Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


12.15 Organic Chemistry and Modern Drug Discovery
Chemical Applications and Practices
97. The total synthesis of a potential drug requires nine steps,
each with a 95% yield. What is the overall yield of the final
drug made from this synthesis?
98. Acetylsalicylic acid, also known as aspirin, is a derivative of
a natural compound found in willow trees. Explain how a
researcher might have originally surmised that an active
substance was to be found in the leaves and bark of the
willow tree.
Comprehensive Problems
99. Why do gasoline manufacturers adjust the gasoline mixture
for automobiles in accordance with the season of the year?
100. Using information from this chapter, describe how the
acidity of salicylic acid, shown below, can be reduced by
modification of the compound via a chemical reaction.
101. Gasoline is delivered to a farmer for use in the farm’s
combines. A cylindrical storage tank with a circumference
of 6.0 ft is used to store the gasoline until it is used. The
farmer uses a yardstick to measure the depth of the gasoline
in the storage tank.
a. How many gallons of gasoline are in the tank if the
yardstick measures the gasoline to a depth of 22 inches?
b. 2,3-dimethylhexane is one of the components in the
gasoline. Draw the structure of this compound.
102. The compound responsible for the odor of wintergreen was
discussed in the chapter.
a. What two compounds (an alcohol and a carboxylic
acid) could be condensed to prepare methyl salicylate?
b. If a company that manufactures a muscle pain relief
cream requires 352 kg of methyl salicylate, how many
liters of the alcohol would be needed? (Assume the
density of the alcohol is 0.789 g/mL.)
H
C
HC
HC
O
Salicylic acid
C
C
H
OH
C
COH
103. In Table 12.5, we mention that one of the fractions distilled
from crude oil is known as petroleum ether. This fraction is
used in the organic research lab to recrystallize reaction
products.
a. Draw and name at least two compounds that could be
found in this distillation fraction.
b. Why does the name of this fraction appear to be a
misnomer?
104. The addition of a halogen to an alkene is a good way to pro-
duce an alkyl halide in the laboratory. But often, a researcher
is interested in adding only one halogen atom to the com-
pound. In such a case, a hydrogen halide is used for the addi-
tion reaction.
a. If a research student fills a 2.5 L flask with 0.25 g gaseous
hydrogen chloride and 0.48 g of gaseous trans-2-butene,
what mass of the addition product can be made?
b. What is the name of that product?
c. What is the concentration, in molarity, of the product in
the flask?
105. The organic chemistry industry of the late part of the 1800s
was interested in creating new dyes for fabrics.
a. What features of an organic compound would make it
suitable for use as a dye?
b. If a particular organic dye was yellow in color, what
range of wavelengths would be reflected by the
compound?
c. What range of energies, in kJ/mol, is associated with
these wavelengths?
Thinking Beyond the Calculation
106. An unknown organic compound is identified. Reaction of
this unknown with two molecules of ethanol produces a
diester. The unknown organic compound can be prepared
by oxidizing 1,2-ethanediol.
a. What is the structure of the unknown?
b. What properties (chemical and physical) would you
predict this unknown to possess?
c. If 10 g of the unknown produce 10 g of the diester, what
is the percent yield of the reaction?
d. When the diester is placed in aqueous solution
containing a small amount of catalyst, the solution
becomes acidic. Provide a balanced reaction that
explains this result.
e. A polymer can be made when the unknown organic
compound is condensed with ethylene glycol
(HOCH
2
CH
2
OH). Draw the structure of this polymer.
538 Chapter 12 Carbon

539
Contents and Selected Applications
Chemical Encounters: Materials in the Hospital
13.1 The Structure of Crystals
Chemical Encounters: X-ray Crystallography
13.2 Metals
Chemical Encounters: Photovoltaic Devices
Chemical Encounters: Dental Amalgams
13.3 Ceramics
Chemical Encounters: Magnetic Resonance Imaging
13.4 Plastics
13.5 Thin Films and Surface Analysis
Chemical Encounters: Heart Defibrillators
13.6 On the Horizon—What Does the Future Hold?
Chemical Encounters: “Green Chemistry”
Modern
Materials
Modern materials affect our lives every
day. Their use in bulletproof vests, auto-
mobiles, and computers saves and pro-
tects us from harm. And, without their
use in the health care industry, we would
surely suffer. Let’s examine a trip to the
hospital to see how modern materials
are used.
Go to college.hmco.com/pic/kelterMEE for online learning resources.
540
A trip to the hospital offers interesting insight
into the products of chemistry. Look around and
you’ll see chemistry that is central to the mission of
the hospital and to the quality of our lives. The emergency
room (ER) nurse who gives an injection, the radiologist who uses
the magnetic resonance imager (MRI), and the doctor who replaces a
hip joint all depend on the products of materials science to do their jobs.
Materials science is concerned with the chemistry and development of substances
employed to make the items we use everyday. What types of materials do you see in the
hospital? The emergency room syringe is made of plastic, as are the bag and the tubing
that holds the intravenous (IV) fluid. The hospital MRI requires a powerful magnetic field
generated with a special type of ceramic material called a superconductor. Hip replace-
ments are often made from metal alloys and plastic polymers that are stronger than the
bone they replace. Each of these products (the plastic, the superconductor, and the metal
alloy) is a recently developed material.
In this chapter we will discuss the chemical features of modern materials. Knowing
about bonding in solids, the properties of metals, and substances called liquid crystals will
lead us to a better understanding of materials science. Along the way, we’ll discover some
new materials that are of great practical use, and we’ll find out that the old materials we
know are also part of the field of materials science. Because many of the modern materials
are solids, we begin our study of modern materials with the structure of crystals.
