Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.

360 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
Lieszkovszky). The peak height at a given mass number, /,„, is the sum of all the
peak height contributions, /„„„ over all gases n:
^m
= 2
['"'/']
^ 2 ['^/lAiTm/i] (23)
n n
where 5„ is the analyzer sensitivity for the gas n,
p„
is the partial pressure for gas
n, and
%„„
is the peak height contribution of gas n at mass number m.
From the figure we can tabulate the signal peaks (in amperes) as follows:
mie
44
40
28
20
18
17
16
15
14
13
12
4
2
1
Jm«(A)
2.20
X
10-'
1.60
X
10-'
5.10
X
10-'
1.72
X
10-'"
1.30
X
lO-'"
2.99
X
10-"
3.50
X
10-'
2.83
X
10-'
5.15
X
lO-'o
2.54
X
10-'"
3.79
X
10-'°
5.80
X
10-"
1.10
X
10-«
5.50
X
10-'"
The total pressure for this spectra is pj ~ 2.0 X 10"^^ torr. The overall sensitiv-
ity of the quadrupole mass spectrometer with the gain of the multiplier adjusted
to 1 X 10^ is 150 A/torr (N2). The calculated sensitivities for the other relevant
gases are
'^[C02] = 160 A/torr
5[Ar] = 132 A/torr
5[C0] = 150 A/torr
5[H20] = 150 A/torr
5[CH4] = 153 A/torr
3.3.4 Techniques for Analysis 361
5[He] = 83 A/torr
5[H2] = 223 A/torr
The analysis should start at the highest mass and continue in descending order of
molecular mass to ensure that all fragment peaks are considered. If we ignore
small contributions from isotopes and doubly ionized species, CO2 has signifi-
cant peaks at mie of
44,
28, 16, and 12 amu. The parent peak is at mass 44 so the
fragmentation ratio, 744 co, = 1- Since there are no other contributions to mass
44,
the partial pressure of
CO2
can be determined from Equation (23),
Pn =
Ln/S„y,„n
= Pco, = 2.2 X 10-9/160 (1) = 1.38 X 10-^1 torr
With this partial pressure and the fragmentation ratios (ynm) for ^^e remaining
peaks,
the fragment peak heights may now be determined from Equation (23),
744,
CO2
=1 '44 = 2.20 X 10-9 A
r28,co = 0.114 /28 = 2.50X10-1^ A
716,0 = 0.085 /i(,= 1.87 X 10-^0 A
yi2,c = 0.06 /i2= 1.32 X 10-^0 A
The CO2 fragment peak heights may now be subtracted from the original signal
levels to give.
mIe
U
40
28
20
18
16
15
14
13
12
4
2
1
hm,
(A)
1.60 X 10"^
4.85 X 10-'
1.71 X 10-"
1.30 X lO-'o
3.31 X 10-'
2.83 X 10-'
5.15 X 10-'"
2.54 X 10-'"
2.97 X 10-'"
5.80 X 10-"
1.10 X 10-8
5.50 X 10-'"

362 Chapter
3.3:
Practical Aspects of Vacuum System Mass Spectrometers
The next peak to be evaluated is that of Ar at mass 40 and at mass 20 (Ar^"^).
Since it has no contributions to the other species it can be subtracted by inspec-
tion. The partial pressure is calculated to be/^Ar = 1-21 X 10"^^ torr.
