Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

80
128
130
132
134
136
138
140
142
144
0.71 Gy
25 h
19 d
11 d
3.9 s
0.1 ms
0.5 s
4.8
min
36 min
2.13 min
32 ky
22 y
235
U
231
Th
227
Ac
223
Fr
219
At
215
Bi
215
Po
211
Pb
211
Bi
207
Tl
207
Pb
211
Po
215
At
219
Rn
223
Ra
227
Th
231
Pa
126
81 82 83 84 85 86 87 88 89 90 91 92 93 94
Number of neutrons
Atomic number
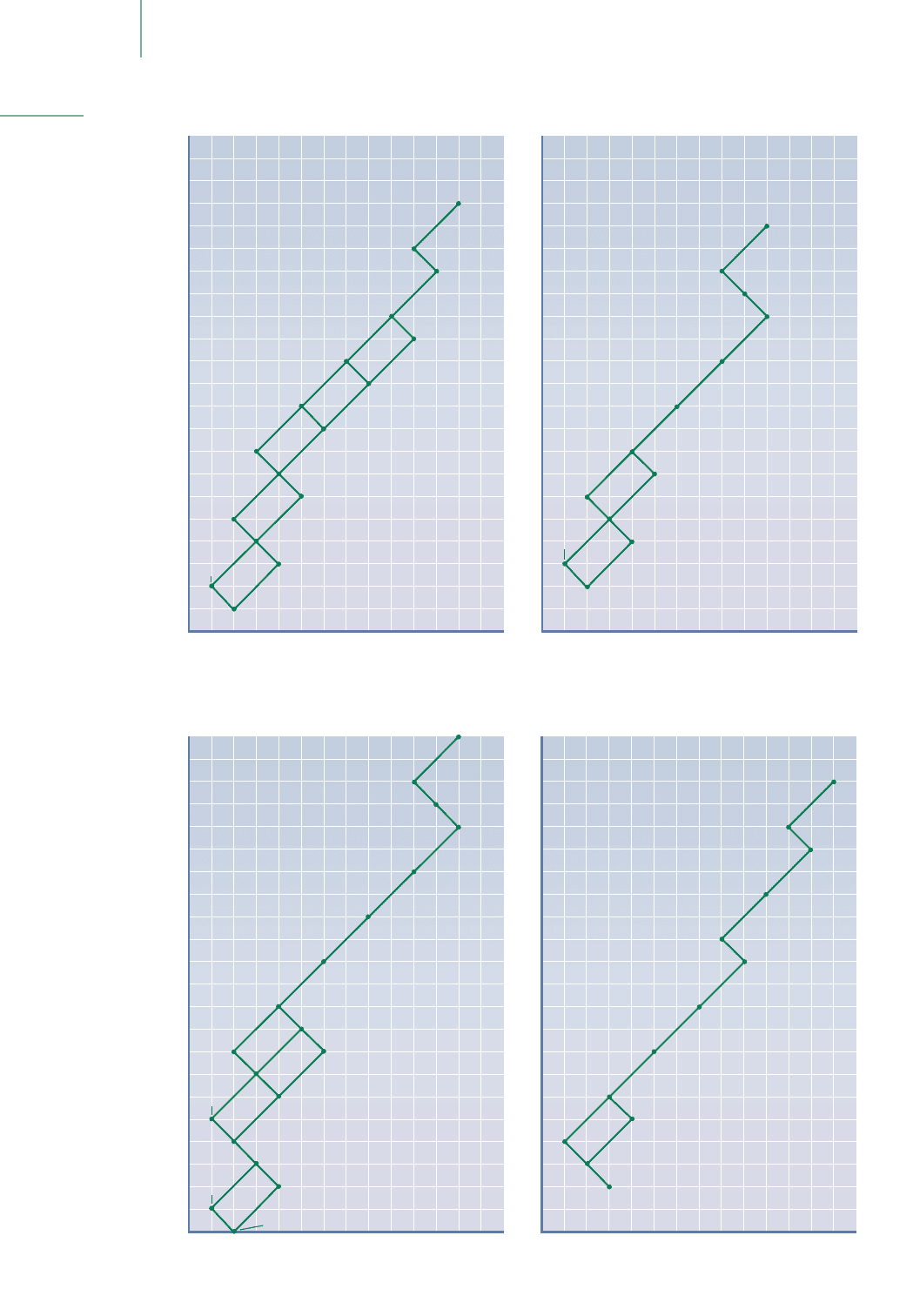
The Uranium-235 Decay Series
778 µs
80
128
130
132
134
136
138
140
142
144
232
Th
228
Ac
228
Th
212
Po
212
Bi
216
At
224
Ra
220
Rn
216
Po
212
Pb
208
Tl
208
Pb
228
Ra
14 Gy
6.7 y
6.1 h
1.9 y
3.7 d
55 s
11 h
164 µs
0.3 µs
3.1
min
126
81 82 83 84 85 86 87 88 89 90 91 92 93 94
Atomic number
The Thorium-232 Decay Series
80
128
130
132
134
136
138
140
142
144
238
U
234
U
234
Th
230
Th
226
Ra
222
Rn
218
Po
214
Pb
214
Bi
210
Tl
218
Rn
218
At
214
Po
210
Po
210
Pb
210
Bi
234
Pa
206
Pb
4.5 Gy
25 d
6.7 h
0.23 My
83 ky
1.6 ky
3.8 d
150 µs
22 y
140 d
1281.3
min
4.2
min
27 min
126
81 82 83 84 85 86 87 88 89 90 91 92 93 94
Number of neutrons
Atomic number
The Uranium-238 Decay Series
206
Tl
3.1 min
80
128
130
132
134
136
138
140
142
144
209
Bi
225
Ac
209
Pb
209
Tl
213
Bi
213
Po
217
At
221
Fr
225
Ra
229
Th
223
Pa
237
Np
233
U
47 min
4.2 ms
20 ms
4.8 min
10 d
7 ky
15 d
0.16 My
2.3 My
27 d
126
81 82 83 84 85 86 87 88 89 90 91 92 93 94
Atomic number
The Neptunium-237 Decay Series
908 Chapter 21 Nuclear Chemistry
FIGURE 21.4
The four natural decay
series. Each decay series
begins at the nuclide
listed at the top right-
hand side of the series
and proceeds to the
lower left-hand side
by nuclear decay. The
half-life of many
of these transitions
are indicated in red
numbers. Gamma
emissions accom-
pany many of these
decays.