Application
C
HEMICAL
ENCOUNTERS:
Materials in the
Hospital
13.1 The Structure of Crystals
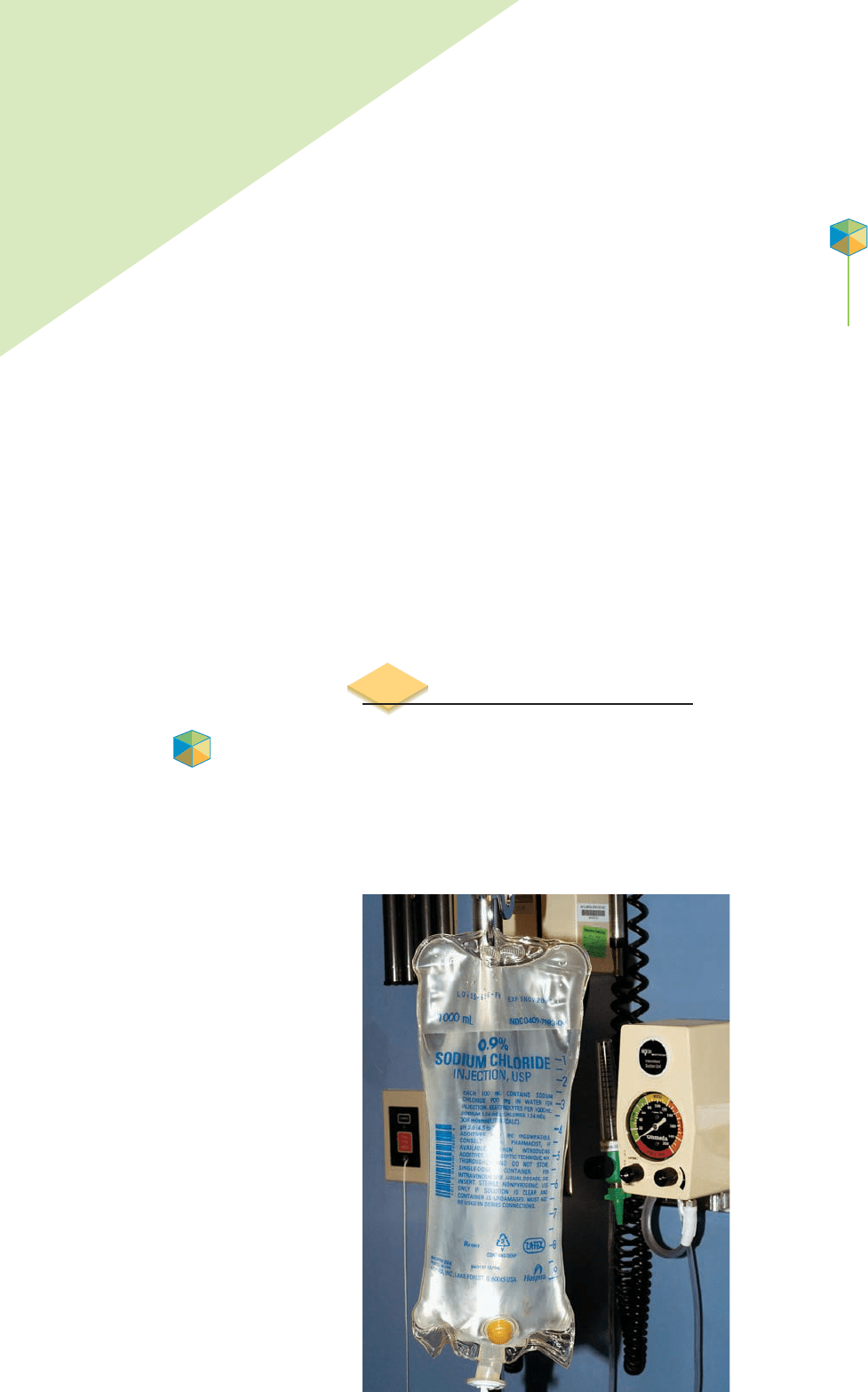
Sodium chloride (table salt) plays an important role in our diet. Medical workers
recognize the essential role that sodium plays in maintaining blood pressure,
allowing the transmission of nerve signals and the transporting of nutrients into
cells. When a nurse reaches for an intravenous saline solution, a bag of sterile
0.9% (w/v) sodium chloride is what she or he gets. Why choose a solution of
sodium chloride? Blood contains 0.9% (w/v) sodium chloride, and this concen-
tration keeps the blood in a patient at the same osmotic pressure (Chapter 11).
An IV saline solution.
Application
Crystalline solid
13.1 The Structure of Crystals 541
In Chapter 8, we discussed the formation of sodium chloride from its elements.
We noted the strength of the ionic bond by focusing on the lattice energy,
786 kJ/mol. To learn more about sodium chloride and other crystalline ionic
compounds, however, we need to explore how the ions come together to make an
ionic crystal. What can the shape of an ionic solid tell us? Knowing the locations
of the ions within an ionic solid enables us to determine the density, the nature
(crystalline or amorphous), and the properties of the solid (see Table 13.1).
