Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

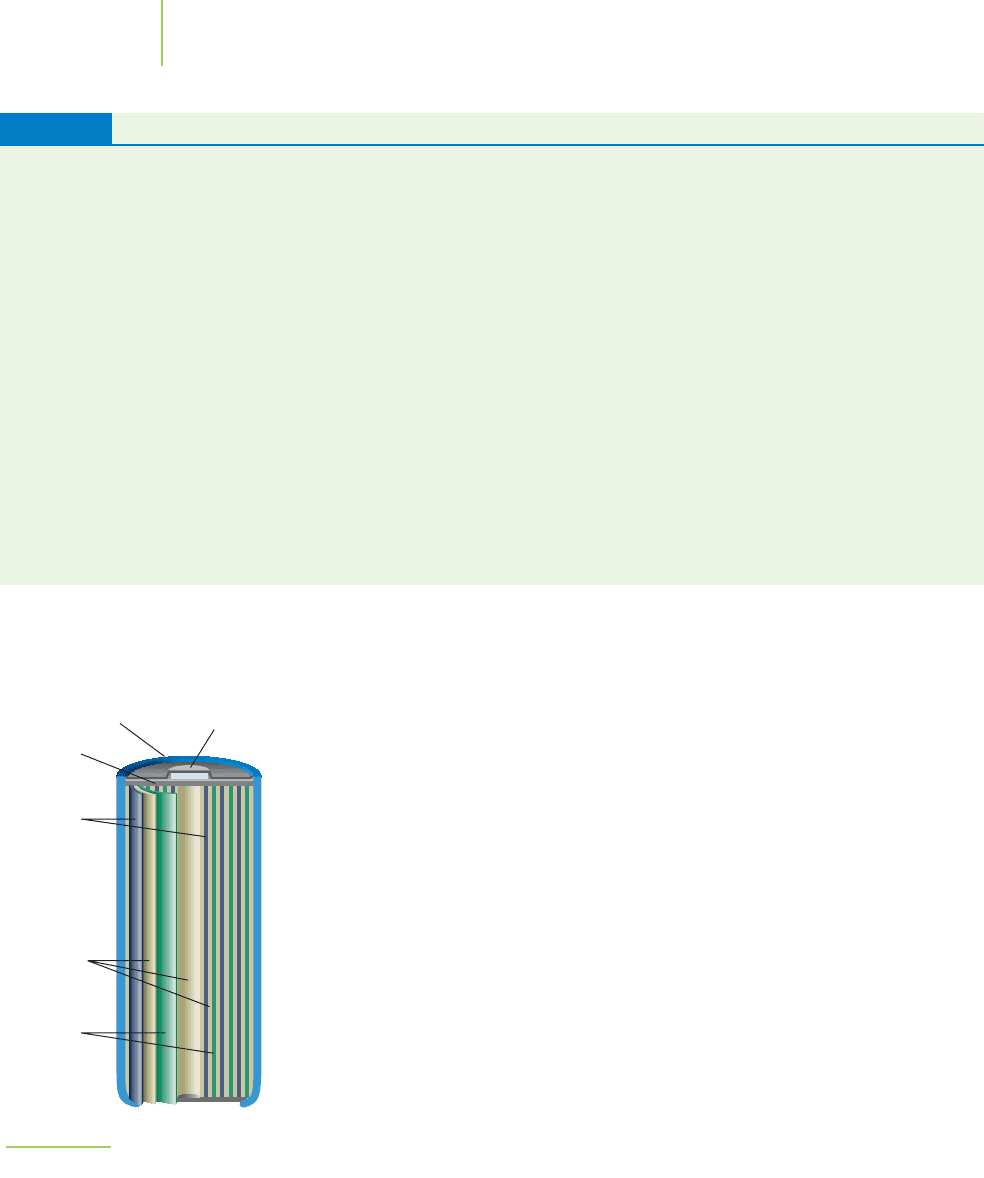
848 Chapter 19 Electrochemistry
Selected Batteries
Zinc–carbon battery Also known as a standard carbon bat-
tery. Zinc–carbon chemistry is used in all inexpensive AA, C,
and D dry-cell batteries. The electrodes are zinc and carbon,
with an acidic paste between them that serves as the
electrolyte.
Alkaline battery Used in common Duracell and Energizer
batteries. The electrodes are zinc and manganese oxide, with
an alkaline electrolyte.
Lithium photo battery Lithium, lithium iodide, and lead-
iodide are used in cameras because of their ability to supply
power surges.
Lead–acid battery (rechargeable) Used in automobiles. The
electrodes are made of lead and lead oxide with a strong
acidic electrolyte.
Nickel–cadmium battery (rechargeable) The electrodes are
nickel hydroxide and cadmium, with potassium hydroxide
as the electrolyte.