Carbon monoxide has a parent peak at mass 28 and has only one significant
fragment peak at mass 12. The partial pressure ispco = 4.85 X 10~^/150 (1) =
3.23 X 10"^^ torr from which the peak height of the C fragment peak is iij =
3.23 X 10"^^ (150) 0.045 = 2.18 X 10"^^ A. Subtracting from the peak heights
gives
m/e
i,„„
(A)
44
40
28
20
18
1.30X10-10
17 2.99X10-11
16 3.31 X 10-9
15 2.83 X 10-9
14 5.15 X 10-10
13 2.54 X 10-10
12 7.92 X 10-11
4 5.80X10-11
2
1.10X10-^
1 5.50X10-10
The next peak in descending order is H2O at mass 18, with fragments at 17 and
16 amu. The partial pressure ispH.o = 1.30 X 10-io/150 (1) = 8.67 X lO-i-^
torr from which the peak height of the fragments are
1*17
= 2.99 X 10-n A and
(16 = 1.43 X
10-1^
A. Subtracting from the remaining peak heights gives
m/e
i„jn
(A)
44
40
28
20

3.3.4 Techniques for Analysis 363
18
17
16 3.30 X 10-'
15 2.83 X 10-'
14 5.15 X 10-"'
13 2.54 X 10""'
12 7.92 X 10-"
4 5.80 X 10-"
2 1.10X10-^
1 5.50 X lO-'O
Methane at mass 16 has significant fragments at 15, 14, 13, and 12 amu. The par-
tial pressurePCH4 = 3.30 X 10-'/153 (1) = 2.16 X 10"" torr from which the
peak heights of the
fragments
are j,5 = 2.83 X 10"' A, I14 = 5.15 X 10-'° A,
ii3 = 2.54 X 10"'° Aand/|2 = 7.92 X 10"" A. Subtracting from the remaining
peaks gives
mie /,„„ (A)
44
40
28
20
18
17
16
15
14
13
12
4 5.80 X 10""
2 1.10X10"^
1 5.50 X 10"'°
364 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
The remaining peaks are unambiguous (if there is no deuterium in the system);
He at mass 4, H2 at mass 2 and the atomic hydrogen fragment at mass 1. The par-
tial pressures are thenpHe = 5.8 X 10"
^
V83(l) = 6.99 X 10"^^ torr and/7H. =
1.1 X 10-^223 (1) = 4.9 X 10"^^ torr.
The sum of the resulting partial pressures in the system are
C02
AT
CO
H2O
CH4
He
H2
p= 1.38 X 10-"
p= 1.21 X 10-"
/7 = 3.23 X 10-"
p =
8.67X
10-'-^
p = 2.16X 10-"
P = 6.99X 10-"
p = 4.90X 10-"
PT
= 2.0 X 10"^^ torr
This example has clearly been adjusted for illustration. The pressures seldom
come out exactly in actual practice. Further, this represents a very simple case of
residual gases in a UHV system. Most often, the spectra are far more compli-
cated, especially if organics are involved. It does, however, illustrate the method-
ology for analyzing any spectra by the fragmentation method.
3.3.5
CALIBRATION
OF
VACUUM SYSTEM
MASS SPECTROMETERS
In general, most mass spectrometer users are only concerned with the qualitative
aspects of the vacuum environment and never calibrate the instrument, but there
are many cases when it is absolutely necessary. Accurate partial pressure measure-
ments for gas composition-sensitive instruments are controlled by the quality of
their calibration. In other words, the measurement is only as good as the calibra-
tion. Considering a high-accuracy calibration, the subsequent demounting of the
instrument, exposure to air, transfer from the calibration system to the operation
system, remounting of the instrument, and finally, the bakeout and degassing re-
quired to reestablish calibration conditions, it is very difficult to maintain an ac-
tual sensitivity near the calibrated sensitivity. Ideally, the calibration system and
the operational system should be one and the same. Unfortunately, this is seldom
the case and the scientific worker must endure the aforementioned scenario pri-
marily because a carefully calibrated instrument is absolutely necessary for good

3.3.5 Calibration of Vacuum System Mass Spectrometers
365
research, development, or process reliability. Several techniques that can be used
to calibrate vacuum instrumentation are presented next. Detailed descriptions of
these methods are provided in Herman [31] and Ellefson and Cain [32].