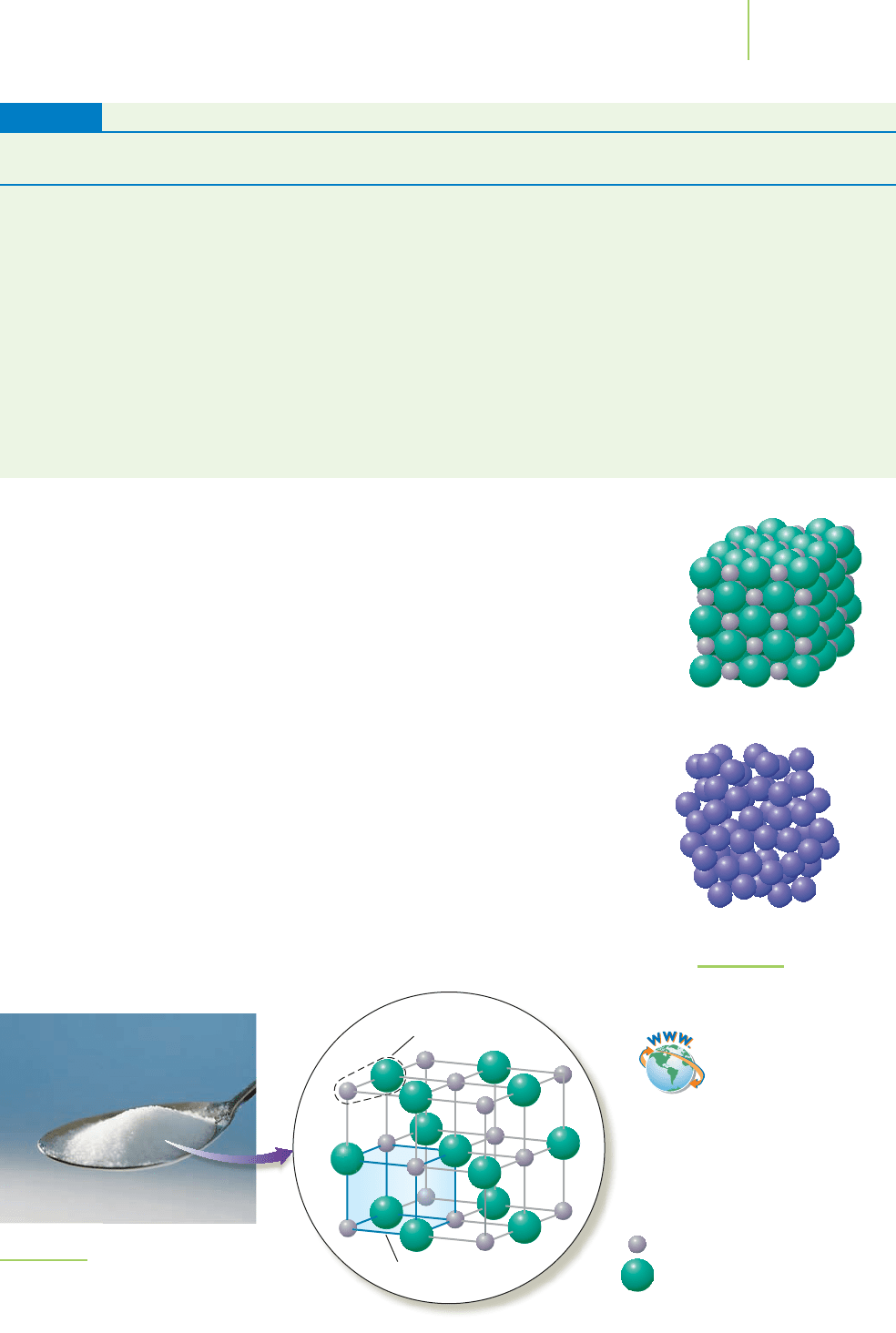
Types of Solids
Why are some compounds, such as table salt or sugar, solid rather than liquid or
gaseous under normal conditions? The atoms, ions, or molecules of a solid are
not free to move significantly relative to each other. The materials are solids
because the intermolecular forces of attraction are strong enough to hold the par-
ticles in a rigid structure. In a
crystalline solid (Figure 13.1), the atoms, ions, or
molecules are highly ordered in repeating units over long ranges. Although they
have fairly rigid and fixed locations of the atoms, ions, or molecules,
amorphous
solids
such as the glass in a window pane lack the high degree of long-range order
(they still have short-range order) found in a crystalline solid. Sodium chloride is
an example of a crystalline solid (Figure 13.2), because it has an ordered structure
with fixed locations for the sodium and chloride ions.
Different Types of Solids and Their Properties
Type of Particles Forces Between
Solid in Solid Particles Physical Properties Examples
Ionic Cations and Electrostatic Hard and brittle, high melting point, NaCl,
anions attraction poor thermal and electrical CaO,
conductivity MgBr
2
Molecular Atoms or London dispersion, Fairly soft, low to moderate CH
4
,
molecules dipole–dipole, and/or melting point, poor thermal and C
6
H
12
O
6
(glucose),
hydrogen bonding electrical conductivity H
2
O, Kr
Network Atoms Covalent bonds Very hard, very high melting point, C (diamond),
covalent typically poor thermal and SiO
2
(quartz),
electrical conductivity SiC, BN
Metallic Atoms Metallic bond Soft to hard, low to high melting Na, Fe, Au, Ag, Al
point, good thermal and
electrical conductivity
TABLE 13.1
Amorphous solid
FIGURE 13.1
Solids can be crystalline or
amorphous.
Unit cell
= Cl
–
= Na
+
Formula
unit (NaCl)
= Cl
–
= Na
+
FIGURE 13.2
Crystals of sodium chloride.
Video Lesson: Types of Solids

Glucose
C
6
H
12
O
6
What does a crystal look like? Try using a magnifying glass or microscope to
look at crystals of table salt or sugar. These two crystalline solids appear as solid
cubes, whereas other solids take the shape of needles, plates, and a host of other
faceted designs. The shape of the crystal you see is a result of the way in which the
atoms and ions are arranged at the atomic level, known as the crystalline lattice.
Some solids, such as table salt (NaCl), are
ionic solids, made up of ionic com-
pounds held together by strong electrostatic forces of attraction between adjacent
cations and anions. Because these forces are formidable, the ionic solids typically
have high melting points. Solutions made from ionic solids conduct electricity, as
we saw in Chapter 4. We referred to them as electrolytes.
On the other hand, some solids, such as glucose (C
6
H
12
O
6
), are molecular
solids
, made up of molecules held in a rigid structure. Despite the structural sim-
ilarity to an ionic solid, the intermolecular forces of attraction between adjacent
molecules are not as strong as the electrostatic forces found in ionic solids. There-
fore, melting points of the molecular solids are not very high. In addition,
because most molecules do not dissociate into ions when they are dissolved, so-
lutions made from molecular solids typically do not conduct electricity. As we’ll
see in this chapter, the properties of molecular and ionic solids are different in
other important ways.
