Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

838 Chapter 19 Electrochemistry
In Step 4 we balance the number of oxygen atoms by adding water molecules
to the product side of the equation.
Cu(s) n Cu
2+
(aq)
HNO
3
(aq) n NO(g) + 2H
2
O(l)
In Step 5 the number of hydrogen atoms is then balanced by adding H
+
to
the reactant side. Note that in both steps 4 and 5, we did not need to modify the
copper half-reaction.
Cu(s) n Cu
2+
(aq)
3H
+
(aq) + HNO
3
(aq) n NO(g) + 2H
2
O(l)
The charges are then balanced in Step 6 by adding electrons to the two half-
reactions. We note that the change in the oxidation number of the element being
oxidized or reduced must equal the number of electrons lost or gained in the
half-reaction. The copper half-reaction has electrons added as a product; the ni-
tric acid half-reaction has electrons added as a reactant. In every redox reaction,
one half-reaction gets the electrons on the right, the other on the left.
Cu(s) n Cu
2+
(aq) + 2e
−
3e
−
+ 3H
+
(aq) + HNO
3
(aq) n NO(g) + 2H
2
O(l)
In Step 7 we make sure that the number of electrons is the same in both half-
reactions by multiplying the half-reactions by an integer. In this case, we multiply
the copper reaction by 3 and the nitric acid reaction by 2. Then, in Step 8, we add
the two reactions together and simplify by eliminating similar items from both
sides of the equation.
3{Cu(s) n Cu
2+
(aq) + 2e
−
}
2 {3e
−
+ 3H
+
(aq) + HNO
3
(aq) n NO(g) + 2H
2
O(l)}
3Cu(s) +6H
+
(aq) + 2HNO
3
(aq) n 3Cu
2+
(aq) + 2NO(g) + 4H
2
O(l)
The result is the balanced redox reaction. One observation and one question can
reasonably arise. The observation is that even though the equation is electrically
balanced, each side is not electrically neutral. That is, we have the same +6 charge
on each side! Now for the question: Where are the negative charges that would
make each side electrically neutral? The answer comes when we remember that
we have written net ionic equations, rather than complete ionic or molecular
equations. In this case, the molecular form comes by adding 6 mol of nitrate ion
(NO
3
−
) to both sides to give
3Cu(s) +8HNO
3
(aq) n 3Cu(NO
3
)
2
(aq) + 2NO(g) + 4H
2
O(l)
In other reactions, spectator ions such as Na
+
or Cl
−
(if they are actually in the
solution!) that are not listed in the net ionic equation make the system electrically
neutral. The key point is that when we write equations in net ionic form, we expect
them to be electrically balanced, not electrically neutral.
3Cu(s) + 6H
+
(aq) + 2HNO
3
(aq)
+ 6NO
3
–
3Cu
2+
(aq) + 2NO(g) + 4H
2
O(l)
+ 6NO
3
–
3Cu(s) + 8HNO
3
(aq) 3Cu(NO
3
)
2
(aq) + 2NO(g) + 4H
2
O(l)
→
→
EXERCISE 19.4 Balancing Redox Equations in Acidic Solutions
Balance the following equation in acidic solution.
Cr
2
O
7
2−
(aq) + NO(g) n Cr
3+
(aq) + NO
3
−
(aq)
Solution
Step 1: Determine the oxidation state on each element in each compound in
the chemical equation. Do we in fact have a redox reaction?
Cr
2
O
7
2−
(aq) + NO(g) n Cr
3+
(aq) + NO
3
−
(aq)
Oxidation states +6 −2 +2 −2 +3 +5 −2
Nitrogen is being oxidized from +2 to +5. Chromium is being reduced from
+6 to +3. This is a redox reaction.
Step 2: Separate the redox reaction into two parts: an oxidation reaction and a
reduction reaction. Include just the compounds that have a change in the oxi-
dation state.
Cr
2
O
7
2−
(aq) n Cr
3+
(aq) (reduction)
NO(g) n NO
3
−
(aq) (oxidation)
Step 3: Balance all atoms except oxygen and hydrogen.
Cr
2
O
7
2−
(aq) n 2Cr
3+
(aq)
NO(g) n NO
3
−
(aq)
Step 4: Balance the oxygen atoms by adding H
2
O to one side in each half-
reaction.
Cr
2
O
7
2−
(aq) n 2Cr
3+
(aq) + 7H
2
O(l)
2H
2
O(l) + NO(g) n NO
3
−
(aq)
Step 5: Balance the hydrogen atoms by adding H
+
to one side in each half-
reaction.
14H
+
(aq) + Cr
2
O
7
2−
(aq) n 2Cr
3+
(aq) + 7H
2
O(l)
2H
2
O(l) + NO(g) n NO
3
−
(aq) + 4H
+
(aq)
Step 6: Balance the charges in each half-reaction by adding electrons to one
side.