3.3.5.1
Static Volume Expansion
The static volume expansion simply involves establishing a pressure that is mea-
surable by a primary standard within a very small volume and expanding that vol-
ume of
gas
into a much larger volume to obtain a substantial reduction in pressure
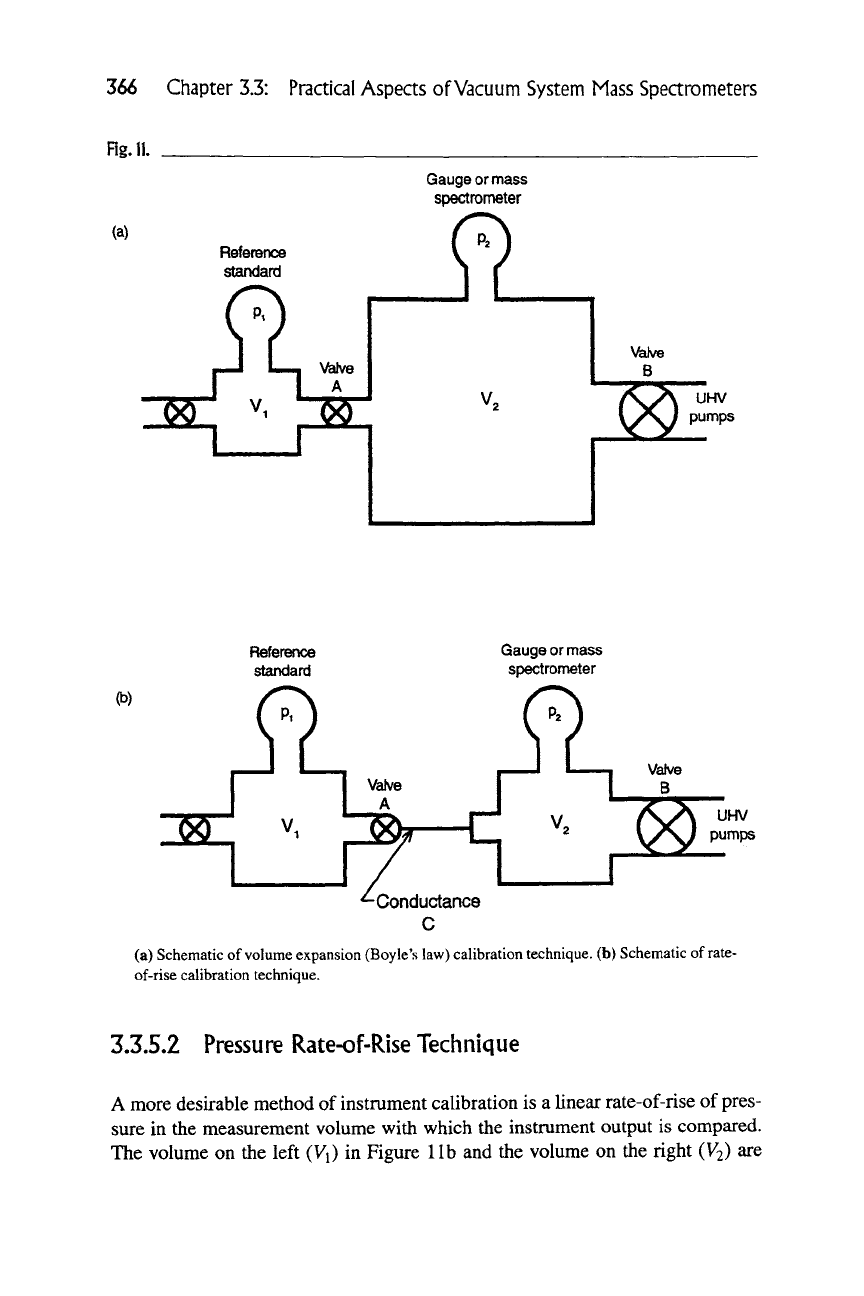
(Boyle's law). Figure 11(a) is a schematic of the technique. The small volume, V^,
typically a few cm\ and the large volume V2, typically > 10^ cm\ are initially
cleaned and evacuated to the UHV range to minimize outgassing contributions to
the signal. The small volume is then pressurized topi = 10"^ or 10~^ torr with
the separation valve closed. The small-volume pressure pi is measured with a
primary standard or a working standard such as a fused silica Bourdon tube , a
capacitance manometer, or a spinning rotor gauge and then the separation valve
(which has a large-diameter bore) is fully opened. The equilibrium pressure then
becomes
PJ = (^4)
Vacuum chamber wall pumping effects such as chemisorption and/or incor-
poration can be serious problems and several runs must be made to saturate the
walls and obtain repeatability. Furthermore, if hydrogen is the calibration gas, the
walls must be significally oxidized to inhibit the seemingly endless hydrogen in-
corporation. This surface condition can be achieved with a bakeout of ~400°C
in an atmosphere of oxygen for several hours. Thicker oxides, of course, can be
obtained using longer times and higher temperatures. Another concern is gauge
pumping. The ordinary ion source in a mass spec operating at an emission current
of ~1 mA may have a pumping speed for N2 approaching 0.1 L/s, which can
have a substantial effect on the pressure diminution. Extrapolation techniques can
be used to estimate the correct expanded pressure, but this is not a totally accept-
able procedure. If wall effects and the gauge pumping are not serious problems,
calibrations as low as 1 X
10 ~^
torr , depending on the gas, can be achieved. If
the two volumes are initially degassed to a base pressure of
1
X 10"^^ torr, wall
outgassing in the static system in V2 may increase the background pressure
two-three decades over the time of the individual measurements. Thus, there is a
1%