21.3 Interaction of Radiation with Matter 909
decrease Z by 2, thus moving the line to the left. But a beta-minus emission in-
creases Z by 1 unit. If beta emission follows an alpha emission, there is a zag back
to the right. The series that begins with
235
U ends with the stable lead-207 iso-
tope, and the
238
U series forms
206
Pb. There are two other naturally occurring
decay series, one that begins with
237
Np to form
209
Bi, and one in which
232
Th
decays to ultimately form
208
Pb.Along the way, these decay chains provide dozens
of radioisotopes that we typically find in our biosphere. This is one of the
sources of naturally occurring radioactive isotopes. Of particular concern is
radon, a radioactive gas that can collect in basements dug into soil that is rich in
uranium ores. Is radon harmful to humans? Understanding the relationship
between radioactivity and human health will help us answer this question.
HERE’S WHAT WE KNOW SO FAR
■
Radioactivity results from the decay of an unstable nucleus.
■
There are three main types of radioactive decay and three common forms of
radioactivity.
■
The alpha particle is a fast-moving helium nucleus.
■
The beta particle is an electron ejected from the nucleus of an unstable atom.
■
The gamma ray is a burst of high-energy electromagnetic radiation.
■
A decay series is a stepwise progression of a radioactive nuclide toward
stability.
21.3
Interaction of Radiation with Matter
Taking a walk on a crisp, sunny day is one of the pleasures of autumn. Any cloud
that blocks the Sun is easily noticed. Not only does the shade reduce the amount
of light hitting your eyes, but your skin also registers the change. The energy ex-
changes, from the infrared through the ultraviolet regions of the electromagnetic
spectrum, are apparent and profound. However, when it comes to detecting the
small amounts of alpha particles, beta particles, and gamma rays that bombard
you on a daily basis, your senses don’t help. For instance, you cannot detect the
alpha particles that radioactive radon, an odorless, colorless, and tasteless gas,
emits.
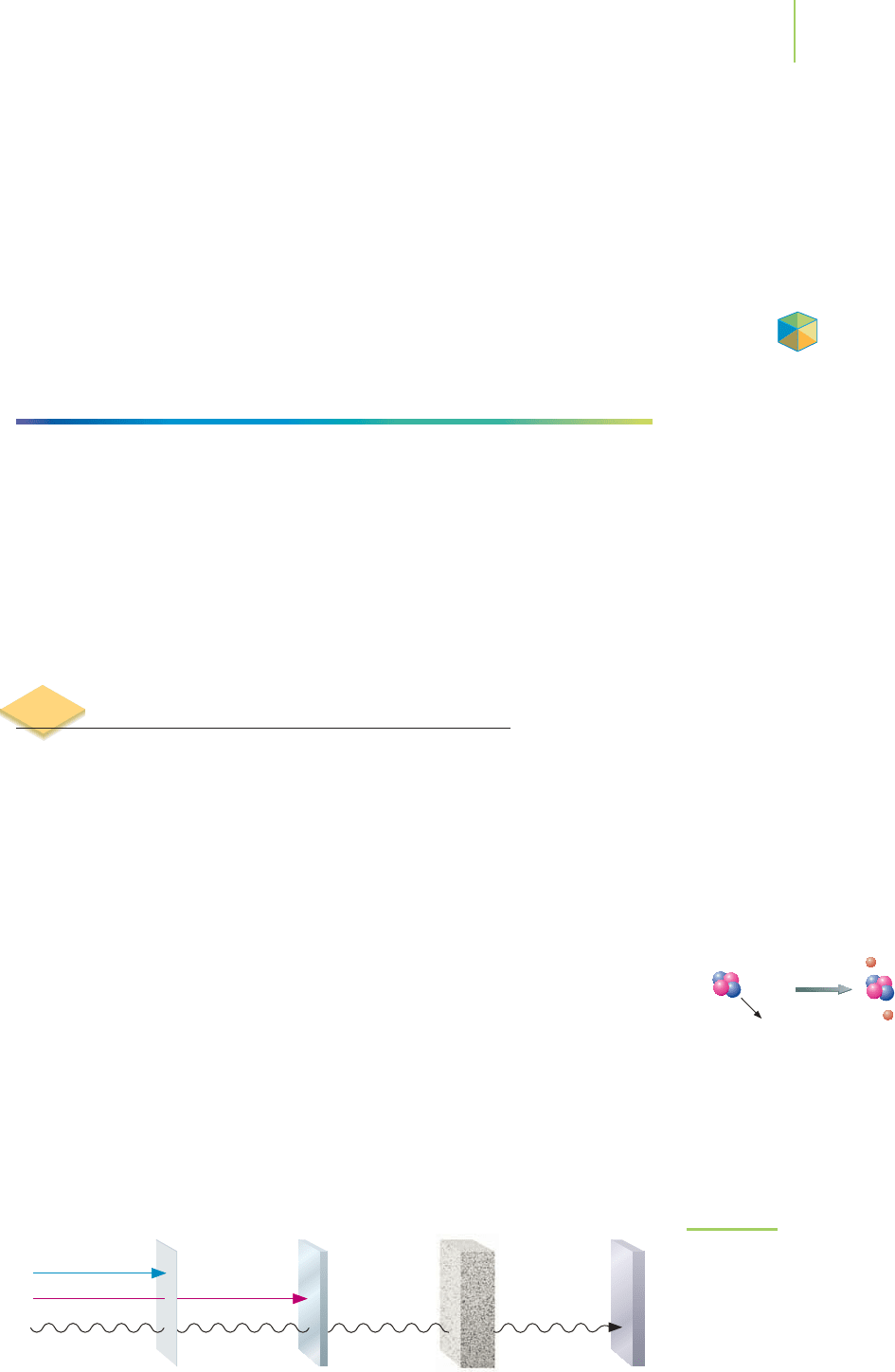
Though seemingly invisible, the different types of radiation form quite a nu-
clear arsenal, as summarized in Figure 21.5. We can envision alpha particles as the
cannon balls of the group. With their greater size and +2 charge, they do not
travel very far before they smash into other atoms. The typical collision results
in the capture of two electrons to form a neutral helium atom. In contrast, beta
particles, being smaller and traveling more rapidly, are the equivalent of high-
velocity bullets. They are able to travel significantly longer distances before a
collision, but they lack the punch of an alpha particle. Gamma rays, with no
mass and no charge, are akin to laser weapons. They shoot great distances
through matter, now and then searing something in their path. What about the
Application
Tissue
α
β
γ
Aluminum Concrete Lead
FIGURE 21.5
Alpha, beta, and gamma radiation differ
in their penetration. Gamma rays are
the most highly penetrating, and alpha
particles the least.
