Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

Application
C
HEMICAL
ENCOUNTERS:
Heart
Defibrillators
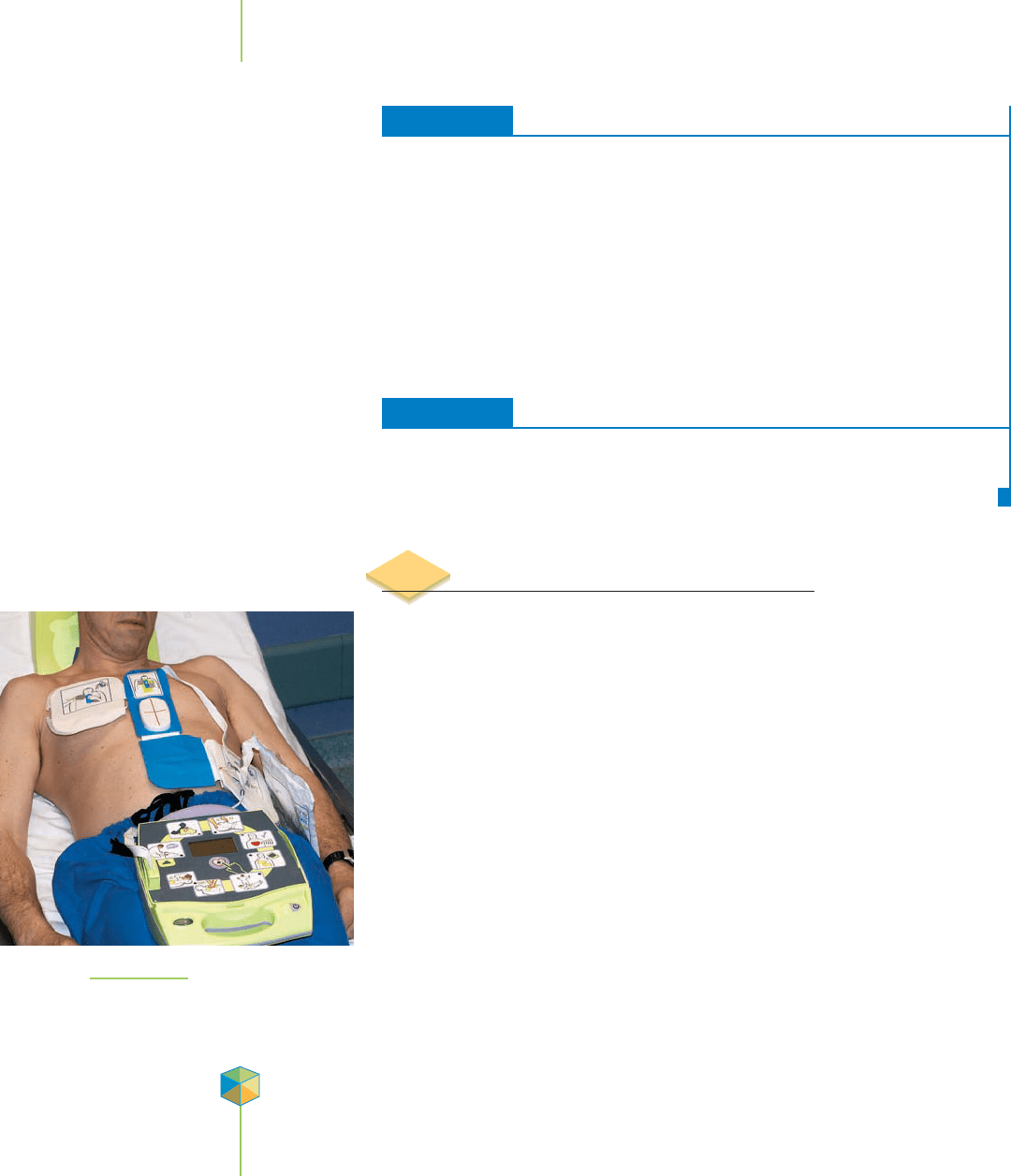
FIGURE 13.28
The portable cardiac defibrillator. The
defibrillator stores electrical energy in
capacitors made with thin films.
568 Chapter 13 Modern Materials
EXERCISE 13.6 Identifying Materials in the Real World
Examine and identify the following items from everyday life. Indicate whether they
are made of a composite material, a plastic, a metal, an alloy, or a ceramic: an iron
bar–reinforced concrete wall, the light switch plate, and a brass doorknocker.
Solution
The reinforced concrete wall is made from iron bars (called re-bar) buried in a mix-
ture of cement and pebbles. Therefore, it is a composite material. The light switch
plate is made from Bakelite, a plastic. Its insulating ability makes it a good choice for
an electrical plate. The doorknocker is made from brass, an alloy. The sheen and
durability of polished brass makes this item luxurious and useful.
PRACTICE 13.6
Identify the materials that make up a floppy disk, the nonstick coating in your fry-
ing pan, and the windshield of your car.
See Problems 55 and 56.
13.5 Thin Films and Surface Analysis
People who experience sudden cardiac arrest (disruption of the normal function
of the heart) are often treated with a portable cardiac defibrillator like that shown
in Figure 13.28. This instrument delivers to the cardiac victim’s heart a powerful
and sudden shock, which can resynchronize the beating cycle of the heart muscle.
The defibrillator contains devices called capacitors that can store large amounts
of electrical energy. At the push of a button a circuit is closed, and the capacitors
release the stored energy as one strong electrical pulse.
One of the most durable capacitors on the market is made with a thin film of
aluminum on either side of a plastic sheet. The two sheets of thin aluminum act
as the positive and negative terminals of a battery. Because a plastic sheet separates
them, the terminals do not allow electricity to pass until a large voltage is applied.
The thin film capacitors are particularly useful in portable devices because they
are self-healing. If a short circuit develops inside the capacitor, the heat generated
causes the aluminum to vaporize, making that portion of the capacitor ineffec-
tive. This reduces the power of the capacitor, but it is still able to function. In a
standard capacitor, a short circuit often destroys the capacitor, which would not
be desirable if you were answering a cardiac arrest emergency.
A thin film is generally defined as a film of any material, typically only 0.1 µm
to 300 µm thick, much thinner than a layer of paint. However, like paint, a thin
film must adhere strongly to the surface on which it is applied. Thin films are
carefully prepared so that the chemical composition is well defined (uniform
thickness, homogeneity, and so on).
