Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


Coordination
Complexes
In 1434, Flemish master Jan van Eyck
painted the Arnolfini Wedding. The
pigments that he mixed and applied to
the painting bring the scene of a newly
married couple to life. The colors we see
in this work are a result of the interaction
of light with electrons in the d orbitals of
transition metals.
868
Contents and Selected Applications
Chemical Encounters: Iron and Hemoglobin
20.1 Bonding in Coordination Complexes
20.2 Ligands
20.3 Coordination Number
20.4 Structure
20.5 Isomers
20.6 Formulas and Names
20.7 Color and Coordination Compounds
Chemical Encounters: Transition Metals and Color
20.8 Chemical Reactions
Chemical Encounters: Cytochromes
Go to college.hmco.com/pic/kelterMEE for online learning resources.
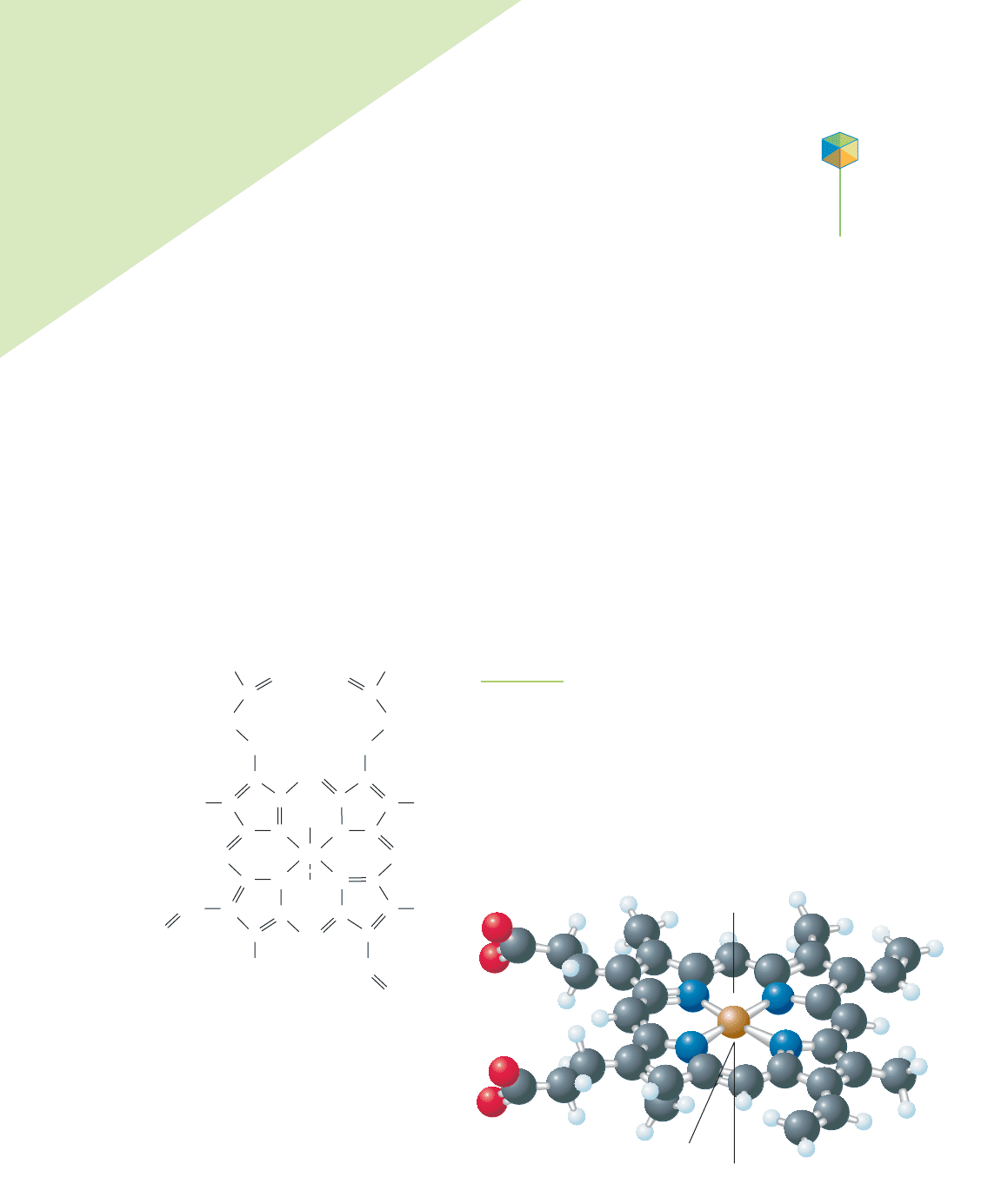
Many of the nutritional supplements we
consume on a daily basis contain iron. Why?
Iron is an essential nutrient with the U.S. recom-
mended daily allowance of 10 mg for men and 15 mg
for women. Even with supplements and recommendations
from nutritional scientists, less than 30% of women aged 12–50
meet their daily recommended allowance of iron. Iron isn’t a vitamin;
rather, it’s called a mineral—the term used for any inorganic element im-
portant for health. It turns out that few items claiming to be rich in iron actu-
ally contain the neutral metal. Instead, they typically contain iron ions in a salt,
often iron sulfate. Why is iron so important to good health? Most of the iron in
the body is contained in a protein known as hemoglobin within the red blood
cells (Chapter 22). Specifically, iron ions are chemically bound in hemoglobin in
a form called a coordination complex.