Crystal Lattices
A structure known as a crystal lattice defines the shapes of crystalline solids. This
crystal lattice marks the position each of the atoms, ions, or molecules within the
crystal. The 3-D grid comprises three pairs of parallel planes that intersect to
makeparallelepipeds (three-dimensional boxes),and in Figure13.3 we see that re-
peating the parallelepiped in three dimensions gives us the lattice for a crystal.
The simplest parallelepiped that can be repeated to produce the entire crystal lat-
tice is the
unit cell (the smallest repeating unit of the lattice). In other words, the
unit cell is the smallest repeating unit of atoms, ions, or molecules that would
describe the entire crystal.Whenever possible, we try to arrange the corners of the
lattice so that they line up with the centers of the atoms, ions, or molecules in
the solid.
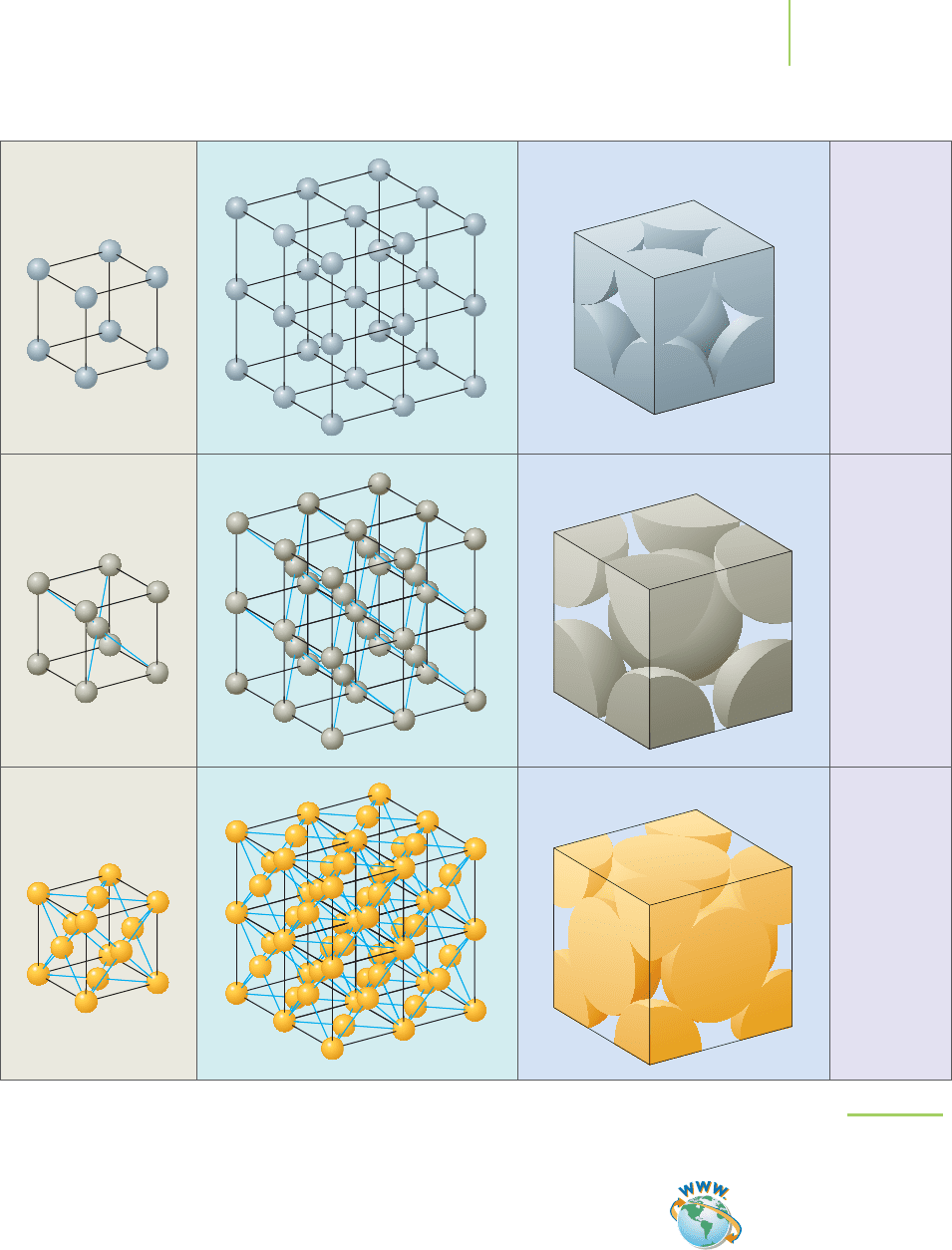
If the parallel sets of planes intersect at right angles to make a cube (as they do
in Figure 13.3), the lattice is known as a cubic lattice. The most basic of the cubic
lattices is made up of the
simple cubic unit cell (also known as the primitive unit
cell), in which the centers of the atoms, ions, or molecules are located only on the
corners of the unit cell. Polonium, a radioactive metal that shows promise as a
thermoelectric power source in space satellites, crystallizes in the simple cubic
structure. The presence of an additional atom, ion, or molecule at the center of
542 Chapter 13 Modern Materials
FIGURE 13.3
Parallelopiped in a cubic lattice.
Visualization: Network Solids
Visualization: Molecular Solids
13.1 The Structure of Crystals 543
Simple cubic
Body-centered cubic
Face-centered cubic
(c)
(b)
(a)
Unit cell Lattice structure Space-filling unit cell Example
Polonium
metal
Uranium
metal
Gold
metal
FIGURE 13.4
Cubic unit cells.
the simple cubic unit cell gives a body-centered cubic (bcc) unit cell. Iron metal
crystallizes as a body-centered cubic structure. If an atom, ion, or molecule is lo-
cated on each of the faces of the cubic lattice, the lattice is called a
face-centered
cubic (fcc) unit cell
. When cooled below –182°C, methane (the gas that heats many
homes) forms crystals that have a face-centered cubic structure. Figure 13.4 illus-
trates each of the unit cells in the cubic lattice group.
