Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


558 Chapter 13 Modern Materials
Whereas metals and plastics can bend
and stretch, ceramic materials are less
ductile. In fact, their ability to withstand compression
makes them ideal candidates for supporting weight,
so ceramics are commonly used as building materials
such as plaster, mortar, and cement.
The formation of plaster and that of mortar are
similar in that a natural material is converted into a
different compound that can be molded before it re-
turns to its natural state. In plaster, the reactions that
take place are based on the dehydration and hydration
chemistry of calcium sulfate (CaSO
4
). To make plaster,
the mineral gypsum (CaSO
4
·2H
2
O) is mined and
ground into a powder. This powder, when heated at
150°C to remove some of the water, is converted into
calcium sulfate hemihydrate (CaSO
4
·
1
⁄
2H
2
O). The hemi-
hydrate, also known as plaster of Paris (after a gypsum
deposit near Paris, France, that had been mined since
the seventeenth century), is then mixed with water to
make a paste. The hemihydrate reabsorbs the water to
re-form the gypsum, which is now in a shape that the
manufacturer has molded.
CaSO
4
·2H
2
O + heat n CaSO
4
·
1
⁄2H
2
O +
3
⁄2H
2
O
CaSO
4
·
1
⁄2H
2
O +
3
⁄2H
2
O n CaSO
4
·2H
2
O + heat
Construction workers use mortar to hold bricks
together in the shape of a wall like that shown in
Figure 13.20. Mortar is made from calcium carbonate
(CaCO
3
, limestone) by heating to a high temperature
in a kiln. The calcium oxide (CaO, lime) that results is
mixed with sand and added to water. This wet paste
is sandwiched between the bricks in a wall. Slowly, the
lime in the paste reacts to form calcium hydroxide
(Ca(OH)
2
, slaked lime). Over time, as the mortar is
“curing,” the reaction of carbon dioxide in the air
returns the slaked lime to limestone.
CaCO
3
+ heat n CaO + CO
2
CaO + H
2
O n Ca(OH)
2
Ca(OH)
2
+ CO
2
n CaCO
3
+ H
2
O
The mortar also reacts with the SiO
2
that is the
main chemical component of sand to form complex
calcium silicates via an acid–base reaction between
the lime (CaO; base) and the sand (SiO
2
; acid).
Cement is a much more complex material than
plaster or mortar, but the chemistry of its hardening is
very similar. Made by heating shale and limestone at
1500°C, the product (called clinker) is ground up in
large rotating chambers containing steel balls until it
has the consistency of flour. Gypsum is often added to
the mixture to improve its hardening properties. The
result is dry cement. Specific amounts of water, sand,
and pebbles are combined with the dry cement to
make concrete. The reactions, shown below, that harden
the cement then begin, trapping the sand and pebbles
inside the mixture. Some of the grains of the mixture
begin absorbing water and react with it (hydration).
Although the rate of hydration is relatively slow for ce-
ment, a poured driveway is often hard enough to use
within a few days. Most cements continue to harden for
up to a year.
Ca
3
SiO
5
+ 3H
2
O n Ca
2
SiO
4
·2H
2
O + Ca(OH)
2
2Ca
3
SiO
5
+ 7H
2
O n Ca
3
Si
2
O
7
·4H
2
O + 3Ca(OH)
2
Ca
3
Al
2
O
6
+ 3CaSO
4
·2H
2
O + 24H
2
O n
Ca
6
Al
2
(SO
4
)
3
(OH)
12
·24H
2
O
NanoWorld / MacroWorld
Big effects of the very small: The chemistry of cement
FIGURE 13.20
Mortar is used to
hold these bricks
together.
A ball mill in a cement plant grinds the material into clinker.
This is just one of the steps taken to produce cement.

13.3 Ceramics 559
Comparison of Ceramics with Other Materials
Hardness is a measure of the relative ability of a material to scratch another; it is
expressed using the Mohs hardness scale. The higher the hardness value, the harder
the material (talc 1, gypsum 2, diamond 10). The coefficient of expansion
indicates the size change upon heating or cooling. A larger value indicates a greater
change in the size of the material as it is heated or cooled. Note that the ceramics
(alumina and zirconia) have relatively high melting points, are hard, and don’t
expand much when heated.
Coefficient of
Melting Point Density Expansion
Material (°C) (g/cm
3
) Hardness (Mohs) (°C
–1
× 10
–6
)
Ceramics
Alumina (Al
2
O
3
) 2050 3.8 9 8.1
Zirconia (ZrO
2
) 2660 5.6 8 6.6
Alloys
Steel 1370 7.9 5 15
Brass 930 8.6 4 20
Metals
Zinc 420 7.1 2.5 35
Sodium 98 0.97 0.4 70
TABLE 13.5
Coating the hull with materials capable of withstanding the heat and resisting ex-
pansion protects the astronauts and the shuttle itself. Even though ceramics alone
can satisfactorily insulate the bottom of the shuttle, they are often glazed with sil-
ica glass. This improves the thermal insulation and the durability of the tiles.
Glass
The glass used to make test tubes for the hospital laboratory is an amorphous
solid ceramic material. Solids such as obsidian, a natural glass ejected from vol-
canoes, shown in Figure 13.21, lack order in the arrangement of the particles that
constitute them. Human-made glass has been known throughout history. For ex-
ample, the ancient Mesopotamians and Egyptians made a very successful busi-
ness out of the preparation of glass objects. Today, we use glass in many different
applications, as illustrated in Table 13.6. The windows on the doors into the hos-
pital, the screens on the computers at the nurses’ stations and the drinking glasses
in the cafeteria are all made of glass.