Within hemoglobin, the iron ions are bonded to other atoms in an elegant
and complex structure known as a heme (see Figure 20.1). The arrangement of
groups attached to the iron enables it to collect an oxygen molecule and later re-
lease it at an appropriate time. Close examination of the heme indicates that the
conformational changes illustrated in Figure 20.2 occur during oxygen binding.
We visited a simpler form of this complex in Chapter 16 when we discussed the
869
Application
C
HEMICAL
ENCOUNTERS:
Iron and
Hemoglobin
Fe
CH
N
+
+
N
C
C
CCH
CC
C
OO
–
O
C
N
C
C
C
CH
CH
N
CH
2
CH
2
CH
3
CH
3
CH
CH
2
CH
2
C
CC
C
C
C
–
O
C
C
CH
CH
2
CH
2
CH
3
CH
3
O
2
binding site
Fe
Histidine
Histidine
FIGURE 20.1
The iron is bonded to other atoms in an elegant and complex structure known
as a heme. The hemoglobin protein uses two amino acid residues to hold the
iron within the protein’s structure. One of the amino acid residues can move out
of the way to allow an oxygen molecule to bind to the iron.
20.1 Bonding in Coordination Complexes
A common feature of bonding within metal complexes is the coordinate covalent
bond
, which we discussed in Chapter 7. Recall that the coordinate covalent bond
forms when both bonding electrons originate from one atom. This means that a
molecule with a lone electron pair (a Lewis base) could potentially be a compo-
nent of a coordination complex. Lewis bases that form a coordinate covalent
bond with a metal or metal ion are known as
ligands. In the aqueous iron com-
plex found in natural water that contains iron, each water molecule donates a pair
of electrons to the iron(III) ion and is therefore the Lewis base. The iron(III) ion
behaves as a Lewis acid, an electron pair acceptor. The resulting bond between the
equilibrium involving oxygen and myoglobin. Without this specific complex
structure, whether in hemoglobin or myoglobin, iron would be incapable of
playing the role of oxygen carrier.
In a typical
coordination complex (or simply complex), a central metal atom
or ion is chemically bonded to several other components. For example, natural
water often contains iron in the form of a chemical combination of an Fe
3+
ion
and six water molecules, [Fe(H
2
O)
6
]
3+
, as shown in Figure 20.3. The bonding,
the structure, and the many biological, medical, and industrial applications of
coordination complexes are the subject of this chapter.
Fe
O
O
O
HH
HH
H
H
H
H
H
H
H
H
O
O
O
870 Chapter 20 Coordination Complexes
Histidine
Protein chain
Iron
Deoxyhemoglobin Oxyhemoglobin
Heme
Bound
oxygen
FIGURE 20.2
Conformational changes take place during the binding of oxygen to the iron ion in hemoglobin.
These changes act like a switch in the hemoglobin protein, activating it and increasing its ability to
bind to three other molecules of oxygen. In the structure shown here, the oxygen molecule (red) is
bound in oxyhemoglobin. When oxygen is not bound to hemoglobin, the protein chains pull the
iron so that the heme sits slightly above the center of the iron ion.
FIGURE 20.3
Natural water often contains
iron in the form of a chemi-
cal combination of an Fe
3+
ion and six water molecules,
[Fe(H
2
O)
6
]
3+
.
HbFe C HbFe C OO
[Fe(H
2
O)
6
]
3+
20.1 Bonding in Coordination Complexes 871
Fe
3+
3+
H
O
H
+
Fe
H
O
H
Carbon monoxide
Oxygen
CO
O O
Application
HbFe O O HbFe O
O
oxygen of water and the iron(III) ion is a coordinate covalent bond. With six
equivalent water molecules donating electron pairs to create six bonds to the cen-
tral Fe
3+
ion, the complete complex ion is [Fe(H
2
O)
6
]
3+
.
In general, we can use the following equation to illustrate the formation of a
coordination complex via donation of a lone pair of electrons from a ligand, L, to
a metal center, M,
M + :L n M–L
The chemistry of hemoglobin is an example of the formation of such a coordi-
nation complex. The iron ion in the hemoglobin molecule forms a bond with an
oxygen molecule and ultimately transports the oxygen molecule from the lungs
to cells throughout the body. Using our shorthand, we can represent the bonding
with oxygen by the following equation:
where Hb represents the complex structure of the hemoglobin molecule. Where
does the oxygen come from? In the lungs, the oxygen
coordinates to the hemo-
globin in a red blood cell—that is, forms a coordinate covalent bond to it. Under
slightly different conditions in the cell, the oxygen is released in the reverse
process and becomes available for respiration.
Carbon monoxide can also coordinate to hemoglobin. In fact, it does so more
strongly than oxygen. A close look at the structure that results indicates that the
lone pair of electrons on the carbon forms the coordinate covalent bond with the
iron in hemoglobin. Equilibrium principles tell us that because the equilibrium
constant for the reaction with CO is much greater (about 200 times greater) than
that for the reaction with oxygen, the presence of small amounts of CO in the
body limits the amount of O
2
that can be carried by hemoglobin. Excessive
amounts of carbon monoxide in the body can result in suffocation. The National
Institute of Occupational Safety and Health (NIOSH) cites an immediate danger
to life with an exposure of 1200 parts per million of CO in air and specifies an
8-hour exposure limit of 35 ppm in air.