How do we make a film of a material so thin? A film so thin is very fragile un-
less it is made directly on a surface. Many techniques have been designed to do
just that. The most common techniques are physical deposition, sputtering, and
chemical-vapor deposition.
Physical deposition relies on sublimation and deposition to place a thin film on
a substrate. A chamber is attached to a vacuum. The material to be made into a
thin film is placed at one end of the chamber, and the substrate is placed at the
other end. The vacuum is turned on, and heat is applied to the material to be va-
porized. When the appropriate heat and pressure have been obtained, the mater-
ial sublimes and moves through the chamber, where it deposits on the substrate.
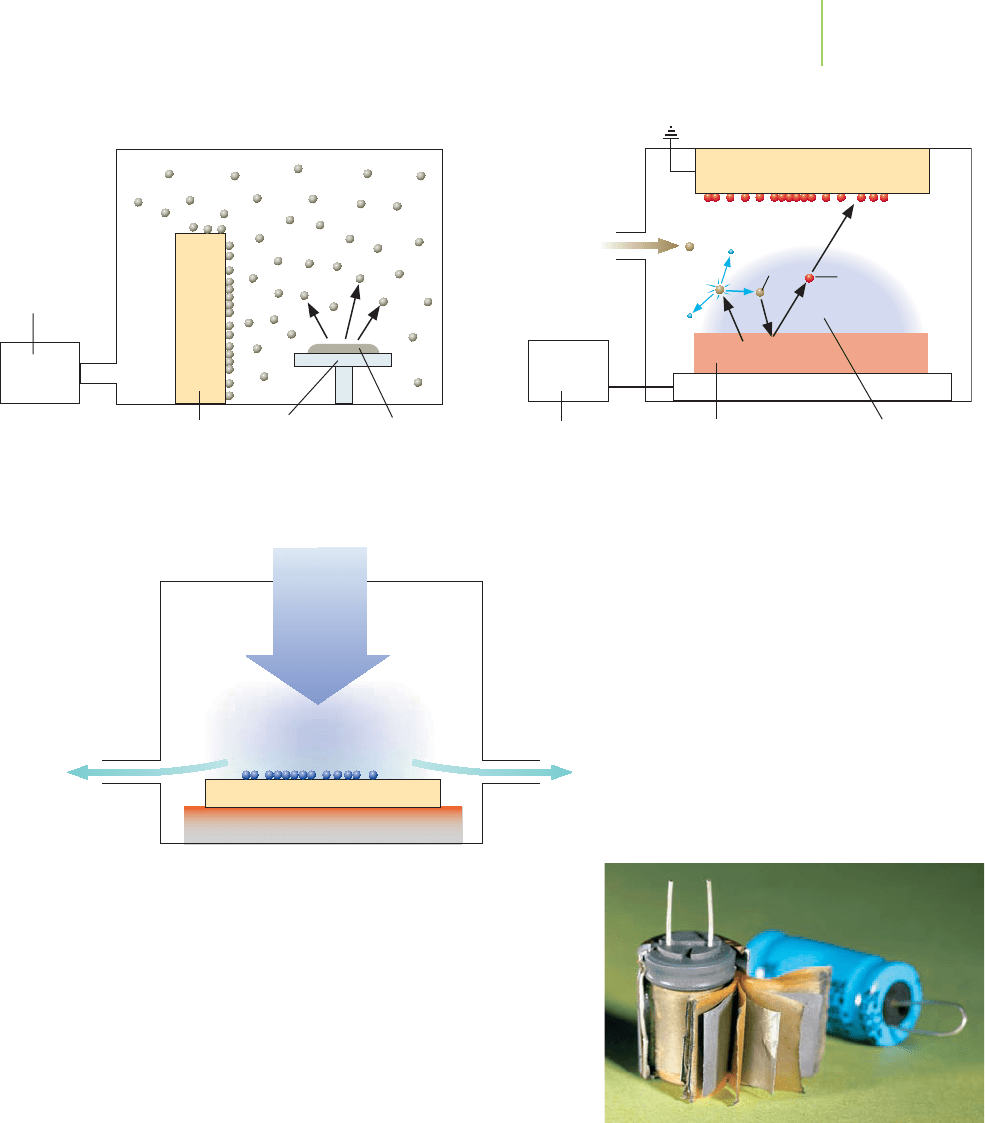
Despite the rotation of the substrate, uniform thickness of the thin
film is difficult to obtain. Typically, thin films of inorganic materials
such as MgF
2
and SiO
2
are made by this method.
In
sputtering, very high voltage is used to move a material from a
negative terminal (the cathode) to a positive terminal (the anode, see
Chapter 19), both surrounded by a low-pressure argon atmosphere.
Inside the device, the high voltage forms argon cations that race to the
cathode. Their impact dislodges metal atoms from the cathode with
extremely high kinetic energy. These ejected atoms fly in all direc-
tions. Some strike the anode and form a thin film. Typically, thin films of metals
(such as silicon, titanium, aluminum, gold, and silver) are made via sputtering
techniques.
In
chemical-vapor deposition, a thin film is prepared when a compound placed
on a surface undergoes a reaction. For example, scratch-resistant sunglasses can
be made by coating the lenses with a carbon-based thin film. In this process, a
mixture of methane (CH
4
) and hydrogen (H
2
) is passed over the lenses. Applica-
tion of intense microwave radiation to this mixture causes the molecules to break
apart into their individual atoms. The carbon atoms re-form into a diamond-like
thin film. The hydrogen atoms react with any carbon that starts to form graphite-
like films. Other methods include the reaction of a metal(IV) halide with hydro-
gen gas to prepare metal films and the reaction of silane (SiH
4
) and ammonia
13.5 Thin Films and Surface Analysis 569
Vacuum
pump
Physical Deposition
Substrate Source
material
Heated
platform
Argon gas
Ar
+
e
–
e
–
e
–
Sputtering
Target
Ground
Power
supply
Magnetic
field
Substrate
Sputtered
target atom
Source
gases
Chemical-Va
p
or De
p
osition
Substrate
Product
gases
Product
gases
Reaction zone
Chemical Vapor Deposition
Capacitors can be made from thin films.
(NH
3
) to make silicon nitride ceramic thin films. This method makes thin films
of metals, alloys, ceramics, and polymers.
