Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

First Thoughts
Coordination Number: To address this question, we must first identify the ligands
and the central metal to which they are attached. In part c, the formula is written
as a neutral compound, not only as the coordination complex. The species in
brackets represents the coordination complex. It is only the coordination complex
that we should consider when answering a question about the coordination
number. Remember that oxalate (ox) is a bidentate ligand.
Geometry: Once we know the coordination number of the complex, we can
determine the geometry of the complex. For coordination number 2 or 6, the
geometry is linear or octahedral, respectively. For four-coordinate complexes,
either tetrahedral or square planar geometry is expected. Remember that metals
with eight d orbital electrons typically form square planar complexes.
Solution
Coordination Number a. 6 b. 2 c. 4 d. 6
Geometry a. Octahedral b. Linear c. Square planar d. Octahedral
Further Insights
Remember that the “coordination” of a ligand to a metal is a covalent bond, and the
same concepts that you learned earlier for covalent bonds still apply. In short, the
metal and ligand mutually attract an electron pair.
PRACTICE 20.2
Determine the coordination number and geometry for each of the following
compounds.
a. [Cr(CO)
6
] b. [Fe(H
2
O)
6
]
2+
c. K
2
[TiCl
4
]d.[Cu(NH
3
)
4
]
+
See Problems 17, 18, and 23–26.
20.5 Isomers
Organic molecules (Chapter 12) are not unique in their ability to form isomeric
structures. Coordination compounds can also form isomers. These types of iso-
mers are divided into two main categories on the basis of whether there is a
change in the structure or in the geometry of the complex. Structural differences
are observed in the linkage isomers, ionization isomers, and coordination sphere
isomers. Differences in geometric isomers arise from the nonspecific nature of
the formation of a coordinate covalent bond
between a ligand and a metal center.
Some ligands coordinate to a metal
through one of several donor atoms. For exam-
ple, the thiocyanate ion (SCN
−
) can coordinate
to a chromium(III) ion either through the
nonbonded pair of electrons on the nitrogen
or through the nonbonded pair of the elec-
trons on the sulfur atom:
878 Chapter 20 Coordination Complexes
Geometric
isomers
Coordination-
sphere isomers
Ionization
isomers
Linkage
isomers
Isomers
Stereoisomers
Structural
isomers
Isomers in coordination compounds.
2+
Cr(H
2
O)
5
NCS
2+
Cr(H
2
O)
5
SCN
Video Lesson: Structures of
Coordination Compounds and
Isomers
These are called linkage isomers. They differ only in the atom that participates in
the coordinate covalent bond with the metal.
Another isomer that is common among the coordination compounds is the
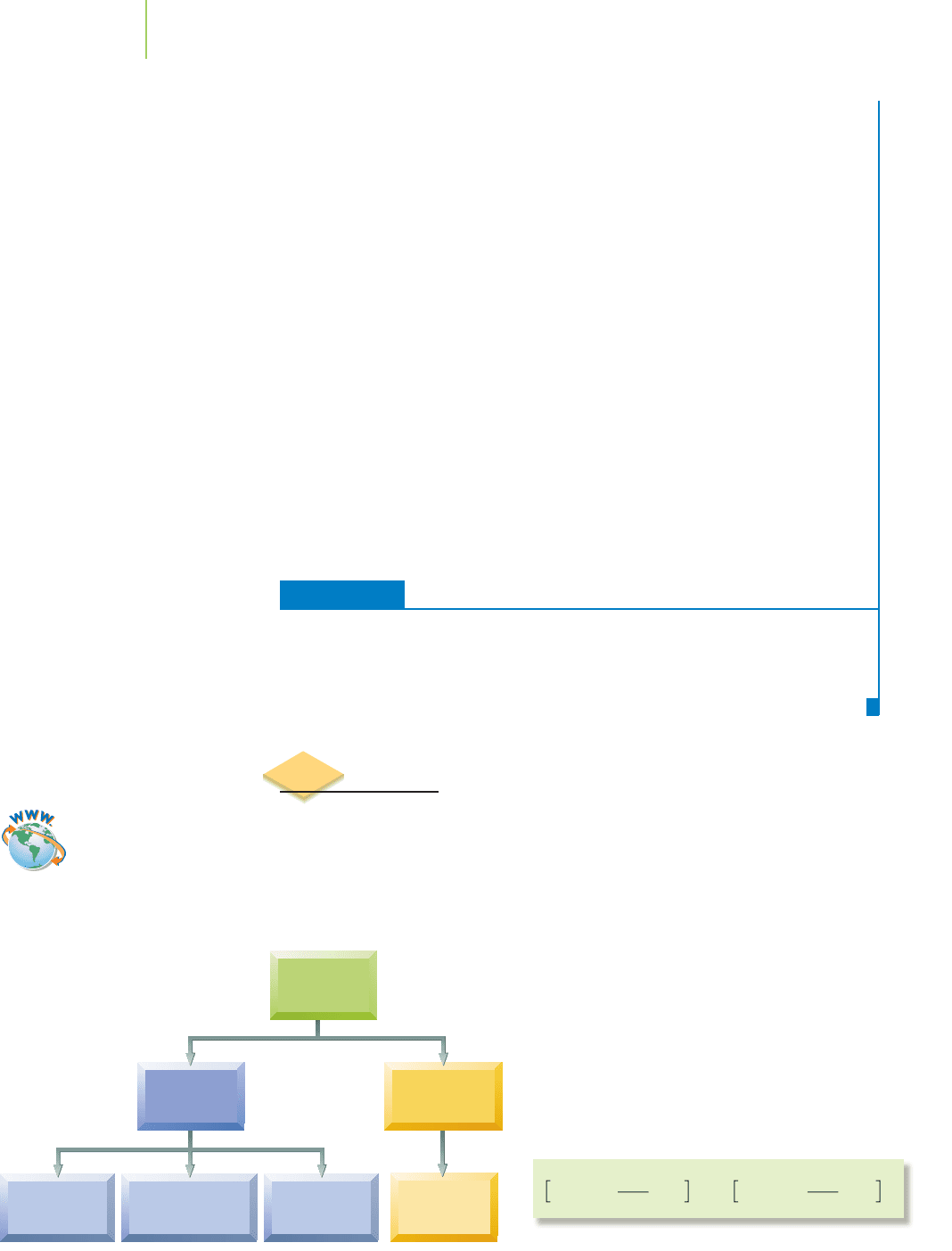
ionization isomer. These are species in which a ligand and an ion (a counter ion)
exchange roles, as with the cobalt complexes shown in Figure 20.15. Note that the
counter ion and a ligand have changed positions in the two compounds. It is that
change that makes these two compounds ionization isomers.