or greater uncertainty in the calibration gas. In practice, the low-pressure cali-
bration limit is probably 1 X 10"^ torr. This technique is not generally used be-
cause of the aforementioned effects and because of its one-decade-or-less range
overlap with ionization-type instruments.
366 Chapter 3.3: Practical Aspects
of
Vacuum System Mass Spectrometers
Rg.ll.
(a)
Reference
standard
Gauge or mass
spectronfieter
(b)
Reference
standard
Gauge or mass
spectrometer
^Conductance
C
(a) Schematic
of
volume expansion (Boyle's law) calibration technique, (b) Schematic
of
rate-
of-rise calibration technique.
3,3.5.2
Pressure Rate-of-Rise Technique
A more desirable method
of
instrument calibration
is a
linear rate-of-rise
of
pres-
sure
in the
measurement volume with which
the
instrument output
is
compared.
The volume
on the
left
(Vi) in
Figure
lib and the
volume
on the
right
(Vi) are

3.3.5 Calibration of Vacuum System Mass Spectrometers
1>(>I
typically small and are initially cleaned and evacuated to the UHV range to mini-
mize the outgassing contribution to the signal. The volume, Vj, is then pressur-
ized from 1 to 1000 torr, which is measured by a reference standard such as a
quartz Bourdon tube, capacitance manometer, or a deadweight tester. The valve is
then opened to the calibrated conductance C, which connects the two
volumes.
The
resulting pressure rise in
V^2»
which has been closed off from its pumps, is then
compared with the instrument output, so that the change in pressure is given by
V2^
= C(p,-p2) (25)
Since/?!
» Pi,
P2(t) = -^t (26)
where/72(0 is the pressure in
V2
at any time t. The conductance, C, is usually se-
lected to give a rate-of-rise that is fast enough to overwhelm gauge pumping, but
slow enough so that instrument response is not a problem. Since
V2
is small and
exposure to the calibration gas for several cycles tends to minimize wall chemi-
sorption and incorporation, wall effects are not a serious problem. Since C is also
small enough thatpi remains constant, the flow in
V2
is assumed to be linear (af-
ter a few seconds). Furthermore, the low-pressure limit for this technique is prob-
ably better than an order of magnitude less than the static expansion technique,
since only a short time is spent in the low-pressure range (e.g., ^1 X 10"^ torr).
