Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


In 2003, over 33.3 million tons of marketable phosphate rock was mined in
the United States, mostly from Florida and North Carolina, according to the U.S.
Geological Survey. In fact, the U.S. production is about 24% of the world’s phos-
phate rock production. Significant amounts of phosphates are also produced in
China and in the Morocco and Western Sahara regions of Africa. This ore is con-
verted into phosphoric acid for use in our society. Table 17.8 indicates the wide
variety of uses of phosphoric acid. Phosphoric acid can be used to convert fluo-
roapatite into a soluble fertilizer, as shown in the following reaction:
2Ca
5
(PO
4
)
3
F + 14H
3
PO
4
10Ca(H
2
PO
4
)
2
+ 2HF
In the human body, phosphate ion is important in maintaining the pH of blood
in a fairly narrow range of 7.3 to 7.5. We will explore how it does so in the next
chapter.
Phosphoric acid is one of two inorganic polyprotic acids that have a world-
wide impact on our ability to convert what nature has given us into products
that sustain and improve our quality of life. Sulfuric acid is the other.
Sulfuric acid is a diprotic acid that is prepared by the Contact process, as we
discussed in Section 16.3. Sulfur is burned in oxygen to form sulfur dioxide,
which is then converted into sulfur trioxide via a catalyst such as vanadium:
S(s) + O
2
(g)
SO
2
(g)
2SO
2
(g) + O
2
(g)
2SO
3
(g)
The sulfur trioxide is then combined with water to give sulfuric acid:
SO
3
(g) + H
2
O(l)
H
2
SO
4
(l)
Amongthe manyusesfor sulfuricacid is a century-oldprocessfor the conversionof
phosphate rock to the fertilizer monocalcium phosphate, Ca(H
2
PO
4
)
2
·H
2
O. The
reaction can be summarized as follows:
2Ca
5
(PO
4
)
3
F + 7H
2
SO
4
+ 3H
2
O
3Ca(H
2
PO
4
)
2
·H
2
O + 7CaSO
4
+ 2HF
Note that the product of this reaction is similar to the one produced by the treat-
ment of phosphate rock with phosphoric acid.
Among the other uses of sulfuric acid is in the manufacture of phosphoric
acid itself from fluoroapatite, with the resulting production of calcium sulfate
dihydrate (CaSO
4
·2H
2
O, also known as gypsum) under reaction conditions that
are different from those in the previous reaction.
2Ca
5
(PO
4
)
3
F + 10H
2
SO
4
+ 20H
2
O
10CaSO
4
·2H
2
O + 6H
3
PO
4
+ 2HF
The gypsum produced in this reaction is quite valuable for use as
Sheetrockandwallboardin theconstructionof homes. Furthermore,
HF is a useful product in the glass industry.
In the reactions we have just shown, the phosphates and sulfates
are present in a variety of forms: as polyprotic acids (H
3
PO
4
and
H
2
SO
4
); as a monobasic salt (it can accept one acidic hydrogen
atom—Ca(H
2
PO
4
)
2
·H
2
O); and as a dibasic salt (it can accept two
acidic hydrogen atoms—CaSO
4
). Another common compound is
calcium phosphate, Ca
3
(PO
4
)
2
,a tribasic salt (it can accept three
acidic hydrogen atoms). Calcium phosphate is one of several cal-
cium salts, including calcium carbonate, CaCO
3
, and calcium cit-
rate, Ca
3
(C
6
H
5
O
7
)
2
·4H
2
O, that many people take daily as a calcium
supplement (Figure 17.18). Each of these species affects the acid concentration of
solutions in predictable ways. Understanding these effects helps us to see how
these acids are employed in manufacturing the products that we use.
748 Chapter 17 Acids and Bases
Uses of Phosphoric
Acid in Manufacturing
Fertilizer
Dentifrices
Soaps
Detergents
Fire control agents
Soft drinks
Incandescent light filaments
Corrosion inhibitors in metals
Organic chemicals such as
ethylene and propylene
TABLE 17.8
FIGURE 17.18
Calcium supplements often contain the
tribasic phosphate ion. This supplement
contains a mixture of calcium-containing
minerals including Ca
3
(PO
4
)
2
and CaCO
3
.