Metallic Crystals
Solid metals are made up of atoms of an element arranged in a crystalline lattice.
If we imagine that the atoms are hard spherical balls, we can get a picture of how
they might be arranged into a crystal. We can visualize this by placing marbles in
a box. Put in just enough marbles to cover the bottom and what happens. To
Video Lesson: Crystal Packing
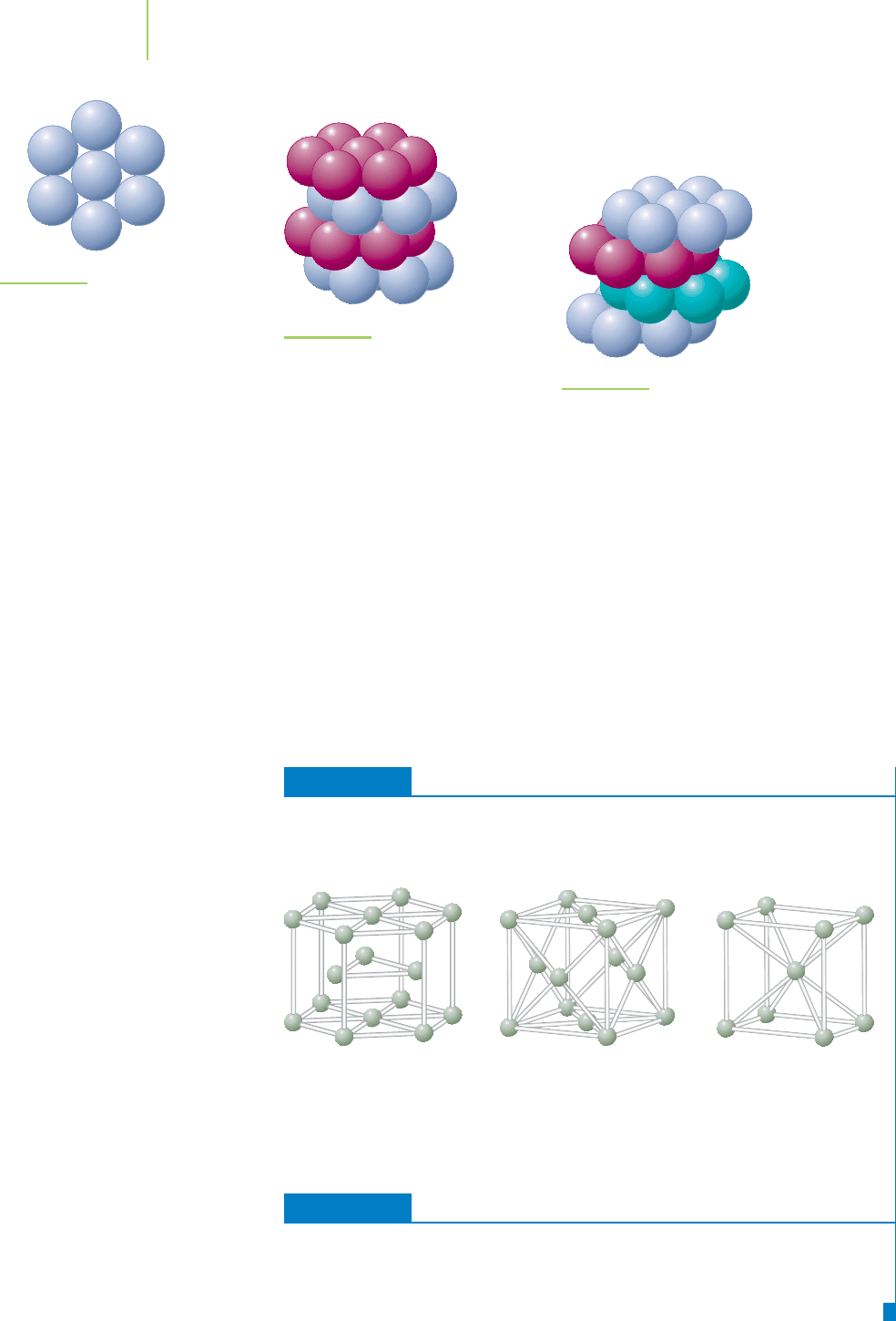
utilize most efficiently all of the space available, the marbles adopt a staggered
arrangement. The way they pack is called a
closest packed structure (Figure 13.5).
What if we want to completely fill a box with marbles? The second layer is also
staggered, but it occupies the dimples in the first layer. However, there are differ-
ent ways in which we can stack the third and subsequent layers of marbles. It is
the placement of this third layer that determines the type of packed structure that
results. If the third layer is placed in such a way that it lines up with the first layer,
what results is a
hexagonal closest packed (hcp) structure whose unit cell is a
hexagonal prism (Figure 13.6). Magnesium and zinc atoms form hexagonal clos-
est packed solids. If the third layer is staggered in the same fashion as the second
layer, so that it doesn’t line up with the first layer, we obtain the
cubic closest
packed (ccp) structure
(Figure 13.7). Metals, such as gold, silver, and copper, adopt
this structure and have face-centered cubic unit cells.
EXERCISE 13.1 Matching
Match each of the following metals with the figure that best represents its structure.
a. copper (fcc) b. iron (bcc) c. iron (hcp)
Solution
Copper (face-centered cubic) is B. Iron (body-centered cubic) is C, and iron (hexag-
onal closest packed) is A.
PRACTICE 13.1
Provide a diagram for each of the following elements in the given unit cell.
a. manganese (simple cubic) b. nickel (fcc) c. tungsten (bcc)
See Problems 5 and 6.