TABLE 19.5
The Chemistry of Some Common Batteries
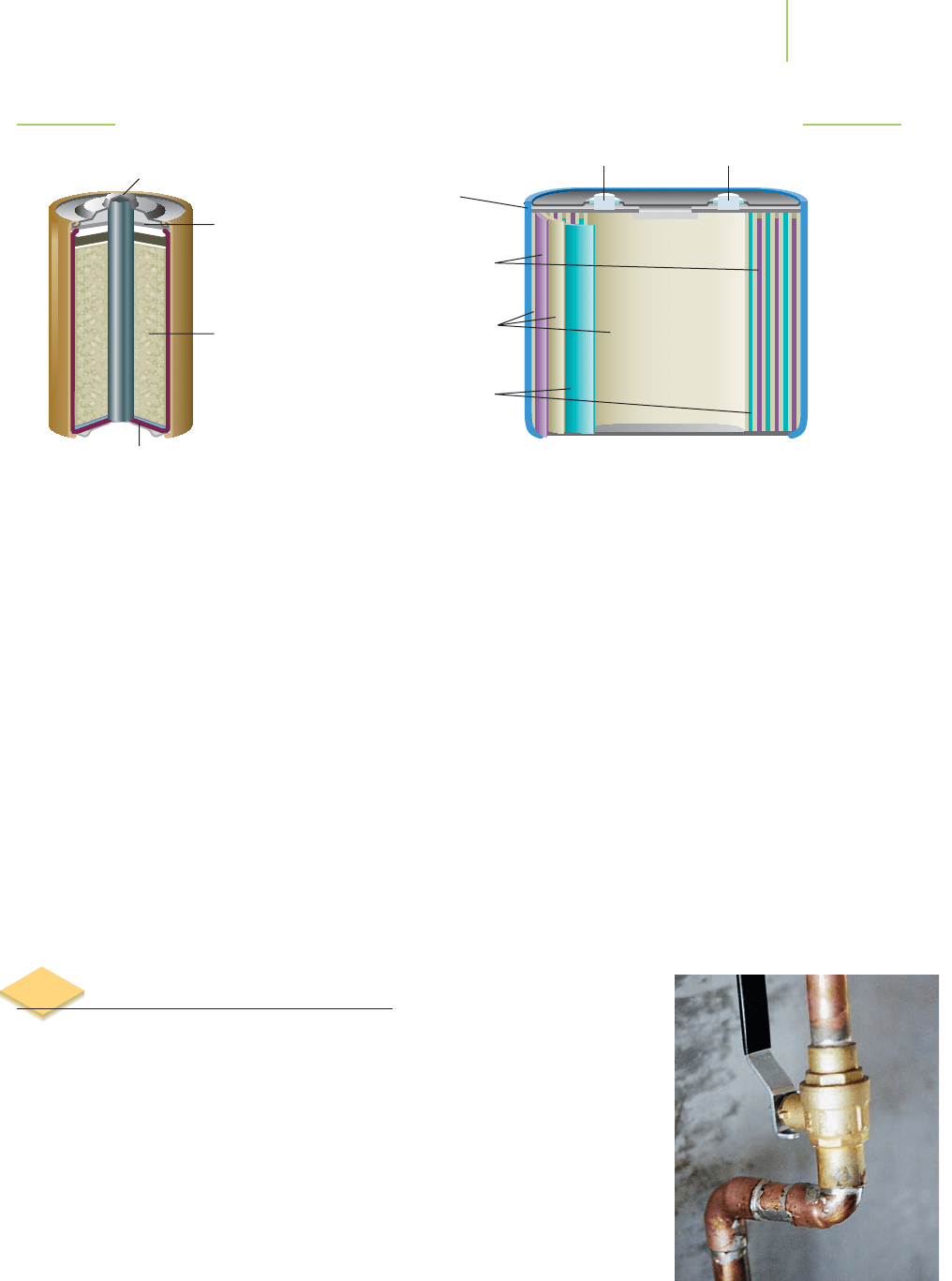
Nickel metal hydride (NiMH) rechargeable batteries are used in many cellular
phones (Figure 19.13). During the charging phase, an external source of electric-
ity causes water in the electrolyte (often aqueous potassium hydroxide) to react
with a rare earth– or zirconium metal–based alloy at what will be the negative
electrode of the battery when it is in operation. This generates hydrogen atoms
that are absorbed into the alloy, and releases hydroxide ions:
Alloy + H
2
O(l) + e
−
n Alloy−H(s) + OH
−
(aq) (reduction)
At the other electrode, which will be the positive electrode when the battery is
powering the phone, nickel hydroxide reacts with hydroxide ions to form nickel
oxyhydroxide, which has nickel in what for it is an unusual +3 oxidation state:
Ni(OH)
2
(s) + OH
−
(aq) n NiOOH + H
2
O + e
−
(oxidation)
When the battery is in use, the hydrogen atoms that were absorbed into the alloy
at the negative electrode are released, combining with hydroxide ions to form
water and supply the electrons that flow through a circuit to power the phone.
Alloy−H(s) + OH
−
(aq) n Alloy + H
2
O(l) + e
−
(oxidation)
At the positive electrode, nickel oxyhydroxide is reduced back to nickel hydroxide
by the electrons that arrive through the circuit, having done their work for us:
NiOOH(s) + H
2
O(l) + e
−
n Ni(OH)
2
(s) + OH
−
(aq) (reduction)
The cycle of charge and discharge can be repeated many times, to power all
the talking and text messaging on the move that is such a pervasive part of mod-
ern life.
A typical nonrechargeable “alkaline” battery for a flashlight (Figure 19.14)
uses the oxidation of zinc metal into zinc ions to generate the electrons for the
electric current:
Zn(s) n Zn
2+
(aq) + 2e
−
(oxidation)
Negative
electrode
(hydrogen-
absorbing
alloy)
Case (–) Cap (+)
Insulator
Positive
electrode
NiO(OH)
Separator
FIGURE 19.13
Nickel metal hydride cell.
Nickel–metal hydride battery (rechargeable) This bat-
tery is rapidly replacing nickel–cadmium because it
does not suffer from the “voltage depression”that nickel-
cadmiums do, in which repeated charging after only
partial discharges prevents it from fully discharging.