The simplest glass is made from quartz (crystalline silica). Figure 13.22 illus-
trates the veryorderedstructure of SiO
2
units in quartz.When heated tothe melting
point (1600°C), some of the silicon–oxygen bonds break and the ordered structure
is disturbed. Rapid cooling of this material results in a highly disordered struc-
ture. The resulting silica glass is composed of SiO
2
units connected in a very
disordered array. Because of the disorder, glass does not have a definite melting
point. Instead, silica glass has a transitional temperature where it begins to soften.
When it is in this softened state, glass can be molded into many different shapes.
Of particular use to the endoscopist at the hospital is the fiber optic line, a
hair-thin wire of plastic-coated glass that can be used to transport light along its
length (Figure 13.23). On the end of the line are a small camera and light. The In-
ternet that connects the emergency room computer to data sources across the
world also uses fiber optic lines to transmit information. How does light bend
along the path taken by the fiber optic wire? John Tyndall (1820–1893) noted that
light could be bent inside a bent stream of water by a property known as total in-
ternal reflectance. Total internal reflectance of the light ensures that any light
inside the glass pipe reaches the end of the fiber optic wire. Because a fiber optic
FIGURE 13.21
Obsidian, a natural glass.
Ancient peoples used obsidian
as a tool because of its
sharpness and durability.
Application
Application
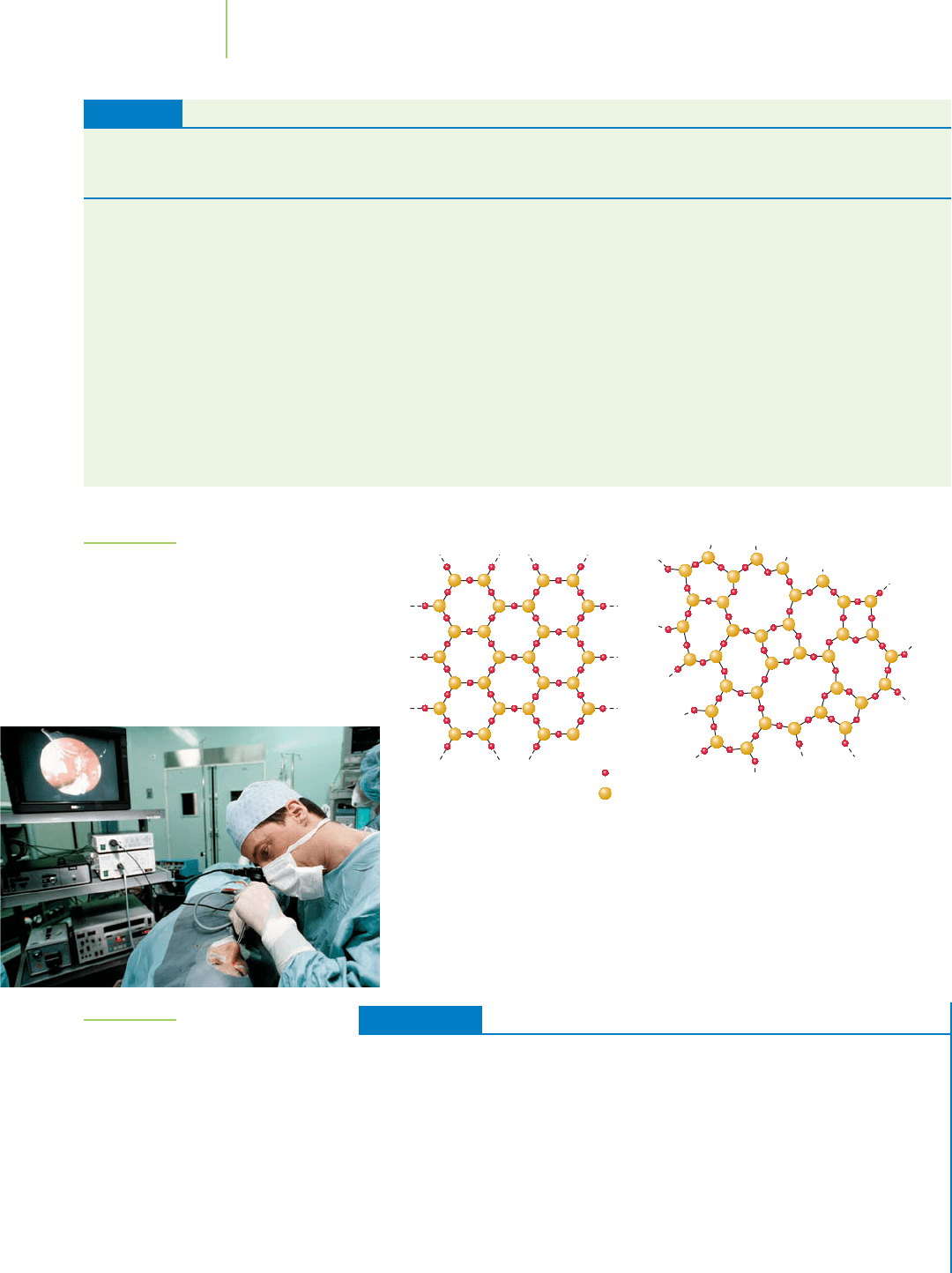
FIGURE 13.22
Silica glass (SiO
2
), also known as quartz glass, or
fused silica. While this type of glass is far superior
in many ways to other types of glass, it is much
more expensive and much more difficult to mold
into useful apparatii.
wire can direct light around corners, the endoscopist can direct the light to
any part of a patient’s interior without the need for large incisions. The in-
credible flexibility of fiber optic wire has increased its use as telephone wire
and for connections between computers and other electronic communica-
tion systems.