FIGURE 20.4
Three important coordination complexes: ferrodoxin, plastocyanin, and Wilkinson’s catalyst. (a) An illustration of
the protein ferrodoxin indicates the location of an Fe
2
S
2
cluster—shown in orange. (b) A drawing of the protein
plastocyanin contains a copper ion—green—held in place by the amino acids in the structure of the protein.
(c) Wilkinson’s catalyst contains a rhodium ion surrounded by triphenylphosphine ligands.
The coordinate covalent bond is very common in compounds involving met-
als and nonmetals. These types of compounds, as shown in Figure 20.4, are often
found at critical reaction centers in biological systems and in many industrial
processes. For example, cytochromes and ferredoxins are iron-containing coordi-
nation complexes in nonanimal biological systems, where they assist in reduction
and oxidation reactions (see Chapter 19) during photosynthesis. In some plants,
nitrogen is converted into a usable form by coordination of an N
2
molecule to a
molybdenum ion in the enzyme nitrogenase. In industrial processes, metal ions
can be used to form coordinate covalent bonds with small molecules in order to
make the small molecule react in certain ways. Wilkinson’s catalyst, shown in
Figure 20.5, is a coordination complex of rhodium used to promote reaction of H
2
with alkenes (in polyunsaturated fats) to make margarine. In another industrial
process used to make the class of molecules known as aldehydes (which are used
in essential oils—volatile compounds that have odors characteristic of plants,
polymer formation, and food additives), carbon monoxide forms a coordination
872 Chapter 20 Coordination Complexes
Rh
Cl
P
P
P
(a) (b) (c)
H
2
(g)
Rh
Cl
P
P
P
HHC
H
H
C
H
H
C
H
H
HHC
H
H
C
H
C
H
FIGURE 20.5
The reaction of hydrogen with an alkene
in the presence ofWilkinson’s catalyst.
This reaction can be used to convert low-
melting-point vegetable oils into semi-
solid fats for use in making margarine.
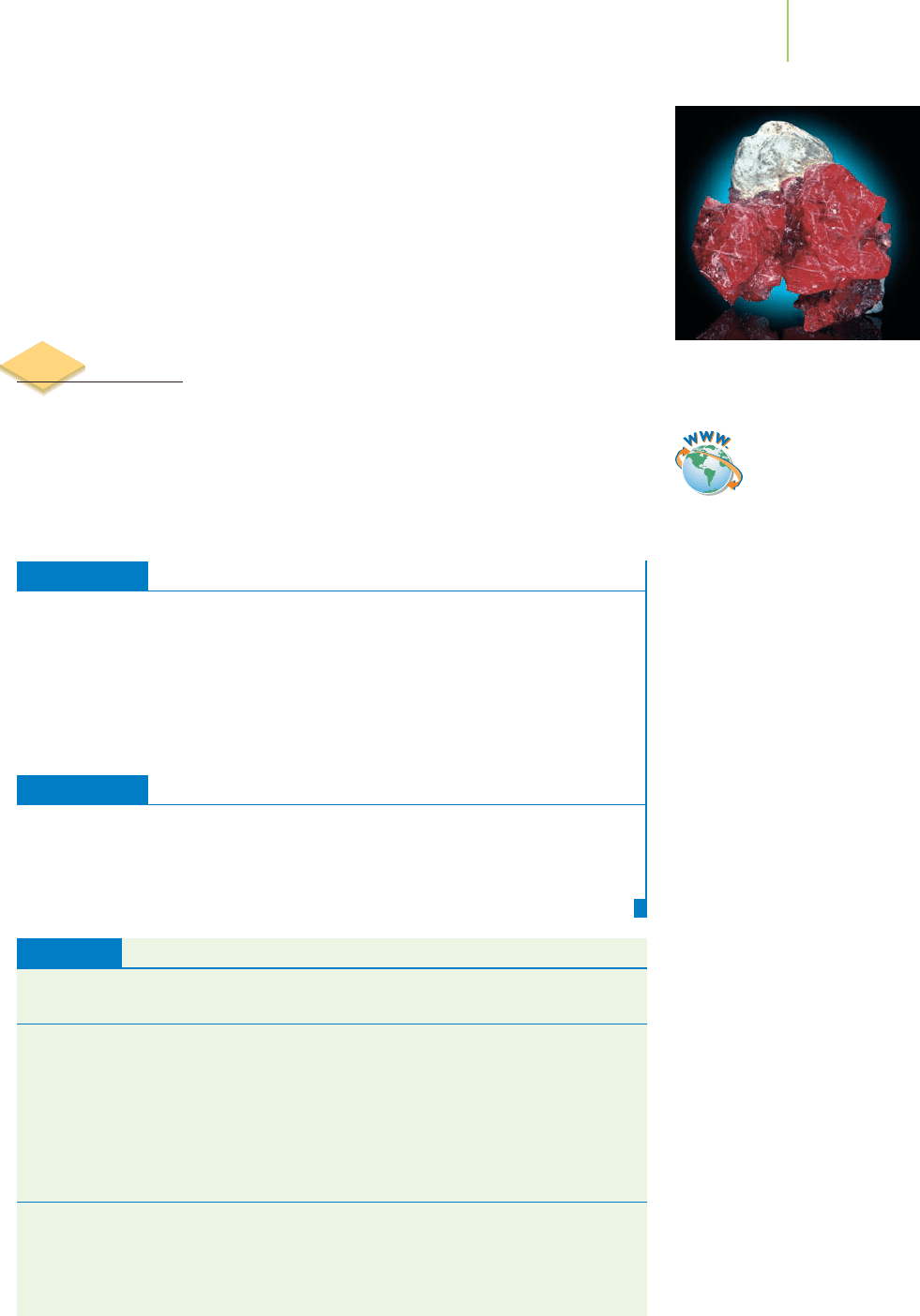
complex with a cobalt atom as it is transformed into the CPO fragment of an
aldehyde.
