Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.

350 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
spectrometer. When the grid was degassed above 400°C, the 16 peak substan-
tially diminished, as predicted from studies of atomic oxygen adsorbed on Ag
[22].
Replacement of the grid with a standard molybdenum grid returned the
spectrum to the more conventional series shown in Figure 3. Minimizing this un-
wanted gas desorption can be accomplished by operating at low emission cur-
rents,
minimizing the grid or collector area, and using materials that have a low
propensity for adsorption.
3.3.3.6 Charge Exchange Mechanisms
In addition to electron impact ionization, dissociation, recombination, sputtering
and
BSD,
charge transfer can also affect the actual ionization volume particle mix.
At normal operational pressures for mass spectrometers (p < 1 X
10 ""^
torr), the
mean free path (1) is sufficiently long that ion-molecular impact is not a signifi-
cant process
(1
< 50 cm), but it does occur and can be a significant signal in some
applications. Essentially, the collision of a positive ion with a gas atom or mole-
cule results in an exchange of
energy,
momentum and direction, but also a change
of charge can occur. An energetic ion can "steal" an electron from a neutral and
become an energetic neutral, leaving behind a slow ion. Actually, the cross sec-
tions for charge exchange vary substantially with the difference in mass between
particles. The transfer is more likely to occur in the proximity of resonance or the
smaller the energy exchanged in a collision. Thus, if the incident ions are the
same species as the target atoms or molecules, the exchange has a higher prob-
ability. The amount of energy transferred is equal to the sum of the kinetic and
ionization energies plus the excitation energy of the target molecule (including
vibration and rotation) less the neutralization energy. The charge transfer may
also include other processes such as dissociation, excitation (with subsequent
light emission), and ion formation. Some examples of exchange are [23,24]