Applications of thin films range from the fabrication of microelectronic de-
vices to use as novel catalysts and as protective coatings. For instance, thin films
of gold that absorb harmful UV radiation from the sun are used to create protec-
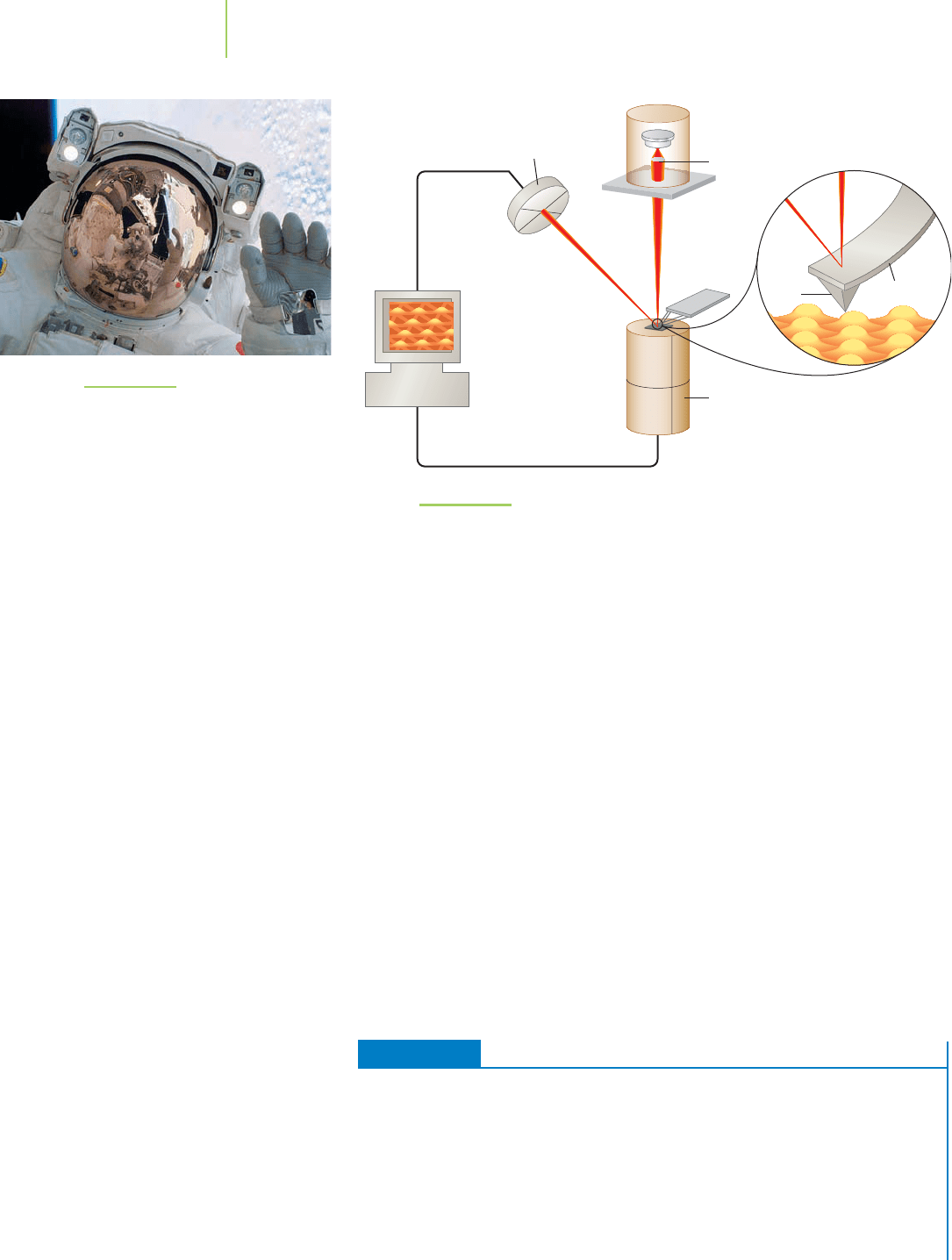
tive eyewear for astronauts in spaceflight (see Figure 13.29).
Thin films are analyzed using a variety of techniques, including scanning tun-
neling microscopy (STM) and atomic force microscopy (AFM). Atomic force
microscopy is similar to STM (Chapter 9) in that an atomic-sized needle (with a
diameter less than 10 nm) is used. However, in AFM, the needle rests on the sur-
face to be explored. A laser is directed at the top of the needle and is used to calcu-
late the distance of the needle from the surface. As the surface is scanned, the nee-
dle moves up and down through the surface’s peaks and crevices. The laser beam
records the changing distance to the surface, and a picture of the surface is pro-
duced (see Figure 13.30). This method is very useful for exploring surface defects.
Modifications of AFM can provide specific information about the surface, such as
the type of atom and the thickness of the film.
EXERCISE 13.7 That’s Pretty Thin
How many atoms of tin (atomic radius = 141 pm) are needed to make a one-atom-
thick thin film that covers an area equal to that of a sheet of paper measuring 8.5 by
11 in? Assume the atoms are aligned in rows and columns as they cover the piece of
paper.
First Thoughts
To answer the question, we need to determine the area of the paper. Then we’ll con-
sider the area occupied by one atom (assuming it is a square that is 282 pm on each
side—the diameter of the tin atom).
570 Chapter 13 Modern Materials
FIGURE 13.29
Soichi Noguchi, a Japanese astronaut,
took a walk in space during the August,
2005, mission of the space shuttle
Discovery. The face shield on his suit is
lowered, showing the gold thin film.
Tip
Cantilever
Computer
Movable
platform
Laser
Photodiode array for
lateral and vertical
deflection measurement
Sample
FIGURE 13.30
A block diagram illustrating atomic force microscopy (AFM). The atomic force microscope
measures the deflection of an atomic-sized needle from the surface of a substrate using
a laser. The data obtained can be used to draw a picture of the hills and valleys on a
substrate.
Solution
8.5 in = 21.59 cm; 11 in = 27.94 cm
Area of paper = 21.59 cm × 27.94 cm = 603.22 cm
2
Area of atom = 282 pm × 282 pm = 79524 pm
2
×
1 ×10
−12
m
1pm
×
100 cm
1m
2
= 7.952 × 10
–16
cm
2
Number of atoms
=
603.22 cm
2
7.952 ×10
−16
cm
2
= 7.6 × 10
17
atoms
Further Insight
That is quite a few atoms, though it is much less than a mole. In fact, this number of
atoms amounts to 1.3 ×10
–6
mol, or 1.5 × 10
–4
g, of tin. The change in mass of the
paper would be less than a milligram!