The coordination complexes of transition metals have some particularly
interesting properties. The color of the complex, such as that found in rubies,
depends on the nature and number of ligands surrounding the metal complex.
The magnetism of coordination complexes, such as the magnetism of the iron
oxide used in videotape, depends on the nature and number of ligands sur-
rounding the metal. The biochemical reactions that are possible with coordina-
tion complexes found in the body are also influenced by the ligands.
20.2 Ligands
A coordination complex such as [Fe(H
2
O)
6
]
3+
consists of a central Fe
3+
metal
ion and coordinated water molecules. The species, such as the water molecules,
that coordinate to the metal are called ligands. Table 20.1 lists some common lig-
ands. Remember that ligands donate a lone pair of electrons to the metal or metal
ion to form the coordinate covalent bond. A metal ion will tend to form coordi-
nate covalent bonds with a specific number of ligands, often either four or six.
EXERCISE 20.1 Identifying Possible Ligands
Which of the following could act as ligands in forming coordination complexes?
a. CH
4
b. N
3
−
c. BH
3
d. CS
2
e. Li
+
Solution
Because each has a nonbonded pair of electrons on one or more atoms, (b) and
(d) could be ligands.
PRACTICE 20.1
Indicate the formula of a coordination complex that might result from the combi-
nation of these metals and ligands.
a. Fe
2+
and 6Cl
−
b. Ni
2+
and 4NH
3
c. Zn
2+
and 6H
2
O
See Problems 3, 4, 9, and 10.
20.2 Ligands 873
Rubies are minerals made of Al
2
O
3
containing less than 1% Cr
2
O
3
as an
impurity.
Selected Common Ligands and Their Names
The atoms shown in red donate a lone pair of electrons to form a coordinate covalent
bond.
Monodentate ligands
Cl
−
chloro Br
−
bromo I
−
iodo
CN
−
cyano NO
2
−
nitro NO
3
−
nitrato*
SCN
−
thiocyanato NO
2
−
nitrito OSO
3
2−
sulfato
SSO
3
2−
thiosulfato O
2−
oxido F
−
fluoro
NH
3
ammine H
2
O aqua NO nitrosyl
CO carbonyl OH
−
hydroxo
Polydentate ligands
H
2
NCH
2
CH
2
NH
2
ethylenediamine (en)
(
−
OOCCH
2
)
2
NCH
2
CH
2
N(CH
2
COO
−
)
2
ethylenediaminetetracetato (EDTA)
−
OOCCOO
−
oxalato (ox)
*The nitrato ligand can be monodentate or bidentate.
TABLE 20.1
Video Lesson: Complexes and
Ligands
Ethylenediamine
H
2
N — CH
2
— CH
2
— NH
2
Some of the molecules and ions that are commonly observed to act as ligands
are shown in Figure 20.6. Oxygen, nitrogen, sulfur, and phosphorus atoms in
molecules are common donor atoms in ligands because they possess a lone pair
of electrons that can be donated to the metal center. Some ligands have non-
bonded pairs of electrons in different locations throughout their structure.
Often, any of these pairs of electrons can be used to create a coordinate covalent
bond. In some cases, only one specific pair of electrons is used to create the
bond. For instance, cyanide (CN
−
) usually binds via the pair of electrons
on the carbon atom. It does so in the “Prussian blue” dye used in dyes
and paints, where the complex [Fe(CN)
6
]
4−
is formed.
Ligands can also use more than one nonbonded pair of electrons to
bond to a metal center. If more than one atom has a nonbonded pair of
electrons, it may use each of those pairs to form independent coordinate
covalent bonds to the metal. In such cases, if the ligand forms two bonds
to the metal, we say it is
bidentate (“two-toothed”); we call it tridentate
(“three-toothed”) if it forms three bonds, and so on. For example, the
bidentate oxalate ion C
2
O
4
2−
can bind to the iron(III) ion in this fashion, as
shown in Figure 20.7. Bathroom stain removers often contain oxalate salts, be-
cause the oxalate ion will coordinate to iron ions in rust and help wash the stain
away. Another common bidentate ligand that donates more than one pair of
electrons is ethylenediamine H
2
NCH
2
CH
2
NH
2
(often abbreviated en), in
which each nitrogen has a lone pair of electrons that can be donated to the
same metal ion.