544 Chapter 13 Modern Materials
A B C
FIGURE 13.7
Cubic closest packed structure.
FIGURE 13.5
Closest packed arrangement
of spheres in two dimensions.
FIGURE 13.6
Hexagonal closest packed
structure.
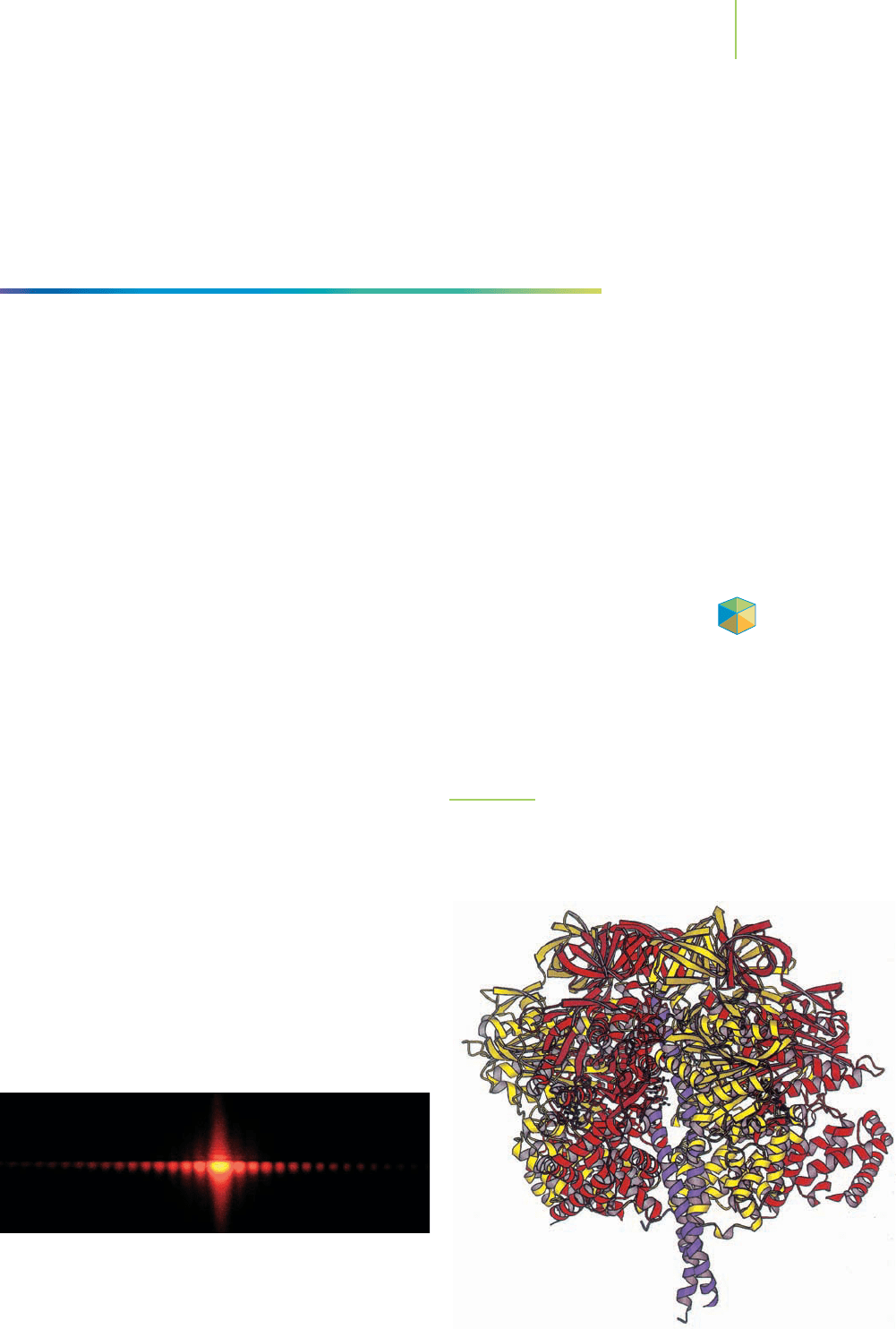
Some metals do not form closest packed structures. For instance, the alkali
metals stack together into body-centered cubic unit cells. Although we can know
how a metal packs into a particular crystalline lattice, the reason why metals pack
in the way they do is not well understood. Predicting the crystalline lattice struc-
ture of a metal is difficult.
Why is it useful to know the structure of the unit cell?
One answer has to do with the enzymes within us. Read on.
HERE’S WHAT WE KNOW SO FAR
■
Solids can be either amorphous or crystalline.
■
Crystalline solids result from highly structured packing of the material.
■
The arrangement of particles within the crystal is as a repeating unit called the
unit cell.
■
The simple cubic, body-centered cubic, and face-centered cubic are examples
of unit cells.
■
In addition, substances can also pack into closest packed structures, which in-
clude the hexagonal closest packed and the cubic closest packed structures.
Crystallography and the Unit Cell
In 1997, the Nobel Prize in chemistry was awarded to Paul D. Boyer, John E.
Walker, and Jens C. Skou “for their elucidation of the enzymatic mechanism un-
derlying the synthesis of adenosine triphosphate (ATP).” Essential to their dis-
covery was the identification of the crystalline structure of an enzyme found in
cows, bovine F1 ATP synthase (Figure 13.8). By determining the structure of this
enzyme, the researchers were able to understand how ATP (a source of energy in
living things) is made. This is just one example of the utility of knowing the crys-
tal structure of a solid. Medical researchers use crystal structures to get a clear
picture of the location where reactions take place, which is called the “active site”
of the enzyme. This information makes it possible to de-
sign drugs that will best influence the activity of the en-
zyme. For instance, the structure of the active site of
HMG-CoA reductase (an enzyme that makes choles-
terol) was used in the development of Rosuvastatin, a
drug that binds to the enzyme and helps slow the forma-
tion of cholesterol. The binding of Rosuvastatin leads to
lower serum cholesterol levels in patients.