560 Chapter 13 Modern Materials
Quartz crystal Glass
Oxygen
Silicon
The Six Main Types of Glass
Resistance to Resistance to
Temperature Corrosive Sample Composition (in addition
Type of Glass Example of Use Shock Chemicals to silica, SiO
2
), Mass %
Soda-lime glass Most common Not good Fair 16% Na
2
O, 7% CaO, 3% MgO,
(90% of all glass) 1% Al
2
O
3,
0.3% K
2
O
Lead crystal Good electrical Not good Fair 24+% PbO
insulator, brilliance
when cut
Borosilicate Light bulbs, bakeware Good Good 12% B
2
O
3
, 5% Na
2
O, 5% Al
2
O
3
Aluminosilicate Resistors Good Good 7.0% B
2
O
3
, 10.4% Al
2
O
3
, 21.0% CaO,
1.0% Na
2
O
Silica Chemical glassware Excellent Good 96% SiO
2
Fused silica Cuvettes, space Excellent Excellent 100% SiO
2
shuttle windows
TABLE 13.6
FIGURE 13.23
The endoscope in action. A fiber optic
cable can be used to explore inside a
body with no need for large incisions.
EXERCISE 13.5 Classifying Common Materials
Classify each of the following common household items as a metal, an alloy, a glass,
or a ceramic: A sidewalk, a 1-oz silver dollar, a bicycle frame, and a perfume bottle.
Solution
The sidewalk is made from concrete. Concrete is the mixture of cement and small
stones or sand. Cement is a ceramic material.
Some coins, such as the 1-oz U.S. silver dollar, are made of nearly pure silver
(>99% pure silver metal). However, most coins (such as the U.S. Golden Dollar), are
made of alloys to help reduce the expense of their manufacture.
The bicycle frame, in order to be light yet durable, is made out of a metal alloy.
The top bicyclists use only aluminum, magnesium, or titanium alloys.
The perfume bottle is typically made from glass. The transparency, esthetic
beauty, and low cost of glass make it an excellent material for bottles.
PRACTICE 13.5
Try some on your own. What class of materials makes up the rims on your car? . . .
a casserole dish? . . . the toilet in your bathroom? ...a wedding band?
See Problems 51 and 52.
Superconductors
Some patients in the hospital enter through the emergency room as cases of
trauma. Often, the patient presents symptoms that are not easily identifiable by
external examination of the body. At this point, physicians call upon devices that
can “see” inside a patient, such as an X-ray machine, which can take images of
bones. Another instrument that can investigate the structure of the organs in the
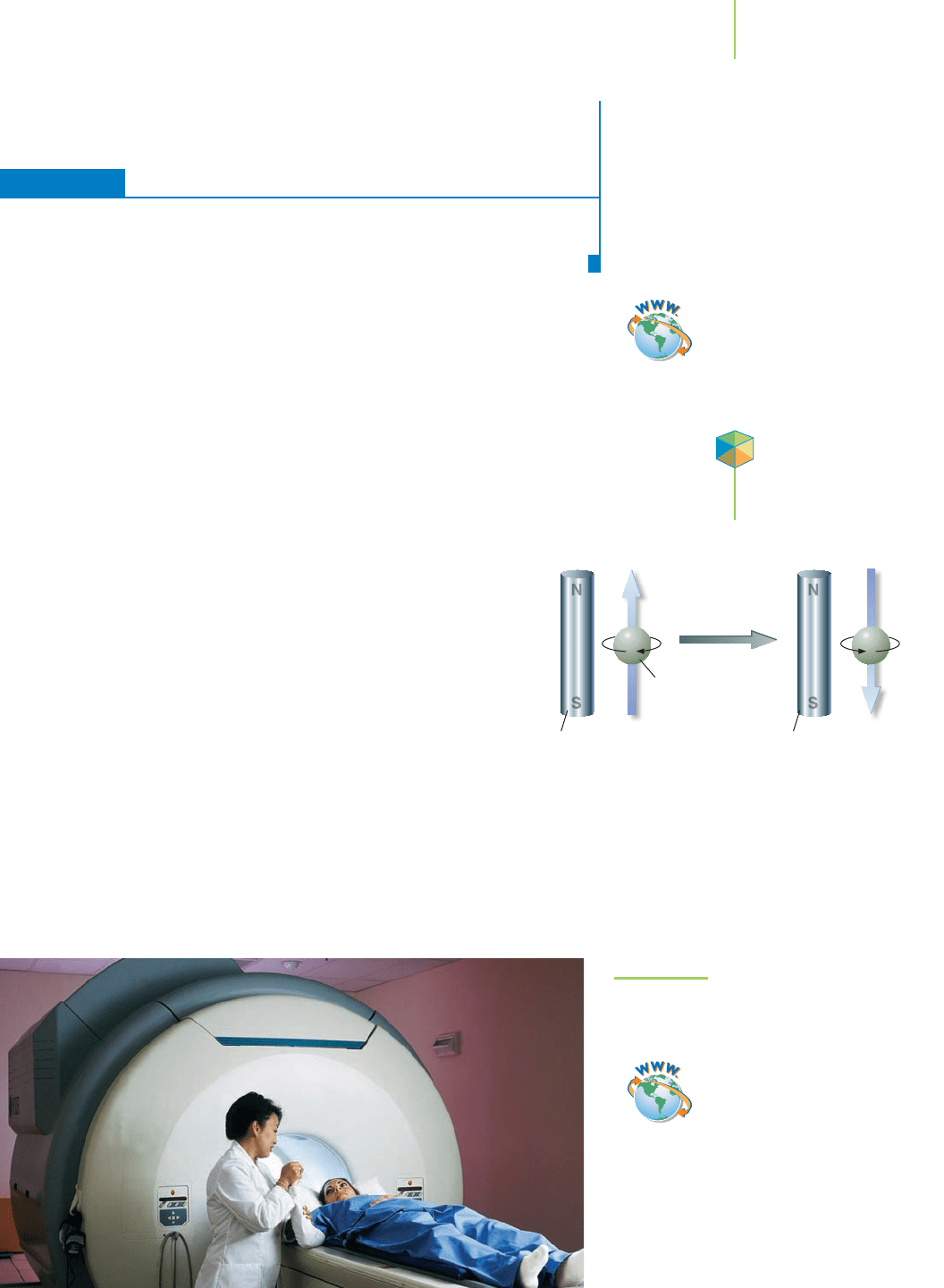
body is the magnetic resonance imager (MRI) shown in Figure 13.24.