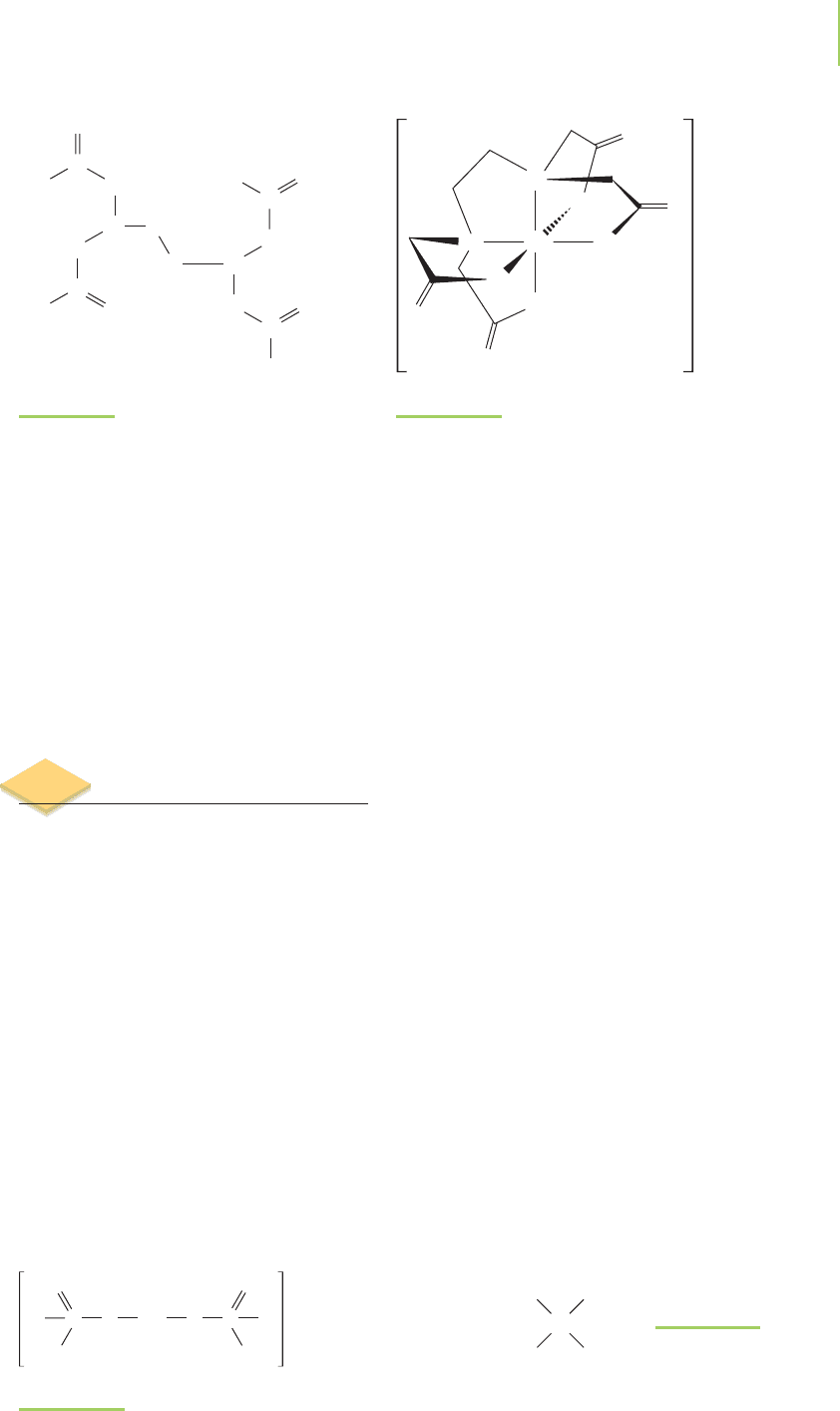
The
polydentate ligands (those that form more than one coordinate covalent
bond to the metal center) are known as
chelates (from the Greek chele for “claw”)
because of the way they clamp onto the metal center and bond tightly. For exam-
ple, the porphyrin ligand is a planar,
tetradentate ligand found in hemoglobin, vi-
tamin B
12
, and chlorophyll, as shown in Figure 20.8. The porphyrin chelates to
iron in hemoglobin, cobalt in vitamin B
12
, and magnesium in chlorophyll. The
chelates are so good at holding the metal firmly within their grasp that it is diffi-
cult to remove the iron from hemoglobin without destroying the hemoglobin
molecule itself. Note that the porphyrin leaves two sites on the metal open so that
it may bind to other ligands. It is the open locations on the metal within the por-
phryin chelate that make chemical reactions possible.
874 Chapter 20 Coordination Complexes
H
H
O
H
H
H
N C OCl
C N
S
FIGURE 20.6
Examples of ligands.
C N
O
O
H
H
H
H
C
C
O
O
N
HH
HH
CC
CC
N
FIGURE 20.7
The oxalate ion is a bidentate ion. Each ox-
alate is capable of donating lone pairs of
electrons from two different atoms to form
two coordinate covalent bonds. Three ox-
alates can bind to one iron(III) ion.
M
L
2
L
1
NN
N
N
FIGURE 20.8
The basic porphyrin structure, show-
ing a metal at the center coordinated
in six different positions. Compare
this structure to that found in the he-
moglobin protein from Figure 20.1.