PRACTICE 13.7
How many moles of nickel (atomic radius = 125 pm) would be needed to coat a
photograph measuring 4.0 in × 6.0 in? Assume that the nickel atoms are arranged
neatly in rows and columns as they cover the photo. How many layers of nickel
atoms would be needed to increase the mass of the photograph by 1.00 g?...by
20.0 g? Is it possible to place a thin film of gold on a photograph measuring 4.0 in ×
6.0 in with only 0.050 mg of Au (atomic radius = 144 pm)?
See Problems 61–66 and 68.
13.6 On the Horizon—
What Does the Future Hold?
Health science professions utilize cutting-edge technology wherever possible to
provide the best care for their patients. Visit the doctor’s office, have your annual
dental exam, or stop by the emergency room, and you’ll notice the use of modern
materials that improve the quality of your care. These materials are so ingrained
in our society that it really would be hard to live our current lifestyle without
them. In fact, fifty years ago we would never have been able to believe that plastics
would stop bullets, that a ceramic bone would be used to repair a fracture, or that
a plastic heart could keep someone alive. With recent advances in the technology
of modern materials, it appears that anything is possible. Just read your newspa-
per to see what’s coming.
“Green Chemistry”
One of the main thrusts in modern materials science is the development of envi-
ronmentally benign technologies, or
Green Chemistry. Replacing toxic catalysts
in manufacturing processes and using aqueous solutions instead of flammable
organic solvents are just two of many things that can be done to make the chem-
ical manufacturing industry more environmentally friendly. Replacing haz-
ardous pesticides with the application of plant proteins to trigger the production
of substances that defend against insects is another approach to helping clean up
the environment. Twelve principles to help guide chemists in crafting a more
environmentally friendly science were articulated by Paul Anastas of the White
House Office of Security and Technology Policy and John Warner of the Univer-
sity of Massachusetts–Boston. Six of those principles are waste prevention, atom
economy (using as much of each reagent as possible in a reaction), reducing the
13.6 On the Horizon—What Does the Future Hold? 571
Application
C
HEMICAL
ENCOUNTERS:
“Green Chemistry”
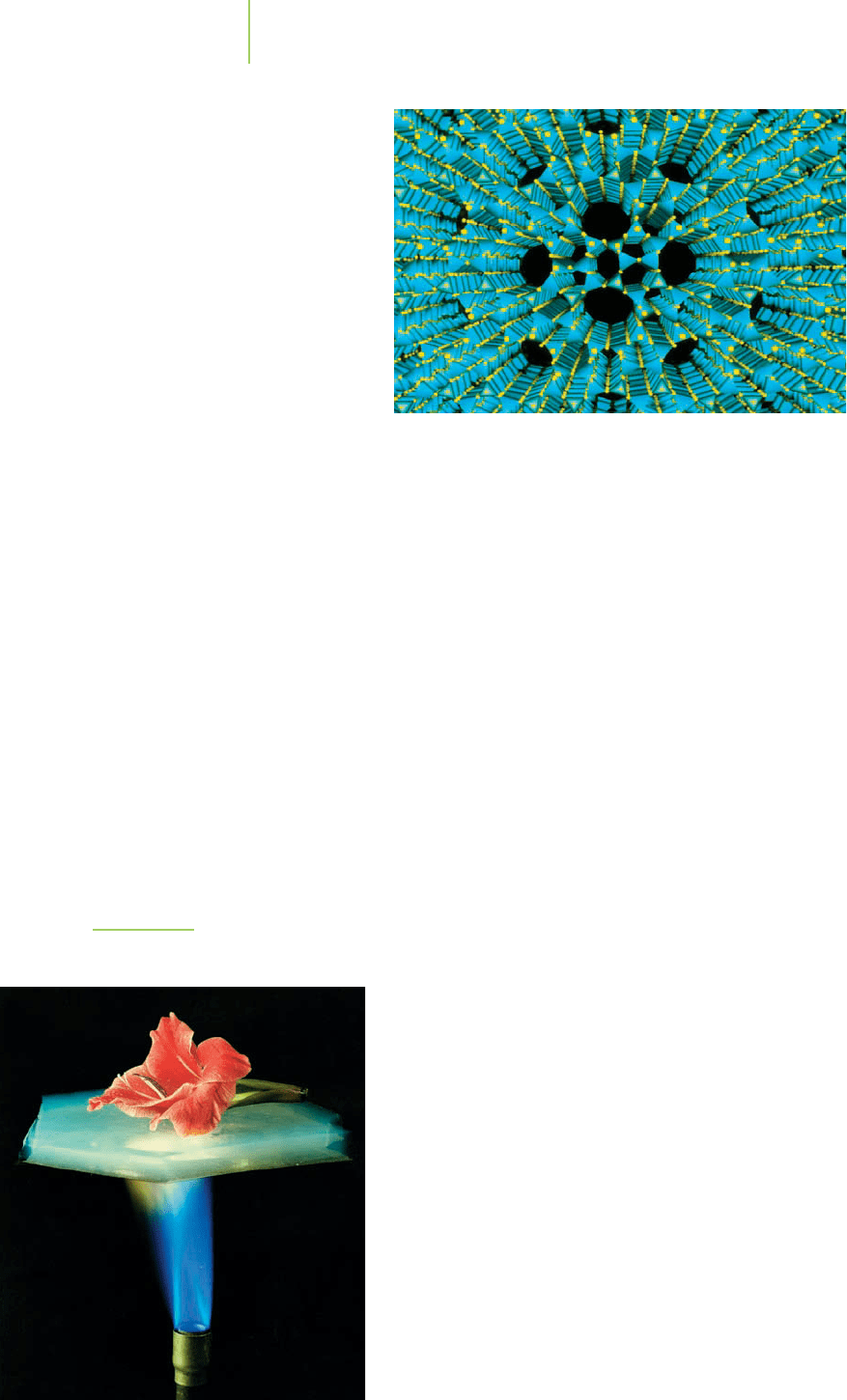
FIGURE 13.31
Hot flame and cool flower. The aerogel
is an excellent insulator for this flower.
572 Chapter 13 Modern Materials
Zeolites show promise as useful
materials. They contain pockets that can
be engineered to hold small molecules.
use of hazardous chemicals, energy efficiency, use of catalytic reactions, and pol-
lution prevention.