TABLE 17.8
The pH of Polyprotic Acids
Phosphoric acid is so common that it represents an important and useful model
for our introduction to the pH of polyprotic acids. We begin by calculating the
pH of 4.0 M aqueous phosphoric acid (neglecting activity effects).
As with any equilibrium problem, the first step is to establish the equilibria of
the species in solution that contribute hydrogen ions. Phosphoric acid can do-
nate three hydrogen ions per molecule, the second being more difficult to donate
than the first because of the negative charge on the resultant dihydrogen phos-
phate anion (H
2
PO
4
−
). The third will be tougher still as a consequence of
the greater charge on the monohydrogen phosphate anion (HPO
4
2−
). This is
reflected in the values for K
a
of each step in the process, in which
K
a
1
is the equi-
librium constant for the first acidic ionization,
K
a
2
refers to the second ionization,
and
K
a
3
describes the third. Table 17.9 lists K
a
values for several polyprotic acids.
K
a
1
values are not only larger than those of
K
a
2
, but they are often considerably so,
and this is frequently true for acids. Water is also a source of hydrogen ions.
H
3
PO
4
H
2
PO
4
−
+ H
+
K
a
1
= 7.5 ×10
−3
H
2
PO
4
−
HPO
4
2−
+ H
+
K
a
2
= 6.2 ×10
–8
HPO
4
2−
PO
4
3−
+ H
+
K
a
3
= 4.8 ×10
–13
H
2
O
OH
−
+ H
+
K
w
= 1.0 × 10
−14
The total concentration of hydrogen ion will equal the sum of the contributions
from all the reactions.
[H
+
]
total
=
[H
+
]
H
3
PO
4
+ [H
+
]
H
2
PO
4
−
+
[H
+
]
HPO
4
2−
+ [H
+
]
H
2
O
Which reactions are important contributors of hydrogen ions to the solution?
The
K
a
1
value is considerably larger than the others, so we may assume that it is
the only important equilibrium. That is,
[H
+
]
total
∼
=
[H
+
]
H
3
PO
4
We will eventually want to prove that the other reactions are not important con-
tributors if we make this assumption.
We may now solve the problem, determining the pH of a 4.0 M solution of
H
3
PO
4
, as we have other equilibrium problems. We’ll remember to test any
assumptions we make along the way.
K
a
1
=
[H
2
PO
4
−
][H
+
]
[H
3
PO
4
]
17.6 Polyprotic Acids 749
K
a
Values for Selected Polyprotic Acids
Formula Name K
a
1
K
a
2
K
a
3
H
3
PO
4
Phosphoric acid 7.4 × 10
−3
6.2 × 10
−8
4.8 × 10
−13
H
3
AsO
4
Arsenic acid 5.0 × 10
−3
8.0 × 10
−8
6.0 × 10
−10
H
2
CO
3
Carbonic acid 4.3 × 10
−7
5.6 × 10
−11
H
2
SO
4
Sulfuric acid >1 1.2 × 10
−2
H
2
SO
3
Sulfurous acid 1.5 × 10
−2
1.0 × 10
−7
H
2
S Hydrosulfuric acid 1.0 × 10
−7
1.0 × 10
−15
H
2
C
2
O
4
Oxalic acid 6.5 × 10
−2
6.1 × 10
−5
H
2
C
6
H
6
O
6
Ascorbic acid 7.9 ×10
−5
1.6 × 10
−12
TABLE 17.9

7.4 ×10
–3
=
x
2
4.0
x = [H
2
PO
4
−
] = [H
+
] = 0.17 M
pH = 0.76
[H
3
PO
4
] = [H
3
PO
4
]
0
− [H
2
PO
4
−
] = 4.0 − 0.17 = 3.83 M
≈
3.8 M
We have made the assumption that “x” is negligible relative to 4.0 M. In this
case,
0.17
4.0
× 100% = 4.3%
Our assumption is valid.
We must now test our assumptions that the other equilibria were not impor-
tant contributors of hydrogen ion to the solution. We may begin with water,
knowing that
[H
+
]
water
= [OH
−
]
total
=
K
w
[H
+
total
]
=
1.0 ×10
−14
0.17
= 5.9 ×10
–14
M
Our assumption that water was a negligible source of hydrogen ion was fine
(with thanks to Le Châtelier!).What about
[H
+
]
HPO
4
2−
, which results from the
loss of a hydrogen ion by H
2
PO
4
−
?