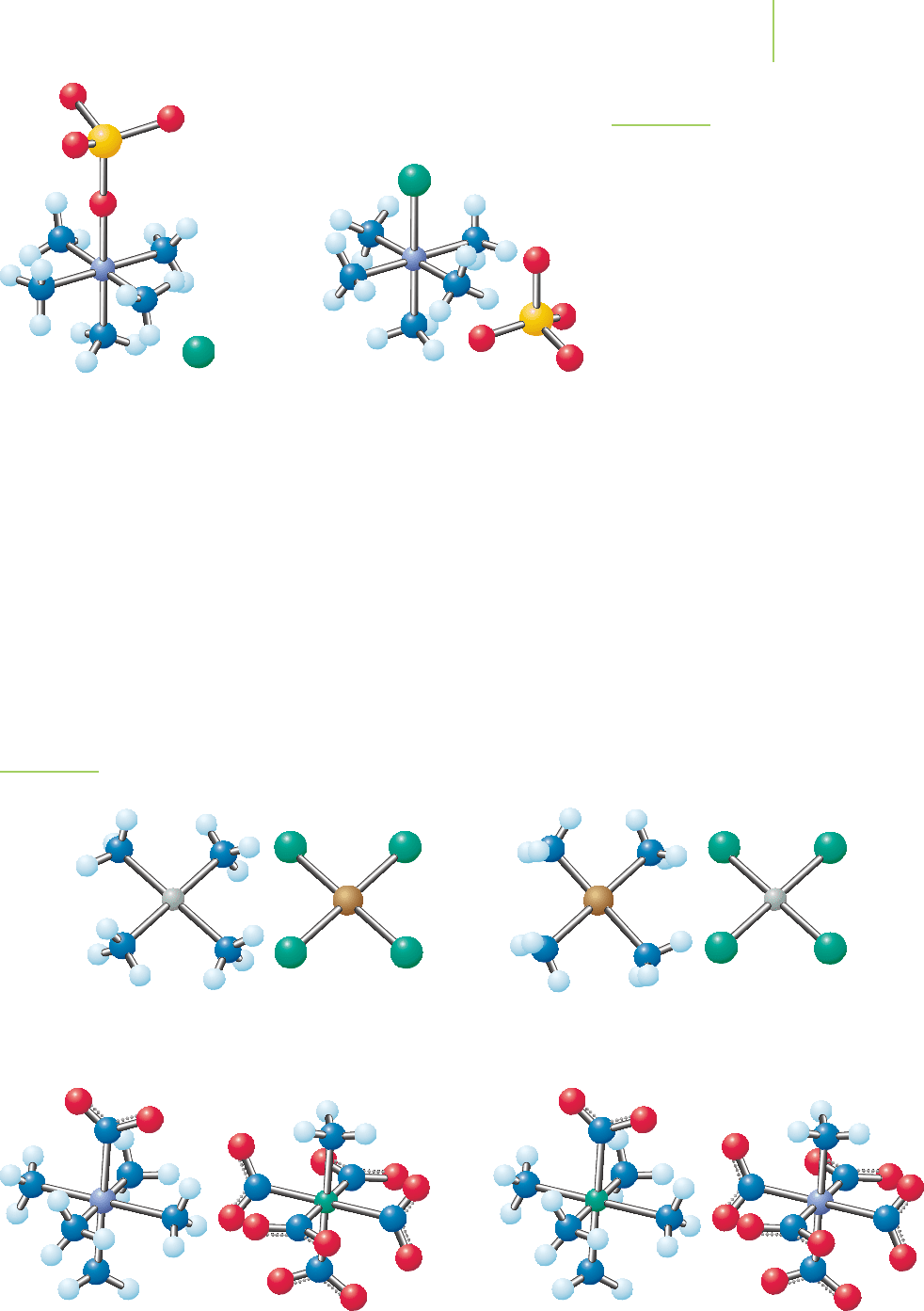
Coordination sphere isomers contain different ligands in the coordination
spheres of cations and anions. In the coordination sphere isomers, both the cation
and anion are coordination complexes. Examples are shown in Figure 20.16. Note
that each of these complexes includes a cation that is a coordination complex and
20.5 Isomers 879
[Co(NH
3
)
5
SO
4
]Cl [Co(NH
3
)
5
Cl]SO
4
FIGURE 20.15
Ionization isomers.
[Pt(NH
3
)
4
][CuCl
4
]
[Co(NH
3
)
5
NO
2
][Cr(NH
3
)(NO
2
)
5
] [Cr(NH
3
)
5
(NO
2
)][Co(NH
3
)(NO
2
)
5
]
[Cu(NH
3
)
4
][PtCl
4
]
FIGURE 20.16
Examples of coordination sphere isomers.
