Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

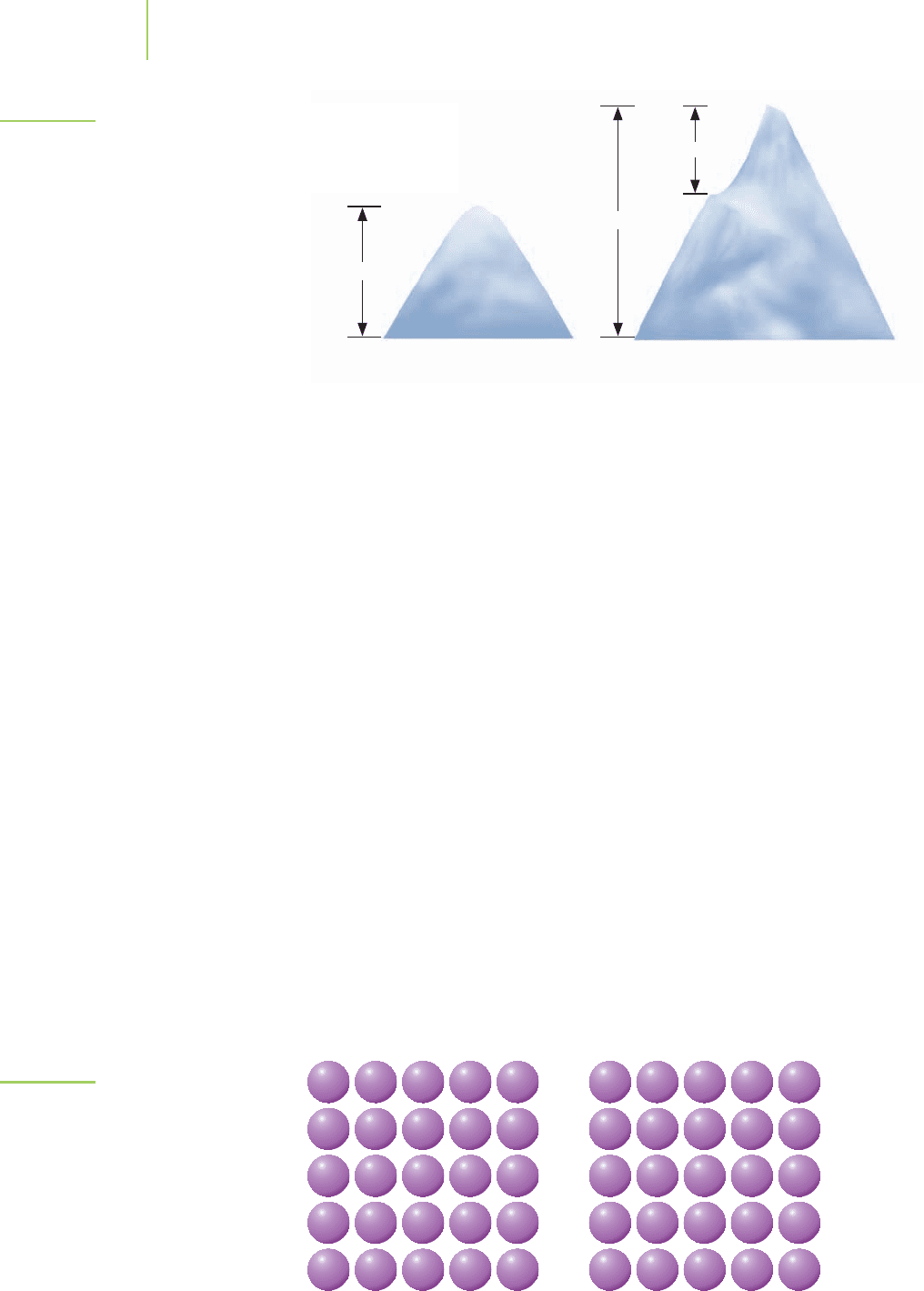
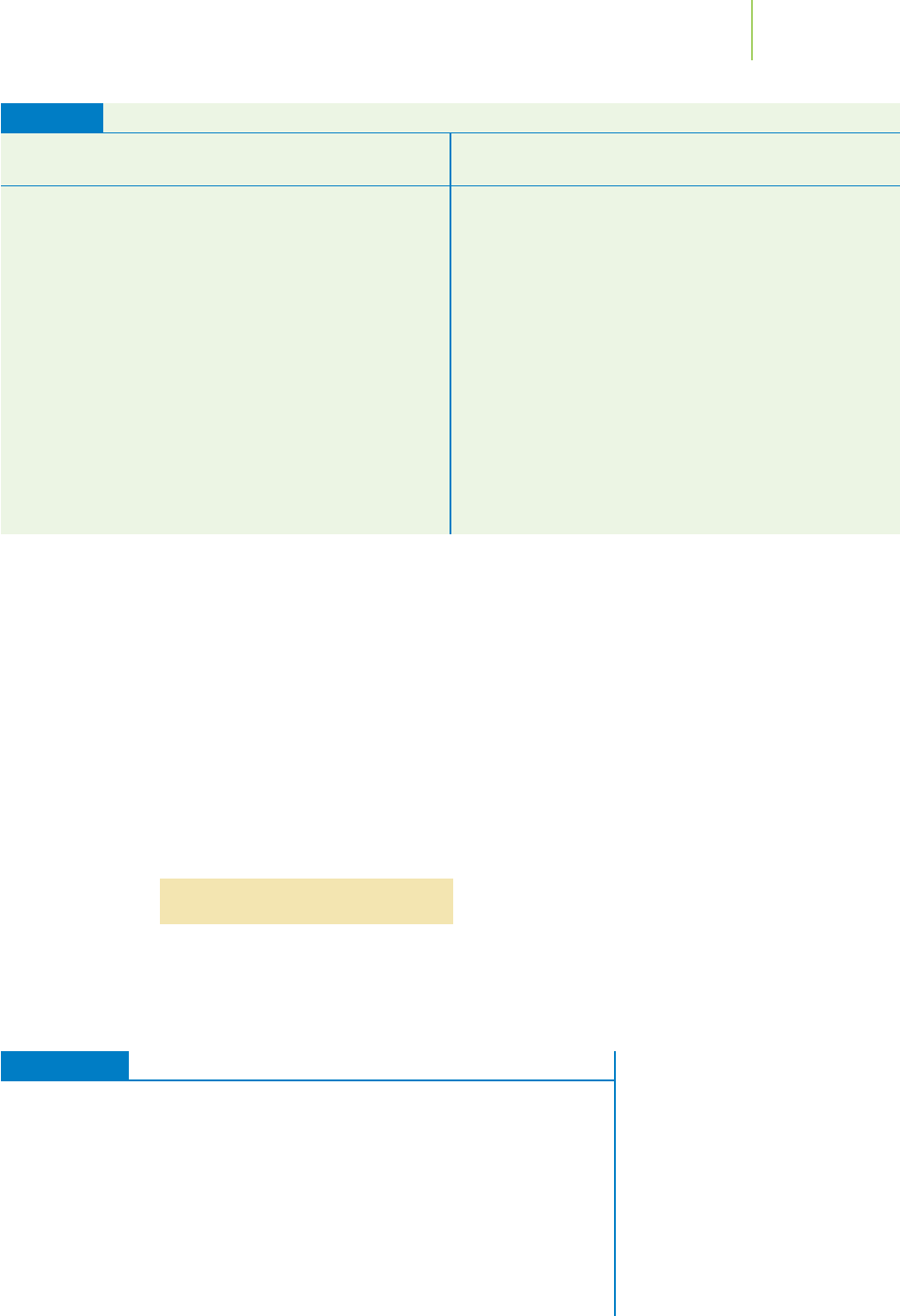
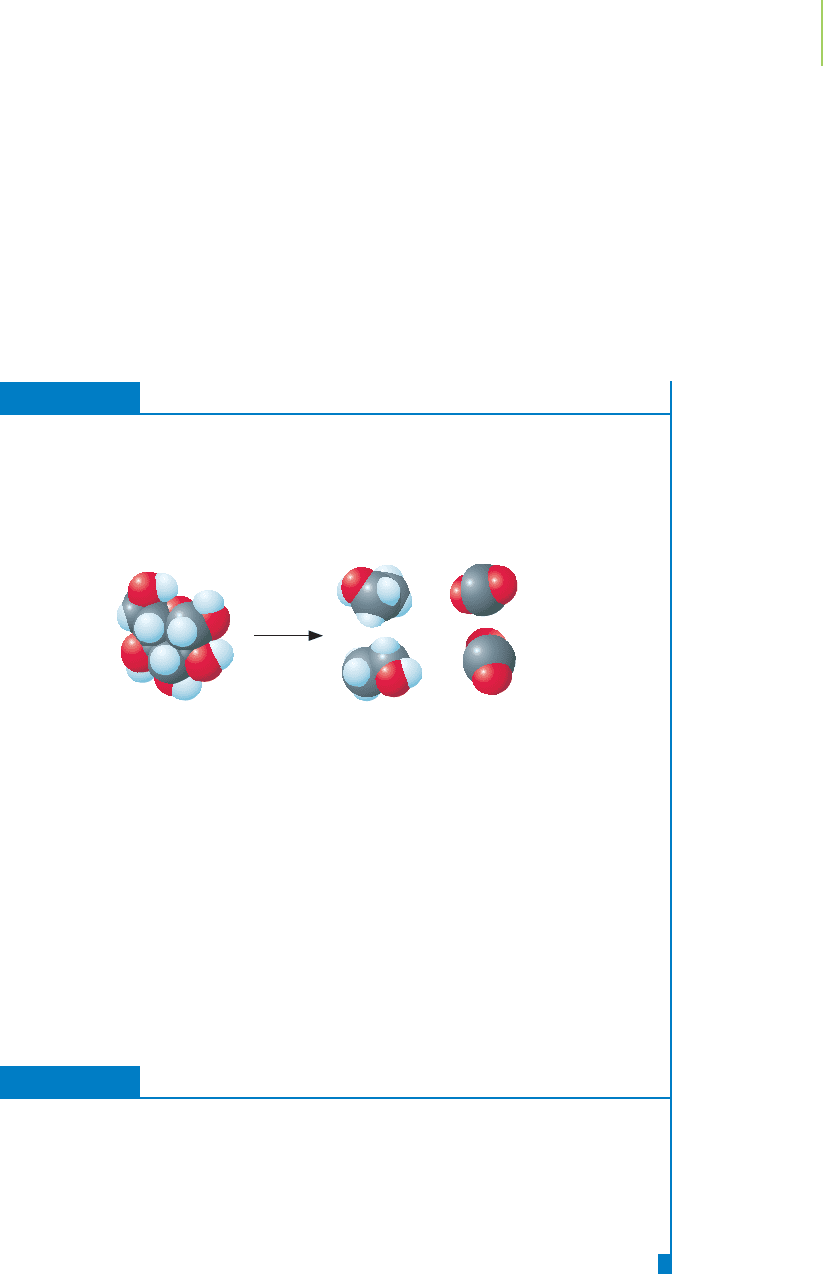
FIGURE 14.19
Adding kinetic energy to a perfect crystal
at 0 K increases the number of micro-
states. The result: The entropy of a com-
pound is always a positive number.