The use of specially engineered zeolites (see Chapter 8) as catalyst compo-
nents is promising. One area where a zeolite catalyst could have great impact is in
the manufacture of phenol (primarily used as to make the resin in plywood
sheets). Research on the use of a zeolite to directly oxidize benzene (C
6
H
6
) to
phenol (C
6
H
5
OH) is currently under way. Typically, the manufacture of phenol
from benzene is a two-step process. The preparation of phenol via this method
generates a lot of by-products. Zeolites may be the perfect solution to this prob-
lem, reducing the large amount of waste generated.
Biopolymers
Research into the modification of natural polymers (biopolymers) has the poten-
tial to have tremendous impact in the health professions. Biopolymers include
nucleic acids (DNA and RNA), proteins (such as enzymes), and some carbohy-
drates (such as cellulose, chitin, and starch). These compounds can be chemi-
cally modified in the laboratory to make biological polymers with interesting
properties.
Biopolymers are environmentally friendly because they degrade (break down
to chemically more benign compounds) in the environment and are often reab-
sorbed by living creatures. For example, dead trees (composed of cellulose and
other natural polymers) decay and become food for other organisms. Cellulose is
biodegradable. The recent use of cellulose instead of Styrofoam “peanuts” as
packaging materials has been an excellent application of Green Chemistry. The
two materials do the same job, acting as cushions for our breakables when
shipped. However, the cellulose peanut can be biodegraded (which greatly re-
duces its volume when added to water), and large quantities of nonrenewable
resources are not needed to manufacture it. Replacing synthetic polymers with
biopolymers helps promote Green Chemistry.
Biopolymers can assist in the delivery of drugs to patients by helping to make
the drugs more readily absorbable by the body. Another recently issued patent
illustrates the use of a biopolymer to enhance the flavor of chewing gum. Because
compounds made by biological systems are often chiral (see Section 12.14), the
use of biopolymers as catalysts in a chemical reaction can provide an environ-
mentally benign method to make medicines.
Aerogels
A special type of glass known as an aerogel has attracted attention lately. The
aerogel shown in Figure 13.31 is, as its name implies, a silica glass whose structure
is mostly air. We can think of the aerogel as a ceramic foam. It is produced by
crystallizing the porous silica structure in a solvent and then removing the solvent
to give a nearly transparent silica structure that has the appearance of soapsuds.
What makes aerogels so interesting is that they have tremendous surface
area. A 1-g sample of an aerogel can have as much as 1000 m
2
of surface area—
equivalent to about 1.4 acre of farmland or one-seventh of the street area taken
up by the Empire State Building in New York City. Combine the high surface area
and extremely low density (0.003 to 0.35 g/mL) with the high resistance to tem-
perature common among the ceramics (some aerogels are completely stable up to
3000°C), and you have a perfect insulator for a spacecraft. In fact, these materials
have significantly greater insulating power than fiberglass. Research is currently
being conducted on the manufacture of flexible aerogels that could be used to
make insulation for homes. Just imagine a firefighter’s coat as thin and light as a
sheet but insulating enough to protect its wearer against the hottest fires.
Key Words 573
The Bottom Line
■
In a crystalline solid, the atoms, ions, or molecules
are highly ordered in repeating units that make up
the lattice of the crystal. Amorphous solids, al-
though their atoms, ions, or molecules inhabit rigid
and fixed locations, lack the long-range order in a
crystal. (Section 13.1)
■
The unit cell is the basic repeating unit that, by
simple translation in three dimensions, can be
used to represent the entire crystalline lattice. (Sec-
tion 13.1)
■
The simple cubic unit cell, the body-centered cubic
unit cell, and the face-centered cubic unit cell make
up most of the crystalline lattices of the metallic el-
ements. (Section 13.1)
■
Metals often adopt a closest packed structure. These
structures include the hexagonal closest packed
structure and the cubic closest packed structure.
(Section 13.1)
■
We can calculate the volume and density of a
unit cell by means of simple geometry. In doing
so, we assume the atoms are hard spheres.
(Section 13.1)
■
Band theory describes why metals are electrical and
thermal conductors, shiny, malleable, and ductile.
(Section 13.2)
■
The valence band and the conduction band result
from the nearly infinite number of atomic orbitals
that overlap to form the molecular orbitals in a
metal. The band gap can be used to assess whether
a compound is a conductor, a semiconductor, or an
insulator. (Section 13.2)
■
Alloys are mixtures of two or more metals. They in-
clude the interstitial and substitutional alloys. Amal-
gams are special alloys of mercury. (Section 13.2)
■
Ceramics, which include glasses and superconduc-
tors, are compounds that contain both ionic and
covalent bonds. (Section 13.3)
■
Plastics, named after their ability to be molded into
shape, are polymeric materials. (Section 13.4)
■
Composite materials are made of an intimate com-
bination of two or more materials. (Section 13.5)
■
“Green Chemistry” is the practice of chemistry via
environmentally benign methods. (Section 13.6)
aerogel A silica glass whose structure is mostly air;
also known as a ceramic foam. (p. 572)
alloy A solution of two or more metals. (p. 556)
amalgam A solution made from a metal dissolved in
mercury. (p. 557)
amorphous solid A solid whose atoms, ions, or mole-
cules occupy fairly rigid and fixed locations but
that lacks a high degree of order over the long
term. (p. 541)
Key Words
band gap A small energy gap that exists between the
valence band and the conduction band (p. 553)
band theory A metal is a lattice of metal cations spaced
throughout a sea of delocalized electrons. (p. 551)
biopolymer A polymer of biological materials, such as
DNA or cellulose. (p. 572)
body-centered cubic (bcc) unit cell A unit cell built via
the addition of an atom, ion, or molecule at the
center of the simple cubic unit cell. (p. 543)

ceramic A nonmetallic material made from inorganic
compounds. (p. 557)
chemical-vapor deposition A thin film prepared when a
compound placed on a surface undergoes a reaction.
(p. 569)
closest packed structure A structure wherein the atoms,
molecules, or ions are arranged in the most efficient
manner possible. (p. 544)
composite material A material made from two or more
different substances. (p. 565)
conduction band The collection of empty molecular or-
bitals on a metal. (p. 552)
conductor A substance that contains a very small band
gap. (p. 554)
crosslinking Reactions that covalently bond two or
more individual strands of a polymer together.
