO
C
C
CH
3
C
HH
OH
C
NH
2
O
C
C
C
C
CH
3
NH
CH
3
OH
H
CC
CC
H
C
N
H
C
H
CCH
H
HO
H
Focus Your Learning 535
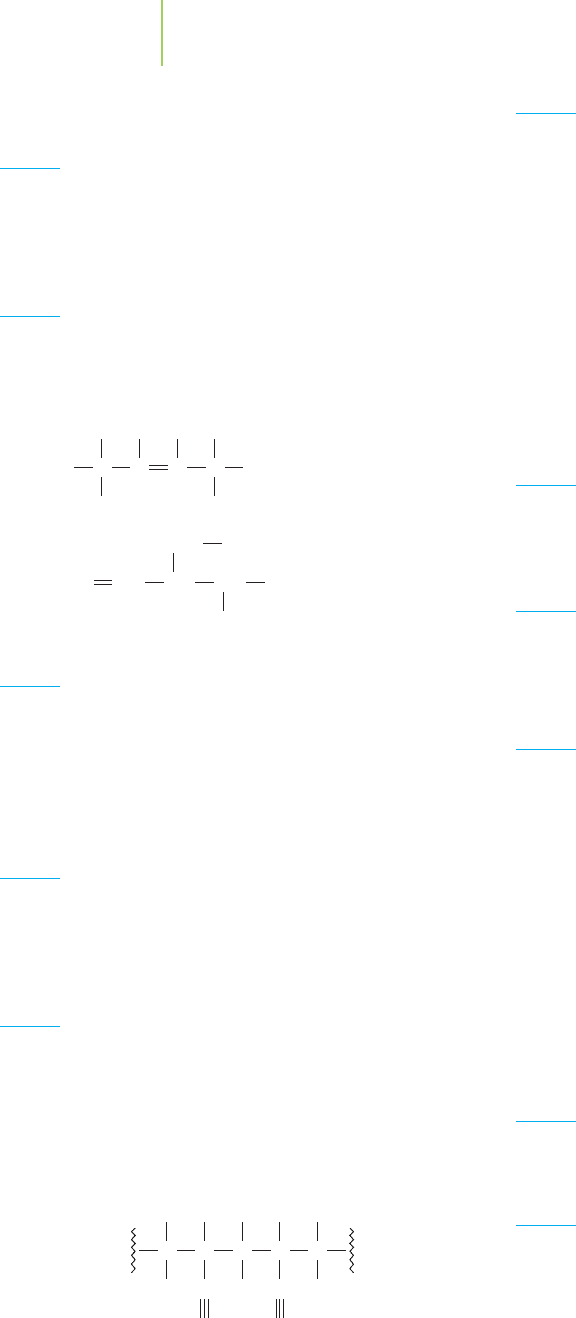
12.8 Ethene, the CPC Bond, and Polymers
Skill Review
55. How many carbon–carbon π bonds are present in each of
these noncyclic molecules?
a. C
3
H
8
b. C
6
H
8
c. C
10
H
8
56. How many carbon–carbon π bonds are present in each of
these noncyclic molecules?
a. C
4
H
8
b. C
5
H
8
c. C
4
H
6
57. Diagram the structure of each of these compounds:
a. 3-Methyl-2-pentene
b. 2-Methyl-4-propyl-3-heptene
58. Name these compounds:
a.
b.
Chemical Applications and Practices
59. One gram of an important hydrocarbon, when completely
combusted, produced 2.93 g of carbon dioxide and 1.80 g of
water. The molar mass of the compound is 30.0. Is this com-
pound an alkane or an alkene? What is the formula of the
compound?
60. Chemists often refer to the addition reaction of alkenes as
“adding across the double bond.” Explain what aspect of the
double bond between carbon atoms favors this process.
61. a. Diagram the structure of propene.
b. Show the balanced equation that represents the conversion
of propene into the alcohol 1-propanol.
62. One way to distinguish an alkane from an alkene is to halo-
genate the double bond. If propene reacted with Br
2
, what
compound would be formed?
63. Polypropylene is an addition polymer with many uses, one of
which is in indoor–outdoor carpeting.
a. What is the structure of the monomer used in this polymer?
b. Diagram a section of polypropylene using four joined
monomers.
64. Clothing materials made from Orlon
®
contain the addition
polymer polyacrylonitrile shown here.
After examining the structure, diagram the structure of the
repeating monomer.
C
H
C
H
H C
C
H
C
H
H C
N N
C
H
H
CH
2
CH CH CH CH
3
CH
3
CH
2
CH
3
C
H
H
C
H
H
C
H
H
H
HC
65. HDPE and LDPE are both plastics that can be recycled.
HDPE is coded as 2, and LDPE is coded as 4. What contrast-
ing structural factor in the molecules of these two polymers
causes their differing properties?
66. Indicate which of these statements is(are) true? For those
that are false, explain why.
a. Addition reactions always involve compounds with
multiple bonds.
b. All addition reactions produce polymers, but not all
polymers are addition polymers.
c. All addition polymers are based on reactions between
molecules with multiple bonds.
d. Addition polymers must contain double bonds.
12.9 Alcohols
Skill Review
67. After looking at the structure of a compound, a student in-
correctly named it 2,5-pentanediol. Provide the correct name
for the compound.
68. Another compound was mislabeled as 1,4-propanediol.
Explain the mistake in this name.
69. Diagram the structure of 1,3-pentanediol. Classify each
—OH group as primary, secondary, or tertiary.
70. Diagram the structure of 2-butanol. Would this alcohol be
classified as a primary, secondary, or tertiary alcohol?