Lithium–ion battery (rechargeable) With a very good
power-to-weight ratio, this is often found in high-end
laptop computers and cell phones.
Zinc–air battery This battery is lightweight and
rechargeable.
Zinc–mercury oxide battery This is often used in hear-
ing aids.
Silver–zinc battery This is used in aeronautical applica-
tions because the power-to-weight ratio is good.
Metal–chloride battery Used in electric vehicles.
Hydrogen fuel cell Used in electric vehicles and to
power the space shuttle.
19.5 Chemical Reactivity Series 849
Anode
(zinc)
Cathode (graphite)
Paste of
MnO
2
,
NH
4
Cl,
and carbon
Separator
FIGURE 19.14
A common dry cell battery.
Aluminum
case
Negative
electrode
Positive
electrode
Positive
terminal
Negative
terminal
Separator
FIGURE 19.15
A lithium ion battery.
When electrons flow back into the battery at the other electrode, they combine
with manganese dioxide:
2MnO
2
(s) + 2H
2
O(l) + 2e
−
n 2MnO(OH)(s) + 2OH
−
(aq) (reduction)
Therefore, the indirect reaction of zinc with manganese dioxide is the source of
the energy that lights the bulb.
Your laptop computer may be powered by a “lithium ion battery” (Fig-
ure 19.15). These batteries use lithium oxide mixed with other metal oxides as the
positive electrode, and crystalline graphite with lithium ions intercalated within
it as the negative electrode. Unlike most conventional batteries, however, the
lithium ion battery is not powered by a redox reaction. Instead, lithium ions
move back and forth within the battery during the cycle of charging and recharg-
ing, accompanied by electrons moving in the external circuit. During charging,
the lithium ions are driven into the graphite cathode by application of the exter-
nal electric current. When the battery is used as a source of power, the ions drift
back to the lithium oxide anode. As the ions leave the cathode, electrons must
travel around the external circuit, ensuring that there is no overall transfer of
electric charge from cathode to anode as the battery is discharged.
19.5 Chemical Reactivity Series
Plumbers often use metal pipes to deliver water from the main water line to your
sink faucet. In fact, plumbers used to use lead pipes, but because the lead in the
pipe can leach into the water and cause heavy metal poisoning, that practice isn’t
followed anymore. Even though plastic pipes made from polyvinyl chloride
(PVC) are much cheaper than metal pipes, there is still a demand for copper
pipes. Why do plumbers use copper for pipes? The demand is mostly due to the
durability of copper compared to plastic, but why don’t they use iron, lithium,
calcium, or magnesium pipes?
The Table of Standard Reduction Potentials (Table 19.3) provides not only
cell potentials but also a ranking of reducing agents and oxidizing agents. This
ranking is called a
reactivity series. Table 19.6 shows a relative listing of some of
the more common metals and includes hydrogen as a reference point. The
strongest reducing agents (those elements that are most easily oxidized) are the
most reactive metals and can be found at the top of the table: Li, Na, and Mg.
Copper pipes in a home transport water
but don’t corrode easily.
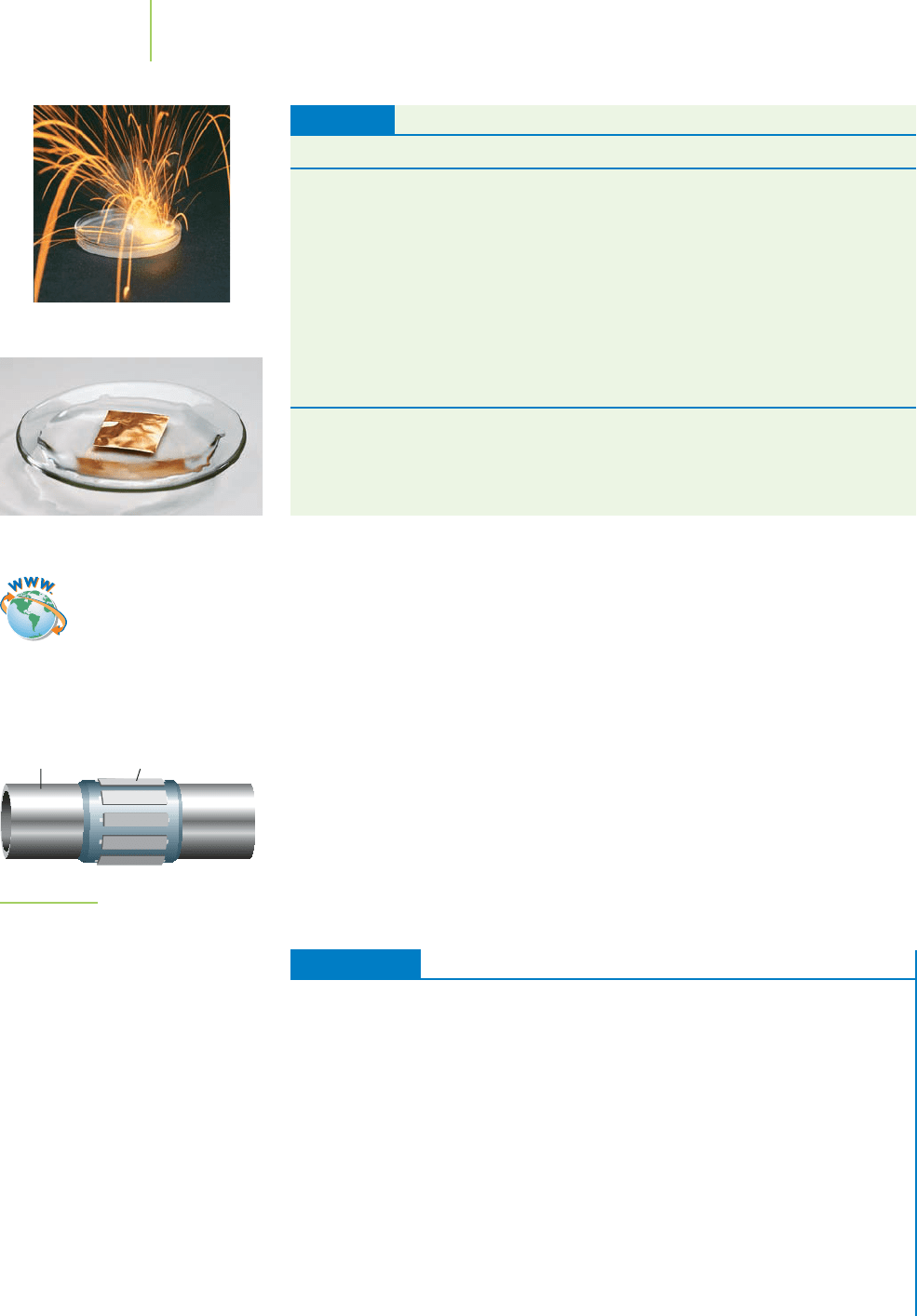
Because these metals are such good reducing agents, you would not want an ear-
ring made out of them; a better choice would be a relatively unreactive metal (and
a weak reducing agent) such as gold, copper, or silver, found at the bottom of the
table. “Fourteen-carat” gold (which means that
14
⁄24 of the sample is gold) is an
alloy of gold, copper, and silver. “Twenty-four-carat” gold is pure gold. The reac-