H
2
PO
4
−
HPO
4
2−
+ H
+
K
a
2
= 6.2 ×10
–8
We showed above that [H
2
PO
4
−
] is equal to 0.17 M. What is [H
+
] in this equa-
tion? It is roughly equal to the hydrogen ion concentration resulting from the first
dissociation, that of H
3
PO
4
,[H
+
]
total
≈ [H
+
]
H
3
PO
4
, and that is also equal to 0.17
M. We can substitute these values into the equilibrium expression to determine
[H
+
]
H
2
PO
4
−
(which equals [HPO
4
2−
]):
K
a
2
= 6.2 ×10
–8
=
[HPO
4
2−
][H
+
]
[H
2
PO
4
−
]
=
[HPO
4
2−
](0.17)
(0.17)
Therefore,
[HPO
4
2−
] =
K
a
2
= 6.2 ×10
–8
M
Also,
[H
+
]
HPO
4
2−
will equal [HPO
4
2−
], because they are both produced in the
same reaction.
[H
+
]
HPO
4
2−
= 6.2 ×10
–8
M
This confirms the notion that only the first equilibrium, the dissociation of
H
3
PO
4
, is an important contributor to [H
+
]
total
.
We can take this one step further and calculate the hydrogen ion concentra-
tion due to the dissociation of the dibasic anion HPO
4
2−
:
HPO
4
3−
PO
4
3−
+ H
+
K
a
3
= 4.8 ×10
–13
H
3
PO
4
H
+
+ H
2
PO
4
−
initial 4.0 M 0 M 0 M
change −x +x +x
equilibrium 4.0 − x +x +x
assumptions 4.0 +x +x
750 Chapter 17 Acids and Bases
In this case, [H
+
]
total
is still 0.17 M and [HPO
4
2−
] = 6.2 × 10
−8
M. Substituting
into the equilibrium expression for
K
a
3
yields
K
a
3
= 4.8 ×10
–13
=
[PO
4
3−
][H
+
]
[HPO
4
−2
]
=
[PO
4
3−
](0.17)
6.2 ×10
−8
x = [PO
4
3–
] = [H
+
]
PO
4
3−
= 1.8 ×10
–19
M
In summary, we have accounted for all of the phosphate species and all of the
hydrogen ion.
[phosphate species] = [H
3
PO
4
] + [H
2
PO
4
−
] + [HPO
4
−
] + [PO
4
3−
]
4.0 M = 3.83 M + 0.17 M + 6.2 × 10
−8
M + 1.8 × 10
−19
M
[H
+
]
total
=
[H
+
]
H
3
PO
4
+
[H
+
]
H
2
PO
4
−
+
[H
+
]
HPO
4
2−
+
[H
+
]
H
2
O
0.17 M ≈ 0.17 M + 6.2 × 10
−8
M + 1.8 × 10
−19
M + 5.9 ×10
−14
M
We note that when the concentration of phosphoric acid is much greater than the
value of
K
a
1
, only the dissociation of phosphoric acid itself contributes signifi-
cantly to the hydrogen ion concentration. The concentration of each phosphate
species changes in solution as the pH changes, with less of the acidic forms
(H
3
PO
4
and H
2
PO
4
−
) and more of the basic forms (HPO
4
2−
and PO
4
3−
) present
at higher pH levels.
EXERCISE 17.14 Concentration of Species in a Polyprotic Acid Solution
Oxalic acid (H
2
C
2
O
4
), which is found in beet leaves, rhubarb, and spinach, is used
in the bookbinding industry, as well as in dye and ink manufacturing. In the analyt-
ical laboratory it can be used as a primary standard against which to determine the
molarity of sodium hydroxide. Using the information in Table 17.9, determine
the pH and [H
2
C
2
O
4
] of a 1.40 M solution of oxalic acid.
H
2
C
2
O
4
(aq)
HC
2
O
4
−
(aq) + H
+
(aq)
K
a
1
= 6.5 ×10
–2
HC
2
O
4
−
(aq)
H
+
(aq) + C
2
O
4
2−
(aq)
K
a
2
= 6.5 ×10
−5
First Thoughts
The major species in solution are H
2
C
2
O
4
and H
2
O. There are a number of equilib-
ria that will occur in the solution. Judging on the basis of the values of
K
a
1
,
K
a
2
, and
K
w
, by far the most significant equilibrium will be the dissociation of H
2
C
2
O
4
.