(p. 563)
crystal lattice A highly ordered framework of atoms,
molecules, or ions. (p. 542)
crystalline solid A solid made from atoms, ions, or
molecules in a highly ordered long-range repeating
pattern. (p. 541)
cubic closest packed (ccp) structure A closest packed
structure made by staggering a second and third row
of particles so that none of the rows line up. (p. 544)
diffraction pattern A pattern of constructive and de-
structive interference after EMR passes through a
solid material. (p. 546)
dope To add an impurity to a pure semiconductor to
alter its conductive properties. (p. 554)
ductile Able to be pulled or drawn into a wire.
(p. 552)
face-centered cubic unit cell A unit cell built via the ad-
dition of an atom, ion, or molecule at the center of
each side of the simple cubic unit cell. (p. 543)
fiber A polymer whose chains are aligned in one di-
rection. (p. 563)
glass An amorphous solid ceramic material. (p. 559)
Green Chemistry The practice of chemistry using envi-
ronmentally benign methods. (p. 571)
hexagonal closest packed (hcp) structure A closest packed
structure made by staggering a second and third row
of particles in such a way that the first and third
rows line up. (p. 544)
insulator A substance that contains a very large band
gap. (p. 554)
interstitial alloy An alloy made by the addition of
solute metals placed in the existing spaces within a
solvent metal. (p. 556)
ionic solids Solids made up of ionic compounds held
together by strong electrostatic forces of attraction
between adjacent cations and anions. (p. 542)
liquid crystal A transitional phase that exists between
the solid and liquid phases for some molecules. Typ-
ically, these molecules have long, rigid structures
with strong dipole moments. (p. 550)
malleable Capable of being shaped. (p. 552)
molecular solids Solids made up of molecules that are
held together by intermolecular forces. (p. 542)
nonbonding molecular orbital A molecular orbital that is
not involved in bonding or antibonding. (p. 551)
n-type semiconductor A semiconductor that contains
electrons (negative charges) in the conduction band.
(p. 554)
phonons The induced vibrations produced in a metal
lattice. (p. 561)
photovoltaic device A device in which light energy can
be used to generate an electric current. (p. 555)
physical deposition The process of sublimation and de-
position used to place a thin film on a substrate.
(p. 568)
plastics A thermally moldable polymer. (p. 563)
p-type semiconductor A semiconductor that is electri-
cally conductive if we apply a small electric field.
The semiconductor contains positive holes in the
valence band. (p. 554)
semiconductor A metal that contains a small band gap.
(p. 553)
simple cubic unit cell An arrangement of particles
within a crystalline solid comprised of six particles
occupying the corners of a cubic box. (p. 542)
sputtering The process of producing a thin film on a
substrate by using high voltage to move a material
from a negative terminal (the cathode) to a positive
terminal (the anode). (p. 569)
substitutional alloy An alloy made by directly replacing
solvent atoms with solute atoms. (p. 556)
superconductor A ceramic whose resistance to the flow
of electrons drops to zero as the temperature is re-
duced. (p. 561)
transition temperature The temperature of a super-
conductor at which the resistance to the flow of
electrons becomes negligible. (p. 562)
tensile strength Resistance to breaking when a sub-
stance is stretched. (p. 563)
unit cell The repeating pattern that makes up a crys-
talline solid. (p. 542)
valence band The collection of filled molecular orbitals
on a metal. (p. 552)
574 Chapter 13 Modern Materials
Focus Your Learning 575
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
13.1 The Structure of Crystals
Skill Review
1. What key feature distinguishes a crystalline solid from an
amorphous solid?
2. Cite an example of a crystalline solid and of an amorphous
solid.
3. Use the descriptive term parallelepiped to define both a crys-
tal lattice and a unit cell.
4. Cite an example of an object made from parallelepipeds that
are visible to the naked eye.
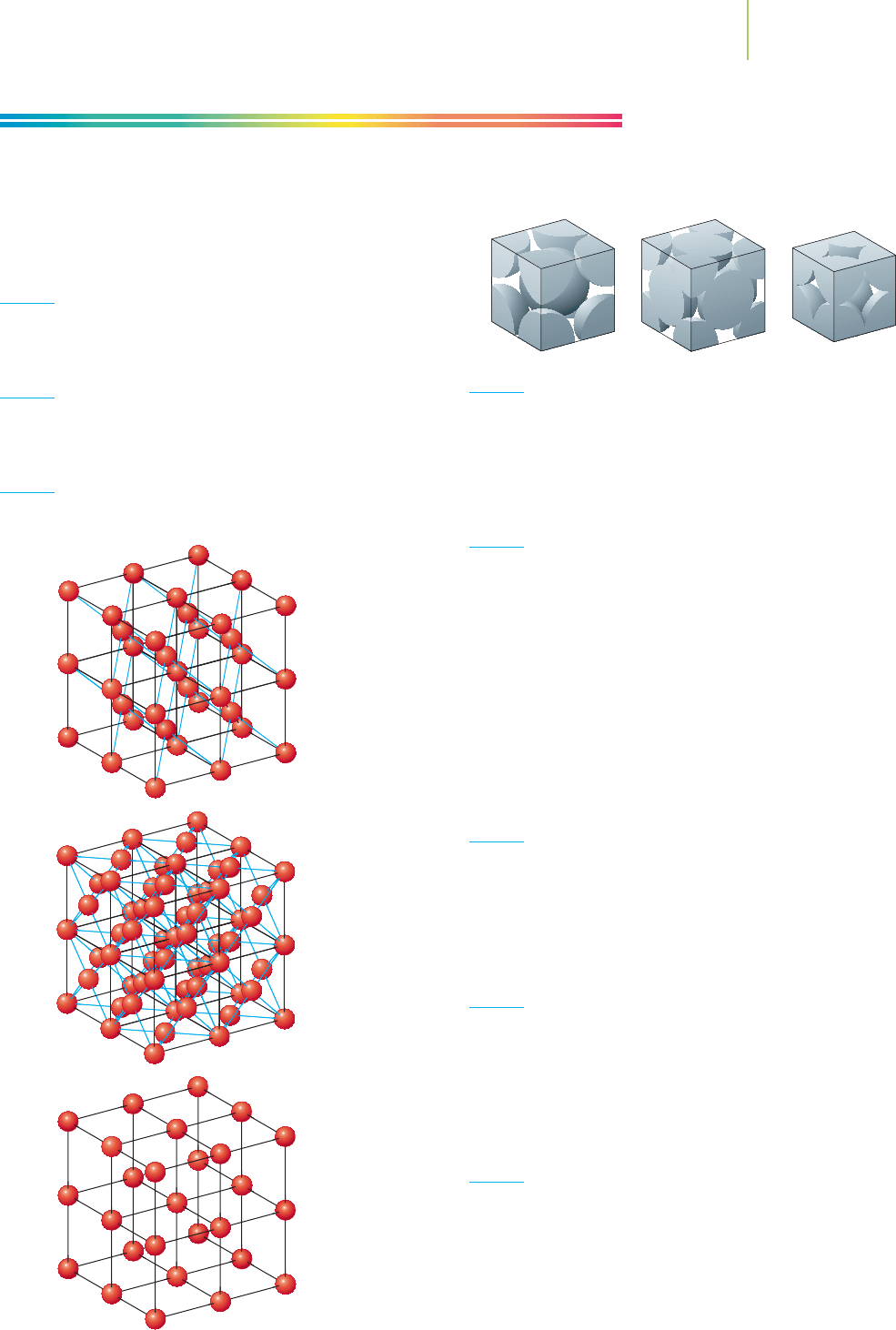
5. Which of these diagrams represents the body-centered cubic
structure of iron?