The rate-of-rise technique has been studied and has been found to be an accept-
able calibration method.
3.3.5.3 Orifice Flow Technique
The orifice dynamic flow method is the primary technique used by the National
Institute for Standards and Technology (NIST) for the calibration of ionization
gauges and mass spectrometers, basically because it can extend the range of cali-
bration several decades below the two methods previously discussed (i.e., from
5 X 10"^ to 5 X
10~^^
torr). The orifice method is a dynamic technique that uses
a flowmeter to provide a known flow Qi into the first of two volumes, which are
pumped at known constant speeds (the two volumes have conductance-limiting
orifices). The second volume, which is the second stage of pressure reduction, is
where the instruments to be calibrated are located for the full range of calibration.
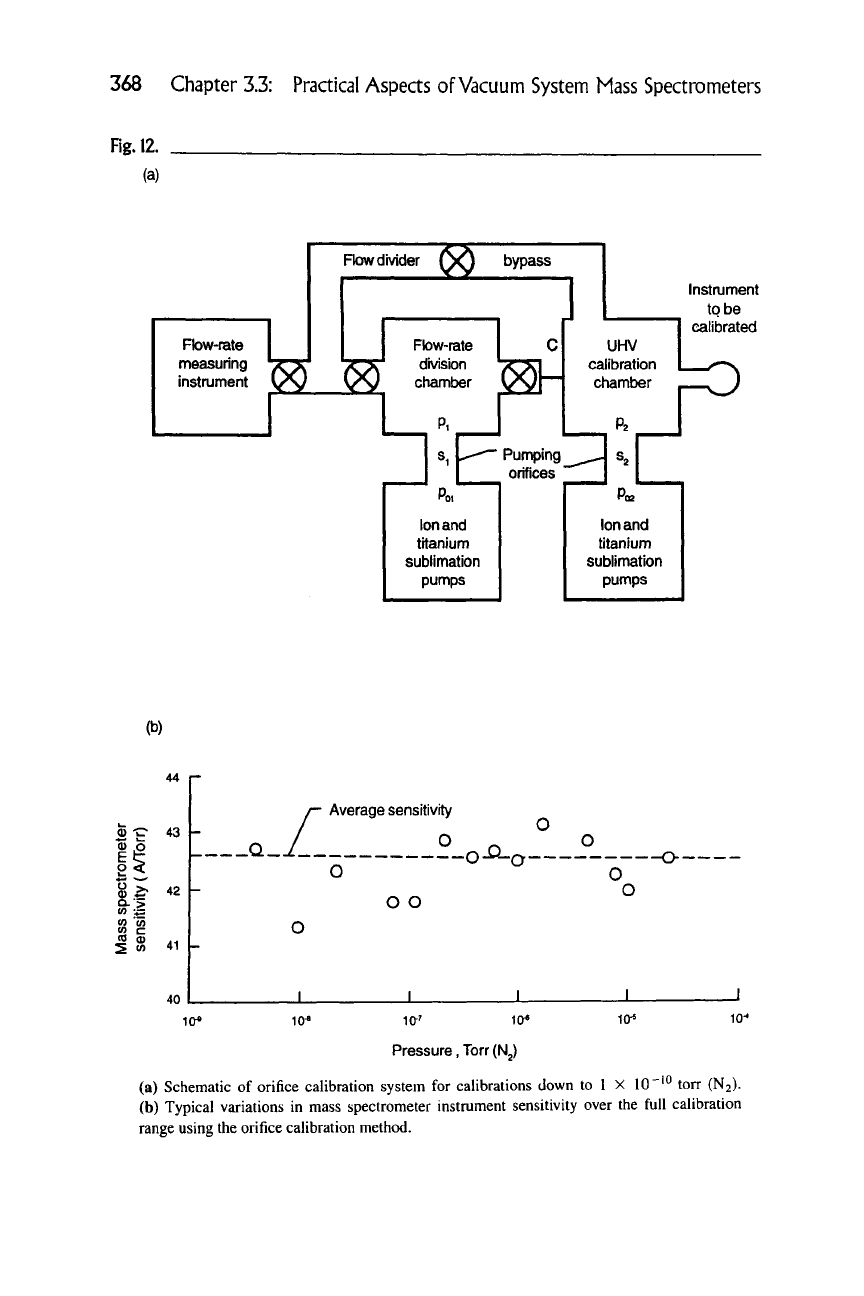
Figure 12(a) is a diagram of the two-staged, ion-sublimation, turbomolecular
pumped orifice calibration system. The entire system goes through a bakeout and
is sufficiently degassed to achieve an ultimate pressure of ~5 X
10 "^^
torr or less
368 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
Fig.