6e
−
+ 14H
+
(aq) + Cr
2
O
7
2−
(aq) n 2Cr
3+
(aq) + 7H
2
O(l)
2H
2
O(l) + NO(g) n NO
3
−
(aq) + 4H
+
(aq) + 3e
−
Step 7: As needed, multiply one or both of the half-reactions by some coeffi-
cient so that the same number of electrons will appear in both half-reactions.
6e
−
+ 14H
+
(aq) + Cr
2
O
7
2−
(aq) n 2Cr
3+
(aq) + 7H
2
O(l)
2{2H
2
O(l) + NO(g) n NO
3
−
(aq) + 4H
+
(aq) + 3e
−
}
Step 8: Add the reactions together and simplify. Note that the electrons on
each side mathematically cancel, indicating the same number of electrons
gained in the reduction as lost in the oxidation.
6e
−
+ 14H
+
(aq) + Cr
2
O
7
2−
(aq) n 2Cr
3+
(aq) + 7H
2
O(l)
4H
2
O(l) + 2NO(g) n 2NO
3
−
(aq) + 8H
+
(aq) + 6e
−
6e
−
+ 14H
+
(aq) + Cr
2
O
7
2−
(aq) + 4H
2
O(l) + 2NO(g) n
2Cr
3+
(aq) + 7H
2
O(l) + 2NO
3
−
(aq) + 8H
+
(aq) + 6e
−
= 6H
+
(aq) + Cr
2
O
7
2−
(aq) + 2NO(g) n 2Cr
3+
(aq) + 2NO
3
−
(aq) + 3H
2
O(l)
19.3 Redox Equations 839

We have 8 water molecules on the right and 2 on the left. This leaves an excess of
6 water molecules on the right, to give us our final balanced equation in basic
solution:
8OH
−
(aq) + CH
3
OH(aq) + 6MnO
4
−
(aq) n CO
3
2−
(aq) + 6H
2
O(l) + 6MnO
4
2−
(aq)
EXERCISE 19.5 Balancing Redox Equations in Basic Solutions
Balance the following equation in basic solution.
I
3
−
(aq) + S
2
O
3
2−
(aq) n I
−
(aq) + SO
4
2−
(aq)
Solution
We may first balance the equation in acidic solution, using our multistep procedure
presented in Figure 19.9.
I
3
−
(aq) + S
2
O
3
2−
(aq) n I
−
(aq) + SO
4
2−
(aq)
PRACTICE 19.4
Balance the following reaction in acidic solution.
ClO
−
(aq) + H
+
(aq) + Cu(s) n Cl
−
(aq) + H
2
O(l) + Cu
2+
(aq)
See Problems 35–38, 47, 48, and 51.
The process we have used to balance the reaction focuses on the use of acidic
solutions. Chemical reactions can also occur in basic conditions. The method we
present to balance basic reactions requires a little chemical sleight-of-hand but
does work effectively. Essentially, we balance the reaction as though it were in an
acidic solution (by adding H
+
as necessary) and then add a quantity of hydroxide
ion (OH
−
) as necessary, to both sides of the equation. Mathematically, we still
have our equality. Chemically, we neutralize the H
+
, getting water on one side
and excess base on the other. Although this does not depict what goes on in the so-
lution (we are starting with a base, not doing a titration!) we correctly end up
with an excess of OH
−
on one side or the other.
We will show the method by looking at the first step in a procedure to analyze
methanol (CH
3
OH) by reaction with the permanganate ion (MnO
4
−
) in base.
Before balancing, we have
CH
3
OH(aq) + MnO
4
−
(aq) n CO
3
2−
(aq) + MnO
4
2−
(aq)
To balance the reaction in basic solution, we first balance it as though it were in
acidic solution. Using our half-reaction technique, we get the following oxidation
and reduction half-reactions:
2H
2
O(l) + CH
3
OH(aq) n CO
3
2−
(aq) + 8H
+
(aq) + 6e
−
(oxidation)
e
−
+ MnO
4
−
(aq) n MnO
4
2−
(aq) (reduction)
We can multiply the reduction reaction by 6 to balance electrons and add the
half-reactions to get the final equation, balanced in acidic solution:
2H
2
O(l) + CH
3
OH(aq) + 6MnO
4
−
(aq) n CO
3
2−
(aq) + 8H
+
(aq) + 6MnO
4
2−
(aq)
To balance in basic solution, we add an amount of OH
−
to each side equal to the
amount of H
+
.
840 Chapter 19 Electrochemistry
The reaction of purple permanganate
ion with methanol.