He+ + H20-^He + H20+ (12)
H2+ + H2O -^ H2 + H2O+ (13)
H20^
+ H->H30+ (14)
H2 + H+->H3^ (15)
Ar++ Ar->Ar +Ar-' (16)
N2''+ Arf->Ar^+N2 (17)
He+ +Xe->He + Xe-^ (18)
Since hydrogen and water are so prevalent in vacuum systems reactions, (12) to
(15) are often observed. The production of
H^"^
is frequently seen in ion sources
especially with environments of hydrogen-containing species, such as hydrocar-
33.4
Techniques
for
Analysis
351
bons.
Argon and other inert gases have been used for many years in charge neu-
tralization cells for forming energetic neutral beams of atomic oxygen and other
species.
3J.4
TECHNIQUES FOR ANALYSIS
3.3.4.1
Partial Pressure Measurement
The accuracy and overall performance of the mass spectrometer is a function of
each section of the mass spectrometer. As an example, consider a quadrupole-
type instrument that is primarily comprised of an ion source, a set of quadrupole
rods for mass filtering, and an electron multiplier for signal amplification (see
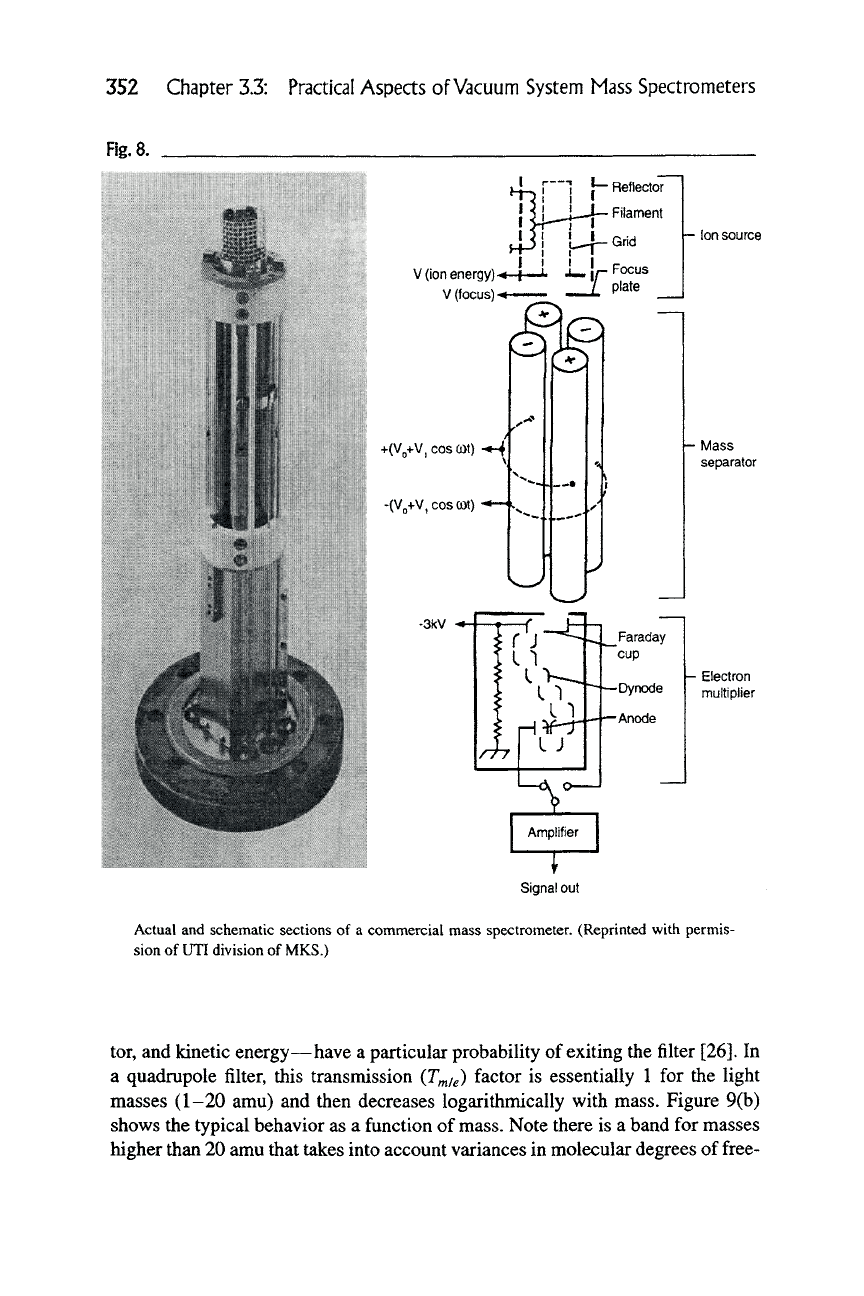
Figure 8).
THE ION SOURCE
For a given ion source geometry, the ionization efficiency is primarily a function
of
the
number of electrons in the atom or molecule. As previously indicated, the
highest probability for ionization of most gases occurs at approximately 70 eV.
The quantity of ions formed and extracted from the source is a function of the
design of the ion source and is characterized by a source factor (ionization effi-
ciency). Flaim and Ownby [25] have determined an equation that approximates
the ionization efficiency (/).
/ = 4.3 X 10-2 ^ „.2. + 0,4 (19)
i
where
Zj
is the number of
electrons
in an atom of
molecule
i and
rii
is the number
of
atoms
in molecule
i.
As an example, consider the water vapor molecule, H2O;
it has 2 atoms of
H (1
electron) and
1
atom of
O
(8 electrons), thus
/[H2O] = 4.3 X 10-2 [2(1) + 1(8)] -f 0.4 = 0.83
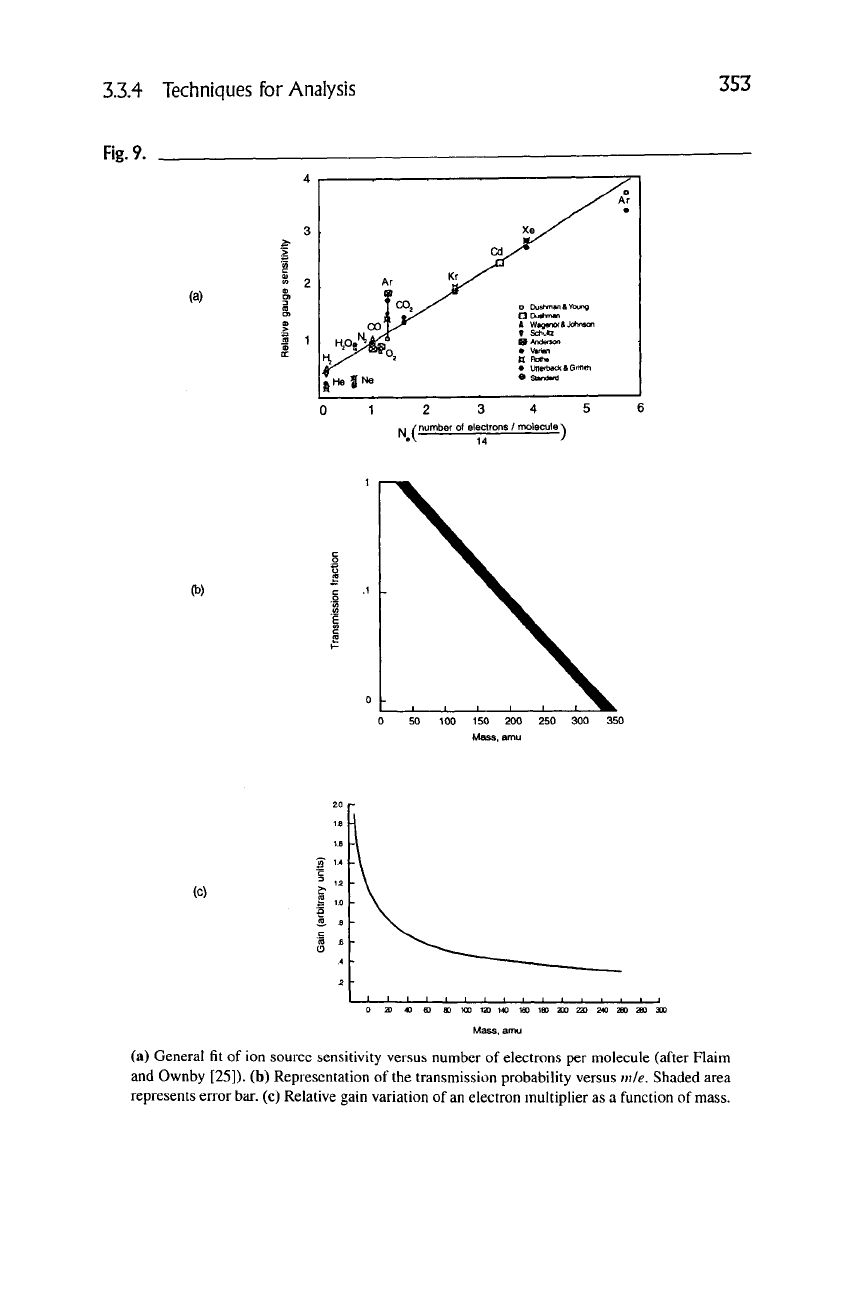
which is the ionization efficiency relative to nitrogen. Figure 9(a) shows the
fit
of
the equation for several atoms and molecules. There is also a geometrical factor