tivity series has social importance beyond its significance to earrings. Underwater
steel pipelines, which contain substantial amounts of iron, electrochemically cor-
rode (the metal deteriorates via oxidation) as a consequence of the interaction of
the iron with water, salt, and oxygen dissolved in the water. This
corrosion can be
minimized by putting, for example, magnesium strips in direct contact with the
pipeline, shown in Figure 19.16. The magnesium, which is more chemically active
than iron, will preferentially oxidize, ideally leaving the iron in its elemental form.
The magnesium, in effect, is sacrificed for the good of the pipeline and therefore
is known as a
sacrificial anode. Other sacrificial anodes include aluminum
wrapped around steel in hot water heaters and zinc coating the propellers and
rudders of ships.
EXERCISE 19.9 Which Is More Reactive?
Iron, especially in the form of stainless steel, can be used in jewelry, such as in the
post of an earring. Where does iron fall on the activity series? Based on the poten-
tial for the oxidation of iron to iron(III) ion, why is iron suitable (or not suitable)
for jewelry?
Solution
From Table 19.3 we see that the oxidation of metallic iron, Fe(s), has a half-reaction
potential of +0.04 V. Metallic copper (−0.34 V), silver (−0.80 V), and gold
(−1.50 V) have negative potentials. From this information we can conclude that
iron is more reactive than copper, silver, or gold. In this sense pure iron would not
be suitable, and you may know that iron spontaneously reacts to make rust when in
contact with moisture and air. Stainless steel, however, is an alloy formulated to in-
hibit corrosion. Some formulas have as much as 18% chromium and 8% nickel
added to the iron. The half-reaction potential for stainless steel is different, and it is
difficult to predict reactivity from the E° values for its constituent elements.
850 Chapter 19 Electrochemistry
Reactivity Series of the Metals
Ion Atom
Ions difficult to displace Li
+
Li Metals that react with water
K
+
K
Ca
2+
Ca
Na
+
Na
Mg
2+
Mg
Al
3+
Al
Zn
2+
Zn Metals that react with acid
Fe
2+
Fe
Ni
2+
Ni
Pb
2+
Pb
Ions easy to displace H
+
H
2
Cu
2+
Cu
Ag
+
Ag Metals that are highly unreactive
Au
3+
Au
TABLE 19.6
Steel
pipeline
Sacrificial
anode
FIGURE 19.16
A sacrificial anode on a steel pipeline.
Reaction of copper with water.
Sodium reacting with water.
Video Lesson: Corrosion and the
Prevention of Corrosion

PRACTICE 19.9
Arrange the following in decreasing order of reactivity:
Na Al Ca Cu
See Problems 63–66.
19.6 Not-So-Standard Conditions:
The Nernst Equation
Native copper and other copper objects can be cleaned with relatively dilute solu-
tions of nitric acid. Concentrated nitric acid is too strong for the job, as Ira
Remsen noted, so ancient coins should not be cleaned with concentrated nitric
acid. A very dilute solution in the hands of a professional, however, can transform
a 1600-year-old coin into a masterpiece that looks as new as the day it was
minted.
How does lowering the concentration of nitric acid change the reactivity? It
does reduce the rate of the reaction (see Chapter 15), but is there an effect on the
potential of the reaction as well? What about the temperature of the reaction? We
know that in general, the rate of a reaction increases as the temperature is raised
(also from Chapter 15). Does cold, dilute nitric acid still react with copper? We
can understand these relationships, which are at nonstandard conditions, by con-
sidering a simpler system in which the copper ion reacts with zinc metal.