The structures of these enzymes were determined by
X-ray crystallography. How does this technique help us
determine the structure of a crystal? We begin by exam-
ining the behavior of light. When light waves pass
through a grating (a series of closely spaced narrow slits),
13.1 The Structure of Crystals 545
FIGURE 13.8
Cartoon of the crystal structure of bovine F1 ATP synthase. Because
there are so many atoms in this enzyme, each strand of the protein is
represented as a ribbon of a different color. Specific atoms are not
included in the structure.
p_13_08
A single light beam from a laser, when passed through a narrow
opening, makes a pattern of dots due to constructive and destructive
interference of the individual photons.
Application
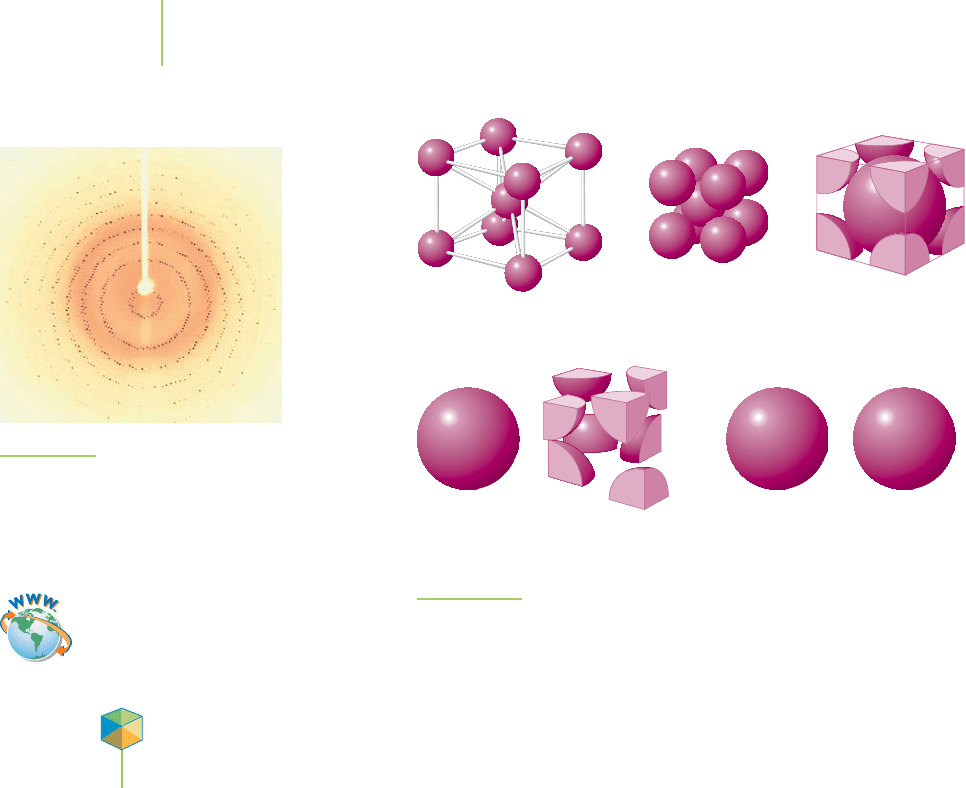
constructive and destructive interference of the waves produces a pattern of light
and dark regions. This
diffraction pattern can be used to determine the position
and widths of the slits. The most useful diffraction pattern occurs when the wave-
length of the light is similar to the widths of the slits. In X-ray crystallography, a
diffraction pattern is generated by using a crystal instead of a series of slits.
Look at the unit cells of the crystals mentioned in the previous section. In-
stead of slits, the unit cells contain a lot of empty space. Because the spaces in the
crystal are on the order of 100 picometers (1 pm = 10
–12
m), radiation of similar
wavelengths is used. X-rays—electromagnetic radiation with wavelengths
near 100 pm—are directed through the crystal and are diffracted as shown in
Figure 13.9. The resulting pattern of high- and low-intensity X-rays is used to de-
termine the position and size of the atoms in the crystal. Our ability to calculate
the positions of the atoms from the crystal diffraction pattern is based on the
work of William Henry Bragg (1862–1942) and William Lawrence Bragg
(1890–1972), a father-and-son team who shared the Nobel Prize in physics in
1915 for their work in X-ray crystallography.
Electromagnetic radiation can pass through a crystal because of the existence
of empty spaces in the unit cell.