Chemical Applications and Practices
71. Phenol is an example of an aromatic alcohol containing six
carbons, six hydrogens, and one oxygen. It is used in produc-
ing disinfectants and some polymers. Diagram the structure
of this important compound.
72. Alcohols can undergo a reaction known as dehydration. In
this reaction, the alcohol loses a molecule of water to form
an alkene. Provide the name of the alkene formed from the
dehydration of each of these alcohols:
a. Ethanol
b. 1-Propanol
c. 2-Propanol
d. 1-Butanol
12.10 From Alcohols to Aldehydes, Ketones,
and Carboxylic Acids
Skill Review
73. Draw the structure of 1-propanol, and identify the product
that would result from oxidation of this compound.
74. Diagram the structure of butanal. What alcohol can be oxi-
dized to produce this compound?
75. Propanoic acid is used to make a type of mold inhibitor. Di-
agram the structure of propanoic acid. What aldehyde could
be oxidized to form this acid?
76. Isopropyl alcohol (2-propanol) is used as the ingredient in
rubbing alcohol. This compound can be oxidized to a ketone,
but not to a carboxylic acid. Draw the structure of the alcohol
and the ketone, and explain why it can’t be used to produce
the corresponding three-carbon carboxylic acid.
536 Chapter 12 Carbon
Chemical Applications and Practices
77. Methyl ethyl ketone is often used as a solvent for organic
compounds. Diagram the structure of this important organic
compound. What secondary alcohol could be oxidized to
form this compound?
78. An unknown compound was oxidized to form pentanoic
acid. The unknown compound was either 2-pentanone or
pentanal. Identify the starting compound.
79. Carboxylic acids are generally considered only weakly acidic.
Show the acid dissociation reaction of propanoic acid in
water.
80. An unknown compound was found to produce an acidic
solution and contained four carbon atoms. The compound
could be prepared from a straight-chain alcohol. Identify the
unknown compound and the starting alcohol.
12.11 From Alcohols and Carboxylic Acids to Esters
Skill Review
81. The ester that produces a banana aroma is called 3-methyl-
butyl ethanoate. Diagram the structures of the starting mate-
rials that produce the ester, and show the balanced equation
for the production of isopentyl acetate.
82. An apple-like aroma can be produced from the combination
of methanol and butanoic acid. Diagram the structure and
name this ester.
83. An apricot aroma can be produced from the ester pentyl bu-
tanoate. Using structures, show the balanced equation that
produces this pleasant fruity aroma.
84. Tristearin is the animal fat associated with beef. The formula
of stearic acid is CH
3
(CH
2
)
16
COOH. Show the reaction be-
tween stearic acid and 1,2,3-propanetriol to form the triple
ester known as tristearin.
Chemical Applications and Practices
85. Linoleic acid is used to form the major oil found in corn. This
unsaturated acid has two double bonds per molecule. The
double bonds are found between carbon atoms number 9
and 10 and between carbon atoms number 12 and 13. The
formula is C
18
H
32
O
2
. Draw the diagram of this linear unsat-
urated carboxylic acid.
86. Oleic acid (a major component of animal fats) contains a cis
alkene and 18 carbon atoms and has the formula C
18
H
34
O
2
.
The alkene is found between carbons number 9 and 10. Draw
the diagram of this linear carboxylic acid.
12.12 Condensation Polymers
Skill Review
87. The simplest amino acid is glycine. Use the structure shown
below to diagram a condensation polymer made from three
glycine monomers. What molecule has been split out as the
polymer forms?
C
O
OH
H
H
H
2
NC
88. Why is the formation of a condensation polymer impossible
in these cases?
a. Ethanoic acid + NH
2
CH
2
CH
2
CH
2
CH
2
NH
2
b. Ethanoic acid + CH
3
CH
2
NH
2
c. HOOC-CH
2
-COOH + CH
3
CH
2
NH
2
Chemical Applications and Practices
89. Thiokol, dating back to the 1920s, was the first synthetic
rubber commercially produced in the United States. It can
be formed as a condensation polymer from the monomer
ClCH
2
CH
2
OCH
2
CH
2
Cl. The reaction takes place when
sodium polysulfide (Na
2
S
2
) reacts with the monomer. Assum-
ing that Na
2
S
2
is a source of —S—S—, draw the structure of
the polymer. What smaller compound is also produced from
the reaction?
90. In addition to water, a by-product from the condensation
polymerization of 5-aminopentanoic acid is common. What
is the structure of this by-product?
NH
2
CH
2
CH
2
CH
2
CH
2
COOH (5-aminopentanoic acid)
12.13 Polyethers
Skill Review
91. Using the information and examples in the chapter, devise
the name of these ethers.
92. Using the information and examples in the chapter, draw the
structure for these ethers.
a. ethyl methyl ether
b. propyl methyl ether
c. butyl ethyl ether
12.14 Handedness in Molecules
Skill Review
93. Indicate whether chirality exists in each of these objects.
a. a pencil c. your textbook
b. your feet d. a fork
94. Indicate whether chirality exists in each of these objects.
a. a CD c. a bottle
b. a butterfly d. a sportscar
95. Identify the chiral center in each of these molecules.
96. Identify the chiral center in each of these molecules.
H
H
H
H
HO
CH
3
C
C
C
H
H
C
C
C
HC
O
C
H
C
H
HH
C
C
CC
H
H
H
H H
CC
C
C
C
O
H
C
Br
H
C
H
H
H
H
HC
HH
C
O
C
C
C
C
C
Br
H
H
H
O
CH
3
O CH
2
CH
2
CH
2
CH
3
CH
3
O CH
3
Focus Your Learning 537