If we put a zinc strip into a solution of copper(II) chloride, we note that a dark
film immediately begins to form on the zinc strip (Figure 19.17a on page 852).
Within an hour, the entire zinc strip has oxidized into the solution, and we are left
with a brown mass of copper metal (Figure 19.17b). We also note the disappear-
ance of the blue color that is characteristic of Cu
2+
in water. This is consistent
with the following half-reactions and overall cell reaction:
Cu
2+
(aq) + 2e
−
n Cu(s) E° =+0.34 V
Zn(s) n Zn
2+
(aq) + 2e
−
E° =+0.76 V
Cu
2+
(aq) + Zn(s) n Zn
2+
(aq) + Cu(s) E°
cell
=+1.10 V
We know from Section 19.3 that we can relate the free energy of a system to the
cell potential:
G =−nFE
We also discussed in Section 14.6 the relationship between free energy change
and the standard free energy change:
G =
G ° + RT lnQ
in which Q = the reaction quotient, the ratio of the concentrations (more prop-
erly, the activities) of the products over the reactants; R is the universal gas con-
stant; and T is the temperature in kelvins. For our current example,
G =
G° + RT ln
[Zn
2+
]
0
[Cu
2+
]
0
We can substitute for
G the expression for the cell potential,
G =−nFE:
−nFE =−nFE ° + RT ln
[Zn
2+
]
0
[Cu
2+
]
0
Dividing each term by −nF enables us to solve for the cell potential at nonstan-
dard conditions:
E = E° −
RT
nF
ln
[Zn
2+
]
0
[Cu
2+
]
0
19.6 Not-So-Standard Conditions: The Nernst Equation 851
This is one form of the Nernst equation, named after Walther Hermann Nernst
(1864–1941), a German chemist who studied the effect of concentration on the
potential of an electrochemical cell. We can write a more general form of the
equation, taking into account the reaction quotient for any process:
E = E
◦
−
RT
nF
ln Q
Because most reactions are conducted at standard temperature (25°C, 298 K), we
can calculate the term
RT
F
.
RT
F
=
8.3145 J·mol
−1
·K
−1
(298 K)
96485 C·mol
−1
= 0.0257
which we can substitute into the Nernst equation in this form:
E = E
◦
−
0.0257
n
ln Q
Although our calculators can easily handle the natural logarithm (“ln”) in the
equation, we typically convert the equation to the more familiar base 10 “log” by
multiplying by 2.3026 (that is,log(10) =1, and ln 10 =2.3026,so log =ln/2.3026).
852 Chapter 19 Electrochemistry
Zn
2+
Cu
2+
Cu
(a) (b)
(c) (d)
2e
–
Zn
Zn
2+
Cu
2+
Cu
Zn
FIGURE 19.17
Zinc metal reacting with copper(II) chloride
solution.
Video Lesson: The Nernst
Equation
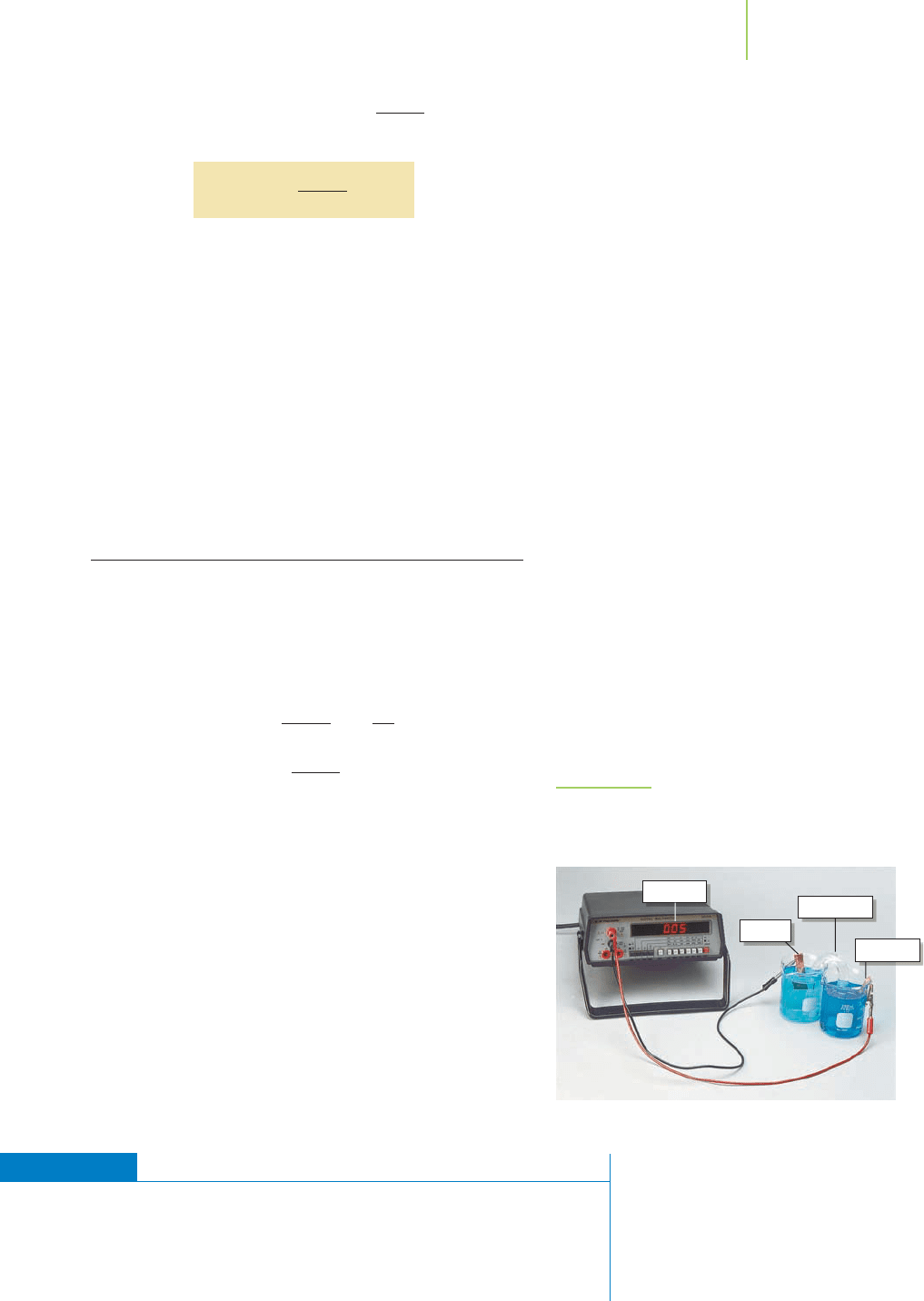
FIGURE 19.18
The concentration cell. The voltage observed in the
copper concentration cell is due to the differences in
concentration of Cu
2+
at the anode and cathode.