Molecule
He
2+
He
0
Collision of an alpha particle and a
molecule results in the formation of an
atom of helium.

Application
Water (neutral) Water (cation radical)
antineutrinos that accompany beta emissions? Having neither a mass nor a
charge, they are not absorbed and are considered harmless.
The type and the energy of the radiation dictate what must be used to shield
us to the greatest extent possible (see Table 21.3). Alpha particles penetrate mat-
ter the least, being stopped by just a few centimeters of air, by the outer layer of
your skin (which is mostly dead cells), or by a piece of paper. Beta particles pen-
etrate more deeply and can pass through several pieces of paper, through a thin
sheet of aluminum, or about a centimeter into your skin. In contrast, gamma rays
can pass right through you. Shielding your body from them requires several
inches of aluminum or lead, and even these may not do the job.
A visit to the dentist reveals an interesting feature of stopping damage from
nuclear radiation. Before an X-ray of a patient’s teeth is obtained, the patient is
typically draped with a sheet of lead. Why is lead one of the materials employed
to shield us from high-energy electromagentic radiation, such as X-rays or nu-
clear radiation? The high density of lead, 11.3g/cm
3
, means that only two inches
of lead will easily shield you from alpha and beta radiation, as well as from most
gamma radiation. However, lead is not unique in this ability. Gold (d =
19.3 g/cm
3
) is more dense than lead and would work even better. Bricks or blocks
of a moderately dense material such as concrete also would do the trick. However,
no dentist is likely to place an apron of gold or a foot of concrete over your ab-
domen before taking dental X-rays. Given its density and price, lead is often the
shielding medium of choice.
Although alpha, beta, and gamma radiation differ in penetration, they are
alike in the effects they produce on the molecular level.All three are known as
ion-
izing radiation
; that is, they are capable of forming ions by knocking electrons out
of atoms. The damage they cause is the reason for their detection with, for exam-
ple, a Geiger counter or film badge.
The consequences of ionizing radiation can be negligible or severe, depend-
ing on how many molecules are damaged inside the body. Although small
amounts of radiation typically lead to only negligible damage that can be
repaired by the body, large doses of radiation can be life-threatening.
How does
ionizing radiation cause damage?
Because between about 50% and 70% of your
body is water, a scenario of particular interest occurs when ionizing radiation
strikes a water molecule. The blow can knock off an electron to form a highly re-
active species with an unpaired electron:
HH
O e
ionizing
radiation
HH
O
910 Chapter 21 Nuclear Chemistry
Differences in Penetration and Shielding for Different Types of Radiation
Penetration Penetration in
Type Examples in Dry Air Skin or Tissue
†
Shielded by Q*
Alpha uranium, plutonium, 2–4 cm 0.05 mm Paper, air, clothing 20
americium
Beta potassium-40, cesium-137 200–300 cm 4–5 mm Heavy clothing ~1
Gamma technetium, cobalt-60 500 m 50 cm Lead, concrete ~1
Fast neutrons Accelerators Several hundred feet High Water, plastic concrete 20
TABLE 21.3
*Q is the relative biological effectiveness, a factor that indicates relative amounts of damage to living tissue.
†
Alpha, beta, and gamma radiation all exhibit a range of energies. The degree of penetration depends on the actual energy.
Use of radiation shield on a patient at
the dentist’s office.
Application
C
HEMICAL ENCOUNTERS:
Radiation and Cancer
Electron density maps of water and the radical
cation of water. Note the decrease in electron
density around the oxygen end of the molecule.

The resulting radical cation (see Chapter 7) undergoes autoprotolysis to form
hydroxyl free radicals:
H
2
O·
+ H
2
OH
3
O
+ ·OH
The fate of the hydroxyl free radicals is particularly damaging to the cells within
your body. If they encounter a molecule of DNA in a dividing cell, they may
damage a section of the genetic code (Figure 21.6). The damage could result in
death of the cell, triggering the formation of mutant proteins (proteins with a
non-natural primary structure; see Chapter 22) or triggering an abnormal func-
tion of the cell leading to cancer. In short, nuclear radiation can be carcinogenic.
Although we do not know exactly what cellular events ensue after a dose of ion-
izing radiation, two things seem clear: (1) The more radiation a person is exposed
to, the greater the likelihood of that person’s developing cancer. (2) These cancers
may not show up until decades after the time of exposure.
Is there a threshold below which radiation is safe? Recent studies have convinc-
ingly demonstrated that the damage inflicted by low-level radiation upon workers
in the nuclear industry and upon World War II nuclear bomb survivors have been
greatly underestimated. Similarly, the link between fetal X-rays (an abandoned
medical practice) and childhood cancer has been established. Fortunately,because
cancer cells grow and divide, they also are susceptible to radiation. Carefully mea-
sured doses of radiation directed at cancerous cells can result in their death.
Overall, the biological effects of nuclear radiation depend on the quantity of
energy transferred to the cells and tissues. In the United States, the
rem or “roent-
gen equivalent in man” is the unit for estimating the damage. Other units that
measure radiation include the
becquerel, curie, roentgen, and rad. Of these, only
the becquerel (Bq) is an SI unit. Scientists in most parts of the world employ two
other SI units, the
sievert (Sv) and the gray (Gy), which are related to the rem and
the rad, respectively, as shown in Table 21.4.
21.3 Interaction of Radiation with Matter 911
Base
damage
Single-strand
break
Double-strand
break
Double-strand
breaks
FIGURE 21.6
Examples of chromosomal damage from
radiation.