12.
(a)
Ion and
titanium
sublimation
pumps
ion and
titanium
sublimation
pumps
Instrument
to
be
calibrated
(b)
II
^ 43
8 ^> 42
(0 0
41
40
Average sensitivity
O
O
O O
O
-o
10*
10^
10-'
10^
10*
10-
Pressure, Torr (N )
(a) Schematic of orifice calibration system for calibrations down to 1 X 10"'^ torr (N2).
(b) Typical variations in mass spectrometer instrument sensitivity over the full calibration
range using the orifice calibration method.

3.3.5 Calibration of Vacuum System Mass Spectrometers 369
in both volumes, although this low background pressure is not absolutely necessary
in the first volume. A flow Qi is admitted in Vj, and the steady-state pressure is
61 + >S'i/7oi Qy
P\
= ^ ^ (2/)
where Si is the conductance-limited orifice in
V2OYS1
«
S^]
and/7oi is the pres-
sure in pump 1. When the valve is opened to V2, the flow Q2 into that volume is
controlled by the calibrated conductance C and given by Q2 = C{pi - pj)- The
steady-state pressure in
V2
is
^ CQi + CSiPoi +
S1S2P02
(28)
^^ S1S2 + CSi
where 52 is the conductance-limited orifice in
V2,
or ^2 «
Sp^,
and
PQ2
is the
pressure in pump 2 (5p and
S^^
are
the intrinsic pumping speeds of pumps
1
and 2).
The magnitude of
Q j
could be a value that permitted a steady-state pressure of the
calibration gas as low as 5 X
10 ~ ^^
ton*.
Thus, the capability of
a
calibration from
5 X 10~^to5 X 10"'^ torr or a range of
5
decades was provided. Once the cali-
bration is complete, this method may also be used to measure outgassing from a
particular volume where Q unknown is Qi. The parameter of interest that charac-
terizes the instrument over the calibration range is the sensitivity. The sensitivity
of a mass spectrometer for
A^2
as determined by an orifice system is shown in Fig-
ure 12(b). The relative variation of this instrument approaches 5%, but with some
care it is possible to achieve
3%.
In general, the conditions under which the cali-
brations are conducted should be nearly identical to those that the instrument
would experience in the system where it is to be used. For example, it is recom-
mended that mass spectrometers in the system have the same geometric metal (or
insulator) envelope around the ion source of the instrument in the calibration sys-
tem, because the electron density around the grid can be substantially different
due to the alteration of potentials in the ion source, resulting in errors exceeding
50 percent.
The total uncertainty of the calibration is governed by several factors. First,
there is the uncertainty in the standard input flow, which may be controlled to
about
1
percent. Then there is an uncertainty in the conductances to the pumps in
Vi and
V2
as well as the conductances between the two volumes. There is the
dif-
ference in gas composition in the pumps compared with the main volumes, but
this should only be a minor concern. Perhaps, the two most significant issues are
that no change occurs in the physical positioning of the instrument elements
(which will substantially change the potentials) and that no chemical contamina-
tion occurs in the instrument transfer back to the operational system (which could
increase BSD effects as well as alter the gain of the multiplier).