To account for that, we multiply the coefficient,
0.0257
n
, by 2.3026, which gives
us the final, common form of the Nernst equation:
E = E
◦
−
0.0592
n
log Q
Using this equation, we can determine the effect of lowering the concentration of
nitric acid on the potential of the copper oxidation.
The Nernst equation can also be used to measure the concentration of a solu-
tion if the standard cell potential and the actual potential are known. Electro-
chemists take advantage of this use of the Nernst equation via ion-selective
electrodes, in which the concentration of an ion such as chloride, ammonium,
cadmium, nitrate, or hydrogen (in a pH electrode) is determined. And there’s
something else that’s interesting about the Nernst equation. For example, let’s
consider the hypothetical redox reaction between copper metal and copper ions
in solution. The potential of the reduction half-reaction and that of the oxidation
half-reaction are the same at standard conditions, and, as we’d expect, no net po-
tential should be noticed for an electrochemical cell containing these reactions.
Cu
2+
(aq) + 2e
−
n Cu(s) E°
red
=+0.34 V
Cu n Cu
2+
(aq) + 2e
−
E°
ox
=−0.34 V
Cu(s) + Cu
2+
(aq) n Cu
2+
(aq) + Cu(s) E°
cell
= 0.00 V
However, what would happen if we increased the concentration of the reactant
copper ions to 2.0 M instead of the standard 1.0 M? Doing so changes the distri-
bution of the species in the reaction. Using the Nernst equation, we can calculate
the result of our modification.
E = E
o
−
0.0592
n
log
1.0
2.0
E = 0.00 −
0.0592
2
log(0.5)
E = 0.00 − (−0.0089)
E =+0.0089 V
The electrochemical potential of the reaction is a nonzero value. By ad-
justing the concentrations of the products and reactants, we have cre-
ated an electrochemical cell. This type of cell is called a
concentration
cell
because the concentrations are driving the potential of the cell (Fig-
ure 19.18). This is the same type of potential that develops across the
membrane of a muscle cell or nerve cell.
How does our cell notation change to reflect the fact that the con-
ditions are not-so-standard? By indicating the concentrations in
parentheses immediately after the species, we can immediately show
how the reaction should be written. For example,
Cu(s) | Cu
2+
(aq) (1.0 M) || Cu
2+
(aq) (2.0 M) | Cu(s)
EXERCISE 19.10 Heart Cell Potential
At the beginning of this chapter, we mentioned the electrical signals in the heart
muscle. Given the differing concentrations of potassium ions inside and outside the
heart cells, what is the electrochemical potential that corresponds to this concentra-
tion gradient? Assume that the electrochemical cell in the body can be represented
by the following cell notation. (In truth, no elemental potassium exists in the
19.6 Not-So-Standard Conditions: The Nernst Equation 853
Cathode
Anode
Potential
Salt bridge
human body. We use this concentration cell as a model merely to estimate the
potential that is obtained in a heart muscle cell.)
K(s) | K
+
(aq) (0.005 M) || K
+
(aq) (0.166 M) | K(s)
Solution
This concentration cell is based on the potassium half-reaction. The complete
reaction is
K(s) + K
+
(aq) n K(s) + K
+
(aq) E°
cell
= 0.00 V
There is one electron involved in the reaction. Using the information from the
Nernst equation, we get a potential of +0.090 V.
E
cell
= E
◦
cell
−
0.0592
n
log
[K
+
product
]
0
[K
+
reactant
]
0
E
cell
= 0.00 −
0.0592
1
log
0.005
0.166
E
cell
= 0.00 −0.0592 log (0.03012)
E
cell
= 0.00 −0.0592(−1.521)
E
cell
= 0.00 −(−0.09005)
E
cell
=+0.090 V
In the heart cell, the sodium gradient provides a potential of −0.052 V. The net
potential across the membrane of the heart cell is +0.038 V.
PRACTICE 19.10
What is the potential of the following electrochemical cell written in shorthand cell
notation? What is the half-reaction listed on the right-hand side? Is this a voltaic
cell? (Assume that the pressure of hydrogen gas is 1.0 atm in each half-cell.)