Units for Measuring Radiation
Measure of Name Abbreviation Definition
Activity becquerel* Bq 1 disintegration per second
Activity curie Ci 1 curie = 3.7 × 10
10
becquerel
Exposure roentgen R 1 roentgen = 2.58 × 10
−4
couloumbs of
charge per kg of air
Absorbed dose radiation absorbed rad 1 rad =1 × 10
−2
J of energy deposited
dose per kg of tissue
Absorbed dose gray* Gy 1 gray = 100 rad
Dose equivalent roentgen equivalent rem Q × absorbed dose
in man
Dose equivalent sievert* Sv 1 sievert = 100 rem
*SI units
TABLE 21.4
The quantity of energy absorbed by tissues is directly related to the time of
exposure to ionizing radiation. In fact, time is an important part of the decision-
making process in medical diagnosis and treatment. For example, prostate cancer in
elderly men is often treated by the implantation of metal “seeds” coated with
125
I
or, more recently,
103
Pd. Why use these nuclides? They are sufficiently radioactive
for only the time needed to control the cancer without creating new cancers.
How
do we know how long they will be radioactive?
The most important measure that
we use to judge the length of time a substance is radioactive is its half-life.
The U.S. Environmental Protection Agency suggests that the average person
receives an annual dose of 0.3 rem of radiation from natural sources. Over the
course of a lifetime, this is predicted to result in 5 or 6 deaths due to cancer per
10,000 people. This sounds shocking until we consider that the rate of deaths due
to cancer from nonradioactive sources is predicted to be about 2000 people per
10,000. Larger doses received in one exposure have a much more deleterious
effect on human health, as shown in Table 21.5. Acute exposures, such as those
that result from accidents in nuclear power plants and those that resulted from
the U.S. bombing of Japan in World War II, cause severe damage to the human
body, often resulting in lifelong health problems and even death.
21.4 The Kinetics of Radioactive Decay
The half-life, t
1/2
, of a radioactive isotope is the period of time it takes for exactly
half of the original nuclei in a radioactive sample to decay. We discussed half-life
in detail in Chapter 15. Table 21.6 shows that half-lives can vary widely among
the radioactive isotopes. They can be as short as a few microseconds and
as long as a few billion years.
How can the half-life tell us how much radioactivity remains after a
given time? Remember from Chapter 15 that after one half-life, one-half
of the sample has reacted and only one-half of the sample remains. After
a second half-life,
1
⁄
2
×
1
⁄
2
=
1
⁄
4 of the sample is left. After 3 half-lives,
1
⁄
2 of
1
⁄
2
of
1
⁄
2,or (
1
⁄
2)
3
=
1
⁄
8, of the sample remains. More generally, then, for n half-
lives, the fraction of the original sample remaining is (
1
⁄
2)
n
. This trend is
shown in Figure 21.7 and is valid for all radioactive decay processes.
Palladium-103, used to coat the seeds implanted in the prostate, de-
cays by electron capture, in which an inner-orbital electron is captured
by a proton in the nucleus to form a neutron. The half-life of Pd-103 is
16.97 days. How long will it take for the radiation to be diminished to
1.00% of its original value so it is considered safe for radiation workers,
the prostate cancer patient, and his family? A look at Figure 21.7 indi-
cates that this will take between 6 and 7 half-lives, or between 102 and
912 Chapter 21 Nuclear Chemistry
Health Effects of Acute Radiation Exposure
Exposure (rem) Health Effect Time to Onset
10 Burns, changes in blood chemistry
50 Nausea Hours
75 Vomiting, hair loss 2–3 weeks
100 Hemorrhage
400 Death Within 2 months
1000 Internal bleeding, death Within 1–2 weeks
2000 Death Within hours
TABLE 21.5
Half-lives of Some
Radioactive Elements
Element Nuclide Half-life
nobelium
250
No 250 s
technetium
99m
Tc 6.0 h
thallium
201
Tl 21.5 h
radon
222
Rn 3.8 d
iodine
131
I 8.040 d
palladium
103
Pd 16.97 d
cobalt
60
Co 5.271 y
hydrogen
3
H 12.3 y
carbon
14
C 5730 y
radium
226
Ra 1.6 × 10
3
y
uranium
238
U 4.5 × 10
9
y
TABLE 21.6
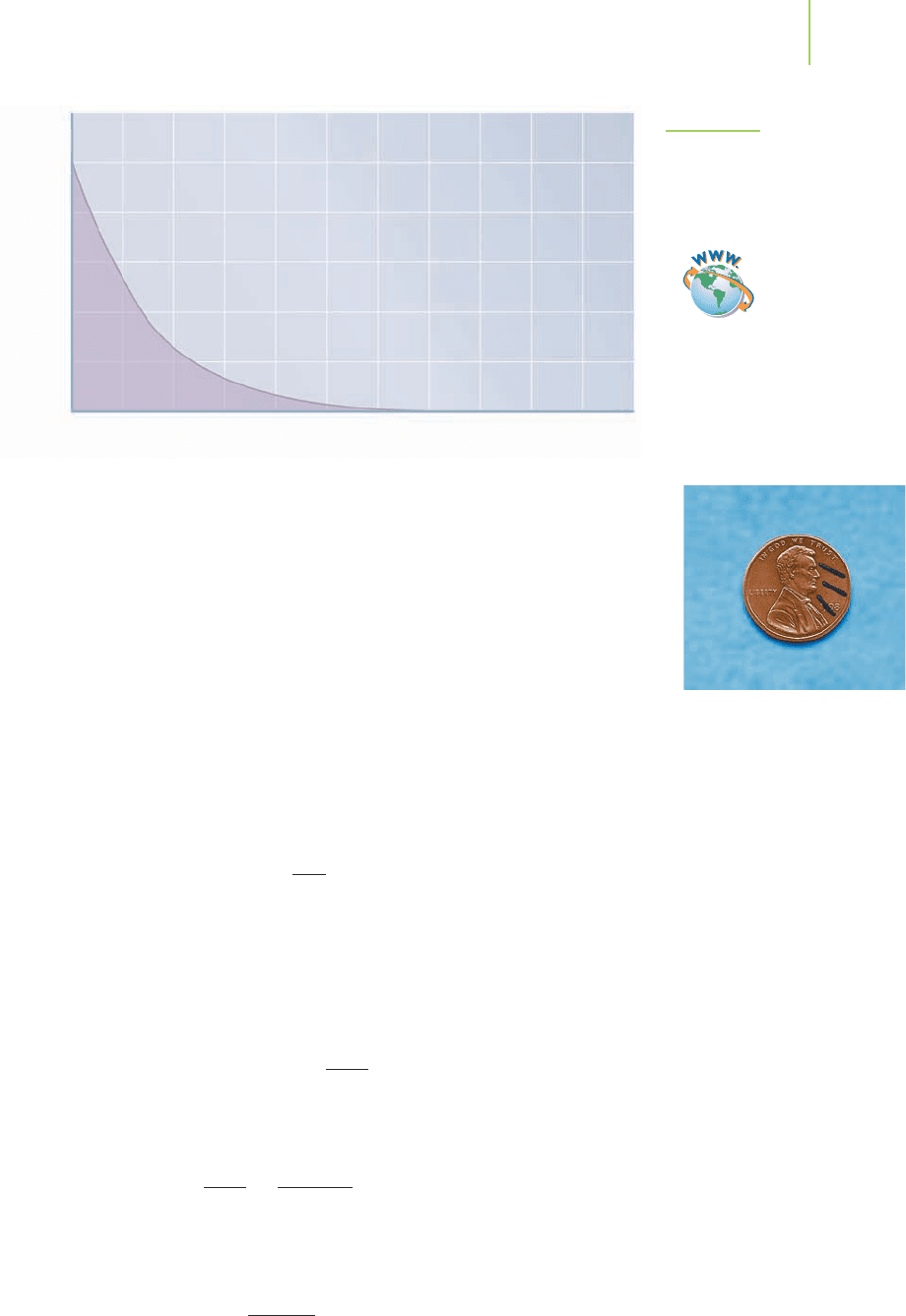
119 days. Such approximations are often sufficient. When necessary, we can also
solve explicitly for n.