(a) Crystal at 0 K. (b) Crystal at some
higher temperature.
Sea level
Mt. McKinley Mt. Everest
5,100 m
8,846.1 m
3,400 m
Base
camp
FIGURE 14.18
Based on the distance traveled by the mountain climbers, Mt. McKinley
appears to be taller than Mt. Everest. However, with our reference point
in place, it is clear that Mt. Everest is much taller.
We can find the answer by returning to our Mt. Everest climbers from Sec-
tion 14.1. Typically, such adventurers climb about 3,400 m (11,400 ft) from the
base camp at the foot of the mountain to reach the summit. Using this informa-
tion, can we accurately say that the mountain is the tallest in the world? Mt.
McKinley in Alaska, with an ascent of 5,100 m (17,000 ft) appears to be taller. In
order to accurately say that Mt. Everest is the tallest, we must consider that the
base camp itself is 5,400 m above sea level. That is, we must establish a reference
point from which to compare the heights, as we’ve done in Figure 14.18. In the
same way, values of entropy are based on some reference point that is common to
all compounds. The
third law of thermodynamics establishes this point for en-
tropy. The law states that the entropy of a pure perfect crystal at 0 K is zero. A
“perfect” crystal, one in which all of the molecules are rigidly and uniformly
aligned, has negligible kinetic energy at 0 K. In other words, if the excess kinetic
energy of the crystal is zero, the crystal has zero entropy. We qualify the phrase
kinetic energy with the word excess because there is still some atomic (electron)
motion in a perfect crystal at 0 K.
We convert this perfect crystal into a collection of molecules at some higher
temperature by adding kinetic energy. This increases the number of microstates,
resulting in an increase in entropy, as illustrated in Figure 14.19. Under the stan-
dard conditions of 298 K and 1 atm, the compound has an associated standard
molar entropy that we designate as S°. This value is the same as that for ∆S
system
at
this temperature and pressure. By measuring the change in entropy from 0 K to
298 K, researchers have determined the standard molar entropies of a wide vari-
ety of compounds (see Table 14.4).
Notethat the magnitudeof thestandardmolar entropiesislarger for those mol-
ecules that are more complex:
S
o
H
2
(g )
= 131 J/mol·K and
S
o
H
2
O(g )
= 189 J/mol·K.
598 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
–+–+–+–+–+
–+–+–+–+–+
–+–+–+–+–+
–+–+–+–+–
–+–+–+–+–+
–+
–+
–+–+–+
–+–+
–+
–+
–+
–+
–+–+–+–+
–+ –+
–+
–+
–+
–+–+–+
–+
–+
(a)
–+–+–+–+–+
–+–+–+–+–+
–+–+–+–+–+
–+–+–+–+–+
(b)
14.4 Calculating Entropy Changes in Chemical Reactions 599
As more and more atoms are incorporated within a compound, the entropy
of the compound increases. Similarly, note that the standard molar entropies
are different for compounds in different states. For example, liquid water
(S° = 70 J/mol·K) has a lower entropy than gaseous water (S° = 189 J/mol·K).
This is understandable, because the molecules in the gaseous state have more
freedom of movement and hence have a greater possible distribution of energies.
Computationally, we use these standard molar entropy values in much the
same way that we used enthalpy values to calculate ∆H° for a reaction. The basic
principles of Hess’s law (see Chapter 5), which we used to calculate the change in
enthalpy for a reaction, also work for determining the change in the entropy of a
reaction. To summarize, the net standard state entropy gain (or loss) for a reac-
tion is calculated by subtracting the total standard state entropy change for the
reactants from the total standard state entropy change for the products:
S
◦
=
nS
◦
products
−
nS
◦
reactants
where n is the stoichiometric coefficient of each of the compounds in the reac-
tion, and indicates that the standard state entropy (S°) is summed. Just as in the
determination of the change in enthalpy of a reaction, S° for a reaction depends
on the way the reaction is written.
EXERCISE 14.6 Calculating ∆ S°
In the oxidation of glucose at 25°C, the products are different from those we con-
sidered early in Section 14.4. In that discussion, glucose reacted at body tempera-
ture. Here, at 25°C, both oxygen and carbon dioxide are gases and water is a liquid.