H
2
(g) | H
+
(aq) (1.0 M) || H
+
(aq) (0.10 M) | H
2
(g)
See Problems 69–72.
When we listen to a battery-powered radio, the sound tends to get softer as
the radio is used. What is happening inside the battery that causes this power
loss? During the progress of the reaction inside the battery, the reactants are being
used up as the products are being formed. According to the Nernst equation, as
the value of Q gets larger, the modification to the standard cell potential (E°) gets
more and more negative and closer and closer to zero. What is the result? As the
reaction proceeds, the potential of the battery decreases as the system inches ever
closer to equilibrium, and the music gets softer. At some point, the battery doesn’t
have enough voltage to run the radio. It has not yet reached equilibrium, but it is
below the threshold that will allow the radio to operate. We say that the batteries
are dead. As we have pointed out already, some batteries can be recharged, be-
cause their discharge reactions can be run in reverse. Rechargeable batteries can
be charged only a finite number of times, typically in the range of 500 to 1000
times for household batteries, because the surfaces of the recharged electrodes do
not form as cleanly as the original surface, and they eventually become too worn
to be useful.
854 Chapter 19 Electrochemistry
The Nernst Equation and the Equilibrium Constant
Measuring the standard cell potential is a very powerful way to solve for the equi-
librium constant of a reaction. Here’s how. When a redox reaction proceeds with-
out any intervention, it will inevitably reach equilibrium. At that point the value
of E
cell
must become zero, and the free energy change for the process also becomes
zero—our thermodynamic definition of equilibrium, introduced in Chapter 16.
When this occurs, the Q value in the Nernst equation is equal to the equilibrium
constant K. We can show this reasoning by using the following equations:
G =
G° + RT ln Q
At equilibrium,
G = 0
so 0 =
G° + RT ln K (note that “Q” has become “K”)
G° = −RT ln K
We know that
G° = −nFE°
Substituting −nFE°
into the previous equation yields
−RT ln K = −nFE
°
Solving for K, we get ln K =
nFE
o
RT
Calculating
F
RT
at standard conditions (as we did for the Nernst equation),
we find that
ln K =
38.92 nE°
Converting from natural ln to base 10 log (by dividing by 2.3026, as we did in
the Nernst equation, yields
log K = 16.9 nE°
To clearly show the connection to the Nernst equation, we will invert 16.9 and
put the result in the denominator.
log K =
nE°
0.0592
We can use the equation in this way. Alternatively, we can take the antilog of
both sides and use it in this way:
K = 10
nE°
0.0592
Which form of the equation you use depends mostly on your comfort level. We
can solve for the equilibrium constant either way. The key point is that our un-
derstanding of the thermodynamic meaning of equilibrium enables us to relate cell
potential to the equilibrium constant. The rest is just manipulating equations to
get where we want to go. Let’s see how we use this relationship to determine
the equilibrium constant for the copper–nitric acid reaction performed by Ira
Remsen. Recall that the reaction is
3Cu(s) +6H
+
(aq) + 2HNO
3
(aq) n 3Cu
2+
(aq) + 2NO(g) + 4H
2
O(l) E °
cell
=+0.62 V
K = 10
nE°
0.0592
K = 10
6(+0.62)
0.0592
= 10
62.8
≈ 10
63
Alternatively,
log
K =
nE°
0.0592
=
6(+0.62)
0.0592
= 62.8
Raising both sides to the power of 10 yields
K ≈ 10
63
19.6 Not-So-Standard Conditions: The Nernst Equation 855
Video Lesson: Electrochemical
Determinants of Equilibria
This is yet another confirmation that the reaction does, in fact, proceed toward
products. As we have this discussion, however, please keep in mind the difference
between thermodynamics and kinetics. Thermodynamics answers the question
“Can a process occur spontaneously?” It says absolutely nothing about speed.
Kinetics addresses the issues of rates and mechanisms. All that our calculations
tell us is that nitric acid can react spontaneously with the copper penny. They
don’t say how fast. That’s a question of kinetics.
EXERCISE 19.11 Equilibrium Constants and Cell Potential
Vanadium(V) ion can be reduced stepwise (that is, to V
4+
,V
3+
and, finally, V
2+
) by
reaction with a “Jones Reductor,” a zinc–mercury amalgam. The reaction for the
reduction of V
5+
to V
4+
ion includes the following two half-reactions:
VO
2
+
(aq) + H
+
(aq) n VO
2+
(aq) + H
2
O(l) E° =+1.00 V
Zn
2+
(aq) n Zn(s) E° =−0.76 V
Calculate the equilibrium constant for this reaction.
First Thoughts
There are a number of steps to solving this problem. One way to figure out what we
need to do in working forward is to start by working backward (where do we want
to be, and how do we get there?). We want the value for K. In order to get that, we
need the value for E°. In order to get that, we need to have a balanced redox equa-
tion for the reduction of VO
2
+
to VO
2+
, in which V
5+
is reduced to V
4+
, and Zn
0
is
oxidized to Zn
2+
. Our order of operations, then, is
1. Balance the redox reaction.
2. Calculate the E° value for the reaction.
3. Calculate the equilibrium constant, knowing the number of electrons
exchanged in the reaction, along with the value for E°.
How might we assess whether the answer we calculate makes sense? At this point,
because we know that the reduction of vanadium ion does occur, our equilibrium
constant should be greater than 1. How much greater will depend on the cell volt-
age and on the number of electrons transferred in the process.