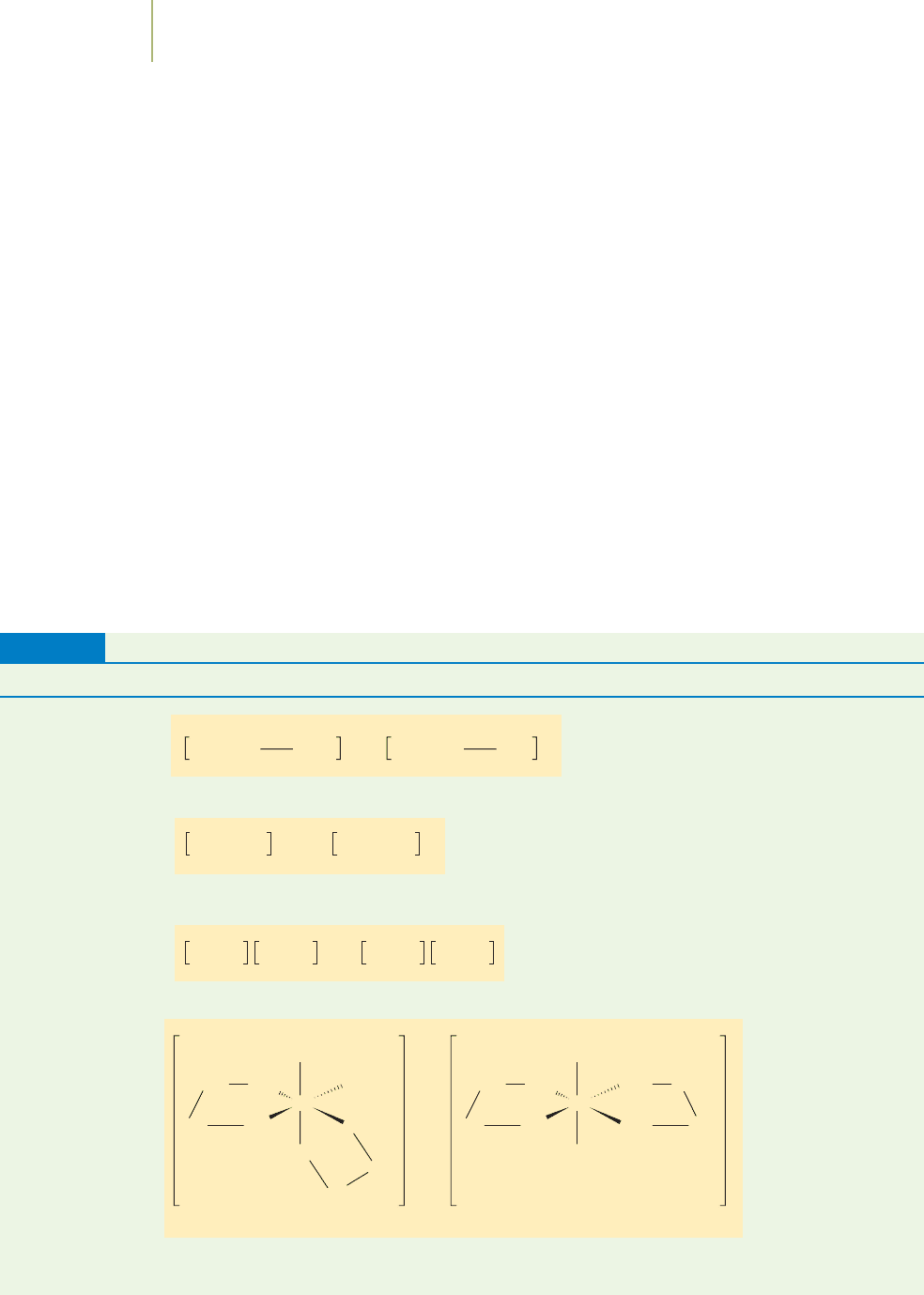
an anion that is a coordination complex. The metal ions in each case have
switched location from the cation to the anion.
Geometric isomers, which we first discussed in Section 12.3, are substances in
which all of the atoms are attached with the same connectivity, or bonds, but
the geometric orientation differs. Square planar four-coordinate complexes of
the general composition MA
2
B
2
and octahedral complexes of the composition
MA
2
B
4
, where M is the metal and A and B are different ligands, can exist as geo-
metric isomers labeled either cis or trans. As shown in Figure 20.17 for the plat-
inum complex [Pt(NH
3
)
2
Cl
2
], the cis isomer has two identical ligands next to
each other. The
trans isomer has two identical ligands on opposite sides of the
metal. Octahedral complexes can exist as cis or trans isomers in complexes such
as [Co(NH
3
)
4
Cl
2
]
+
(Figure 20.18). In fact, in the days when Alfred Werner
(1866–1919) first defined the nature of coordination complexes (for which he
won the 1913 Nobel Prize in chemistry), he noted the two isomers of
[Co(NH
3
)
4
Cl
2
]Cl. He called one the Praseo complex and the other the Violeo
complex. These two isomers were identified easily, and named to reflect their
identification, because the difference in their color was quite evident. To be sure,
the color of a coordination complex is markedly influenced by the geometric
arrangement of the ligands around the metal center. Table 20.2 gives a summary
of the various isomers common among coordination compounds.
880 Chapter 20 Coordination Complexes
Isomers of Coordination Complexes
Isomer Example Explanation
Linkage Ligand binds through different donor atoms
Ionization Anion and ligand interchanged
Coordination sphere Distribution of coordinating ligands differs
Geometric Orientation of
ligands around the
metal center differs
TABLE 20.2
+
Co(NH
3
)
5
NO
2
+
Co(NH
3
)
5
ONO
Pt(NH
3
)
3
Cl Br Pt(NH
3
)
3
Br Cl
Co(en)
3
Cr(ox)
3
Cr(en)
3
Co(ox)
3
+
CH
2
NH
2
NH
2
NH
2
Co
cis
Cl
Cl
CH
2
CH
2
CH
2
NH
2
+
CH
2
NH
2
CH
2
Cl
NH
2
CH
2
Co
trans
Cl
NH
2
CH
2
NH
2
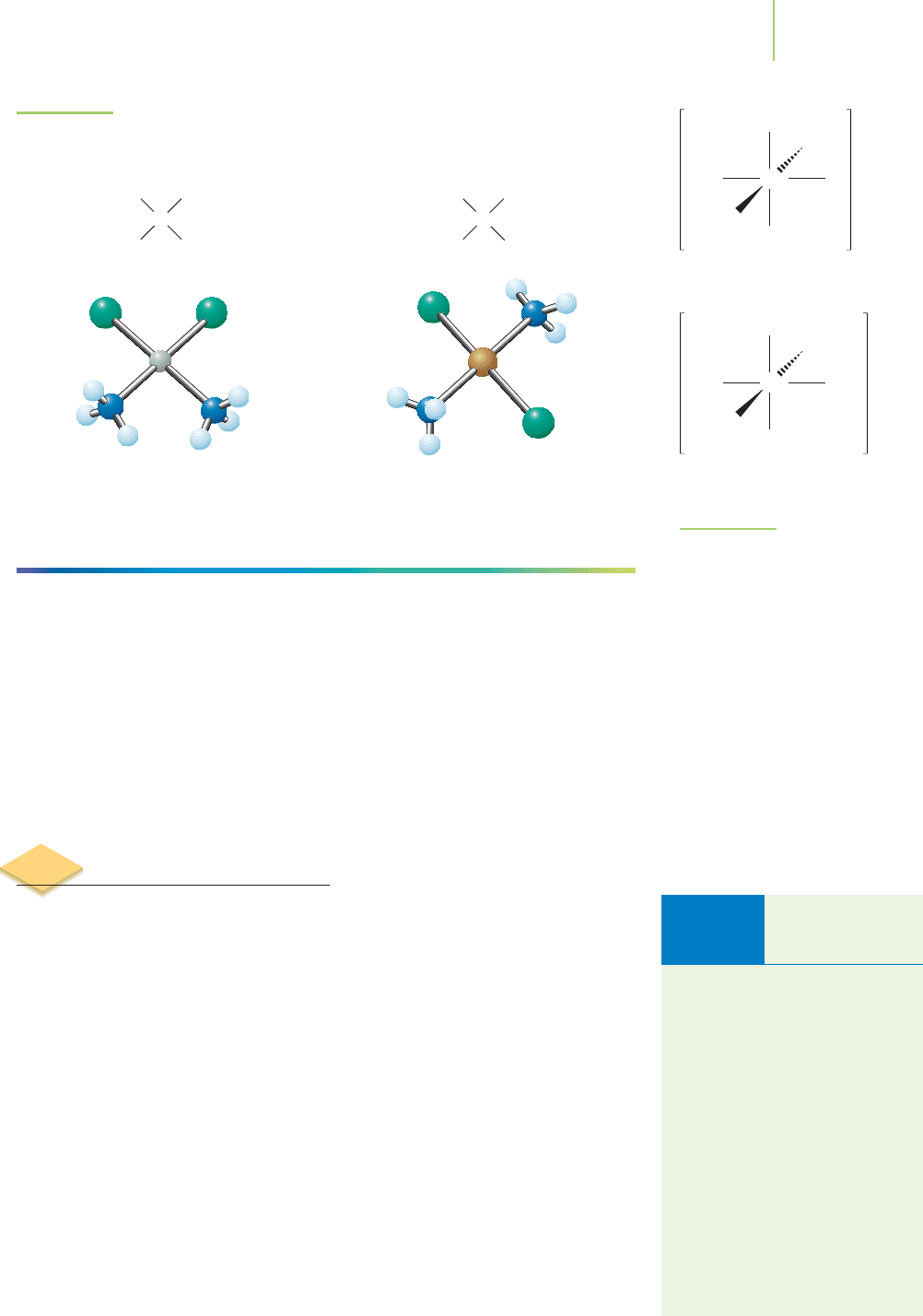
FIGURE 20.17
Cis and trans isomers are geometric isomers. In the cis isomer shown here, the two chloro ligands
are on the same side of the complex. The chloro ligands are on opposite sides in the trans complex.
HERE’S WHAT WE KNOW SO FAR
■
Coordination complexes are present in simple metal ions in solution and as
the reaction center in many biological molecules.
■
A Lewis base that donates a lone pair of electrons to a metal to form a coordi-
nate covalent bond forms a coordination complex.
■
The coordination numbers of various metal centers are commonly observed
to be 2, 4, or 6.
■
Common coordination geometries are linear, tetrahedral, square planar, and
octahedral.
■
A variety of isomer classes are observed for metal complexes.
20.6 Formulas and Names
Coordination compounds are quite varied in their structure, bonding, ability
to form isomers, and other features. For this reason, assigning specific names to
these compounds must be done with care. Just as in naming binary compounds
(Chapter 2) and organic compounds (Chapter 12), IUPAC rules will guide us in
constructing the proper name.
Formulas
Coordination complexes (the metal center and the ligands) can be neutral, an-
ionic, or cationic in nature. To ensure continuity from one structure to another,
we often write the formula of a coordination compound in accordance with a set
of rules. Those rules are outlined in Table 20.3.
Nomenclature
Systematic names for coordination compounds are constructed in accordance