Use the table of thermodynamic values in the appendix to assess the change in en-
tropy for this reaction under standard conditions.
C
6
H
12
O
6
(s) + 6O
2
(g) n 6CO
2
(g) + 6H
2
O(l)
First Thoughts
If the water were written as a gas, we would predict a very large increase in the num-
ber of moles of gaseous products, leading to the prediction of a positive entropy
Standard Molar Entropies and Free Energies for Selected Elements and Compounds at 25.0°C
∆
f
H° ∆
f
G° S° ∆
f
H° ∆
f
G° S°
Substance (kJ/mol) (kJ/mol) (J/mol·K) Substance (kJ/mol) (kJ/mol) (J/mol·K)
Br
2
(l) 0 0 152
Br
2
(g) +31 +3 245
HBr(g) −36 −53 199
CaF
2
(s) −1220 −1167 69
CaCl
2
(s) −796 −748 105
CaCO
3
(s) −1207 −1129 93
C(graphite) 0 0 6
C(diamond) +2 +32
CO
2
(g) −394 −394 214
CO
2
(aq) −414 −386 118
CH
4
(g) −75 −50 186
C
2
H
2
(g) +227 +209 201
C
2
H
4
(g) +52 +68 220
C
2
H
6
(g) −85 −33 230
CH
3
OH(l) −239 −166 127
TABLE 14.4
C
2
H
5
OH(l) −278 −175 161
CH
3
Cl(g) −84 −60 234
HCl(g) −92 −95 187
HCl(aq) −168 −131 55
H
2
(g) 0 0 131
Fe
2
O
3
(s) −826 −740 90
N
2
(g) 0 0 192
NO(g) 90 87 211
NO
2
(g) 33 51 240
NH
3
(g) −46 −16 192
H
2
O(g) −242 −229 189
H
2
O(l) −286 −237 70
NaOH(s) −426 −379 64
H
2
SO
4
(aq) −909 −745 20
ZnO(s) −348 −318 44

Application
C
HEMICAL
E
NCOUNTERS:
Pyruvate
and Lactate
change for the reaction. However, in this reaction as written, the number of moles
of gas remains constant, and we are unable to assert that the entropy change would
be positive from that standpoint alone. However, we might predict that the entropy
of the system would increase slightly, because we are forming six molecules of a liq-
uid where we had one molecule of solid reactants. We can use the summation equa-
tion to calculate the change in entropy for the reaction.
S
◦
=
nS
◦
products
−
nS
◦
reactants
Solution
From Table 14.4 we obtain S° values for each of the compounds in the reaction.
Using the equation shown above, we can calculate the value of S°:
6 mol CO
2
× 214 J/mol·K = 1284 J/K
6 mol H
2
O × 70 J/mol·K = 420 J/K
Total S°
products
= 1704 J/K
1 mol C
6
H
12
O
6
× 212 J/mol·K = 212 J/K
6 mol O
2
× 205 J/mol·K = 1230 J/K
Total S°
reactants
= 1442 J/K
Total S°
products
= 1704 J/K
−Total S°
reactants
=−1442 J/K
∆S° = 262 J/K
The reaction has an increase in entropy.
Further Insight
Does this answer make sense? Although the number of molecules of gas is the same
for the reactants and the products, we can reasonably assert that the gain in entropy
is due to the formation of six molecules of liquid from every molecule of solid. The
effect is not as profound as for the formation of a gas (∆S° = 262 J/K versus ∆S° =
974 J/K), but it does contribute, as in this case. Our answer makes sense.
PRACTICE 14.6
Predict the sign of ∆S° for each of these reactions. Then calculate the values of ∆S°.
You will need to use the table of thermodynamic values in the appendix. Do your
calculations agree with your predictions?
CH
3
OH(l) + HCl(g) n CH
3
Cl(g) + H
2
O(g)
3O
2
(g) n 2O
3
(g)
See Problems 46 and 48–52.
14.5 Free Energy
In exercising muscles, the level of oxygen often is a limiting reagent. As the mus-
cles consume glucose to provide the energy needed to contract and relax, the
limited supply of oxygen forces a buildup of pyruvate (see Figure 14.20). This
molecule can continue on the typical glycolysis pathway toward complete oxida-
tion, but with limited oxygen this does not happen. Instead, the exercising muscle
obtains energy by converting pyruvate into lactate (a reduction!) by the pathway
shown in Figure 14.21. The reduced form of a biological molecule known as
NAD
+
(nicotinamide adenine dinucleotide) is oxidized in the process. (Remem-
ber that we must have an oxidation and a reduction for a redox reaction to occur.)