2H
2
O(l) + CH
3
OH(aq) + 6MnO
4
−
(aq) n CO
3
2−
(aq) + 8H
+
(aq) + 6MnO
4
2−
(aq)
+ 8OH
−
(aq) + 8OH
−
(aq)
8OH
−
(aq) + 2H
2
O(l) + CH
3
OH(aq) + 6MnO
4
−
(aq) n CO
3
2−
(aq) + 8H
2
O(l) + 6MnO
4
2−
(aq)
The iodine changes from an oxidation state of −
1
⁄3 in I
3
−
to −1inI
−
. (reduction)
The sulfur changes from an oxidation state of +2 in S
2
O
3
2−
to +6 in SO
4
2−
.
(oxidation)
The balanced half-reactions in acidic solution are
2e
−
+ I
3
−
(aq) n 3I
−
(aq)
5H
2
O(l) + S
2
O
3
2−
(aq)n 2SO
4
2−
(aq) + 10H
+
(aq) + 8e
−
We multiply the reduction equation by 4 to equalize the electrons gained and lost in
the reduction and oxidation half-reactions
4{2e
−
+ I
3
−
(aq) n 3I
−
(aq)} = 8e
−
+ 4I
3
−
(aq) n 12I
−
(aq)
5H
2
O(l) + S
2
O
3
2−
(aq) n 2SO
4
2−
(aq) + 10H
+
(aq) + 8e
−
We add the two half-reactions to get the final, balanced equation in acidic solution.
5H
2
O(l) + S
2
O
3
2−
(aq) + 4I
3
−
(aq) n 2SO
4
2−
(aq) + 12I
−
(aq) + 10H
+
(aq)
To balance in base, we add 10OH
−
to both sides.
5H
2
O(l) + S
2
O
3
2−
(aq) + 4I
3
−
(aq) n 2SO
4
2−
(aq) + 12I
−
(aq) + 10H
+
(aq)
+ 10OH
−
(aq) + 10OH
−
(aq)
We cancel our the extra waters to give the equation that is now balanced in base.
10OH
−
(aq) +S
2
O
3
2−
(aq) + 4I
3
−
(aq) n 2SO
4
2−
(aq) + 12I
−
(aq) + 5H
2
O(l)
Is the equation electrically and atomically balanced? Check to make sure that the
charge is the same on both sides of the equation and that there is the number of
atoms on each side. If one or both of these checks do not work, then we’ve made a
mistake balancing the equation.
PRACTICE 19.5
Balance the following equation in basic solution.
MnO
4
−
(aq) + Mn
2+
(aq) n MnO
2
(s)
(Hint: MnO
2
can be written as the product in both an oxidation and a reduction
half-reaction.)
See Problems 39–42 and 52.
Manipulating Half-Cell Reactions
We have learned to balance redox reactions by recognizing that they include elec-
tron loss and electron gain.Although calculating the spontaneity of an individual
half-reaction may lead to the conclusion that the half-reaction is spontaneous,
this is only half of the picture. Because a redox reaction is simply the sum of an
oxidation half-reaction and a reduction half-reaction, we determine the spon-
taneity of the resulting reaction only by including both. Remember that we are
using the SHE as our reference point in these calculations.
We can then say that for two half-reactions, the sum of their change in free en-
ergy should be equal to the free energy change for the complete redox reaction:
∆
rxn
G° = ∆G
1
° + ∆G
2
°
If we substitute the equivalent expression (−nFE°) for ∆G°, the equation
becomes
−n
rxn
FE
rxn
° =−n
1
FE
1
° +−n
2
FE
2
°
19.3 Redox Equations 841

Because the value of F is a constant, and because the number of electrons was the
same when we balanced the reaction (n
1
= n
2
= n
rxn
), all terms but the cell po-
tentials cancel:
E
rxn
° =E
1
° + E
2
°
This means that if the redox reactions are written so that one is a reduction and
one is an oxidation, and the cell potential for the oxidation reaction relative to the
SHE has the sign opposite that of its half-reaction potential when written as a re-
duction, then the two half-reaction potentials are additive for a balanced redox
equation.
E
rxn
° =E
red
° + E
ox
°
From that conclusion, we can say, for example, that if copper is oxidized in a re-
action, we write it as an oxidation, and just as we reverse the standard free energy
value,
∆G°, when we reverse a reaction, we reverse the E°, as follows:
Cu
2+
(aq) + 2e
−
n Cu(s) E° =+0.34 V (reduction)
Cu(aq) n Cu
2+
(aq) + 2e
−
E° =−0.34 V (oxidation)
This notion of reversing the cell potential relative to the SHE when we reverse the
reaction gives a consistent picture of the thermodynamic relationship between
∆G°
and E°. In addition, it is a reminder that until 1953, when IUPAC changed its pro-
tocol to list half-reaction potentials as reductions, they were formerly listed as
oxidations, with opposite signs relative to the SHE.
We know that the nitric acid−copper reaction that we introduced at the be-
ginning of this section is spontaneous, as evidenced by its effect on Ira Remsen’s
lungs, hands, and pants. Let’s see how we can manipulate the half-reactions to
confirm this. In Table 19.3, we notice that one of the reactions is the reverse of the
desired reaction.