(
1
⁄2)
n
= 1/100 = 0.0100
Taking the natural logarithm (ln) of both sides yields
n[ln(
1
⁄2)] = ln(0.0100)
n(−0.693) =−4.605
n = 6.645 half-lives
Solving for the time needed, we find that
t = 6.645 half-lives × 16.97 days/half-life
= 113 days
There is another approach based on the understanding that radioactive iso-
topes decay via first-order kinetics. Recall from Chapter 15 that the relationship
among concentration, time, and half-life for any first-order process is shown by
ln
[A
t
]
[A
i
]
=−kt
where A
t
= the amount of substance remaining
A
i
= the initial amount of substance
k
= the first-order rate constant for the reaction (in this case, the decay)
t = time
We also know that the rate constant for a first-order reaction can be determined by
k =
0.693
t
1/2
This means that we can solve explicitly for the rate constant if we are given the
half-life, t
1/2
. In our example,
k =
0.693
t
1/2
=
0.693
16.97 day
= 0.04084 day
−1
Assuming that we start with A
i
= 1.000, we can determine that if we only have 1%
remaining, A
t
= 0.0100. Then
ln
[0.0100]
[1.00]
=−0.04084t
−4.605 =−0.04084t
113 = t
so t = 113 days, and we obtain the same answer (113 days) by either method.
21.4 The Kinetics of Radioactive Decay 913
0
2000
4000
6000
8000
10,000
12,000
01234567891011
Number of half-lives
Number of radon atoms
FIGURE 21.7
The kinetics of radioactive decay. The half-life
of a radioisotope, such as radon, is the time
required for half of the nuclei in the radio-
active sample to decompose.
Three palladium-103 “seeds,”
which are used for the treatment
of prostate cancer, easily fit on
the top of a penny.
Visualization: Half-Life of Nuclear
Decay
Video Lesson: Rates of
Disintegration Reactions

But when would the entire Pd-103 sample be gone? This is a question we
cannot precisely answer, although we can come very close. Why are we unable to
tell when all of the Pd-103 is gone? After each half-life, half of the number of
radioactive atoms remaining from a previous half-life are still remaining. Sooner
or later, after a very large number (roughly 80) of half-lives have passed, only two
radioactive atoms remain for every mole we started with. Half-life is a statistical
measure. Probabilities, which don’t apply with a sample size of two, will not
accurately tell the rate of the reaction.
EXERCISE 21.4 Half-life Calculations: Here Today, Gone Tomorrow?
After exercising on a treadmill, a patient was given thallium-201 for a diagnostic
scan of his heart (Figure 21.8). How long will it take for 95.0% of the thallium to
have decayed? The half-life of thallium-201 is 21.5 hours.
Solution
As discussed previously, we can solve the problem in two ways. Using the first
method, we find the number of half-lives that pass until 5.0%, or a fraction of 0.050,
of the Tl-201 remains.
(
1
⁄2)
n
= 0.050
n[ln(
1
⁄2)] = ln(0.050)
n(–0.693) = –3.00
n = 4.32 half-lives
t = 4.32 half-lives × 21.5 hours/half-life = 93 hours
Using the second method, we proceed as follows:
k =
0.693
t
1/2
=
0.693
21.5h
= 0.0322 h
−1
ln
[0.050]
[1.00]
=−0.0322t
−3.00 =−0.0322t
93 hours = t
PRACTICE 21.4
The half-life of
198
Au is 2.69 days. How long would it take for 99% of a gold-198
sample to decay?
See Problems 39–42 and 47–48.
The previous problem involved percentages. But you also can work half-life
problems given a starting mass or a starting number of atoms. Both are measures
of the radioactivity present. Similarly, you can work half-life problems given a
unit of activity such as the number of disintegrations per second (becquerels) or
curies, because these also measure the amount of radioactivity and are propor-
tional both to the mass and to the number of atoms. Such variations in units re-
flect the differing needs of real-world situations where you are likely to encounter
radioisotopes.
The half-life of a radioactive isotope can also help you to determine how
long the radioisotope will be useful or, perhaps, to weigh the hazards associated
with that particular isotope. For example, would you rather be around a
radioisotope that decayed to 1% of its activity in 10 seconds or one that did so in
10 centuries? For the latter, the rate of decay is much lower, and you would be
914 Chapter 21 Nuclear Chemistry
FIGURE 21.8
Proton
Neutron
14
N
bombarded with far fewer alpha particles, beta particles, or gamma rays in a given
time period. This principle is important in the use of technetium in medical
imaging. We have noted that technetium-99m is a gamma emitter. It has a half-
life of 6.01 hours, which is long enough for a medical procedure, but short
enough that the substance doesn’t persist very long.
99m
43
Tc →
99
43
Tc +
0
0
t
1/2
= 6.01 h
The product nuclide,
99
43
Tc
, is still radioactive. However, with its half-life of
213,000 years, the activity of Tc-99 as a beta emitter is low. In the two and a half
weeks it takes for the majority of Tc-99 to be completely eliminated from the
body, it does little damage.