The MRI works by measuring the absorption of energy by a particular type of
nucleus in the radio frequency range of the electromagnetic spectrum. However,
in order for the absorption to be noticed, the nucleus must be aligned with a
strong magnetic field. The size of the magnetic fields required is much greater
that what can be obtained with a bar magnet. In fact, the bulk of the
MRI is occupied by a superconducting magnet that can generate
these large magnetic fields. What is a superconductor and how does
it find use as a magnet?
A
superconductor is a type of ceramic material that acts quite
differently than a metal. The conduction of electrons in metals is
met with resistance. We use this property to toast bread when the
NiChrome (an alloy of nickel, chromium, and, often, iron and other
metals) coils of a toaster get quite hot and exhibit their characteris-
tic orange glow, as a consequence of their high electrical resistance.
We might imagine that copper wires could be used to toast bread,
too. However, copper melts when you put enough voltage through
it to get it hot enough to toast bread. NiChrome wire, on the other
hand, has a high melting point and won’t melt inside your toaster.
Why do ceramics and metals such as NiChrome get hot when
electrons flow through them? The resistance is due to a combina-
tion of impurities in the alloy and to the existence of induced quan-
tized vibrations (called
phonons) in the metal lattice. Resistance
13.3 Ceramics 561
Application
C
HEMICAL ENCOUNTERS:
Magnetic Resonance
Imaging
KELTER/MOSHER 1/e
329670_ula_13_07.eps
11/22/05
size: 17p0 x 9p3
External
magnetic field
+ Energy
Nucleus
External
magnetic field
A nucleus acts as a tiny bar magnet and aligns with an
external magnetic field. If the nucleus then absorbs en-
ergy in the radio frequency range, it can flip over and
align against the external magnetic field. This higher
energy state is unstable, and as the nucleus realigns
with the external magnetic field, it gives off the energy
it absorbed.
FIGURE 13.24
The magnetic resonance imager at work.
The MRI generates powerful magnetic fields
using a superconducting ceramic.
Video Lesson: CIA
Demonstration:
Superconductivity
Visualization: Magnetic
Levitation by a Superconductor

H
c
c
b
a
H
c
H
c
H
c
C
C
C
C
C
C
C
C
H
c
H
b
H
b
H
a
(a)
(b)
H
a
H
a
562 Chapter 13 Modern Materials
A nuclear magnetic resonance spec-
trophotometer can be used to obtain
information on the structure of a
molecule. A hydrogen atom spectrum
of ethylbenzene illustrates the infor-
mation that is obtained. This informa-
tion can be used to build the struc-
ture of a molecule.
increases as the vibrations of the metal lat-
tice increase (the metal gets hotter), and
eventually the metal can reach its melting
point or combust. For instance, if too
much current is placed into the NiChrome
wires in your toaster, they will catch fire.
When metals are cooled, the resistance
to the flow of electrons drops until it
reaches a constant positive value. A super-
conductor acts differently. As the tempera-
ture of the superconducting ceramic is
lowered, the resistance to the flow of elec-
trons decreases, as in metals. However, at
the
transition temperature, the resistance to
the flow of electrons becomes negligible.
What does “negligible” mean to us and how
does that make a superconductor useful?
No resistance implies that no energy is lost
in the movement of electrons along the su-
perconductor’s lattice. If the coil in your
toaster were made from a superconducting
wire, the electrons could pass through it
without resistance, and it wouldn’t get hot.
It wouldn’t toast either. With a superconducting wire, electricity could be deliv-
ered to your home efficiently and very inexpensively (gone would be the need for
the step-down transformer on the telephone pole near your home). This same
lack of resistance also implies that a superconductor can carry extremely large
electric currents. This is exactly what is needed in the MRI.
To generate the sizable magnetic field needed in the MRI, a large electric cur-
rent is placed in a coil of superconducting ceramic wire. As the electrons travel
around the coil, they generate the magnetic field perpendicular to the flow of
the electrons. In order to be superconducting, the ceramic must be kept cold,
typically at the temperature of boiling liquid helium (4 K). However, ongoing
research on “high-temperature”superconductors may one day eliminate the need
for liquid helium. This would be quite beneficial because helium is a nonrenew-
able resource. Table 13.7 lists some superconductors and the
temperature at which each is superconducting.
The MRI is actually based on a key instrument in chemical
research: the nuclear magnetic resonance spectrophotome-
ter (NMR). Within nuclei of the same type (here, all hydro-
gen nuclei) a particular range of frequencies is commonly
absorbed. Moreover, specific electromagnetic environments
Comparison of Superconducting Materials
The record for the highest superconducting temperature for a ceramic
material is held by a complex mixture of atoms (Hg
0.8
Tl
0.2
Ba
2
Ca
2
Cu
3
O
8.33
).
The noninteger subscripts are used in these formulas to show a simple
ratio of the atoms. In order for us to observe the superconductive proper-
ties of the commonly used superconductors, they must be immersed in
liquid helium (boiling point = –268.9°C, or 4.2 K).
Highest Superconducting
Material Temperature (K)
Hg
0.8
Tl
0.2
Ba
2
Ca
2
Cu
3
O
8.33
138 (record)
HgBa
2
Ca
2
Cu
3
O
8
133–135
YPd
2
B
2
C23
LuNi
2
B
2
C 16.6
Tm Ni
2
B
2
C11
ErNi
2
B
2
C 10.5
Nb
0.6
Ti
0.4
9.8
Lead (fcc) 7.196
Tantalum (bcc) 4.47
Aluminum (fcc) 1.175
Platinum (fcc) 0.0019
TABLE 13.7

within a molecule cause individual nuclei to absorb slightly different frequencies
of radio waves. When a compound is placed in the NMR, these specific frequen-
cies are recorded. Then they can be used to identify the environments within a
molecule. With a very powerful magnetic field, chemists can use this information
from the NMR to determine the structural formula for a molecule.