H
2
C
2
O
4
(aq)
HC
2
O
4
−
(aq) + H
+
(aq)
initial 1.40 0 0
change −xxx
final 1.40 − xxx
assumptions 1.40 xx
As always, we will test our “negligible ionization” assumption (1.40 ∼ x ≈ 1.40).
However, because K
a
is not less than 10
−2
× [H
2
C
2
O
4
]
0
, our assumption may well
not be valid.
Solution
K
a
1
=
[HC
2
O
4
−
][H
+
]
[H
2
C
2
O
4
]
= 6.5 ×10
–2
=
x
2
1.40
x = [H
+
] = [HC
2
O
4
] = 0.302 M
17.6 Polyprotic Acids 751
Oxalic acid
C
2
H
2
O
4
Testing the 5% rule, we find that
0.302
1.40
× 100% = 21.5%!
This confirms our prediction based on K
a
and [H
2
C
2
O
4
]
0
. Therefore,
[H
2
C
2
O
4
]
=
[H
2
C
2
O
4
]
0
but rather equals “1.40 − x.”
6.5 ×10
–2
=
x
2
1.40 − x
We can solve using the quadratic formula, as we did in Section 16.5. Clearing the
fraction and setting equal to zero, we get
x
2
+ 0.065(x) − 0.091 = 0
where a = 1, b = 0.065, and c =−0.091. Solving yields
x =
−0.065 ±
(0.065)
2
− 4(1)(−0.091)
2(1)
x = 0.271 M
[H
+
] = [HC
2
O
4
−
] = 0.271 M
[H
2
C
2
O
4
] = 1.40 − 0.271 ≈ 1.13 M
pH =−log(0.271) = 0.57
Checking our math, we find that
K
a
1
=
(0.271)
2
1.13
= 0.0650
Further Insights
Does our answer make sense? Although we classify oxalic acid as “weak” because its
K
a
1
value is less than 1 (and its
K
a
2
value is even smaller,) it is still stronger than
many common weak acids such as acetic and citric acids. It is therefore reasonable
that this relatively concentrated solution should have a low pH.
PRACTICE 17.14
Determine the pH of an aqueous 0.200 M H
3
PO
4
solution.
See Problems 59, 60, 63, and 64.
We have seen that we can get a sense of what the pH of a solution will be by
assessing the competing equilibria that occur in solution. Using this understanding,
the pH of seemingly complex systems can be solved in a structured and mean-
ingful fashion. We can extend this understanding to salts that contain an anion
and cation that have acid–base behavior.
17.7 Assessing the Acid–Base Behavior
of Salts in Aqueous Solution
Salts, such as NaCl, NH
4
NO
3
, and NaNO
2
, are ionic compounds. When they
dissociate in water they may exhibit acid–base behavior. The key questions you
need to ask when assessing whether a salt will be acidic, basic, or neutral in aque-
ous solution are
What are the acid–base properties of the cation and anion parts of
the salt?
and Which is more influential, the acid strength of the cation or the base
752 Chapter 17 Acids and Bases
Video Lesson: Acid–Base
Properties of Salt Solutions

strength of the anion? Whichever is stronger will determine whether the salt solu-
tion is acidic or basic.
Sodium nitrite (NaNO
2
) is an important example because it is a food additive
that helps retard spoilage in meat and also is used in many industrial applica-
tions, including the production of nitrogen-containing dyes as well as anticorro-
sion agents. Its use in the food industry has been restricted by the Food and Drug
Administration to 200 parts per million in meat and poultry that is ready for
sale, because sodium nitrite was implicated in the 1970s as a possible precursor
for some cancer-causing compounds. The salt essentially completely dissociates
in aqueous solution to give Na
+
and NO
2
−
ions. These are the main species in
solution in addition to H
2
O. What are the acid–base properties of each of these
species?
Remember the conjugate acid–base relationships:
■
Strong acids and bases have weak conjugates. Na
+
and other alkali and
alkaline earth metal ions exhibit no important acid–base properties.
■
The nitrite ion (NO
2
−
) is the conjugate base of the weak acid HNO
2
(K
a
=
4.6 × 10
−4
). Remember that weak is a relative term. HNO
2
is weak compared
to HNO
3
, which has K
a
1, but is far stronger than HCN (K
a
= 6.2 ×10
−10
).