From the balancing process steps 8 and 9:
3{Cu(s) n Cu
2+
(aq) + 2e
−
}
2{3e
−
+ 3H
+
(aq) + HNO
3
(aq) n NO(g) + 2H
2
O(l)}
3Cu(s) +6H
+
(aq) + 2HNO
3
(aq) n 3Cu
2+
(aq) + 2NO(g) + 4H
2
O(l)
From Table 19.3:
Cu
2+
(aq) + 2e
−
n Cu(s) E° =+0.34 V
3H
+
(aq) + HNO
3
(aq) + 3e
−
n NO(g) + 2H
2
O(l) E° =+0.96 V
To make the half-reactions from the table look like the half-reactions in the redox
reaction, we need to reverse the copper reduction and write it as an oxidation. As
we pointed out above, reversing the reaction also changes the sign on the poten-
tial of the reaction, or, in this case, the half-reaction.
Cu
2+
(aq) + 2e
−
n Cu(s) E° =+0.34 V (reduction)
Cu(aq) n Cu
2+
(aq) + 2e
−
E° =−0.34 V (oxidation)
However, no modification of the potential is necessary when we double or triple a
half-reaction because the number of electrons involved in the process also doubles or
triples. The free energy, however, does change when we multiply the equation,
because
G
◦
=−nFE
◦
, and if, as in this case, we triple n,
G
◦
will triple as
well. This also is consistent with our discussion of thermodynamics in Chapters 5
and 14.
Cu(aq) n Cu
2+
(aq) + 2e
−
E° =−0.34 V (oxidation)
3Cu(s) n 3Cu
2+
(aq) + 6e
−
E° =−0.34 V (oxidation)
Table 19.4 lists the key aspects of manipulating half-reactions.
842 Chapter 19 Electrochemistry
For the copper–nitric acid reaction, the reaction potential is determined by adding
the nitric acid reduction (+0.96 V) to the copper oxidation (−0.34 V). The resulting
potential is positive (+0.62 V), identifying the reaction as a spontaneous process, as
is consistent with Ira Remsen’s experience.
3{Cu(s) n Cu
2+
(aq) + 2e
−
} E° =−0.34 V
2{3e
−
+ 3H
+
(aq) + HNO
3
(aq) n NO(g) + 2H
2
O(l)} E° =+0.96 V
3Cu(s) +6H
+
(aq) + 2HNO
3
(aq) n 3Cu
2+
(aq) + 2NO(g) + 4H
2
O(l) E°
cell
=+0.62 V
EXERCISE 19.6 Dissolving Gold
Nitric acid can be used to dissolve copper. Can nitric acid dissolve gold at standard
conditions? The unbalanced reaction is shown here.
HNO
3
(aq) +Au(s) n Au
3+
(aq) + NO(g)
First Thoughts
What we’re really asking in this problem is “Is the reaction shown spontaneous?”
To determine the spontaneity we can use Faraday’s equation, which requires us to
know the potential of the reaction. We can obtain this by combining properly
balanced half-reactions, which have reduction potentials given in Table 19.3.
Solution
The two unbalanced half-reactions of interest are
Au(s) n Au
3+
(aq)
HNO
3
(aq) n NO(g)
We can balance them in acidic solution (because of the presence of nitric acid) and
combine them to give
Au(s) n Au
3+
(aq) + 3e
−
(oxidation)
3e
−
+ 3H
+
(aq) + HNO
3
(aq) n NO(g) + 2H
2
O(l) (reduction)
3H
+
(aq) + HNO
3
(aq) +Au(s) n NO(g) +Au
3+
(aq) + 2H
2
O(l)
Table 19.3 lists the potential relative to the SHE for each half-reaction written as
a reduction. We can reverse the gold reduction reaction because the metal is
19.3 Redox Equations 843
Important Points for Potentials of Redox Reactions
• Just as free energies can be combined for chemical reactions, an oxidation and a re-
duction half-cell potential can be combined to produce a chemical equation.
• Just as you reverse the sign of ∆G° when you reverse a chemical reaction, you re-
verse the sign of E° when you reverse a half-cell reaction.
• Although ∆G° values depend on the coefficients in the chemical equation (that is,
when you double the coefficients, you double ∆G°), E°values do not. In a half-
reaction, if the coefficients change, the number of electrons, n, will change as well,
in essence canceling the effect of the change to the coefficients.
• Because of the negative sign in
G
◦
=−nFE
◦
, electrochemical cell reactions with a
positive voltage are spontaneous. (Recall from Chapter 15 that the rate is not related
to the spontaneity of the reaction.)