13.4 Plastics
Today’s hospitals are completely different from those of 50 years ago. Advances in
the field of medicine from open-heart surgery to the use of more powerful and
useful medicines are among the most dramatic changes. Walk into a hospital
and what do you see? Plastics. Everywhere you look,
plastics have found a use. The
nurse, the physician, and the staff work with plastics in the form of gloves,
smocks, masks, and surgical booties. During the time in the hospital, the patient
is exposed to plastic in the form of syringes, tubing, sterile packaging, bandages,
and even the chairs in the waiting room.
What is plastic? All plastics are polymers (see Chapter 12), but not all poly-
mers are plastics. The DNA and protein within us, for example, are naturally
occurring polymers that are not plastics. Plastics are polymers that can be
molded into a shape and then hardened in that form. In 1907, the U.S. chemist
Leo Baekeland prepared the first completely synthetic plastic. Named after its in-
ventor, bakelite could be formed easily into almost any shape. After it hardened,
bakelite was tough, durable, and resistant to heat. Bakelite was used in the early
half of the 1900s as a lightweight counterpart to steel. Like most plastics, it also
works as a good insulator, which increases its utility as handles for frying pans,
spatulas, electrical plate covers, and other household items.
The successes with Bakelite prompted the preparation of many more types of
plastics, including polyethylene, saran, Teflon, nylon, neoprene, and a host of oth-
ers. Today, our world is inundated with plastics, which are used wherever possible
because they are light in weight and low in cost (see Table 13.8).
Why are there so many different types of plastics? The short answer
is that different types of plastics have different properties. Differing
properties suit different uses. A rigid plastic would not make a good
climbing rope because it does not bend. A stretchable plastic wouldn’t
make a good ruler or calculator housing. Therefore, we make different
plastics for different specific applications. The properties of a plastic
are related to the way it is manufactured and to its composition. By
controlling the polymerization reactions, we can make short polymer
chains (with lower melting points) or long polymer chains.
Crosslinking,
or linking the chains of adjacent polymer strands together, bestows
strength on the overall plastic. Orienting the chains in parallel makes
stretchable fibers.
Fibers
Some airplanes are made largely of plastic—not the same type of plastic that is in