with a set of rules. Those rules are outlined in Table 20.4. Let’s use these rules to
name a few examples. Consider that we are interested in naming the neutral com-
plex [PtCl
2
(NH
3
)
2
]. Step 1 from Table 20.4 indicates that we should name the
compound using the rules in step 3. The ligands are named alphabetically before
20.6 Formulas and Names 881
cis-Diamminedichloroplatinum(II)
Pt
ClCl
NH
3
NH
3
trans-Diamminedichloroplatinum(II)
Pt
Cl
Cl
NH
3
NH
3
NH
3
H
3
N
H
3
NCo
Cl
Cl
NH
3
cis
FIGURE 20.18
Cis and trans isomers are also
possible in octahedral complexes.
NH
3
H
3
N
H
3
NCo
NH
3
Cl
Cl
trans
Rules for Writing
Formulas for Coordi-
nation Compounds
1. The metal is written first,
followed by the ligands.
2. Anionic ligands are written
before the neutral ligands,
each in alphabetical order.
3. The complex is enclosed in
brackets.
4. Polyatomic ligands, such as
NH
3
or NO
2
−
, are enclosed
in parentheses.
5. Ionic compounds containing
coordination complexes are
written in the traditional way:
cation on the left, anion on
the right.
TABLE 20.3
TABLE 20.3
we name the metal. Thus the ammine ligand is named before the chloro ligand.
Moreover, there are two of each of these ligands, so we should write down
diamminedichloro
without any spaces. Then, in step 3d, we write the name of the metal and its
charge in parentheses immediately afterward. Because the charge on the entire
complex is zero, and the complex is neutral, we do not add a suffix to the name:
diamminedichloroplatinum(II)
The name we have created for the formula indicates the number and type of each
ligand, and the metal and its oxidation state. If we knew the three-dimensional
arrangement of the atoms in this complex, we could also include that in-
formation in the name. For example, if we knew that the structure indi-
cated a cis arrangement of the atoms, we could designate that by writing
cis-diamminedichloroplatinum(II). Without that knowledge, though, we can
provide only the name of the formula.
If we wish to name [Co(NH
3
)
6
]Cl
3
, we can do so by following the rules. In
step 1, we note that the compound is made up of a cationic complex and some
anionic counter ions. Step 2 in Table 20.4 directs us to write the name of the com-
plex (the cation). The ligands are named first, like this:
hexaammine
Then in step 3d we name the metal and its charge. Again, we do not add a suffix,
because the complex is a cation:
hexaamminecobalt(III)
Finally, steps 6 and 7 tell us to add the counter ions by naming them as we did in
Chapter 3. Note that we don’t add a prefix to their name.
hexaamminecobalt(III) chloride
An anionic complex is not much different. Suppose we wished to name
K
2
[NiCl
4
]. In step 1, we note that the anion is a complex and the cation is a
counter ion. Accordingly, we name the cation first.
potassium
882 Chapter 20 Coordination Complexes
Rules for Naming Coordination Compounds
1. Name the cation,and then the anion. If the complex is neutral, name it using step 3.
2. Is the cation a complex ion?
3. Ye s – name it using these rules, and then skip to step 5. No – skip to step 4.
a. Name the ligands first using Table 20.1.
b. Name the ligands in alphabetical order. Anionic ligands often end in “o,” as in bromo,
hydroxo, and sulfato.
c. If more than one ligand of the same type is present, a prefix indicates the number of
units. For simple ligands, the prefixes di-, tri-, tetra-, penta-, and hexa- are used. For
complex ligands, the prefixes bis-, tris-, tetrakis-, pentakis-, and hexakis- are used.
d. Name the metal last, with its oxidation number in parentheses in Roman numerals.