Solution
The balanced half-reactions and overall cell reaction are
2VO
2
+
(aq) + 4H
+
(aq) + 2e
−
n 2VO
2+
(aq) + 2H
2
O(l) E° =+1.00 V
Zn(s) n Zn
2+
(aq) + 2e
−
E° =+0.76 V
2VO
2
+
(aq)+4H
+
(aq)+Zn(s) n2VO
2+
(aq)+Zn
2+
(aq)+2H
2
O(l) E°=+1.76V
K = 10
nE°
0.0592
K = 10
2(+1.76)
0.0592
= 10
59.5
≈ 10
60
Alternatively,
log K =
nE°
0.0592
=
2(+1.76)
0.0592
= 59.5
Raising both sides to the power of 10 yields
K ≈ 10
60
856 Chapter 19 Electrochemistry
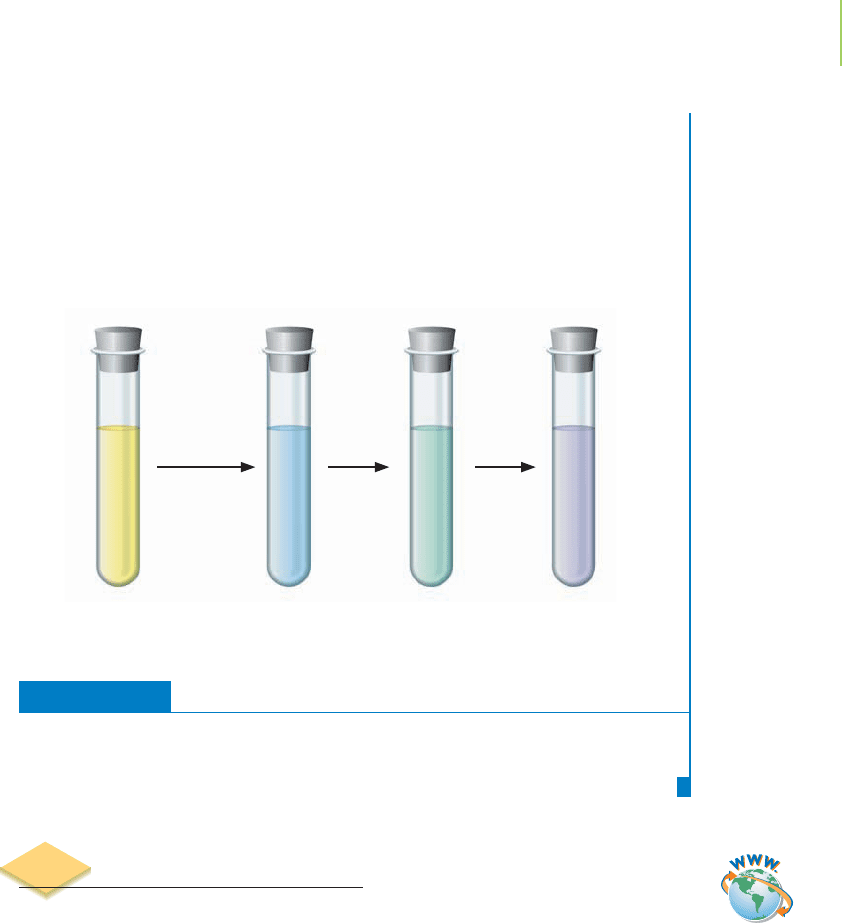
Zn is added. Time
VO
2
+
VO
2+
Time
VO
+
V
2+
Further Insights
Does our answer make sense? The cell voltage is very high and positive in this two-
electron transfer, so our equilibrium constant is large, indicating that the reduction
of vanadium proceeds essentially to completion.
Here are pictures of the vanadium solutions, showing each ion from V
5+
(left-
most picture) to V
2+
(rightmost picture). We will discuss the reasons why transition
metal ions in solution have color, and often change color when reduced or oxidized,
in the next chapter.
19.7 Electrolytic Reactions 857
The varying oxidation states of vanadium.
PRACTICE 19.11
What is the equilibrium constant for the reaction of copper metal with zinc ion,
discussed at the beginning of this section?
See Problem 68.
19.7 Electrolytic Reactions
Some metals, including copper, gold, and silver, are found in their pure elemental
state in the environment. On the other hand, aluminum metal, based on its reac-
tivity (Section 19.5) is found only chemically combined in ores such as bauxite
(hydrated aluminum oxide; Al
2
O
3
· H
2
O or Al
2
O
3
· 3H
2
O). In fact, aluminum,
largely in the form of the aluminum oxides and silicates, makes up 8.1% of the
Earth’s crust. However, we know that pure aluminum can be produced, because it
is a major component in so many common products: the can in which we store
our soda, the wrap in which we put our fish for freezing, and the lightweight
bicycle we ride down the street. How is aluminum metal made from bauxite?
The basic process, called electrolysis, entails passing a current through a solu-
tion of metal ions in an electrochemical cell in the direction opposite to the spon-
taneous reaction. Doing so forces the nonspontaneous reaction to occur. This
process, also known as
electrowinning, is responsible for the manufacture and
purification not only of aluminum but also of many other metals.
Al
3+
+ 3e
−
n Al
Cu
2+
+ 2e
−
n Cu
Ag
+
+ e
−
n Ag etc....
Electrowinning is the most inexpensive method for making aluminum and
magnesium metals. Electrowinning of metals such as aluminum and magnesium
Video Lesson: Electrolytic Cells
Tutorial: Electrolytic Cells