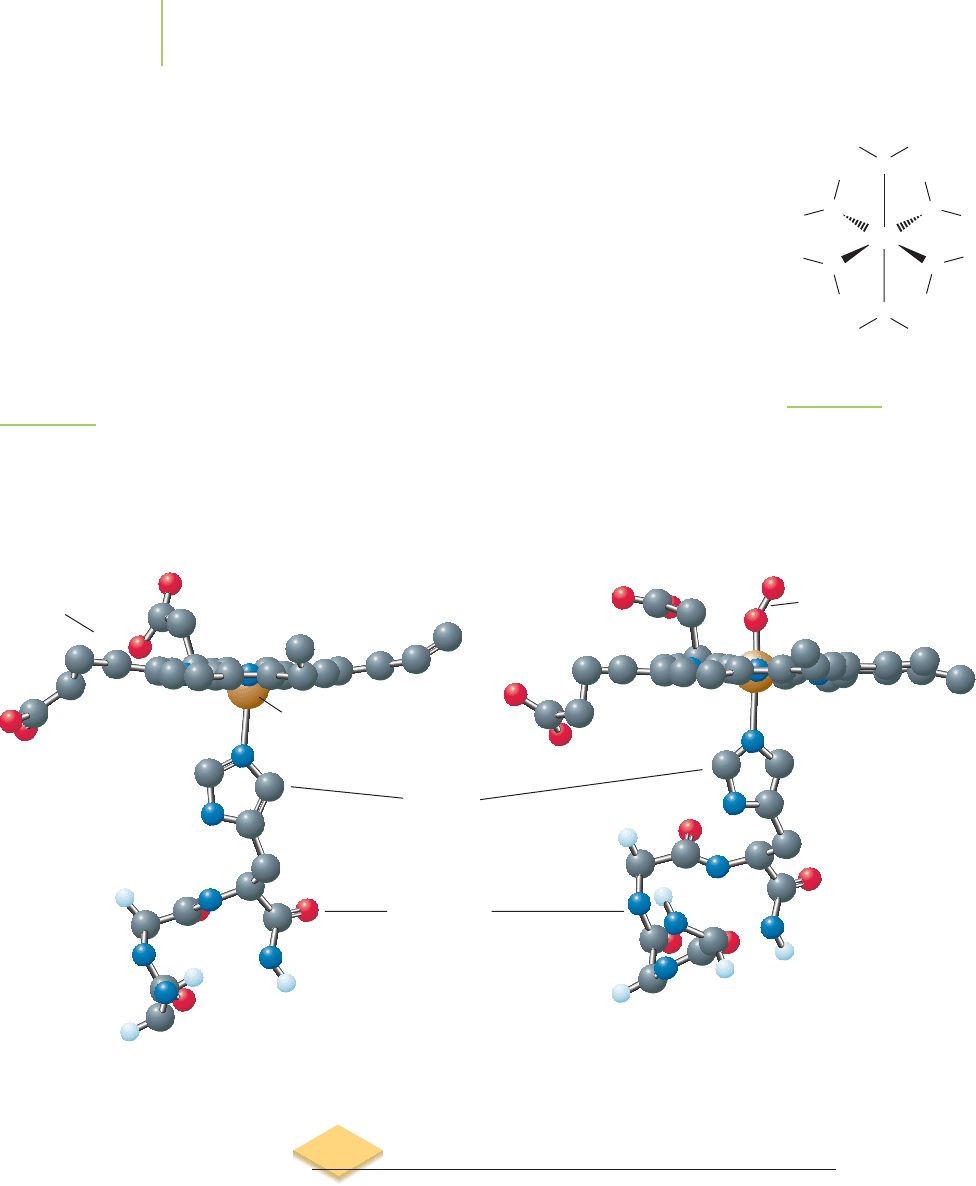
The most important industrial ligand, discussed in Chapter 17 and 18, is
ethylenediaminetetraacetate, which is abbreviated EDTA
4−
. The structure of this
tetraanion, shown in Figure 20.9, illustrates that both nitrogen atoms, and one
oxygen atom from each end of the compound, have lone pairs of electrons. It acts
as a chelate by forming up to six coordinate covalent bonds to a metal center, de-
pending on the size of the metal ion. For example, EDTA
4−
forms six bonds to
calcium ions, as shown in Figure 20.10. The exceptionally large formation con-
stant for the reaction of EDTA
4−
with many metal ions illustrates that the forma-
tion of these bonds is quite favorable.
20.3 Coordination Number
The number of donor atoms to which a given metal ion bonds is known as its
coordination number. Coordination numbers of 4 and 6 are most common, but
coordination numbers as low as 2 and as large as 8 are not unusual. For example,
in the film development process, excess silver ion is removed from photographic
film by using the thiosulfate ion (S
2
O
3
2−
), which forms a coordination complex
with a coordination number of 2 (see Figure 20.11). In another example, cis-
platin, a prominent anticancer drug, has at its center a platinum(II) ion with a
coordination number of 4, as shown in Figure 20.12. As we discussed earlier,
when an Fe
3+
ion is present in water, it is surrounded by 6 water molecules in a
complex, [Fe(H
2
O)
6
]
3+
. And iron’s coordination number is 6. If iron forms a
complex with the oxalate ligand, [Fe(C
2
O
4
)
3
]
3−
, its coordination number is still
6, because each oxalate ligand donates two electron pairs.