your soda bottle, but a plastic that is made in roughly the same manner. The type
of plastic that makes up the wings of some planes is a polymeric fiber. A
fiber,
some examples of which are shown in Table 13.9 on page 566, is a polymer whose
chains are aligned in one direction. Fibers have a high
tensile strength (they
stretch without breaking when you pull them), a property that is good for, among
other products, airplane wings. Airplane wings need to be able to stretch when
the plane rides through turbulence. If the plastic weren’t stretchable, the air-
plane’s wings could snap off.
13.4 Plastics 563
Parallel chains improve
the strength of a polymer.
Crosslinking the chains makes
the polymer even stronger.
564 Chapter 13 Modern Materials
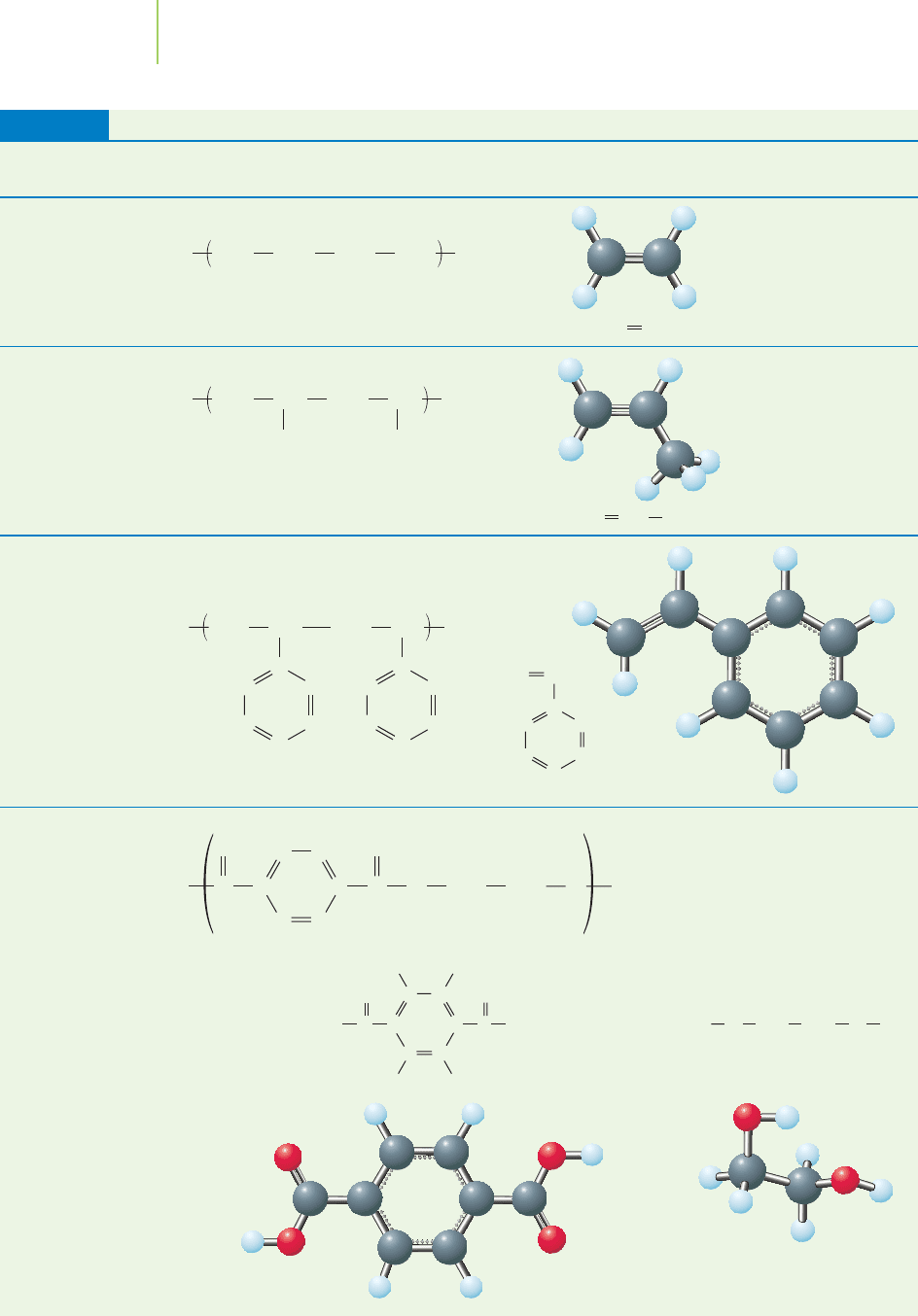
Selected Plastics and Their Chemical Composition
Plastic (and
representative uses) Structure Monomer
Polyethylene (plastic
bags, sheeting)
Polypropylene
(car parts, food
packaging)
Styrofoam
(coffee cups)
Polyethylene
terephthalate
(soda bottles)
CH
2
CH CH
2
CH
3
CH
3
CH
n
CH
2
CH
2
CH
2
CH
2
n
TABLE 13.8
CH
2
CH
2
OO HH
C
C
C
C
CCCOH
HH
HH
HO
O
C
O
CH
2
CH
2
CH
2
CH CH
3
CHH
2
C
C
C
H
CH
CH
HC
HC
C
HC
HC
C
H
CH
2
CH CH
2
CH
CH
CH
C
HC
HC
C
H
CH
CH
n
CHHC
CH
n
HC
CC
C
O
C CH
2
O CH
2
O
O
13.4 Plastics 565
Other plastic fibers can be spun into thread and then woven into textiles. Put
a price tag and a label on them, and you could sell them in the clothing store.
Nylon and polyester, when made into clothing like that shown in Figure 13.25, are
two very common plastic fibers. Fibers do have drawbacks, however. They stretch
only in the direction in which they are aligned, so if you pull on a fiber in the
wrong direction, it tends to come apart quite easily. To alleviate this problem,
the fiber can be manufactured with the addition of a different material. The re-
sult is a
composite material that possesses the strengths of both components.
Composite materials don’t need to be made from only plastics. In fact, a brick
wall is an example of a composite material (cement and ceramic bricks). Another
example is the 50/50 cotton/polyester blend used to make shirts.
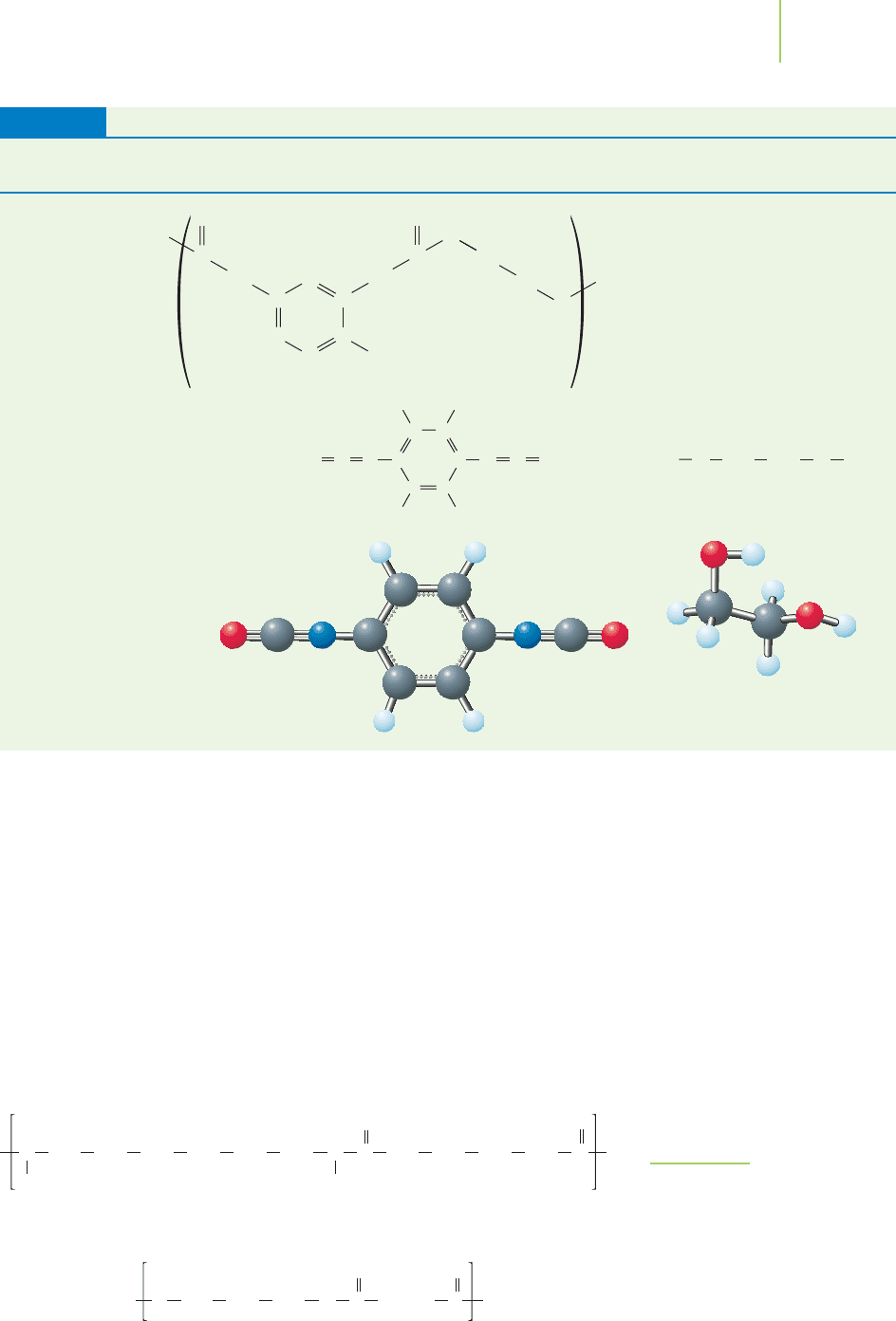
(Continued)
Plastic (and
representative uses) Structure and Monomer
TABLE 13.8
Polyurethane
(foam, gaskets,
bearings)
CH
2
CH
2
OO
H
H
C
C
C
C
HH
HH
CC
NNCOO
C
n
H
C
HC
C
O
C
H
NH
O
O
CH
3
NH
C
C
CH
2
CH
2
C
C
O
CH
2
N
H
NC
H
Nylon [6,
6]
Polyester [3
,16]
n
n
CH
2
CH
2
CH
2
OOCCH
2
CH
2
(CH
2
)
14
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
O
O
C
O
C
O
FIGURE 13.25
Nylon and polyester.