TABLE 19.4
Connective tissue
Nerve
Papillae
Nucleus
Blood supply
Electrically conductive
tissue
Application
C
HEMICAL
ENCOUNTERS:
The Electric Eel
being oxidized. We can use the potentials of the half-reactions to calculate the
cell voltage.
Au(s) n Au
3+
(aq) + 3e
−
E° =−1.50 V
3e
−
+ 3H
+
(aq) + HNO
3
(aq) n NO(g) + 2H
2
O(l) E° =+0.96 V
3H
+
(aq) +HNO
3
(aq) +Au(s) n NO(g) +Au
3+
(aq) +2H
2
O(l) E°
cell
=−0.54 V
The negative value for the cell voltage indicates that the reaction of nitric acid and
gold is not spontaneous. Nitric acid doesn’t dissolve gold.
Further Insights
You’ve probably noticed that you don’t have to balance a redox reaction in order to
determine the standard voltage of the reaction. This is because the voltage of a reac-
tion is not dependent on the stoichiometry of the equation. Simply adding the half-
reaction potentials (as long as one is an oxidation and the other a reduction) yields
the voltage of the resulting reaction. However, it is good practice to balance the
equation so that we can tell how many electrons are exchanged. We will need this in-
formation if our system is at nonstandard conditions, which is most of the time.
A process for dissolving gold was known during the early days of the alchemists.
Because metals like gold and platinum wouldn’t react with strong acids, such as ni-
tric acid, they were known as the royal metals. However, there existed a solution that
would dissolve gold and platinum. These metals were soluble in a mixture of one
part nitric acid and three parts hydrochloric acid known as aqua regia, or royal
water. Aqua regia is used commercially as a first step in separating platinum from
gold that is combined with other metals in their ore mixtures. Both metals are ini-
tially dissolved and then selectively precipitated from solution.
PRACTICE 19.6
Determine the cell voltage at standard conditions for the following redox reactions:
CH
4
(g) + H
2
O(g) n CO(g) + H
2
(g)
Ag(s) + H
2
O
2
(aq) n H
2
O(l) +Ag
+
(aq)
See Problems 27 and 28.
19.4 Electrochemical Cells
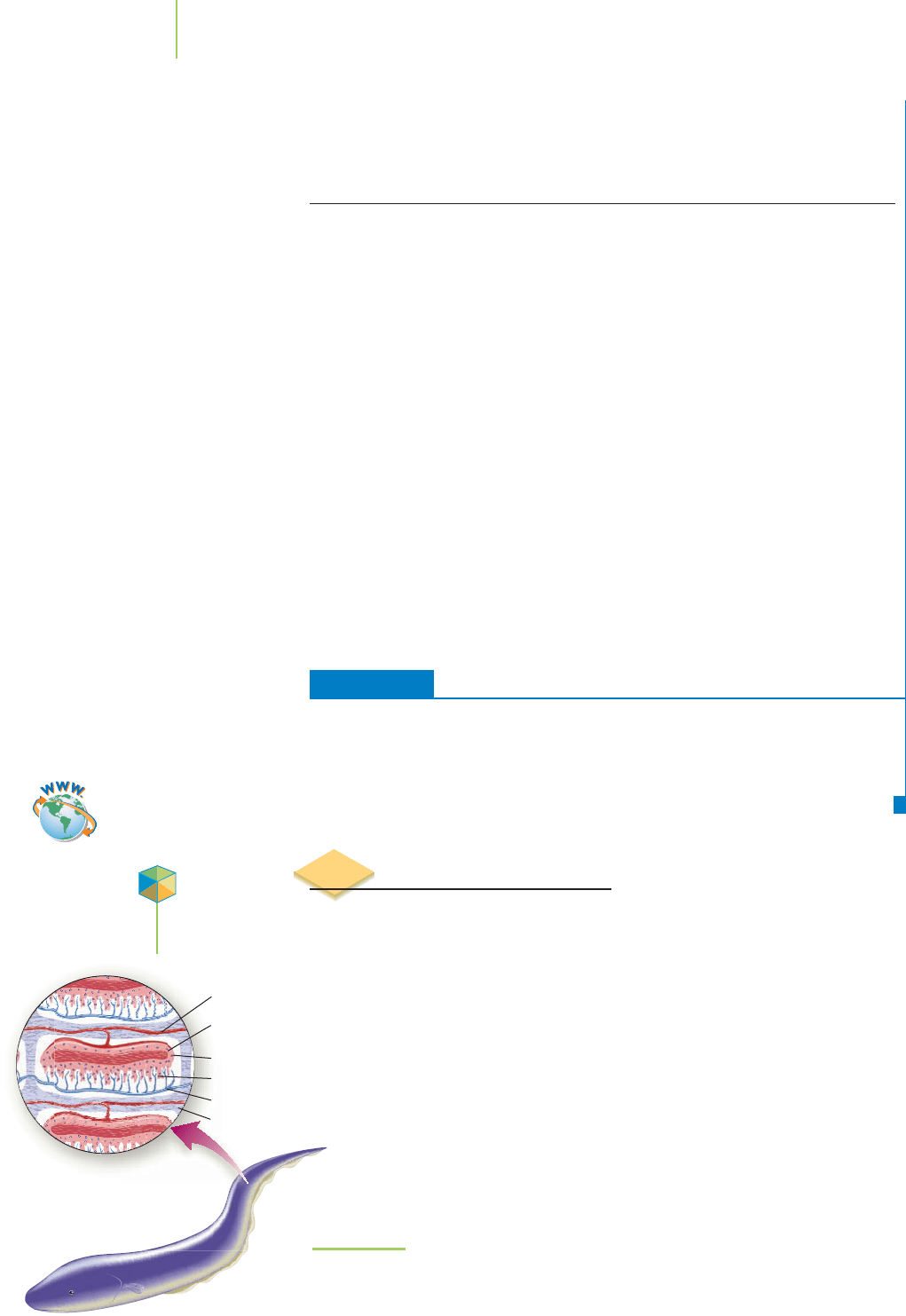
It may be “shocking” to learn, but it’s true: The electric eel is a formidable oppo-
nent. When startled, stepped on, or hunting for food, the eel can deliver up to
1 ampere (1 coulomb of charge per second) at 600 V, enough electrical energy
to stun or even kill large animals. (To be accurate, the electric eel isn’t
an eel. It lives in fresh water and is really a fish.) How does the electric
eel generate the electricity used in hunting?
The powerful shock that the eel can deliver is produced by 5000 to
10,000 specialized cells, called electroplates, in its tail (see Figure 19.10).
Using biochemical processes, the eel charges up the electroplates in
much the same manner as muscle and nerve cells are charged. Then,
when a nerve impulse is sent to the tail, the electroplates discharge their
stored potential. Can you think of an electrical storage device similar to