a.
6. Which of these diagrams represents the face-centered cubic
structure of strontium?
7. How many complete atoms would you expect to find in a
unit cell made from the simple cubic unit cell, but with addi-
tional atoms placed along every edge of the unit cell?
8. How many complete atoms would you expect to find in a
unit cell made from the simple cubic unit cell, but with addi-
tional atoms placed only at the face of two of the sides of the
unit cell?
9. Without any further information, indicate whether each of
the following three solids is most likely to be molecular, ionic,
or metallic: Solid A is the only one of the three that conducts
electricity in the solid state. Solid B melts when placed in
boiling water. Solid C dissolves in water, and the resulting
solution conducts electricity.
10. As part of a display of fruit, a grocer places two types of ap-
ples (red and yellow) in a pattern. The apples are to be placed
in a slanted display with a wooden frame. The first layer is
made of red apples, and the second layer is placed above the
first in the “dimples” or gaps. Show how the third layer of red
apples would be placed if the grocers, remembering their
chemistry, wanted to make the display resemble hexagonal
closest packing.
11. Calculate the volume of a fcc unit cell with each of the fol-
lowing atomic radii:
a. 125 pm b. 210 pm c. 302 pm
12. Calculate the volume of a bcc unit cell if it is composed of
atoms with each of the following radii:
a. 200 pm b. 183 pm c. 164 pm
Chemical Applications and Practices
13. Suppose the label on a can of soup reports 220 mg of sodium
per serving of soup. How many grams of NaCl will be present
in one serving of the soup?
14. When passing through the small openings within crystal
structures, X-rays diffract into a pattern. Say a researcher is
deriving the structure of an interesting protein by using
X-rays with a frequency of 1.94 × 10
19
s
−1
. What is the
wavelength, in nanometers, of the X-rays?
15. The metal nickel has several remarkable uses. When alloyed
with other metals, it can form a type of “memory metal”
called nitinol, which is a nearly 1:1 mixture of nickel and
titanium. Nickel crystallizes in a face-centered cubic arrange-
ment. The density of nickel is 8.90 g/cm
3
. From this informa-
tion, calculate the radius of an atom of this coinage metal?
(Currently the percentage of nickel in a U.S. “nickel” coin is
approximately 25%.)
(
c
)(
a
)(
b
)
b.
c.

16. The element iridium is currently an important part of an
extinction theory concerning dinosaurs. Unusual deposits
of iridium indicate that a large, iridium-containing meteor
may have hit our planet, resulting in a dust cloud from the
impact blocking the light from the sun. Iridium crystallizes
in a face-centered cubic structure. What is the density of this
corrosion-resistant metal whose radius is 136 pm?
17. Titanium metal and its oxide have a wide variety of uses. Its
relatively light weight and strength make it ideal in some
alloys. Titanium dioxide is found in paints and some lip-
sticks. Pure metallic titanium crystallizes in a body-centered
cubic structure. Titanium has a density of 4.5 g/cm
3
. What is
the radius of an atom of titanium? What percent of the unit
cell is empty space?
18. Metallic chromium has an atomic radius of 125 pm. Judging
on the basis of the density of chromium (7.2 g/cm
3
), which
of three cubic unit cells (simple, face-centered, or body-
centered) would you predict for chromium?
19. One way to verify the numerical value of Avogadro’s number
is to use X-ray data and unit cell calculations. Although the
reasoning is the same for any selection, use the following
specific information about copper to make the verification.
Copper crystallizes with a face-centered unit cell with an
edge length of 3.6 × 10
−10
m. The density of copper is ap-
proximately 8.9 g/cm
3
.
20. A hypothetical metal was determined to have a fcc unit cell
and a density of 0.185 mol/cm
3
. What is the calculated radius
of the metal?
21. Assuming that the following metals crystallize in the fcc unit
cell, which would you predict to have the greater density,
Co (radius = 125 pm) or Rh (radius =135 pm)?
22. Assuming that the following metals crystallize into a bcc unit
cell, which would you predict to have the greater density,
V (radius = 131 pm) or Cr (radius = 125 pm)?
13.2 Metals
Skill Review
23. Identify the metals in the following list of elements.
B,Na,Al,Ca,Cr,Se,Sb,Bi
24. List two physical characteristics that would enable you to dif-
ferentiate between calcium and carbon (graphite).
25. How many valence electrons are found in an atom of potas-
sium? How many molecular orbitals are found when three
atoms of potassium combine?
26. How many valence electrons are found in an atom of cal-
cium? How many molecular orbitals are found when three
calcium atoms combine?
27. The following three energies represent the band gap values
for three samples. Which of the values in this list is most
likely to be that of a metal?...that of a semiconductor? . . .
that of an insulator? 85 kJ/mol, 450 kJ/mol, 2.5 kJ/mol.
28. Which of these energies would you expect copper metal to
possess as its band gap: 0.06 kJ/mol, 73 kJ/mol, or 510 kJ/mol?
29. The particular types of alloys known as amalgams use mer-
cury as a component. Explain, using the delocalized electron
576 Chapter 13 Modern Materials
bonding model, how the metal components in any alloy
allow the atoms of different metals to bond.
30. How does the amalgam picture you created in Problem 29
allow for varying ratios of components, unlike the set ratio of
typical compounds?
Chemical Applications and Practices
31. A grocery stocker wishes to illustrate the two types of alloys
to his friends by using red and green apples to repressent
atoms. Explain how this would be accomplished.