566 Chapter 13 Modern Materials
Common Plastic Fibers
Fiber Structure
Kevlar
(note interactions between strands)
Nylon
(note interactions
between strands)
Polyester
TABLE 13.9
Carbon fibers
(note graphite-like structure)
N N N N N N N
N N N N N N N
O
O
NH
O
O
N
H
N
H
O
O
O
O
NH
O
O
HN
NH
O
O
N
H
O
O
NH
O
O
C
O
N
H
N
C
O
H
C
O
N
H
N
C
O
H
C
O
N
H
N
C
O
H
C
O
N
H
N
C
O
H
O CH
2
CH
2
CH
2
O (CH
2
)
14
n
CC
O
O
13.4 Plastics 567
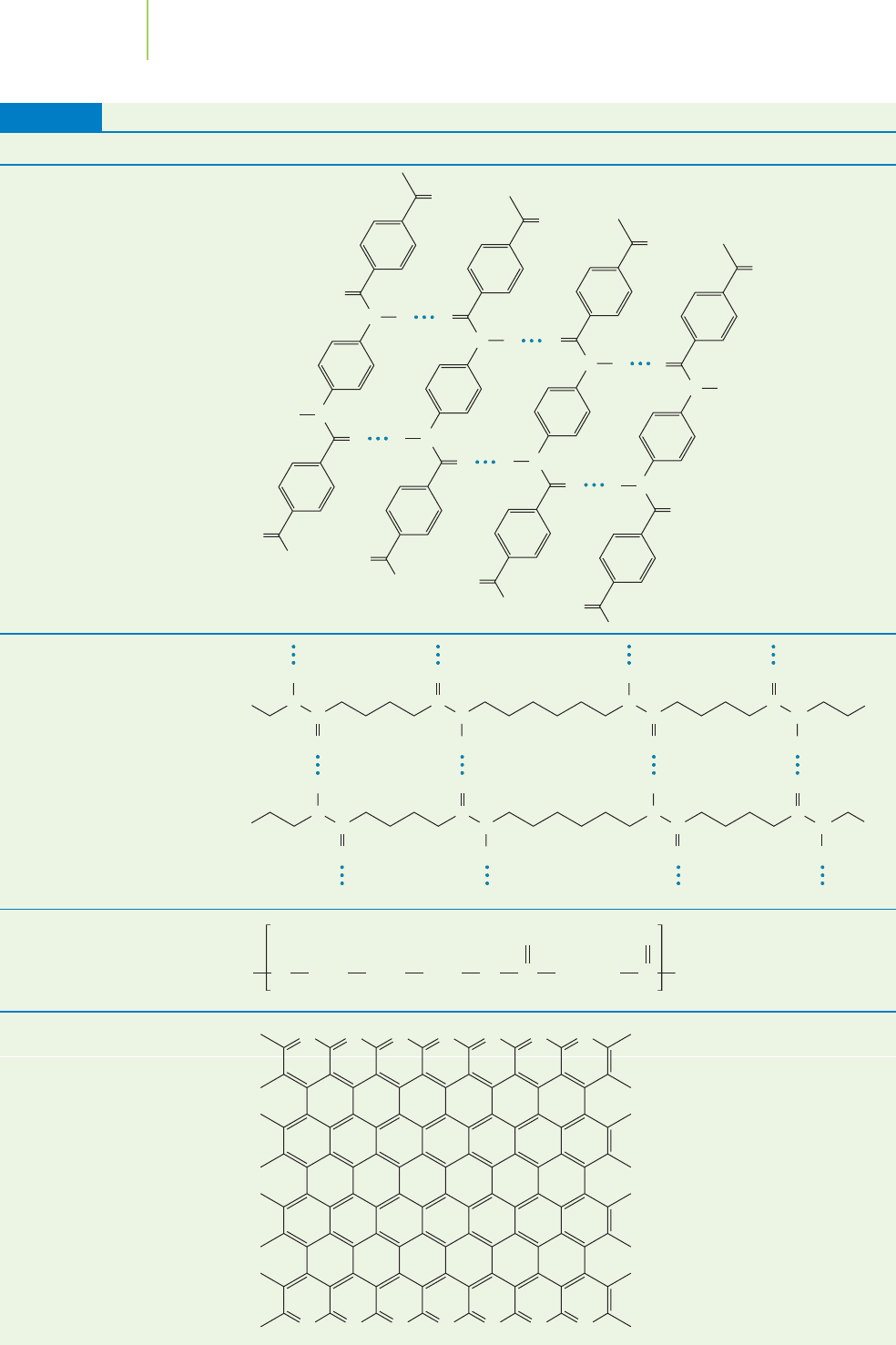
Aramid (short for aromatic amide)
plastics such as Kevlar®, shown in Fig-
ure 13.26, are an example of a composite
fiber that is particularly strong. What makes
this material so strong? The adjacent poly-
mer chains in the material are held together
by hydrogen bonds. If the fiber is mixed
with an epoxy resin when it is manufac-
tured, it becomes even stronger. The fibers
are then woven together to make fabric that
is light, flexible, and so strong that it can
even stop bullets, enabling police depart-
ments to use them as bulletproof vests.