The NO
2
−
ion will act as a weak base.
■
Water has relatively little acid–base effect.
Therefore, an aqueous solution of NaNO
2
should be slightly basic. We can
now determine how basic by applying our understanding of equilibrium.
The Relationship of K
a
to K
b
Consider an aqueous 0.500 M NaNO
2
solution. The process in which the nitrite
ion (or any base) reacts with water to produce the conjugate acid and hydroxide
ion is called
base hydrolysis. The important equilibrium is
NO
2
−
(aq) + H
2
O(l)
HNO
2
(aq) + OH
−
(aq) K
b
= ?
Note that this equation describes the equilibration of a base with water. What is
the value for K
b
? When we look in Appendix 5, we do not find an entry for NO
2
−
.
However, we do find a value for the K
a
of nitrous acid:
HNO
2
(aq)
H
+
(aq) + NO
2
−
(aq) K
a
= 7.0 × 10
−4
Is it possible to relate the two equilibria to get a value for K
b
of the nitrite ion
hydrolysis? The short answer is yes. If we take the expression for K
b
and multiply
by
[H
+
]
[H
+
]
, we get
K
b
=
[HNO
2
][OH
−
]
[NO
2
−
]
×
[H
+
]
[H
+
]
=
[HNO
2
][OH
−
][H
+
]
[NO
2
−
][H
+
]
Because K
w
= [H
+
][OH
−
], we can substitute K
w
into the equation, which gives
K
a
=
[HNO
2
]K
w
[NO
2
−
][H
+
]
If you write the mass-action expression for K
a
for the ionization of nitrous acid,
you might note that
[HNO
2
]
[NO
2
−
][H
+
]
is equal to 1/K
a
. This means that the K
b
expres-
sion may be rewritten as
K
b
=
K
w
K
a
or, as more often cited,
K
w
= K
a
× K
b
17.7 Assessing the Acid–Base Behavior of Salts in Aqueous Solution 753
Application

This means that we can determine the K
a
or K
b
value for the conjugate of any
weak acid or base, given its equilibrium constant. For the nitrite ion,
K
b
=
K
w
K
a
=
1.0 ×10
−14
7.0 ×10
−4
= 1.4 ×10
–11
We may now determine the pH of this weak base as we would any other weak base
in solution. We will do this as Exercise 17.15.
EXERCISE 17.15 pH of a Salt with a Cation with No Acidic Properties
Determine the pH of a 0.500 M NaNO
2
solution.
First Thoughts
As we discussed previously, the Na
+
cation has no acid−base properties, and the
hydrolysis of the NO
2
−
ion will produce OH
−
ions, leading to a basic solution.
NO
2
−
(aq) + H
2
O(l)
HNO
2
(aq) + OH
−
(aq)
K
b
=
[HNO
2
][OH
−
]
[NO
2
−
]
= 1.4 ×10
−11
We may now proceed as with any other weak-base problem.
NO
2
−
(aq) + H
2
O(l)
HNO
2
(aq) + OH
−
(aq)
initial 0.500 −00
change −x −xx
final 0.500 − x −xx
with assumption 0.500 −xx
In this exercise,“x” = [HNO
2
] = [OH
−
].
Solution
1.4 ×10
–11
=
x
2
0.500
x = [OH
−
] = [HNO
2
] = 2.6 × 10
−6
M
This passes the 5% test (K
b
is so small!).
pOH =−log(2.6 ×10
−6
) = 5.59
pH = 14 − 5.59 = 8.41
Further Insights
Does the answer make sense? We have a weak base, and the pH is indicative of this.
Therefore, our calculation is reasonable.
PRACTICE 17.15
Determine the pH of a 0.250 M sodium acetate (CH
3
COONa) solution.
See Problems 75–82.
What happens when both the cation and the anion have acid–base properties?