600 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
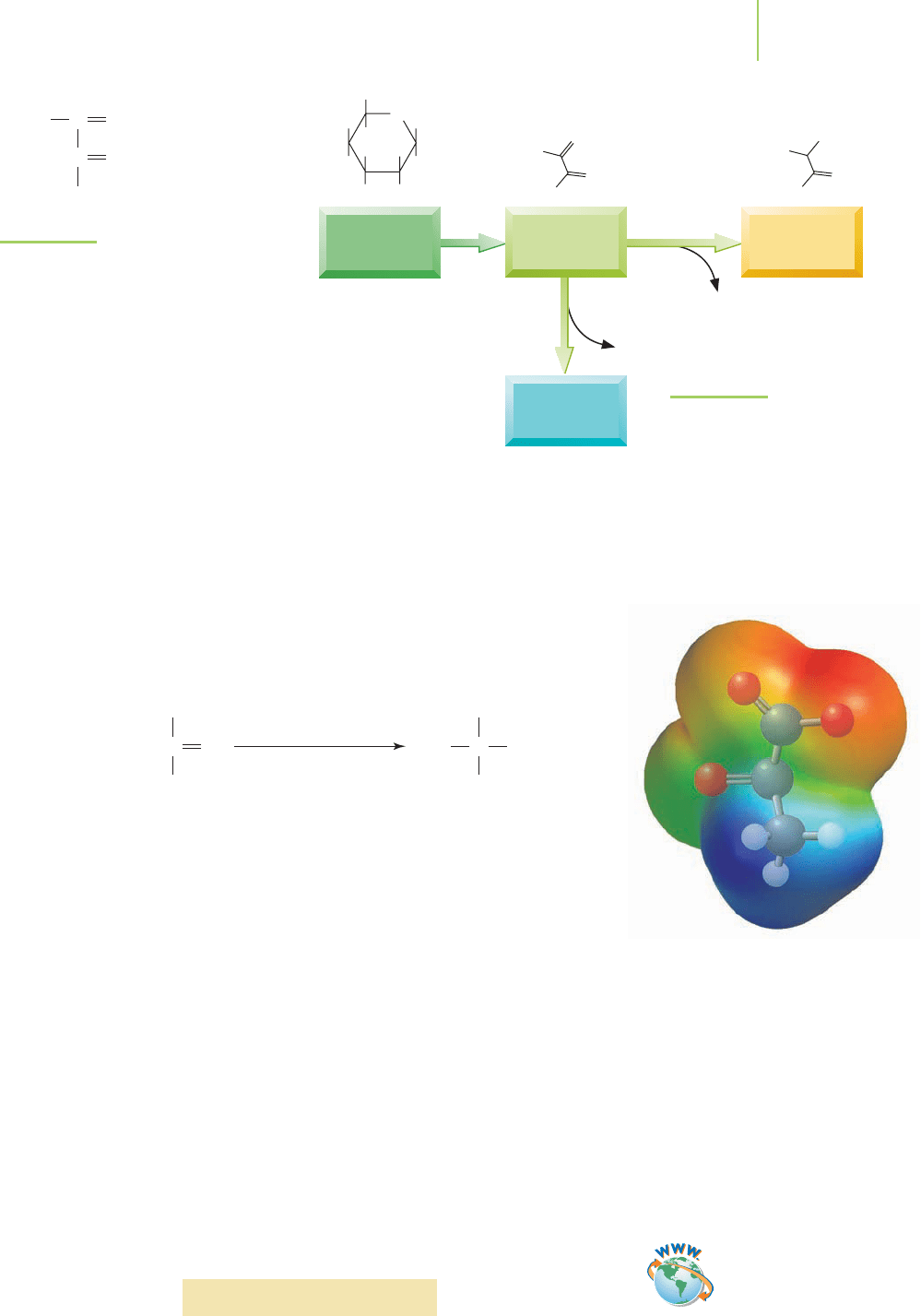
Glucose Pyruvate Lactate
Carbon
dioxide
Aerobic
Anaerobic
Energy
Energy
HO
O
O
–
O
O
HO
O
OH
OH
OH
CH
2
OH
O
–
Pyruvate
Unfortunately, lactate is potentially a metabolic dead end in humans; further ca-
tabolism of lactate may not occur. Eventually, the lactate can be changed back to
pyruvate by the reverse reaction and then catabolized in the presence of oxygen.
While early research indicated that the body signals the presence of excess lactate
(and a decrease in oxygen in the muscles) with a burning sensation, recent results
seem to contradict this claim.
The reaction of pyruvate to make lactate is energetically favorable. Some
energy is harvested from the pyruvate. However, qualitatively, it is difficult to
predict this by just looking at the reaction. Determining the spontaneity of the
reaction by simply looking at the states of the reaction is also difficult.
After some calculations, we can determine the value of S° (that is, S
system
),
but we also have to determine the entropy of the surroundings (S
surroundings
) in
order to calculate the change in entropy of the universe (system + surroundings)
and, therefore, the spontaneity of the reaction. A simpler and more useful way
to determine reaction spontaneity was identified by Josiah Willard Gibbs
(1839–1903), professor of mathematical physics at Yale from 1871 to 1903.
Gibbs showed that calculating a property he called the
free energy (G) makes
possible the straightforward determination of reaction spontaneity. The equation
is indicative of two ideas that we have discussed.
■
Idea 1: An increase in the number of microstates available to the system favors
an increase in the entropy of the universe.
■
Idea 2: An exothermic reaction increases the kinetic energy of the surround-
ings, therefore increasing the range of motions of its particles, leading to an
increase in the entropy of the surroundings and favoring an increase in the
entropy of the universe.
The
Gibbs equation is G = H − TS
where H is the enthalpy of the system, T is the temperature in kelvins, and S is the
entropy of the system. We are interested in the change in the free energy of the
system, so the Gibbs equation becomes
G = H
system
− TS
system
at constant temperature and pressure.