(1) If the complex is negatively charged, the name of the metal ends in -ate.
(2) If the complex is positively charged or neutral, no suffix is added to the metal name.
4. Name the cation using the conventions described in Chapter 3.
5. Is the anion a complex ion?
6. YES – Name it using the rules in step 3, and then stop. NO – Go to step 7.
7. Name the anion using the conventions described in Chapter 3, and then stop.
TABLE 20.4
Video Lesson: Naming
Coordination Compounds
20.7 Color and Coordination Compounds 883
The complex anion is named using step 3. The ligands go first (note that there are
four of them):
potassium tetrachloro
And the metal is part of an anion, so it gets the suffix -ate:
potassium tetrachloronickelate(II)
K
2
NiCl
4
Co(NH
3
)
6
Cl
3
Co(NO
2
)
2
(NH
3
)
42
SO
4
Examples of coordination compounds. See if you can name them all.
EXERCISE 20.3 Naming Compounds
Give the name for each of these compounds.
a. [Cr(NH
3
)
2
(en)
2
]SO
4
b. (NH
4
)
3
[Fe(CN)
6
]
First Thoughts
Naming coordination compounds requires us to identify the oxidation states of the
metal center in addition to understanding and knowing the charges on the individ-
ual ligands and ions. To answer this question accurately, separate the compounds
into their two halves (cation and anion). For the half containing the metal ion,
determine its oxidation state, and then use the rules in Table 20.4 to name it.
Solution
a. diamminebis(ethylenediamine)chromium(II) sulfate
b. ammonium hexacyanoferrate(III)
Further Insight
The names for these compounds have a different sound to them, compared to the
simpler compounds we worked with in Chapter 3. Just remember to follow the rules
in Table 20.4, and be sure that you know the names of the ligands from Table 20.1.
PRACTICE 20.3
Name each of these compounds.
a. [CrCl
2
(NH
3
)
4
]
2
SO
4
b. Na
2
[Ni(CN)
4
]
Draw the formula for each of the following coordination compounds.
c. calcium hexafluoroferrate(II)
d. tetraamminedicarbonylmanganese(II) sulfate
See Problems 37–40.
20.7 Color and Coordination Compounds
Many painters create images that both catch our eye and induce a feeling in our
mind. The goal of the painter is often to portray more than just a staged scene.
Van Gogh’s painting Starry Night (see Figure 20.19) evokes a certain sense of
wonder. The effect is generated by the brush strokes and the colors applied to the
canvas. Are chemical compounds responsible for the color of paint? The answer
is a resounding yes. Colors used in paintings are often constructed from ancient
Video Lesson: Color and
Transition Metals

formulations that include compounds of transition metals. One of the general
characteristics of transition metals is the color of their compounds. Compare
table salt (NaCl), baking soda (NaHCO
3
), chalk (CaCO
3
), and Epsom salts
(MgSO
4
) to ruby (with Cr
2
O
3
), emerald (with Cr
2
O
3
), and rust (Fe
2
O
3
· nH
2
O, or
hematite). Chromium was so named because of the variety and brilliance of the
colors of its compounds.
What causes compounds that contain transition metals to
have such striking colors?
Transition Metals and Color
Because color is a common feature of transition metal compounds, it seems rea-
sonable that there must be some common characteristics that give rise to their
colors. The presence of color in most transition metal compounds can be attrib-
uted to the presence of partially filled d orbitals in the compounds and to the in-
fluence of the coordination environment on the energies of the d orbitals. Let’s
see how the color of transition metal compounds is related to fundamental
atomic structure principles.
We perceive color when our eye detects light rays that differ from the ordinary
distribution of those present in white light. For example, a sweater appears blue
if white light strikes it, colors complementary to blue are absorbed, and the re-
maining light is reflected. A glass of fruit punch may appear red if white light
strikes it and the colors complementary to red are absorbed. The remaining light,
which appears red to our eye, is transmitted through the liquid and gives the liq-
uid its red color. Color, then, is the array of light rays that our eyes observe being
reflected from or transmitted through an object.
There are many objects that can absorb or transmit certain wavelengths of
light but not be “colored.” This occurs when the light being absorbed or trans-
mitted is outside of the visible region (400–700 nm) of the electromagnetic spec-
trum. Recall from Chapter 6 that ultraviolet (UV) light has shorter wavelengths,
typically defined as light in the wavelength range of 200–400 nm. A photon of UV
light carries a lot of energy and may be damaging to substances it strikes. Infrared
light has longer wavelengths than visible light.
Given our understanding of light (Chapter 6), we can begin to ask questions
about how a compound can appear to be colored.
For example, what characteris-
tic change occurs in a substance when light is absorbed?
Answering this question
will enable us to understand why lime (CaO) is colorless but rust (Fe
2
O
3
) is col-
ored. Several fundamental principles are involved.
884 Chapter 20 Coordination Complexes
FIGURE 20.19
Van Gogh’s Starry Night uses the colors
of transition metals to evoke the feeling
of magic in the night sky.
Application
C
HEMICAL
ENCOUNTERS:
Transition Metals
and Color
750 nm
650 nm
O
Y
R
GV
B
580 nm
430 nm
490 nm
400 nm
560 nm
The color wheel indicates the range of
wavelengths that corresponds to each
color.