That is, what if the cation can react to supply hydrogen ion to the solution and the
anion can supply hydroxide ion? In a broad sense, we can determine whether the
solution will be acidic or basic from the relative strengths of the acidic and basic
754 Chapter 17 Acids and Bases
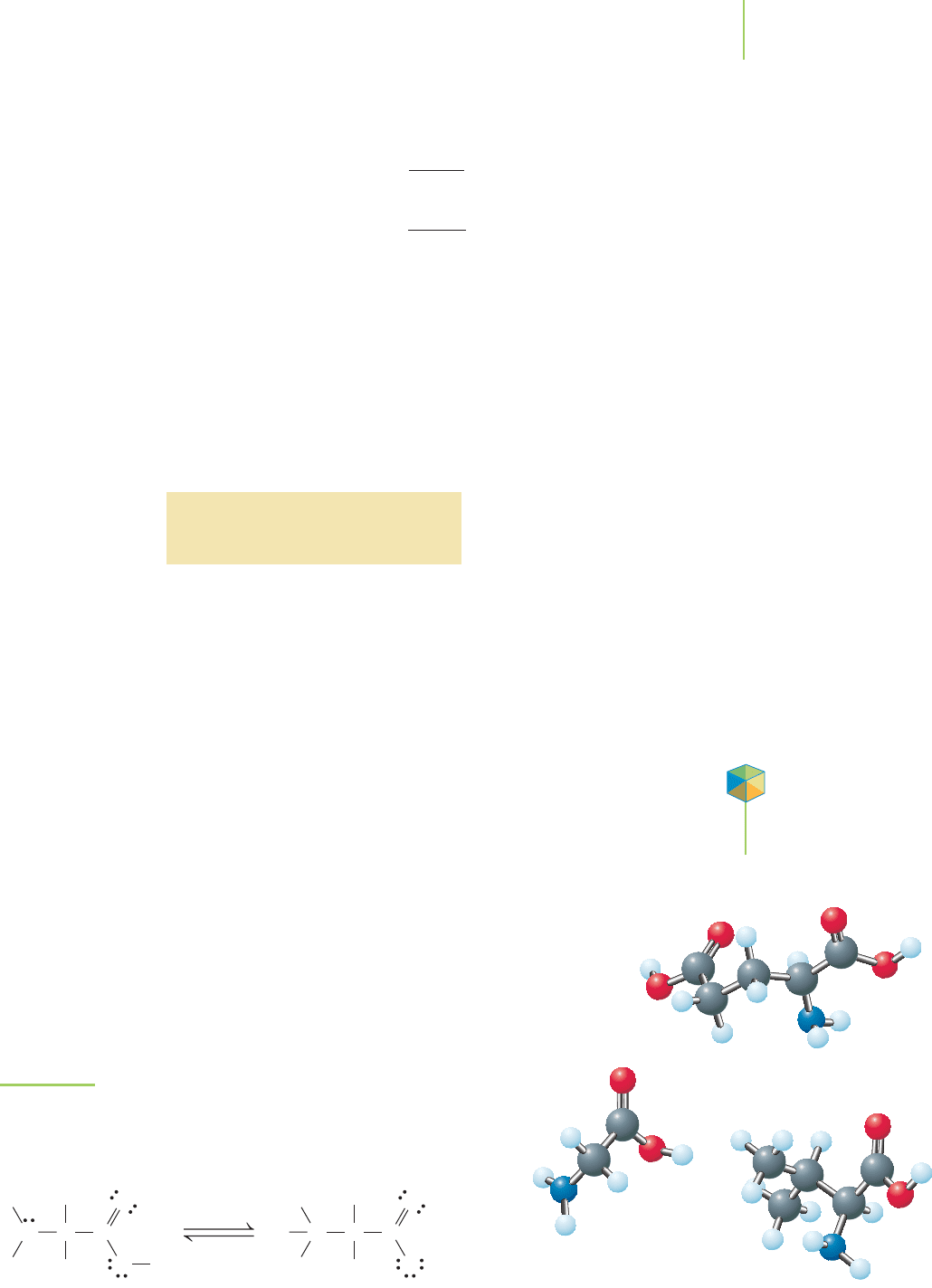
FIGURE 17.19
Amino acids are soluble in water because they often exist as
zwitterions—doubly charged amino acids. As shown here with alanine,
this can occur because the ONH
2
is a stronger base than the OCOO
−
,
so the hydrogen ion will move from the COOH to the amine group.