21.5
Mass and Binding Energy
By now you might be a bit suspicious. Many nuclei are unstable and sponta-
neously decay. Significant amounts of energy are released during alpha, beta,
and gamma emission—enough to ionize molecules or to kill cancer cells—but we
haven’t talked about the source of the energy.
Where does it come from, and how
does this help explain the decay processes that nuclei undergo?
The place to start to find an answer is with Einstein’s famous equation,
E = mc
2
in which the constant, c, is equal to the speed of light. This relationship illustrates
that a particular mass, when completely converted, is equivalent to a surprisingly
large quantity of energy. Any chemical reaction that is accompanied by a loss in
energy, such as in an exothermic reaction, actually also has a corresponding loss
in mass. However, the mass losses are so minuscule that we cannot detect them
using conventional instruments. In contrast, the changes in mass due to a nuclear
reaction, though tiny, are quite measurable.
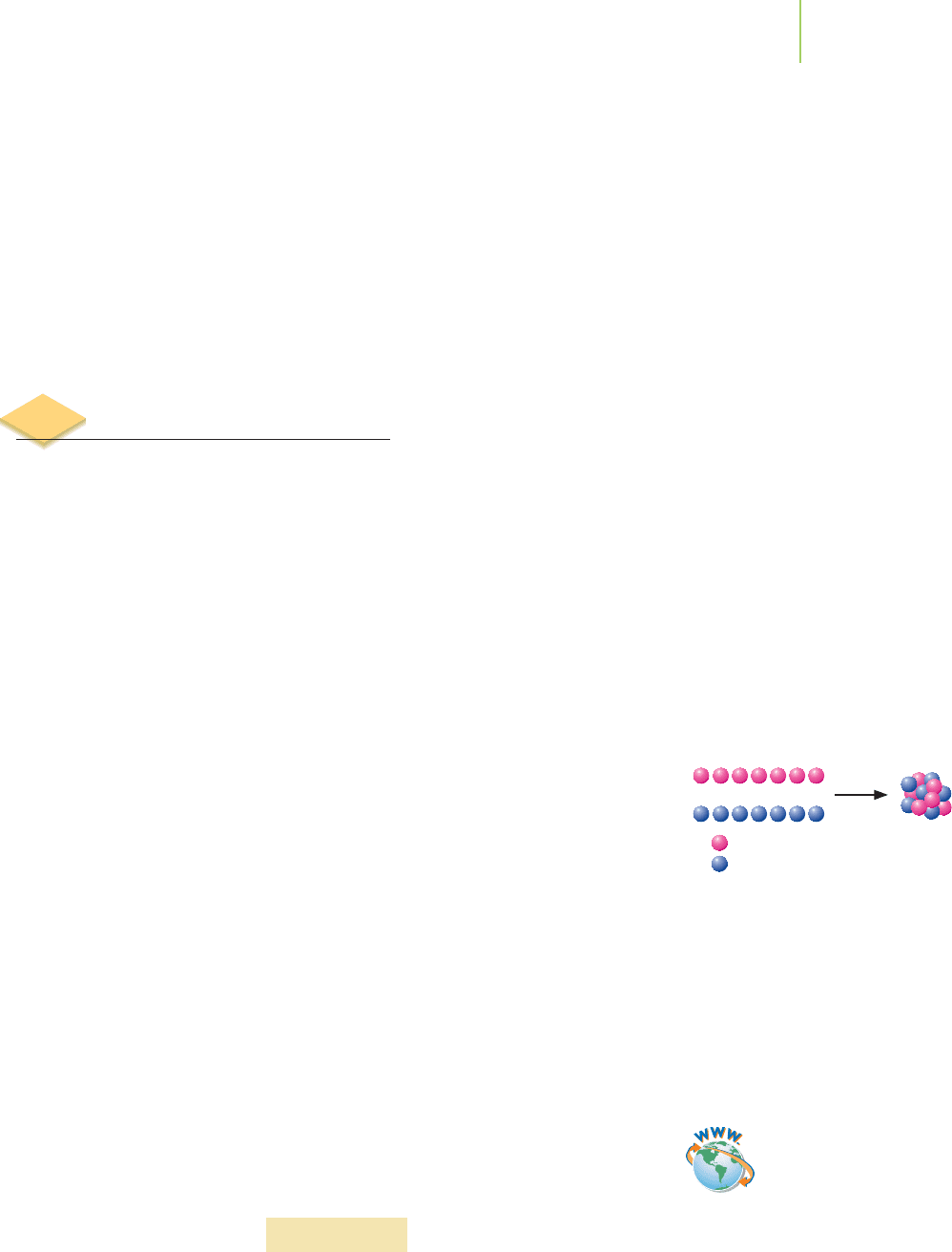
Let’s explore this connection by examining the formation of nitrogen-14
from its individual nuclear particles:
7p +7n →
14
N nucleus
A mole of nitrogen-14 nuclei (without any electrons) weighs 13.99540 g. What is
the mass of 7 separate moles of protons (1.00727 g/mol) and 7 mol of neutrons
(1.008665 g/mol)?
7 mol of protons × 1.00727 g/mol = 7.05089 g
7 mol of neutrons × 1.008665 g/mol = 7.060655 g
Total mass = 7.05089 g + 7.060655 g = 14.11154 g
This exceeds the mass of a mole of nitrogen-14 nuclei by (14.11154 g−13.99540 g) =
0.11614 g. This
mass defect, the mass difference between the individual protons
and neutrons and the composite nucleus, was used in binding the protons and
neutrons together within the nucleus. The
binding energy, expressed as a positive
number, is the energy required to dismantle the nucleus into its individual pro-
tons and neutrons. To ensure that our calculations give us positive numbers for
binding energy, we typically use Einstein’s equation in a different form:
∆E = |∆m|c
2
where ∆E is the binding energy and |∆m| is the absolute value of the change in
mass in kilograms. We use kilograms because our SI unit of energy, the joule,
is defined as kg·m/s
2
. For nitrogen-14, using our mass defect of 0.11614 g
(= 1.1614
×
10
−
4
kg), the binding energy can be calculated as
∆E = |∆m|c
2
= |–1.1614
×
10
−
4
kg|
×
(2.9979
×
10
8
m/s)
2
= 1.04
×
10
13
J
21.5 Mass and Binding Energy 915
Seven protons and seven neutrons form
the nucleus of nitrogen-14.