What makes a metal ion have a certain coordination number? It is primarily a
function of the nature of metal ions. Large metal ions have space around them
within which more ligands can fit. Not as many ligands can fit around a small
metal ion. Other factors, such as the charges on the ligands and metal and the
electron configuration of the metal ion, determine the most likely coordination
number for a metal ion.
20.3 Coordination Number 875
C
O
CH
2
C
CH
2
O
H
2
C
H
2
C
C
O
O
O
O
N
H
2
CN
C
O
O
CH
2
FIGURE 20.9
EDTA
4−
O
NCa
O
O
O
O
O
N
O
O
2
FIGURE 20.10
EDTA
4−
coordinated to calcium ion.
3
SS
O
O
AgO SSO
O
O
FIGURE 20.11
A two-coordinate complex of silver.
Pt
NH
3
NH
3
ClCl
FIGURE 20.12
Four-coordinate cisplatin.
20.4 Structure
Mercury in the environment poses a particular threat to life, both to aquatic
species and to those species that consume them. The most insidious form of mer-
cury is dimethyl mercury, [Hg(CH
3
)
2
]. Transformed from mercury metal in the
environment, dimethyl mercury is a very toxic product. The mercury(II) ion in
this complex coordinates with the methyl groups, :CH
3
−
, to form the complex
[H
3
COHgOCH
3
]. Given the VSEPR rules, the complex is linear, with a
COHgOC bond angle of 180°. This structure is typical of metals with a coordi-
nation number of 2.
876 Chapter 20 Coordination Complexes
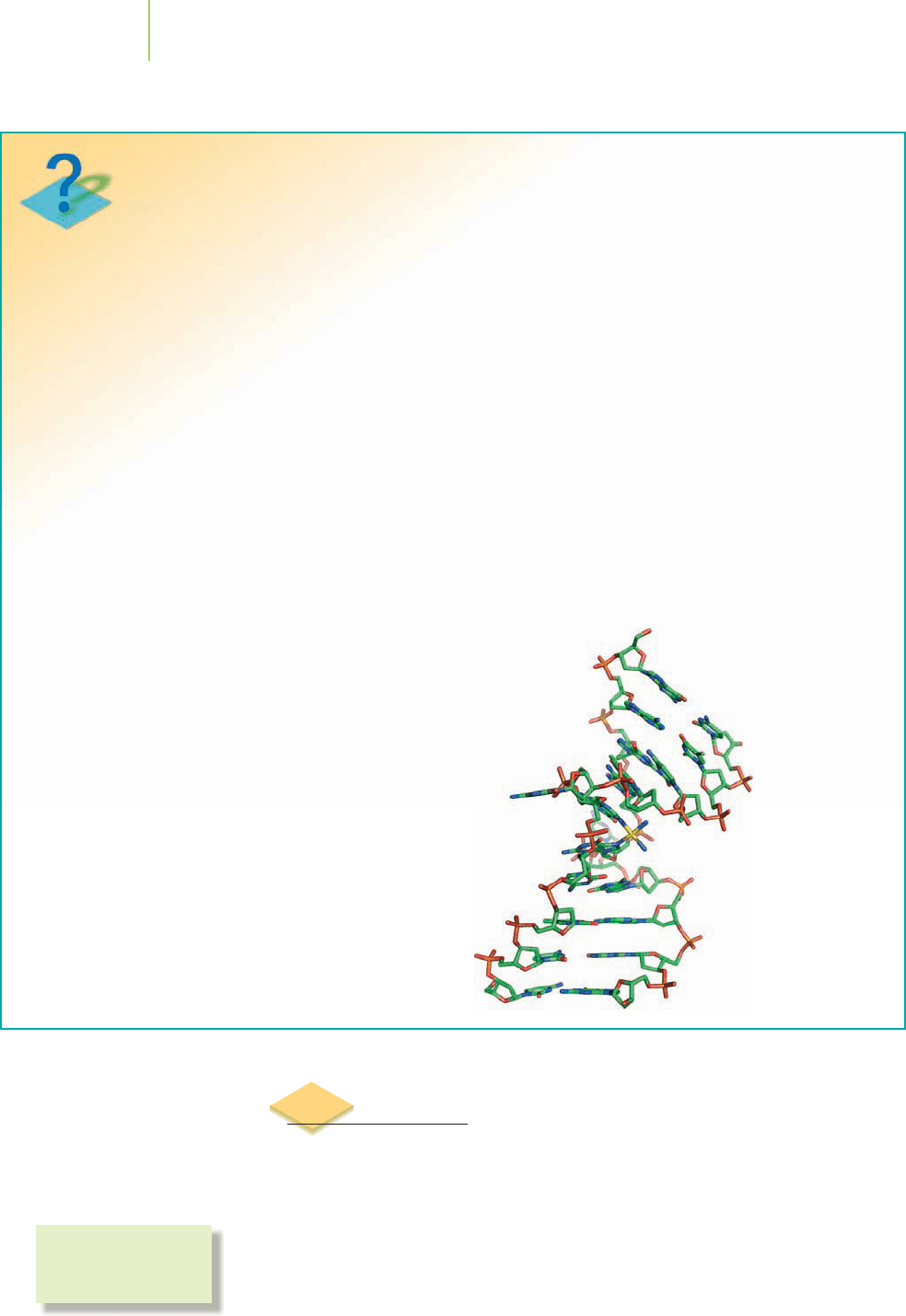
Cancer is an often devastating disease. Research has
made significant strides in understanding the disease in
its many forms, including advances in detection, treat-
ment, and care that have improved the quality of life for
its victims. One prominent drug used in cancer treat-
ment, especially testicular and ovarian cancers, is cis-
platin, a coordination complex of platinum(II) that is
shown in Figure 20.12. The anticancer activity of cis-
platin was discovered as a result careful observation and
basic interpretation of an experiment that had nothing
to do with fighting cancer. But good science nonetheless
led to the discovery.