the electric eel’s that we use every day? The electrochemical cell seems to
fit the definition. We’ll explore the electrochemical cell in this section
844 Chapter 19 Electrochemistry
FIGURE 19.10
Electroplates in the tail of the electric eel develop a potential charge that
can be delivered to shock its prey.
(Drawing by Rick Simonson.)
Video Lesson: Electrochemical
Cells

Na
+
Na
+
Na
+
Na
+
NO
3
–
NO
3
–
NO
3
–
NO
3
–
The salt bridge.
FIGURE 19.12
Using the electrochemical cell to light a bulb.
and learn how we can make use of the electrical en-
ergy of chemical reactions.
Electrochemical Cells in the Laboratory
Redox reactions, such as the dissolving penny, can
take place inside a beaker just like other reactions.
However, we can harness the energy from the elec-
trons that are exchanged in the redox reaction if we
modify the experimental setup. Take a look at what
happens when we separate the copper penny from
the nitric acid shown in Figure 19.11. The brown
fumes produced by the subsequent oxidation of NO
to NO
2
by O
2
still waft from the beaker, and the cop-
per ions are still generated. The redox reaction is still
working. Examine the experimental setup closely to see why it works. We have
3Cu(s) +6H
+
(aq) + 2HNO
3
(aq) n 3Cu
2+
(aq) + 2NO(g) + 4H
2
O(l) E°
cell
=+0.62 V
followed by the oxidation of NO to the poisonous NO
2
gas:
2NO(g) + O
2
(g) n 2NO
2
(g) ∆H° =−112 kJ
The oxidation half-reaction is in the beaker on the right, and the reduction
half-reaction is on the left. The two beakers are connected with a wire that trans-
fers the electrons from beaker to beaker. At both ends of the wire is an electrode.
The electrode in the oxidation reaction is called the anode, and the electrode in
the reduction reaction is called the cathode. Remember, electrons are one of the
products of the copper oxidation. They need someplace to go. Providing a wire
for them to travel into the nitric acid reduction reaction keeps the reaction run-
ning. If we open the circuit on the wire, the reaction stops. The wire is an essen-
tial part of this electrochemical cell.
The tube labeled
salt bridge in the diagram contains an electrolyte such as
potassium chloride or sodium nitrate and allows ions to pass from beaker to
beaker. The salt bridge is needed because as electrons move from the beaker on
the right (the oxidation reaction) to the beaker on the left (the reduction reac-
tion), a strong positive charge will develop at the anode as more of the copper
penny becomes Cu
2+
. A similar thing happens in the other beaker; the H
+
is
being removed to make NO and water, and the cathode becomes negatively
charged. The developing charges attract the electrons as they move away from the
19.4 Electrochemical Cells 845
Copper
electrode
Copper
cell
Electrode
Salt bridge
Nitric acid
cell
FIGURE 19.11
The electrochemical cell. Ira Remsen’s ob-
servations are still valid in this setup. The
nitric acid still acts on the copper. Note
the presence of the salt bridge and the
electrodes.