Video Lesson: Binding Energy
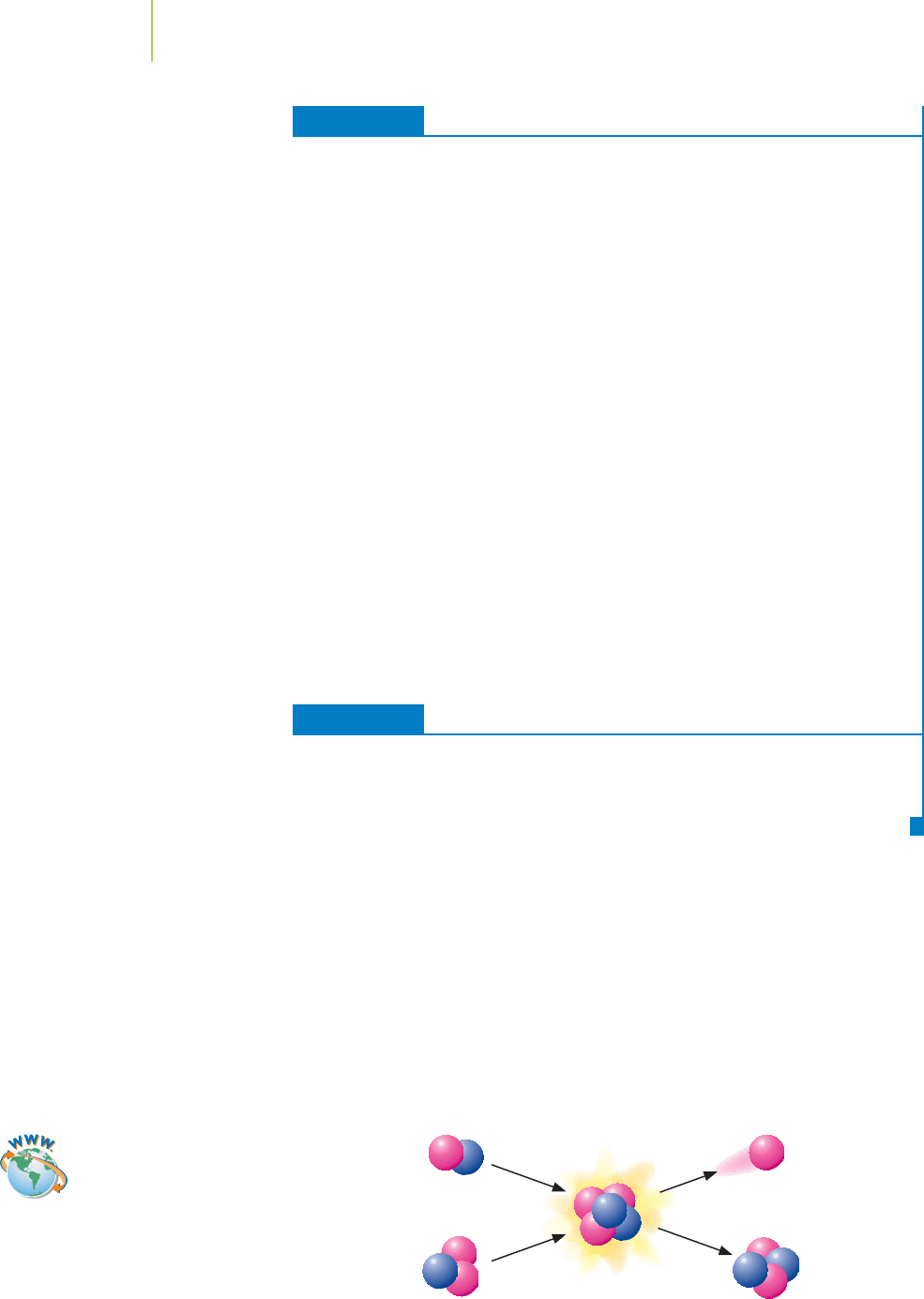
EXERCISE 21.5 The Energy Advantage of Nuclear Reactions
When bombarded with neutrons, uranium-235 can split to form bromine-87,
lanthanum-146, and three neutrons. The masses reported here are those of the bare
nuclei.
235
92
U +
1
0
n →
87
35
Br +
146
57
La + 3
1
0
n
grams/mole 234.9936 1.008665 86.90156 145.8944 3.0260
Calculate the mass defect and the energy released in kJ/mol.
Solution
The mass defect is the difference in mass between the ending and starting materials.
∆m = (86.90156 + 145.8944 + 3.0260) − (234.9936 + 1.008665)
=−0.1803 g/mol =−1.803
×
10
−4
kg/mol
The energy equivalent to this mass is ∆E = |∆m|c
2
.
∆E = |∆m|c
2
= |–1.803
×
10
−4
kg/mol |
×
(2.9979
×
10
8
m/s)
2
= 1.621
×
10
13
J/mol = 1.621
×
10
10
kJ/mol
This is many orders of magnitude greater than the energy released by an equivalent
mass of materials in a combustion reaction.
Even though this problem uses the masses of the bare nuclei, the problem could
have been solved using the atomic masses, because the number of electrons on each
side of the equation remains constant. In radioactive decay processes that involve a
change in the number of protons, the mass of the electrons must also be considered
in the calculation of the mass defect, when atomic masses are used.
PRACTICE 21.5
Calculate the energy released in the following process.
224
88
Ra →
220
86
Rn +
4
2
He
See Problems 53 and 54.
Some atoms are more thermodynamically stable than others. A table of
binding energies shows only that the values generally increase as the atoms get
heavier. However, if you recalculate the binding energy for each atom and report
the values per nucleon (proton or neutron), the stabilities pop right out at you.
Nuclei with greater binding energies per nucleon are more stable. Note in Fig-
ure 21.9 that the lightest elements, those with mass numbers of 20 or less, have
the lowest binding energies per nucleon. In comparison with iron, helium simply
does not have enough nucleons to be as strongly glued together. The process that
liberates energy on the sun,
fusion (or nuclear fusion), is energetically favorable
because lighter elements such as helium are joined to form heavier ones that have
a more favorable binding energy per nucleon.
916 Chapter 21 Nuclear Chemistry
Deuteron
Energetic
neutron
Triton Helium
nucleus
Fusion
reaction
Nuclear fusion.