Lactate dehydrogenase
HO C
COO
H NAD
CH
3
H
C
COO
ONADH
CH
3
14.5 Free Energy 601
OC
O
OC
CH
3
FIGURE 14.20
Pyruvate is a product of the
glycolysis pathway.
FIGURE 14.21
Pyruvate cannot continue oxidation
(aerobic pathway) without oxygen.
Instead, energy is derived by the con-
version of pyruvate to lactate (anaer-
obic pathway).
Video Lesson: Gibbs Free Energy
This equation doesn’t seem very useful at first. However, the value of the
change in the free energy (G) of a process is related to S
universe
by the equation
shown below. In other words, the free energy change of a process can be used to
determine the spontaneity of that process. Because of the negative sign in the re-
lationship, negative free energy changes (G =
−) imply spontaneous processes,
and positive free energy changes (G =+) indicate nonspontaneous processes
Table 14.5 lists the outcomes of the change in free energy based on the relation-
ship between H and S.
S
universe
=
−G
T
Therefore, the Gibbs equation can be used to calculate the change in free en-
ergy for a process, because we know how to calculate ∆H and ∆S using tabulated
values (see Table 14.4). All we need, then, is the temperature of the process and
the application of some assumptions. First, as a requirement of the Gibbs equa-
tion, we must assume that the pressure remains constant. We also have to assume
that both ∆H and ∆S are temperature independent, which is not always a good
assumption, especially for ∆S.
Let’s reexamine the conversion of pyruvate to lactate under anaerobic condi-
tions. Qualitatively, we cannot use the change in the number of moles of gas from
reactants to products to help us assess whether the reaction will be spontaneous,
because there are no gases in the reaction.
However, because the values of H and S have been measured for this reaction,
we can determine the change in free energy. In this system, H = –78.7 kJ/mol
and S = –88.75 J/mol·K. On the basis of these data alone, we identify the reac-
tion as exothermic with a decrease in system entropy. What is the value of G for
the reaction at 298 K?
G = H − TS
G =−78,700 J/mol − (298 K ×−88.75 J/mol·K)
G =−52,253 J/mol =−52.3 kJ/mol
Our calculations show that the free energy of this reaction decreases. One thing
to keep in mind during such calculations is that in order to use the Gibbs equa-
tion, we must modify the values of H and S so that they have the same units.
In our calculation, we converted them into units of J/mol and J/mol·K.
We previously mentioned that the change in free energy is negatively related
to the change in the entropy of the universe. That is, a negative value for the
Lactate dehydrogenase
HO C
COO
H NAD
CH
3
H
C
COO
ONADH
CH
3
602 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
Effect of Enthalpy, Entropy, and Temperature on the Spontaneity
of a Process
H S Low Temperature High Temperature
++G =+; nonspontaneous G =−; spontaneous
+−G =+; nonspontaneous G =+; nonspontaneous
−+G =−; spontaneous G =−; spontaneous
−−G =−; spontaneous G =+; nonspontaneous
TABLE 14.5
Lactate
change in free energy implies a spontaneous process. For a reaction, such as the
conversion of pyruvate to lactate, in which the change in free energy is a negative
value (G =−), the entropy of the universe has a positive value (S
universe
=+).
The reaction is spontaneous under standard conditions. We might not have pre-
dicted this solely on the basis of our examination of S° for the reaction. Simi-
larly, a process can be considered nonspontaneous if there is an increase in the
free energy of the system (G =+). This means that if the reaction, as written, is
nonspontaneous, the reverse reaction is spontaneous. For instance, the formation
of lactate from pyruvate is spontaneous (a lowering of free energy), but the for-
mation of pyruvate from lactate is nonspontaneous.
EXERCISE 14.7 Spontaneity and Biochemical Reactions
Yeast cells operate under anaerobic conditions to convert glucose to pyruvate, and
pyruvate to ethanol and CO
2
. Humans are unable to convert pyruvate to ethanol
and must make lactate instead. What is the value of the change in the free energy
(G) for the conversion of glucose to ethanol at 25°C? (Assume that both the
enthalpy and the entropy were also determined at 25°C.)
C
6
H
12
O
6
(s) n 2C
2
H
6
O(l) + 2CO
2
(g) H =−70.0 kJ/mol
Glucose Ethanol S = 534 J/mol·K
Solution
G = H − TS
G =−70,000 J/mol − (298 K) × (534 J/mol·K)
G =−229,132 J/mol
G =−229 kJ/mol
Because the entropy is given in J/K·mol, the units of the enthalpy have been changed
(from kilojoules to joules) so that the two values can be added easily. The reaction is
spontaneous, with a rather large negative free energy change.
PRACTICE 14.7
Calculate ∆G at −10°C and at 45°C for the ice-to-water phase transition. Which do
you predict to be spontaneous? Do your calculations agree with your predictions?
(Assume that the enthalpy and entropy are temperature independent.)
H
2
O(s) n H
2
O(l) H = 6.01 kJ/mol
S = 22.0 J/mol·K
See Problem 56.