parts of the salt. For example, we can view ammonium cyanide (NH
4
CN) as un-
dergoing two important equilibrium reactions:
NH
4
+
(aq)
NH
3
(aq) + H
+
(aq) K
a
=
K
w
K
b(NH
3
)
= 5.6 ×10
–10
CN
−
(aq) + H
2
O(l)
HCN(aq) + OH
−
K
b
=
K
w
K
a(HCN)
= 1.6 ×10
–5
Based on the equilibrium constants for the reactions, the CN
−
ion is a much
stronger base than the NH
4
+
ion is an acid, and we would therefore expect the so-
lution to be basic. However, unlike the previous example using NaNO
2
, in which
only the nitrite ion had any acid–base behavior, here both the cation and the
anion could contribute to the pH of the solution, and we must take both equilib-
ria into account. In the sense that we have one substance that acts as an acid and
one that acts as a base, this is similar to an amphiprotic substance. The derivation
for the pH of a substance that has both acid and base properties, such as this type
of salt or an amphiprotic substance such as Na
2
HPO
4
, is fairly complex. The re-
sulting formula, however, is simple and useful:
[H
+
] = (
K
a(NH
4
+
)
× K
a(HCN)
)
1/2
pH =
1
⁄2
(pK
a(NH
4
+
)
+ pK
a(HCN)
)
If the concentration of the substance is greater than 0.100 M (true with
amphiprotic substances as well), the pH of the aqueous salt solution is approxi-
mately concentration independent. Let’s use these equations to calculate the pH
of a 0.800 M NH
4
CN solution.
[H
+
] = (
K
a(NH
4
+
)
× K
a(HCN)
)
1/2
= (5.6 × 10
−10
× 6.2 × 10
−10
)
1/2
= 5.9 × 10
−10
M pH = 9.23
Amino acids are biologically vital compounds that have the ability to act as an
acid and as a base within the same molecule. That is, one part of the molecule is
acidic and a different part of the molecule is basic. Amino acids can polymerize
into large units to form proteins such as hemoglobin (which transports oxygen),
pepsinogen (which digests other proteins), and human growth hormone (which
promotes normal growth). Amino acids are water soluble because they carry
both positive and negative charge in aqueous solution. An example of how this
can happen, involving the amino acid alanine, is shown in Figure 17.19. When
dissolved in water, the carboxylic acid group loses a hydrogen ion, and the amine
group gains a hydrogen ion.Why does this happen? The —NH
3
is a stronger base
than the —COO
−
, so the hydrogen ion will move from the COOH to the amine
group. Ions that are doubly ionized in this way are called
zwitterions. They can act
in the same way as any other amphiprotic substance, donating a hydrogen ion to
water or accepting one from water.
17.7 Assessing the Acid–Base Behavior of Salts in Aqueous Solution 755
Application
C
HEMICAL ENCOUNTERS:
Acid–Base Properties
of Amino Acids
N CC
CH
3
H
H
H
H
O
O O
NH CC
CH
3
H
H
H
O
Neutral form Zwitterion
Glutamic acid
Valine
Glycine
Some amino acids.

EXERCISE 17.16 pH of Zwitterions
Given the following reactions and equilibrium constants, calculate the pH of a so-
lution of 0.200 M alanine.
CH
3
(NH
3
+
)COO
−
(aq)
CH
3
(NH
2
)COO
−
(aq) + H
+
(aq)
K
a
2
= 1.4 × 10
−10
CH
3
(NH
3
+
)COO
−
(aq) + H
2
O(l)
CH
3
(NH
3
+
)COOH(aq) + OH
−
(aq)
K
b
2
= 2.2 × 10
−12
First Thoughts
The donation of hydrogen ion by alanine is favored over its acceptance of a hydro-
gen ion. We therefore would expect the solution to be somewhat, though not
strongly, acidic.
Solution
We must find
K
a
1
for the second equation, just as in the ammonium cyanide case
described previously.
K
a
1
=
K
w
K
b
2
=
1.0 ×10
−14
2.2 ×10
−12
= 4.5 ×10
–3
[H
+
] = (
K
a
2
× K
a
1
)
1/2
= (1.4 × 10
−10
× 4.5 × 10
−3
)
1/2
= 7.9 × 10
−7
M
pH = 6.10
Further Insights
At this pH, alanine exists primarily as the zwitterion and is therefore electrically
neutral. This is called the isoelectric pH. Each amino acid has a different isoelectric
point, depending on its own acid–base properties. Chemists and biologists make
use of the different isoelectric points (Figure 17.20) to separate amino acids in an
electric field by changing the pH, because each amino acid will respond to the elec-
tric field differently depending on its isoelectric point.