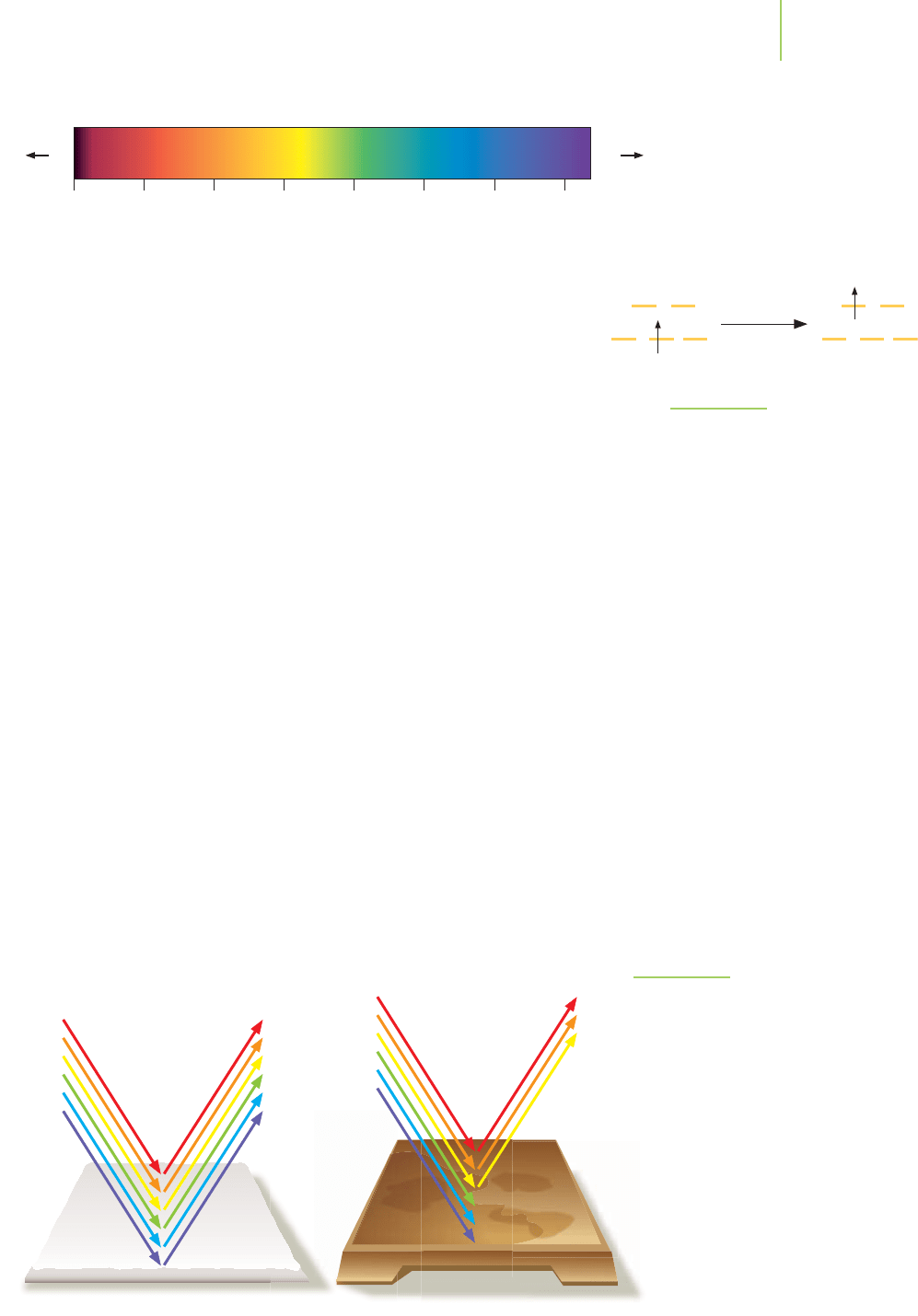
Absorption of a photon of visible light by a compound results in the
excitation of an electron from a low energy orbital to a higher energy or-
bital within the substance. In order for the photon to be absorbed, the
difference in orbital energy levels must match the energy of the photon
absorbed. Finally, the excited electron must be able to be excited to the
higher energy orbital—that is, the higher energy orbital must not be full. For ex-
ample, when visible light strikes rust, an electron in a lower energy orbital ab-
sorbs a photon of blue light. The electron is excited to a higher energy orbital.
(Usually the high-energy electron dissipates its energy as heat and relaxes back to
its original energy level, ready to absorb again!) Orange light is reflected, and that
is what our eyes detect. Rust is orange-colored.
In many transition metal compounds, of which rust is one, the same process
occurs. What electronic transitions take place to allow the absorption of visible
light? The d orbitals of the transition metal are often arranged such that they
have a slight difference in energy. One example is shown in Figure 20.20. This
difference in energy is roughly that of a photon in the visible region of the elec-
tromagnetic spectrum. If the d orbitals are partially filled, as they are in the tran-
sition metal ions, a photon can cause an electron in the lower energy orbitals to
jump to an empty (or partially filled) higher energy d orbital.
Let’s use this information to compare the colors of rust (Fe
2
O
3
) and chalk
(CaCO
3
). The iron(III) ion in rust has the electron configuration [Ar]3d
5
. It con-
tains partially filled d orbitals as its valence orbitals. These d orbitals on the metal,
surrounded by a field of oxide ions, are split into different energy levels in ways
that we will discuss in the next section. When visible light strikes rust, some wave-
lengths of light are absorbed, and an electron is excited to a higher empty energy
level. The calcium ion in lime is isoelectronic with argon. It does not have par-
tially filled d orbitals, cannot absorb visible light energy, and reflects all of the vis-
ible light to our eyes. It appears to be white, as shown in Figure 20.21.
20.7 Color and Coordination Compounds 885
400450500550600650750
IR UV
700
Wavelength (nm)
RYGBVIO
The visible spectrum.
Light
absorbed
FIGURE 20.20
A slight difference in the energy of the
five d orbitals on a transition metal in a
complex allows visible light to be ab-
sorbed. This causes an electron to be ex-
cited to the higher-energy d orbitals.
Lime Rust
FIGURE 20.21
The color of lime and that of rust are a result of
the absorption of specific wavelengths of light
in the visible region of the electromagnetic
spectrum. Calcium ions in lime do not possess
electrons in d orbitals and cannot absorb light
in the visible region. Iron ions in rust have d or-
bital electrons and do absorb visible light.
d
xy,
d
x
2
–y
2
d
xy
d
x
2
–y
2
Crystal Field Theory
We can understand the nature of the d orbital splitting from careful analysis of
geometry and d orbital shape. In a free, gaseous metal atom or ion, there is no dif-
ference in energy among the orbitals of the same sublevel. We say that these
orbitals, such as the 3d orbitals on gaseous iron, are degenerate. But something
happens when we bring other atoms (such as the negatively charged anions) up
close to the metal. They cause distortions in the orbitals of the metal. In a six-
coordinate octahedral complex, for example, ions approach the central metal
from each end of the three coordinate axes. Their orientation causes the orbitals
on the metal to lose their degenerate nature.