A Second Way to Calculate ∆G
Just like enthalpy and entropy, free energy is a state function. Remember from
Chapter 5 that when we are determining the value of a state function, it doesn’t
matter how we calculate it because the end result is independent of the path. To
illustrate this, we return to the trek up Mt. Everest. Climbers can take either the
14.5 Free Energy 603
+
more popular south route up the Khumbu icefall or the north route along the
ridge to the summit. No matter which way the climbers ascend, no matter how
many steps there are in each of the two routes, and no matter how long their as-
cent takes the climbers, if they make it to the top they have climbed to a point
29,035 ft above sea level. As we saw before, altitude change is a state function. It
doesn’t matter how two climbers arrive at the summit of Mt. Everest; their change
in altitude from the base camp to the top is the same.
Because free energy is a state function, we can obtain G by using the same
method we used to calculate H. Adding a series of reactions, each with a known
free energy, is often a good way to arrive at a particular calculation of the free en-
ergy, because many reactions cannot be performed directly in the lab. For exam-
ple, the combustion of carbon, as occurs in our charcoal grills, can never give just
carbon monoxide but, rather, always results in a mixture of oxides. For another
example, say we were interested in the calculation of G for the formation of ice
from steam at –18°C (255 K) and 1 atm, another reaction that is difficult to do in
the lab. We could sum the equations (and the free energy terms) that describe the
stepwise conversion from gas to liquid and from liquid to ice to arrive at ∆G for
the process. Note that the free energy terms are specific to this temperature:
H
2
O(g) → H
2
O(l) G =−13.6 kJ/mol
H
2
O(l) → H
2
O(s ) G =−0.39 kJ/mol
H
2
O(g) → H
2
O(s ) G =−14.0 kJ/mol
We see that the process of converting water vapor to ice has a negative change in
free energy at –18°C. What does this value of ∆G tell us, and does it agree with
what we expect for this system? Under these conditions (1 atm, –18°C), the for-
mation of ice from steam is spontaneous, whereas the converse process, sublima-
tion of solid to the vapor, is not.
The summation procedure is not limited to phase changes. For example,
glycogen (a polymer of glucose used as storage for quick energy release) is de-
graded to glucose-1-phosphate (Glu-1-P) when the body signals that energy is
needed. In order for this derivative of glucose to be used in glycolysis, it must first
be converted to glucose-6-phosphate (Glu-6-P) by a two-step process. The
hydrolysis reaction of each of these phosphates has been extensively studied and
the free energy change noted. What is the net free energy change for the direct
conversion of glucose-1-phosphate to glucose-6-phosphate? On the basis of our
previous discussion, we can calculate ∆G:
Glu-1-P + H
2
O n glucose + phosphate G =−21 kJ/mol
glucose + phosphate n Glu-6-P + H
2
O G =+14 kJ/mol
Glu-1-P n Glu-6-P G =−7 kJ/mol
604 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
Glu-1-P
Glu-6-P
Application
The net reaction is spontaneous. The reverse of this reaction, the conversion of
glucose-6-phosphate into glucose-1-phosphate, is nonspontaneous with ∆G =
+7 kJ/mol. Does the result make sense? If the body needs energy, production of
glucose-6-phosphate should be spontaneous.
As you can see, we can use the summation method for determining the spon-
taneity of a reaction in cases where the free energy of each in a series of reactions
is known. In some cases, we can even measure the spontaneity of supposed reac-
tions or processes that we do not wish to perform in the laboratory. The summa-
tion of a series of reactions makes possible the quick and easy calculation of the
spontaneity of a reaction.
Free Energy of Formation
We can also calculate the spontaneity of a reaction using standard free energies
of formation (
f
G°). The standard molar free energy of formation (
f
G°) is the
change in the free energy of 1 mol of a substance in its standard state as it is made
from its constituent elements in their standard states. The equation relating the
standard free energy of formation for any compound is analogous to the equa-
tion written for the standard enthalpy of formation. For example,
H
2
(g) +
1
⁄2O
2
(g) n H
2
O(l)
The standard molar free energy of formation,
f
G°, for all elements in their stan-
dard states is zero, just as it is for the standard molar enthalpy of formation,
f
H°.
Recall from our previous discussion on the third law of thermodynamics that the
standard molar entropy of an element, S°, is not equal to zero.
The tabulation of
f
G° values is given in Table 14.4 and in the appendix.
Access to this and similar tables enables us to determine quickly the free energy
change for an unknown reaction. In much the same manner that we use S° and
H°, we can find the change in the standard free energy of a reaction. The sum of
the free energies of formation for the reactants is subtracted from the sum of the
free energies of formation for the products:
G
◦
=
n
f
G
o
products
−
n
f
G
o
reactants
where n is the stoichiometric coefficient of each of the compounds in the
reaction.
EXERCISE 14.8 I’m Hungry. What’s for Dinner? Free Energy and Spontaneity
In a fasting organism, there is no more glucose left to make energy. Glycogen sup-
plies become depleted. The organism must find a way to make molecules that can be
catabolized for energy. One such source of energy is found in the amino acid ala-
nine. This molecule is converted to pyruvate for use in the production of energy
(remember that pyruvate is an intermediate during the complete oxidation of
glucose). Given the equation for the production of pyruvic acid, the acidic form of
pyruvate, from alanine, as well as the values of the free energy of formation for both,
determine the spontaneity of this reaction under standard conditions.