Visualization: Electrochemical
Half-Reactions in a Galvanic Cell
Visualization: Galvanic (Voltaic)
Cells

beaker. Without the salt bridge, the electrons would stop this type of movement.
We must have a salt bridge in our electrochemical cell design so that the entire
system can remain electrically neutral. The finished electrochemical cell produces
the same reaction that Ira Remsen observed (nitric acid acts upon copper), but
the movement of the electrons through the wire can be used to light a bulb, as
shown in Figure 19.12. We have harnessed the electrons as electrical energy.
EXERCISE 19.7 Alkaline Batteries
Some electrochemical cells are constructed using an alkaline electrolyte. Here are
two half-cells that can be employed:
Cd(OH)
2
(s) n Cd(s)
NiO
2
(s) n Ni(OH)
2
(s)
a. Based on the information in the standard reduction potentials table (Table 19.3),
write the two oxidation−reduction reactions possible from these half-
reactions, and calculate their cell potentials.
b. Which could be used as the basis of an electrolytic cell? ...ofa galvanic cell?
What conditions are required to obtain these specific cell potentials? (Recall
our discussion in Section 19.1.)
c. When used as a voltaic cell, this set of reactions makes a useful battery that can
power portable appliances and tools. Judging on the basis of the elements that
make up the galvanic cell, can you identify the name of this type of battery?
What significance does the reverse reaction have?
Solution
a. The two half-cell reactions are
Cd(OH)
2
(s) + 2e
−
n Cd(s) + 2OH
−
(aq) E° =−0.81 V
NiO
2
(s) + 2H
2
O(l) + 2e
−
n Ni(OH)
2
(s) + 2OH
−
(aq) E° =+0.49 V
If the first reaction is reversed, E°
cell
= (+0.81 V) + (+0.49 V) =+1.30 V.
Cd(s) + NiO
2
(s) + 2H
2
O(l) n Cd(OH)
2
(s) + Ni(OH)
2
(s) E° =+1.30 V
If the second reaction is reversed, E°
cell
= (−0.81 V) + (−0.49 V) =−1.30 V.
Cd(OH)
2
(s) +Ni(OH)
2
(s) n Cd(s) + NiO
2
(s) +2H
2
O(l) E° =−1.30 V
In both cases, the OH
−
(aq) and the electrons cancel when the half-cell reac-
tions are added.
b. To obtain these voltages, the reactions must be run at 25°C at 1 atm pressure
(standard conditions). The concentration of OH
−
(aq) must be 1 M (solids
have a constant concentration). The first oxidation–reduction reaction could
form the basis of a galvanic cell, because E°
cell
is positive. The second would be
electrolytic, because E°
cell
is negative.
c. This voltaic cell is better known as a NiCad battery. The reverse electrolytic
reaction proceeds well, because once formed, the reaction products remain in
contact with the electrodes. Therefore NiCad batteries can be recharged by
running the cell in reverse by applying a reverse voltage greater than the cell