Does our answer make sense? We asserted that the solution should be somewhat
acidic because the acid-producing equilibrium is favored over the base-producing
equilibrium. Food for thought: What would you expect to be the pH if the value of
K
a
for the hydrogen ion–producing reaction in a particular salt is the same as the
value of K
b
for the base-producing equilibrium of the salt? Can you think of any
examples of this?
PRACTICE 17.16
What is the pH of a 0.150 M alanine solution?
See Problems 83 and 84.
The principles that we have introduced for the evaluation of the pH of a salt
can be extended from the two cases we have dealt with to a third case, in which
the anion has no basic properties but the cation does have acidic properties. The
thinking—that is, the questions that we raise—is the same. These questions
about the nature of the equilibria in solution represent the unifying problem-
solving theme in this chapter as well as the previous one dealing with chemical
equilibrium.
Table 17.10 qualitatively summarizes the effect of the cation and of the anion
on the pH of a salt.
756 Chapter 17 Acids and Bases
FIGURE 17.20
Isoelectric focusing can be used to purify
and identify a mixture of compounds.
For example, a sample of proteins from a
particular cultivar of barley can be sepa-
rated into the individual “bands” shown
here. The proteins have been stained
purple with a dye.
Photograph by Maria Sulman
17.8 Anhydrides in Aqueous Solution
We started this chapter saying,“Home is where the heart is.” The “home” that we
talked about was not only our individual residence but ourselves and our world.
In a sense, our final application, the set of reactions that cause acid deposition
from the atmosphere, is a proper place to conclude the discussion, because the
Earth is our communal home. To understand acid deposition, of which acid rain
is one form, we need to understand the reactions of acidic and basic anhydrides
(also known as acid and basic oxides) with water.
Basic anhydrides are binary compounds formed between metals with very low
electronegativity and oxygen (see Section 17.2 for review of why these reactions
would form bases). Strong bases are formed when Group IA and Group IIA
anhydrides (an =“without” + hydro =“water”) react with water. Metal hydrox-
ides are often prepared this way instead of by reaction of the metal with water,
which can sometimes be violent, as with the reaction of cesium with water to
produce cesium hydroxide and hydrogen gas. Examples of anhydride and water
reactions are
Li
2
O(s) + H
2
O(l)
2LiOH(aq)
CaO(s) + H
2
O(l)
Ca(OH)
2
(aq)
Acid anhydrides are binary compounds formed between nonmetals and oxy-
gen. Examples are SO
2
,SO
3
,NO
2
,P
4
O
10
, and CO
2
. These compounds react with
water to form acids, their acid strength being related to the electronegativity of
the nonmetal combined with oxygen. One example is the reaction of sulfur diox-
ide generated in industrial smokestacks with water:
SO
2
(g) + H
2
O(l)
H
2
SO
3
(aq)
The sulfurous acid generated in this reaction is not strong. However, dust in the
air can catalyze the reaction between SO
2
and oxygen:
2SO
2
(g) + O
2
(g)
2SO
3
(g)
In a reaction analogous to the Contact process, the sulfur trioxide reacts with
water vapor in the air to form sulfuric acid:
SO
3
(g) + H
2
O(l)
H
2
SO
4
(aq)
The acid that is formed can fall to Earth on a variety of surfaces, including snow,
rain, and fog, and deposit on trees, lakes, and the like, and that is why we call the
process
acid deposition.
Nitrogen and oxygen released from the tailpipes of motorized vehicles during
operation can react to form nitric oxide, which then slowly reacts with atmos-
pheric oxygen to form nitrogen dioxide:
N
2
(g) + O
2
(g)
2NO(g)
2NO(g) + O
2
(g)
2NO
2
(g)
17.8 Anhydrides in Aqueous Solution 757
Effect of Cation and Anion of the Acidity of a Salt
Cation Anion Aqueous Solution Example
Acidic Neutral Acidic NH
4
NO
3
Neutral Basic Basic Na
2
CO
3
Neutral Neutral Neutral NaCl
Acidic Basic Depends on the relative
strength of each
TABLE 17.10
Application
C
HEMICAL
ENCOUNTERS:
Acid Deposition and
Acid-Neutralizing
Capacity
Acid deposition can severely damage the
environment.