High tensile strength and low density make
Aramid plastics better than steel.
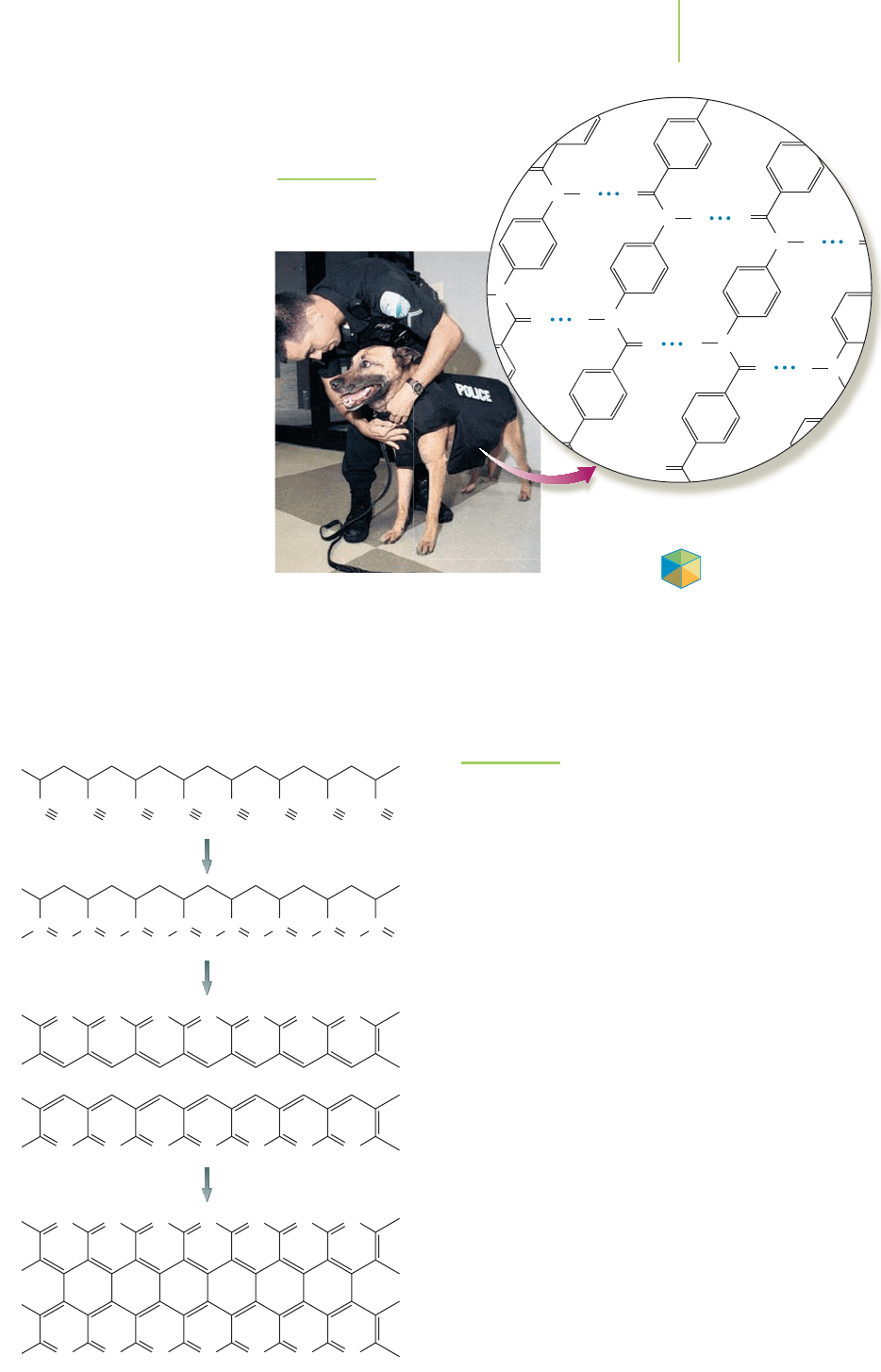
Carbon fiber is another of the compos-
ite materials. A polymerization reaction of
acrylonitrile gives the polymer polyacry-
lonitrile (PAN) via the reactions illustrated
in Figure 13.27. Heating PAN in air to
300°C oxidizes the polymer. To finish the
preparation of a carbon fiber, the oxidized
PAN is heated to 3000°C, resulting in a structure that is very similar to graphite
and is extremely strong. It is three times stronger than high-tensile steel, but only
one-sixth as dense. This makes carbon fiber composites the perfect choice for air-
planes, boats, and other structural components (light, yet extremely strong).
NH
O
N
N
H
O
O
HN
NH
O
N
H
O
NH
O
O
FIGURE 13.26
Aramid polymers and
the bulletproof vest.
N N N N N N N
N N N N N N N
N N N N N N N
N N N N N N N
N N N N N N N
C C C C C C C C
N
Polyacrylonitrile
Heat
700°C
400–600°C
N
CC
N
C
N
C
N
C
N
C
N
C
N
C
FIGURE 13.27
Reactions that make carbon fiber.
Application