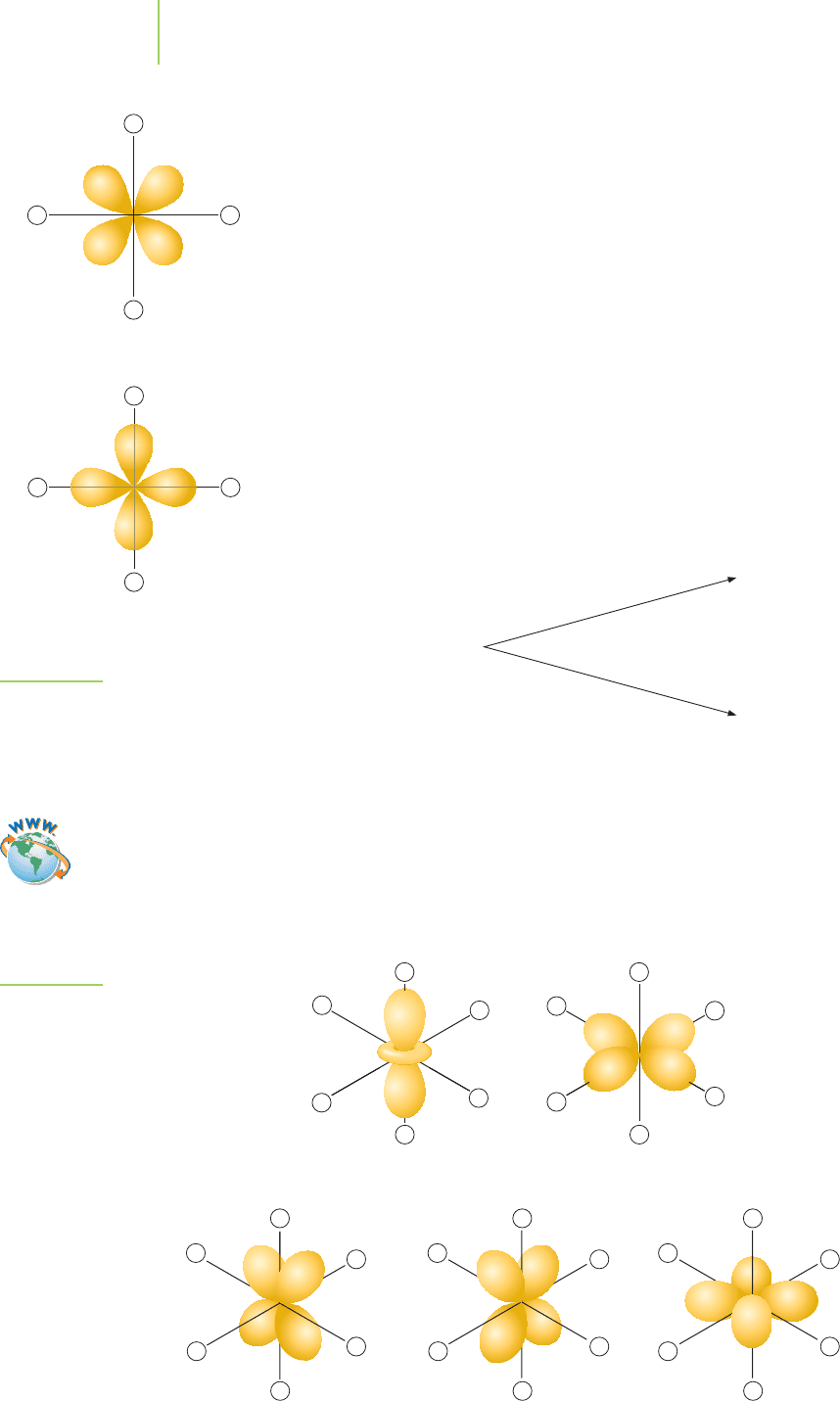
Let’s look just at the xy plane in an octahedral complex. The octahedral lig-
ands are oriented along the x axis and the y axis. The d orbitals on a metal at
the center of our plane are oriented in specific ways. Which electron would
have lower energy, an electron in a d
x
2
−y
2
orbital pointed directly at a negatively
charged ligand or an electron in a d
xy
orbital pointed between the negatively
charged ligands? Using Figure 20.22 as a graphical guide, we can answer this
question. Because like charges repel, an electron in a d
x
2
−y
2
orbital would be
higher in energy. We would represent this on an energy-level diagram as follows:
886 Chapter 20 Coordination Complexes
d
xy
d
x
2
–y
2
d
yz
d
xz
d
z
2
–
–
–
–
–
–
–
–
–
–
–
–
–––
–
–––
–
–
–
–
–
–
–
–
–
–
–
FIGURE 20.23
d orbitals in an octahedral crystal field.
Ligands interacting
with a d
x
2
–y
2 orbital
__
_
_
__
_
_
Ligands interacting
with a d
xy
orbital
FIGURE 20.22
As ligands approach the d orbitals, they
affect the relative energy levels of the
5d orbitals. Some, like the d
x
2
– y
2
orbital,
have an increased energy due to the
repulsion of similar charges.
Octahedral Crystal Field Splitting
For a metal center in an octahedral crystal field, an electron in a d
x
2
−y
2
orbital
would be at higher energy than an electron in a d
xy
orbital. We can extend this
analysis to three dimensions and include all d orbitals as shown in Figure 20.23.
Note that the d
x
2
−y
2
and d
z
2
orbitals are pointed right at the negatively charged lig-
ands, but the d
xy
, d
xz
, and d
yz
orbitals are pointed between the axes. The d
xy
,d
xz
,
and d
yz
orbitals will be at lower energy than the d
x
2
−y
2
and d
z
2
orbitals. Based on
Video Lesson: Crystal Field
Theory
the symmetry of the system, the d
xz
, d
yz
, and d
xy
orbitals are at the same energy
level. Although it is less obvious, the d
x
2
−y
2
and d
z
2
orbitals are at the same energy
level, which is higher than that of the d
xy
, d
xz
, and d
yz
orbitals.
In an octahedral array of anions or ligands, the d orbital energies of the cen-
tral metal are split into a lower energy set (d
xz
,d
xy
,d
yz
) that we label the t
2g
set and
an upper energy set (d
x
2
−y
2
, d
z
2
) that we label the e
g
set, as shown in Figure 20.24.
The energy difference between the t
2g
orbitals and the e
g
orbitals in the octahedral
field, the
crystal field splitting energy, is given the symbol ∆
o
, where the subscript
“o” indicates the splitting energy for an octahedral complex. The magnitude of
∆
o
, the difference in energy between the t
2g
and e
g
orbitals, depends on the nature
of the central metal and the nature of the ligands.