First Thoughts
The existence of ∆
f
G° values for both the starting materials and the products means
that we can use the summation equation to calculate the standard free energy
change for the reaction.
14.5 Free Energy 605
Video Lesson: Standard Free
Energy Changes of Formation
Solution
1 mol glutamic acid × −174 kJ/mol = −174 kJ
1 mol pyruvic acid × −111 kJ/mol = −111 kJ
Total
f
G°
products
=−285 kJ
1 mol ketoglutaric acid ×−195.5 kJ/mol = −195.5 kJ
1 mol alanine ×−88.5 kJ/mol = −88.5 kJ
Total
f
G°
reactants
= −284 kJ
Total
f
G°
products
= −285 kJ
−Total
f
G°
reactants
= −(−284 kJ)
G° = −1kJ
Judging on the basis of our calculations, the change in free energy is negative and
the reaction is spontaneous.
Further Insight
Pyruvate is so important to the production of energy that its formation is vital to
the survival of an organism. Because of this, reactions that make pyruvate are typi-
cally spontaneous. One way to obtain pyruvate is from a source of alanine, as shown
here. Where does the organism get alanine? Because alanine is an amino acid, we
might predict that it comes from stores of excess alanine. However, there are few
stores of this amino acid in the body, so it must come from another source. In par-
ticular, alanine can be obtained from the degradation of existing proteins (which
contain alanine and other amino acids). Although there are other ways to make
pyruvate, the only way to obtain pyruvate when an organism is starved is to con-
sume proteins. And the consumption of proteins means that muscle tissue is destroyed.
For this and other reasons, starvation diets are not the best way to lose weight.
PRACTICE 14.8
Use the table of thermodynamic values in the appendix to determine whether each
of these oxidations is spontaneous. You may need to balance the equations.
C
6
H
12
O
6
(s) + 6O
2
(g) → 6CO
2
(g) + 6H
2
O(g)
CH
3
CH
2
CH
3
(g) + O
2
(g) → CO
2
(g) + H
2
O(g)
Mg(s) + N
2
(g) → Mg
3
N
2
(s)
See Problems 59–62.
606 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
Ketoglutaric acid
–195.5
f
G˚ (kJ/mol)
Alanine
–88.5
f
G˚ (kJ/mol)
Glutamic acid
–174
f
G˚ (kJ/mol)
Pyruvic acid
–111
f
G˚ (kJ/mol)
O
OHC
HHC
HHC
O
OHC
O
C
H
HHC
NH
2
HC
HO
O
C
O
OHC
HHC
HH
H
C
NH
2
OHC
O
C
H
HHC
OC
HO
O
C
++
Glucose
CHO
OHH
HHO
H
H
OH
OH
CH
2
OH
HPO
4
2–
ATP + H
2
O ADP + HPO
4
2–
+
Glucose-6-phosphate
G = 13.8 kJ/mol
CHO
OHH
HHO
H
H
OH
OH
CH
2
OPO
3
2–
H
2
O+
Glucose
CHO
OHH
HHO
H
H
OH
OH
CH
2
OH
ATP+
Glucose-6-phosphate
G = –16.7 kJ/mol
CHO
OHH
HHO
H
H
OH
OH
CH
2
OPO
3
2–
ADP+
G = –30.5 kJ/mol
HERE’S WHAT WE KNOW SO FAR
■
Entropy and free energy are state functions.
■
The change in entropy and free energy for a reaction can be determined by
subtracting the sum of these quantities for the reactants from the sum for the
products.
■
Negative ∆G values are indicative of spontaneous processes.
■
The Gibbs equation (∆G = ∆H – T∆S) can be used to determine the spon-
taneity of a process.
■
Like enthalpy changes, ∆S and ∆G for a multi-step process are simply the sum
of the entropy or free energy changes for each individual process.
14.6 When ∆G = 0; A Taste of Equilibrium
We’ve already mentioned that the complete oxidation of glucose in the body pro-
duces ATP. This molecule is a source of potential energy to force nonspontaneous
reactions in the body to become spontaneous. For example, the preparation of
glucose-6-phosphate from glucose and phosphate is nonspontaneous. Coupling
the hydrolysis of ATP with the production of glucose-6-phosphate helps to make
the overall process spontaneous. In this way, the ATP molecule is used to force a
reaction to become spontaneous. Although the majority of the ATP in the body
comes from the oxidation of glucose, ATP can be made in many other ways. One
such way is through the reaction of two molecules of ADP (adenosine diphos-
phate), which can occur in vigorously active muscles. When the supply of glucose
runs low and the level of ATP in the muscle drops, muscle cells gather energy by
converting ADP to ATP and AMP. This enables the cells to continue their activity.
Let’s examine more closely the preparation of one molecule each of ATP and
AMP (adenosine monophosphate) from two molecules of ADP. What is the first
thing that you note about the reaction as written?
2ADP n ATP + AMP ∆G ≈ 0 kJ/mol
14.6 When ∆G = 0; A Taste of Equilibrium 607
Application
C
HEMICAL
E
NCOUNTERS:
ATP Formation