32. A grocery stocker creates a stack of soup cans to display two
different kinds of soups. Judging on the basis of the pattern
in which they are stacked, which type of alloy do they appear
to represent?
33. Sodium metal is very reactive, yet under controlled condi-
tions its ability to absorb heat on a per-gram basis can be very
useful. Some nuclear reactor designs make use of sodium in-
stead of water as a heat transfer agent.
a. How many valence electrons does sodium have?
b. Using band theory, explain why thermal energy causes
the electrons in sodium, like other metals, to populate
antibonding orbitals.
34. Show the construction of a molecular orbital diagram from
the combination of two sodium atoms, of three sodium
atoms, and of four sodium atoms.
35. Semiconductors can also be used in lasers. If a laser were
constructed and it produced a wavelength of 820 nm, what
would be the band gap energy (in joules per photon)?
36. What would be the band gap energy (in kilojoules per mole)
for a laser whose wavelength was 720 nm?
37. Doping silicon allows for the production of n-type and
p-type semiconductors. Would doping silicon with indium
make an n- or a p-type semiconductor? Explain the basis for
your answer.
38. Silicon and selenium both have semiconductor properties.Ex-
plain why doping both with arsenic would have opposite re-
sults in terms of producing n-type or p-type semiconductors.
39. The blue color of the Hope diamond is due to small amounts
of boron in the carbon lattice. Predict what electronic prop-
erties this diamond must have.
40. What properties would you expect to find in a diamond
doped with small quantities of arsenic in the carbon lattice?

13.3 Ceramics
Skill Review
41. Explain why the bonding and resulting “band gap”in ceramic
materials make them good insulators.
42. Describe the difference between the bonding in the glass in a
window and the plaster in a wall.
43. Most compounds melt over a very small temperature range
that can even be used to help identify the compound. How-
ever, glass materials do not have such a small temperature
range for melting. Explain why the melting temperature for
glasses is replaced with a transition temperature.
44. Color, brittleness, and other properties of glass can be influ-
enced by the presence of impurities. However, the glass used
in fiber optics must be based on very pure SiO
2
. Explain how
this material, in the form of glass fibers, can be used to send
messages using light.
45. The frequency used for a typical MRI scan is in the range of
250 MHz. What are the wavelength and energy (in joules per
photon) for this technique?
46. a. Metals are known for their ability to conduct electricity.
However, the structure and bonding in metals also cause
resistance to conductance. Explain how phonons con-
tribute to the resistance.
b. What causes resistance to change at the transition tem-
perature in superconductors?
Chemical Applications and Practices
47. Silicates are formed from the connections between tetrahe-
dral structures made of a silicon atom bonded to four oxygen
atoms. Diagram this tetrahedral anion with a −4 charge.
48. Joining of the tetrahedral silicates typically occurs through
sharing of one of the oxygen atoms from one tetrahedron to
another. Draw a diagram of how the SiO
4
−4
ion could be used
to produce two different substances.
49. Glass containers are known to be relatively inert. In fact, cor-
rosive acids are typically stored in glass containers. However,
hydrofluoric acid (HF) will react with glass and must be
stored in polyethylene containers. This property of HF has
been used as a method of glass etching. (However, the fumes
produced are extremely harmful.) In the reaction shown
here, how many grams of glass would react with 10.0 g of HF?
4HF(aq) + SiO
2
(s) → 2H
2
O(l) + SiF
4
(g)
50. Safety glass is found in automobiles manufactured in the
United States. It consists of a “polymer sandwich”of a layer of
plastic between two glass layers. Using the known properties
of plastics and glass, explain why this arrangement may help
reduce some impact injuries in automobile accidents.
51. During a typical day, you are likely to come in contact with
the materials discussed in this chapter. Classify each of the
following as a metal, an alloy, a glass, a plastic, or a ceramic
(more than one answer may be appropriate).
a. the body of the pen you use;
b. a contact lens;
c. the frames on a pair of glasses.
52. Classify each of the following as a metal, an alloy, a glass, a
plastic, or a ceramic:
a. a milk jug;
b. the body of the spark plug in an automobile engine;
Focus Your Learning
577
c. the frame of an automobile;
d. an etched plaque.
Section 13.4 Plastics
Skill Review
53. What distinguishing property of plastics forms the basis of
this quote? “All plastics are polymers, but not all polymers are
plastics.”
54. Define the term plastic. Provide an example of a plastic and
an example of a polymer that isn’t a plastic.
55. Determine whether each of the following everyday items
contains a plastic, a metal,an alloy, a glass, or a ceramic (more
than one answer may be appropriate).
a. the frame of a compact mirror;
b. a mirror;
c. the body of the computer you use;
d. a drinking cup used at the dentist’s office.
56. Determine whether each of the following everyday items
contains a plastic, a metal, an alloy, a glass, or a ceramic:
a. a CD case;
b. the outer body of a cell phone;
c. a leisure suit;
d. a burner on a stove.
Chemical Applications and Practices
57. Some manufacturers are using composites to obtain desir-
able properties for the exterior of new automobiles. Describe
what advantages a composite material might provide for
automobile exteriors. What disadvantages might exist?
58. Why would a manufacturer consider constructing an air-
plane wing using composite materials?
59. Most hydrocarbon polymers make poor electrical conduc-
tors, although there are some that do conduct electricity.
Explain (noting the types of bonds in the polymers) why this
property arises in polymers.
60. The construction of a polymer made from ethyne (C
2
H
2
)
could conceivably be quite advantageous. Considering the
fact that a resulting polymer could contain a nearly infinite
string of alternating single and double bonds, what advan-
tage could you foresee to such a polymer?
Section 13.5 Thin Films and Surface Analysis
Skill Review
61. Approximate the number of gold atoms that would make up
the thickness of a gold film on an astronaut’s visor. Assume
that the film is 215 nm thick and that the radius of a gold
atom is 144 pm.
62. How many moles of gold would be needed to prepare a thin
film that is 576 pm thick covering an area similar in size to
a postage stamp (1 in by 1 in)? The radius of a gold atom is
144 pm.
63. How many atoms of silver (atomic radius =145 pm) must be
deposited to completely cover an area that measures 2.54 cm
by 2.54 cm? Assume the layer is only one atom thick.
64. A notecard that measures 3 in by 5 in is coated on one side
with a thin film of copper (atomic radius = 128). How much
will this coating add to the mass of the notecard?