How much empty space is there in a crystal? We
talk about how the spheres are packed as closely together as possible, but in our
unit cells we seem to have a lot of gaps. To determine the amount of empty space,
we need to know how much space the atoms in a unit cell occupy and how much
space the entire unit cell occupies. The difference between these two values is
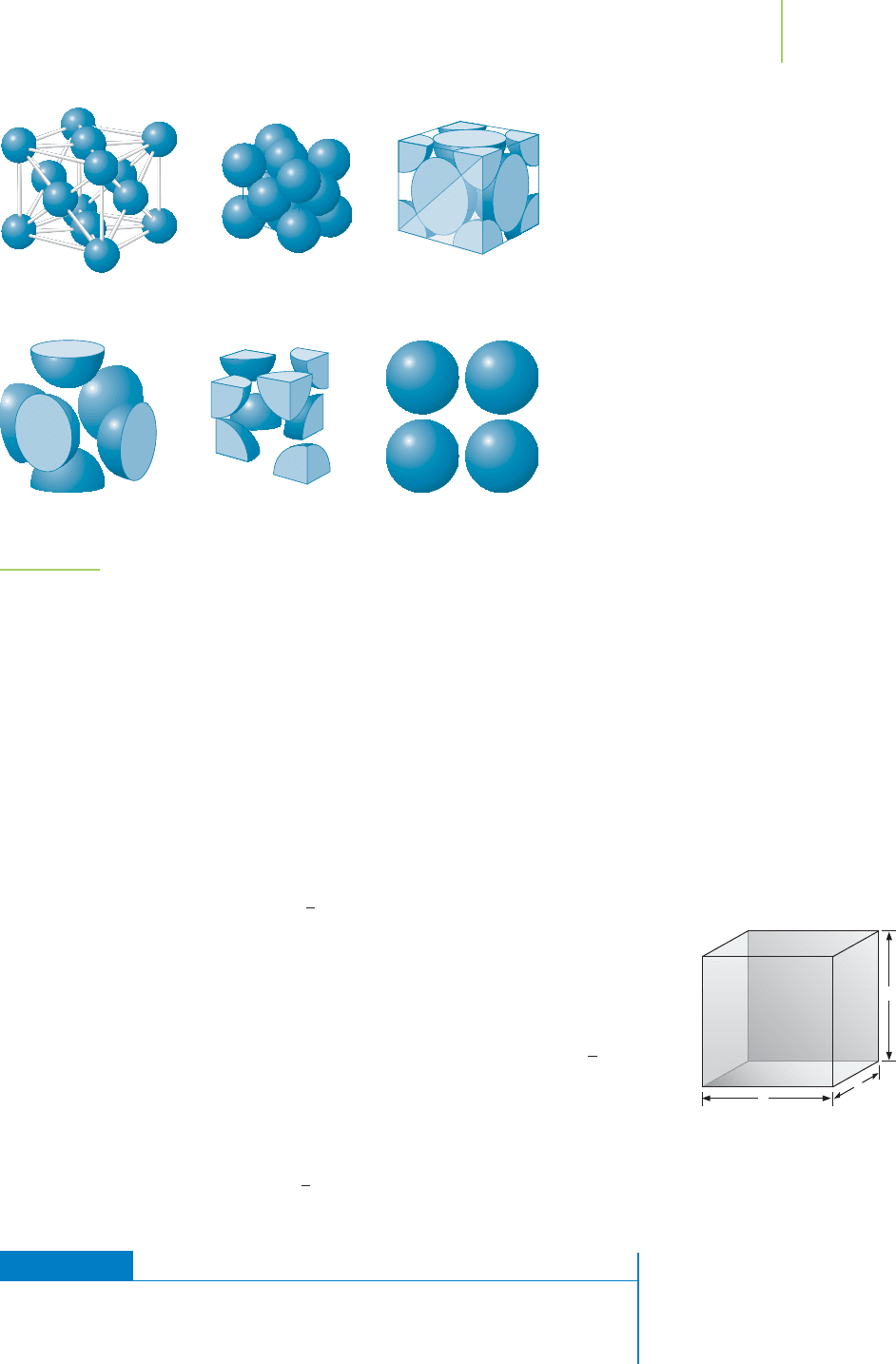
the empty space. How many complete atoms occupy the unit cell? To simplify the
process, consider the atoms as hard spheres. Figure 13.10 shows the bcc unit cell,
which contains one complete atom in the center of the unit cell. At the corners of
the cell are
1
⁄8 “pieces” of atoms. Because there are eight corners in the unit cell,
there must be the equivalent of one complete atom. The unit cell for a body-
centered cubic structure contains two complete atoms: the central one and the
eight
1
⁄8’s that make up the corners. We can go through a similar process for the
primitive cell (one complete atom) and the face-centered cubic cell (four com-
plete atoms).
546 Chapter 13 Modern Materials
FIGURE 13.9
A pattern of reflected X-rays is captured
after interaction with a crystal. The
location of the dots in this diffraction
pattern can be used to determine the
structure of a compound.
(b) (c)
(a)
(
d
)
1 atom + 8 parts 2 atoms
=
FIGURE 13.10
Body-centered cubic unit cell.
Application
C
HEMICAL ENCOUNTERS:
X-ray Crystallography
Video Lesson: Crystal Structure
Because we can determine how many atoms occupy a unit cell, knowing the
volume of the unit cell enables us to find the amount of empty space in the cell.
What is the volume of a unit cell? We can calculate this for each type of unit cell.
In the face-centered cubic unit cell (Figure 13.11), we see that the diagonal across
one face of the cube is 4 times the radius of the atom (4r) and that the height and
width of the cell are the same distance (d). To find the length d, we use the
Pythagorean Theorem (a
2
+ b
2
= c
2
) and solve for the distance:
d
2
+ d
2
= (4r)
2
2d
2
= 16r
2
d
2
= 8r
2
d = r
√
8 = 2.828r
Therefore, the distance along one of the faces of the fcc unit cell is 2.828 times the
radius of the atoms. Note that the distance along the edge of the unit cell is greater
than twice the radius of the atoms. This implies, correctly, that the atoms in the
corners of the unit cell do not touch the atoms in the other corners.
The volume of any parallelepiped is determined by multiplying the length times
the width times the height. For the fcc unit cell, the volume would then be
(r
√
8)
3
,or
22.63r
3
. To illustrate this equation, gold metal crystallizes in the cubic closest
packed structure (with fcc unit cells). The volume of the unit cell for gold metal
whose radius is 144 pm is
r = 144 pm
Volume
= (144
√
8)
3
= 6.76 ×10
7
pm
3
EXERCISE 13.2 There’s a Lot of Room in Here
Copper, silver, and gold are known as the coinage metals because of their use in
making coins. Each of these metals forms a face-centered cubic solid. What per-
centage of a fcc unit cell is occupied by empty space?
13.1 The Structure of Crystals 547
Volume = a × b × c
a
b
c
The volume of any parallelipiped is
equal to the height times the width
times the length.
(d)
6 parts + 8 parts 4 atoms
=
(b) (c)(a)
FIGURE 13.11
Face-centered cubic unit cell.