Visualization: Nuclear Fusion
Video Lesson: Nuclear Fusion
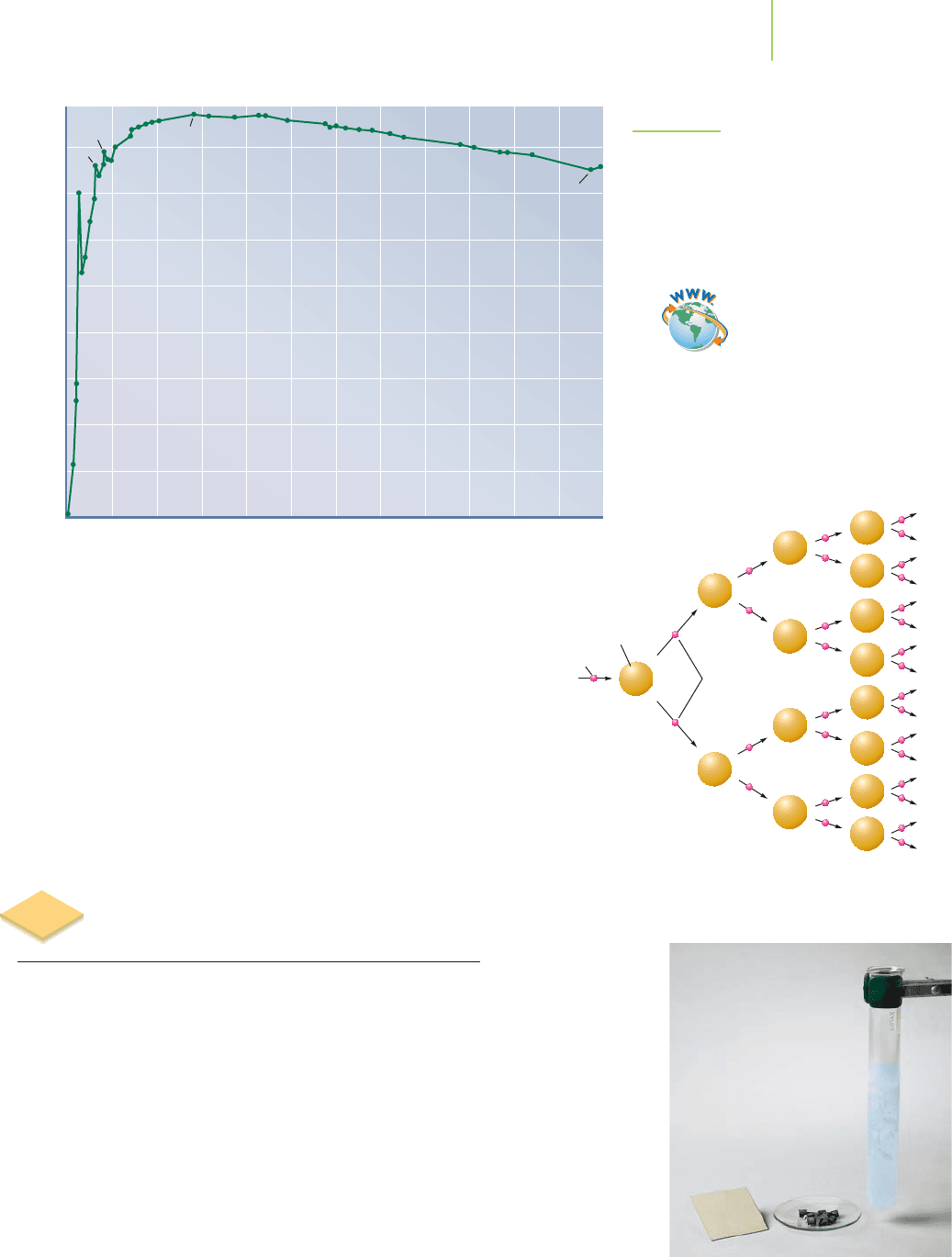
The heaviest elements also have somewhat lower stability. Ura-
nium, with so many protons and neutrons packed into its nucleus,
has a lower binding energy per nucleon than iron or cobalt. As we
will see in Section 21.7, it is energetically favorable to split heavier
nuclei into smaller ones via
fission (or nuclear fission) to form nuclei
with a more favorable binding energy per nucleon.
Finally, look at the maximum of the curve and you will find
elements with the most stable nuclei—those with mass numbers
around 60, such as iron and nickel. Other elements also have sur-
prisingly high binding energies given their mass number, such as
4
He (an alpha particle),
12
C, and
16
O. Alternatively, one might argue
that the values for
6
Li and
14
N are surprisingly low.
21.6
Nuclear Stability and
Human-made Radioactive Nuclides
“Here today, gone tomorrow” doesn’t apply to most of the atoms that make up
our world. That is fortunate for us. In fact, the majority of the atoms on our
planet that are here today can be expected to be here tomorrow. Although it may
be hard to locate a specific atom from one day to the next, you can be reasonably
sure that it is still here. Why? Most atoms on Earth are not radioactive.
Which factors seem to affect nuclear stability? Measurements indicate that
nature favors even numbers of protons. Elements such as helium, oxygen, iron,
and lead that have even atomic numbers tend to be more abundant than their
odd neighbors. For example, of the eight elements that make up over 99% of
Earth’s total mass, only one (aluminum) has an odd atomic number. Still more
favored are nuclei that have even numbers of both protons and neutrons. Perhaps
the most dramatic case is
4
2
He, the alpha particle. Given this stability, it is not sur-
prising that helium is the second most abundant element in the universe.
Experimental data confirm that certain numbers of either protons or neu-
trons (called “magic numbers”) are favored: 2, 8, 20, 28, 50, 82, and 114. The ele-
ments helium (Z
=2), oxygen (Z =8), calcium (Z = 20), and nickel (Z = 28) have
21.6 Nuclear Stability and Human-made Radioactive Nuclides 917
Two neutrons
from fission
Nucleus
Neutron
Nuclear fission.
Oxygen (shown here in liquid form), cal-
cium, and nickel have magic numbers of
nucleons.
0 20 40 60 80 100 120 140 160 180 200 220 240
1
H
2
H
3
H
3
He
6
Li
7
Li
12
C
16
O
56
Fe
235
U
4
He
1
2
3
4
5
6
7
8
9
Average binding energy per nucleon (MeV)
Number of nucleons in nucleus, A
FIGURE 21.9
The binding energy per nucleon reaches a
maximum near iron, atomic number 26.
Visualization: Nuclear Fission
Video Lesson: Nuclear Fission