Dr. Barnett Rosenberg at Michigan State University
investigated the effect of an electric current on a culture
of Escherichia coli bacteria. This bacterium, commonly
found in the gastrointestinal tracts of many living crea-
tures, is often used in initial biochemical studies because
of its ready availability. Rosenberg observed that when an
electric current was applied to solutions of the bacteria,
cell division in the vicinity of a platinum electrode was
inhibited. Studying this interesting result further, he
recognized that it was not due to the electric current,
which was the focus of the investigation. Noting that a
compound known as cis-diamminedichloroplatinum
(cisplatin) was being produced in the vicinity of the plat-
inum electrode used for his experiment, Rosenberg rea-
soned that the cisplatin must be responsible for inhibit-
ing the cell division. It was later determined that
this compound, when given to cancer patients, can sig-
nificantly reduce the size of their tumors and can even
cause the disease to go into remission. Since 1970, the
survival rate for testicular cancer patients has increased
from 10% to over 90%.
How does cisplatin exhibit this remarkable biologi-
cal activity?
Cisplatin is a four-coordinate, square planar
complex, as is commonly observed for many metal com-
How do we know?
What is the nature of the structure, bonding,
and reactivity in cisplatin?
A drawing of the
crystal structure of
cisplatin bound to a
short piece of DNA.
Dimethylmercury
CH
3
— Hg — CH
3
plexes with eight d orbital electrons. The platinum(II)
metal center is fairly unreactive, which allows the neutral
cisplatin complex to remain largely intact through injec-
tion, circulation, and penetration into the nucleus of a
cancerous cell. The chloro ligands are eventually replaced
by water molecules. This provides an opportunity for the
platinum center to coordinate to DNA molecules. Ulti-
mately, the platinum center binds to a nitrogen atom
from each of two guanine units in a single DNA strand
(see Chapter 22). The geometry of the ligands around
the platinum center is vital to this biological activity. In
fact, the chloro ligands must be cis to each other—on the
same side of the complex. Once coordinated to the gua-
nine nitrogens, the inert platinum center remains bound,
tying two points on the DNA strand together. Such bind-
ing inhibits reproduction of DNA during cell division
and restricts use of the DNA for normal cellular func-
tions. Ultimately these effects hinder the growth of the
cancer cells.
We observe that two geometries are common for metals that have a coordina-
tion number of 4. Those geometries, tetrahedral and square planar, are shown in
Figure 20.13. The
tetrahedral geometry is the one predicted by VSEPR (see
Section 8.4) when there are four electron sets around the central atom. In this
geometry, the bond angles are close to 109°. The
square planar geometry arises as
a consequence of the electron configuration of the metal ion. Square planar geom-
etry,with bond angles of 90°, is often observed for metal ions such as Ni
2+
and Pt
2+
that have an outer nd
8
electron configuration. That is, they have eight electrons
in their outermost d orbitals. An example of the square planar geometry is found
in the anticancer drug cisplatin, which we introduced in the previous section. The
square planar geometry is crucial in the interaction of this drug with DNA.
The final geometry common to the coordination compounds is the
octahedral
complex, an example of which is shown in Figure 20.14. In this geometry, the six
ligands occupy equivalent positions around the metal center. For example, hexa-
aquairon(III), [Fe(H
2
O)
6
]
3+
, includes six water ligands symmetrically surround-
ing the central Fe
3+
ion. We can think of the ligands as sitting at positions on each
end of the coordinate x, y, and z axes with bond angles of 90°. The iron in hemo-
globin, shown in Figure 20.1, occupies a nearly octahedral coordination environ-
ment with four sites occupied by nitrogen atoms from a porphyrin ligand. One
site is occupied by a histidine (an amino acid that makes up one part of the he-
moglobin molecule), and the final site is occupied by a molecule of oxygen.
EXERCISE 20.2 Coordination Number and Geometry
Give the coordination number of the central metal and predict the geometry of the
following compounds.
a. [Al(H
2
O)
6
]
3+
b. [Ag(CN)
2
]
−
c. Na
2
[PdCl
4
] d. [Co(ox)
3
]
3−
20.4 Structure 877
Coordination number = 4
Square planar
90°
109.5°
Tetrahedral
FIGURE 20.13
Common geometries with a
coordination number of 4.
Coordination number = 6
90°
90°
Octahedral
FIGURE 20.14
The octahedral geometry; all six posi-
tions in this geometry are identical.
Mercury occurs naturally in rocks, such
as coal. For this reason, the largest con-
centrations of mercury in the environ-
ment are near coal mines, power plants
that use coal to produce electricity, and
wetlands that trap sediments and
precipitates from the atmosphere.