Tetrahedral Crystal Field Splitting
A tetrahedral ligand field can be viewed as one with the metal ion at the center of
a cube and ions at alternate corners of a cube, with the coordinate axes going
through the centers of the faces of the cube as shown in Figure 20.25. In this
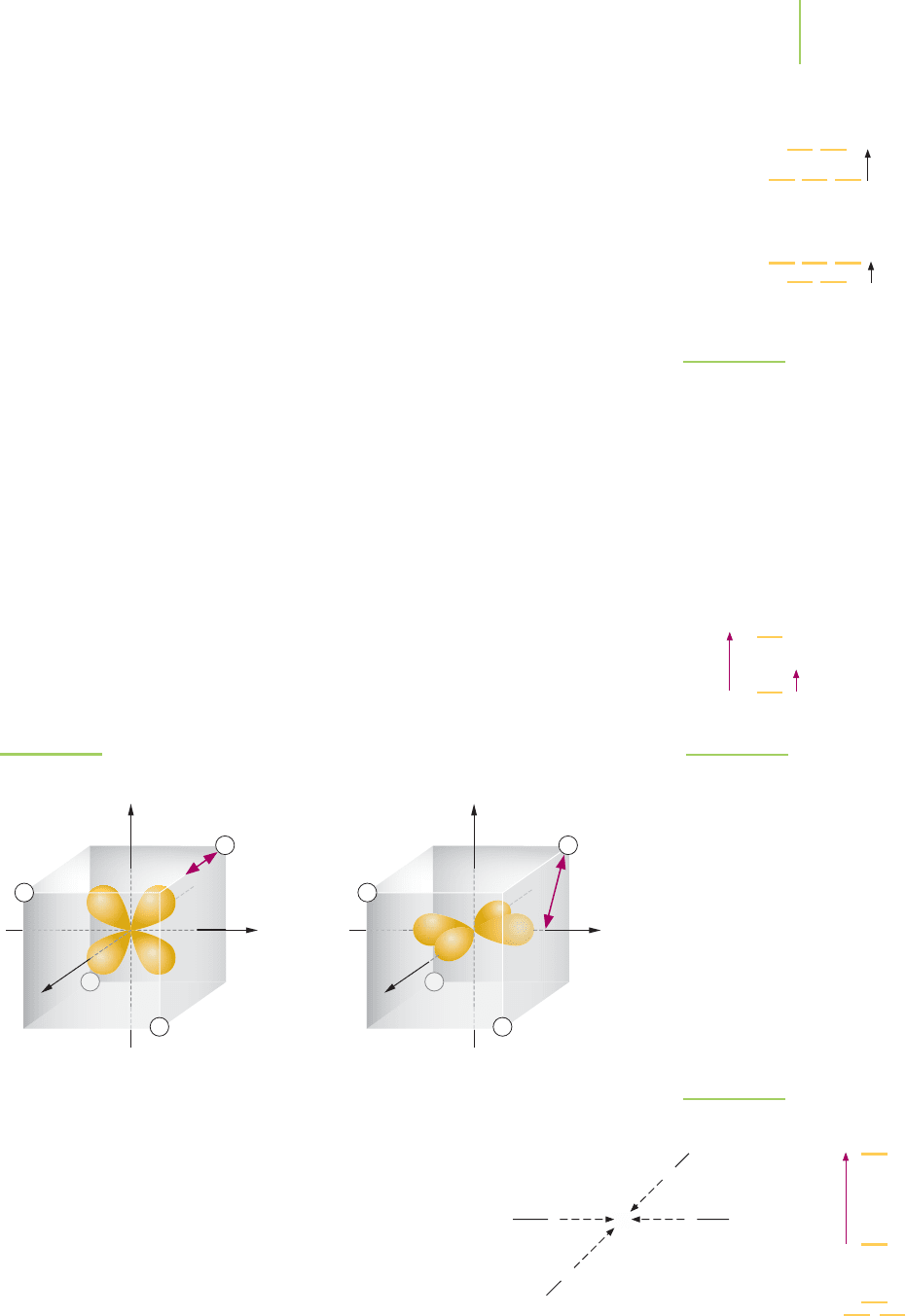
situation, none of the d orbitals of a central atom are pointed at the ions. The d
xy
,
d
xz
, and d
yz
lobes are closer to the ions than the d
x
2
−y
2
and d
z
2
lobes. Thus the d
xy
,
d
xz
, and d
yz
orbitals are at a higher energy than the d
x
2
−y
2
and d
z
2
orbitals. As
shown in Figure 20.25, the tetrahedral crystal field splitting energy, ∆
t
, where the
subscript “t”indicates a tetrahedral complex, is qualitatively the inverse of the oc-
tahedral field splitting diagram. We label the lower orbital set “e” and the upper
orbital set “t”. As the comparison in Figure 20.26 shows, the magnitude of the
splitting, ∆
t
, is estimated to be
4
⁄
9 the size of ∆
o
, so the magnitude of tetrahedral
crystal field splitting is smaller than that of octahedral field splitting.
20.7 Color and Coordination Compounds 887
Tetrahedral crystal field splitting
∆
t
e
t
Octahedral crystal field splitting
t
2g
∆
o
e
g
FIGURE 20.24
Crystal field splitting of metal d orbitals for
octahedral and tetrahedral geometries.
Square Planar Crystal Field Splitting
We can define the square planar geometry as one with the
four ligands in the xy plane as in an octahedral complex,
but with the two opposing ligands on the z axis com-
pletely removed. The crystal field effect in the xy plane
remains the same, but the effect in the z direction is re-
moved. This results in stabilization of the d orbitals with
z axis components. The resulting crystal field splitting
diagram is shown in Figure 20.27. The difference in en-
ergy between d
xy
and d
x
2
− y
2
is still ∆
o
. The other energy
differences are considerably smaller.
d
x
2
–y
2
z
x
y
d
yz
–
–
–
–
z
x
y
–
–
–
–
FIGURE 20.25
Tetrahedral crystal field splitting (arrows show the distance from orbital lobe to ions).
∆
o
∆
t
d
xy
d
x
2
–y
2
FIGURE 20.26
The value of ∆
t
is
4
⁄9 that of
∆
o
, the distance in energy
from the d
x
2
–
y
2
to the d
xy
orbital.
FIGURE 20.27
Square planar coordination complex.
∆
o
–y +y
+x
–x
L
L
L
L
M
Ligand arrangement of a
square planar complex
d Orbital energies in a
square planar comple
x
d
xy
d
x
2
–y
2
d
z
2
d
yz
d
xz