potential.
PRACTICE 19.7
Draw the electrochemical cell for each of the redox reactions given in Practice 19.6.
What is the potential of the galvanic cell? ...ofthe electrolytic cell?
See Problems 57–59.
846 Chapter 19 Electrochemistry
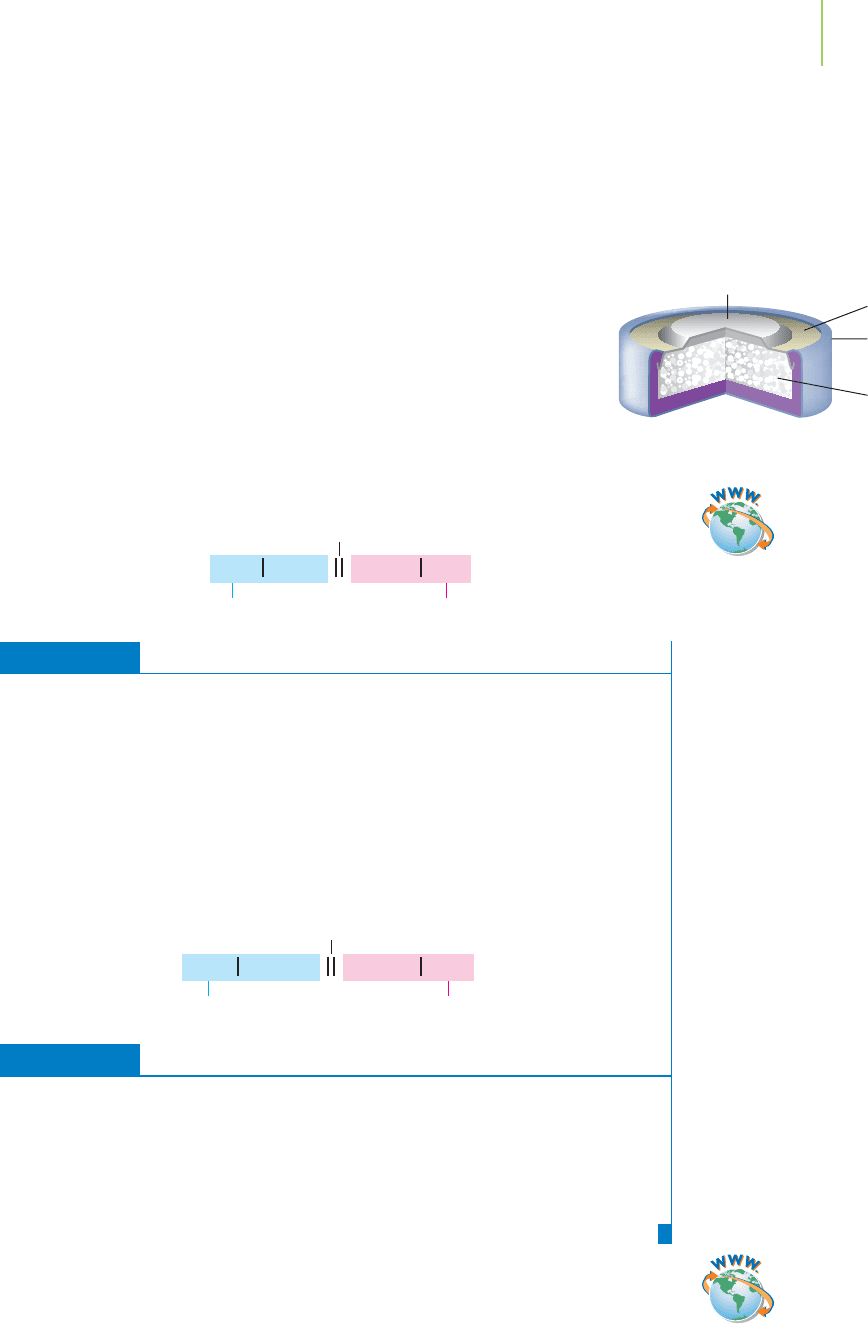
Mg(s) Mg
2+
(aq)Cu
2+
(aq) Cu(s)
Bridge
Anode Cathode
Zn(s) ZnO(s)Ag
2
O(s) Ag(s)
Bridge
Anode Cathode
Cathode
Insulation
Anode
(zinc container)
Paste of Ag
2
O
in a medium of
KOH and ZnO
Cutaway view of button battery.
Cell Notation
Because the balanced chemical equations require a lot of time to write, shorthand
notation (called
cell notation) is often used when describing electrochemical cells.
It may look hard to do, but cell notation is as easy as knowing your ABCs—that
is, anode, bridge, cathode. Consider the reaction that takes place in the silver
oxide cell found in pacemaker batteries:
Ag
2
O(s) + Zn(s) n 2Ag(s) + ZnO(s) E° =+1.86 V
A closer look at the half-reactions tells us that silver is being reduced
and zinc is being oxidized. The silver metal must be the cathode, and
the zinc metal must be the anode. We write each half-reaction, and
then, without even balancing them, we construct the overall short-
hand notation.
Zn(s) n ZnO(s) (oxidation, anode) Zn(s) | ZnO(s)
Ag
2
O(s) n Ag(s) (reduction, cathode) Ag
2
O(s) | Ag(s)
EXERCISE 19.8 Shorthand Notation
Write the shorthand cell notation for the following voltaic cell.
Cu
2+
(aq) + Mg(s) n Cu(s) + Mg
2+
(aq)
Solution
Our goal is to write our ABCs—anode, bridge, and cathode. In order to do this, we
need to know which half-reaction is the oxidation and which is the reduction. The
copper ion gains two electrons, so it is reduced. The copper is the cathode. The mag-
nesium loses two electrons, so it is oxidized. It is the anode. We now have enough
information to write the cell notation:
19.4 Electrochemical Cells 847
PRACTICE 19.8
Create a galvanic cell using these half-reactions, and write the cell notation. Check
to make sure your reaction is written as a spontaneous redox reaction.
Fe
2+
(aq) + 2e
−
n Fe(s)
Al
3+
(aq) + 3e
−
n Al(s)
See Problems 55 and 56.
Batteries
Commercial batteries come in many shapes and sizes and are based on a wide va-
riety of chemical processes (Table 19.5). The best battery for any application is
usually chosen by considering power output, cost, convenience, size, and whether
the battery will be rechargeable. The chemistry of commercial batteries is often
rather complex compared to the simple electrochemical cells used in the teaching
lab to convey the basic principles.
Visualization: Zinc/Copper Cells
with Lemons
Video Lesson: CIA
Demonstration: The Fruit-
Powered Clock
Video Lesson: Batteries