similar to a gauge constant that reflects the efficiency in which the ions are ex-
tracted from the source and directed to the mass filter.
THE MASS FILTER
When the ions enter the mass
filter,
those ions that match the selected filtering
conditions—i.e., those which have the right mass to charge ratio, entrance vec-
352 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
Rg.8.
Reflector
Filament
V (ion energy)-<-f
V (focus) -^
• Ion source
+(VQ+V, cos cot)
-(Vo+V,COSCDt)
\-
l\/lass
separator
-3kV -»
|— Electron
multiplier
Actual and schematic sections of a commercial mass spectrometer. (Reprinted with permis-
sion of UTI division of MKS.)
tor, and kinetic energy—have a particular probability of exiting the filter [26]. In
a quadrupole filter, this transmission (T^/e) factor is essentially 1 for the Ught
masses (1-20 amu) and then decreases logarithmically with mass. Figure 9(b)
shows the typical behavior as a function of
mass.
Note there is a band for masses
higher than 20 amu that takes into account variances in molecular degrees of free-
33.4 Techniques for Analysis
353
Fig.
9.
(a)
Ar
^HefNe
Kr
V
Cd
Xe/^
O Dushman 4
Vexing
a Dushman
A Wag«nor& Johnson
V Sc^ula
0 Anderson
• Vkrion
H Roihe
• UttefbackiGriflim
9 Standwd
Ar
•
N.(
number of electrons / molecule '\
(b)
100 150 200 250 300 350
Mass,
amu
(C)
(a) General
fit
of ion source sensitivity versus number of electrons per molecule (after Flaim
and Ownby [25]). (b) Representation of the transmission probability versus mie. Shaded area
represents error
bar.
(c) Relative gain variation of
an
electron multiplier as a function of mass.
354 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
dom, ion velocity (time in the filter), rf frequency, and potential uniformity in the
radial and axial dimensions. As shown in the figure, the transmission decreases to
about 0.5 at 75 amu.
THE ION DETECTOR
On exiting the mass filter, the ions are collected by a Faraday cup or amplified by
an electron multiplier. A well-designed Faraday cup should completely collect all
ions and not gain or lose any signal due to secondary emission of electrons. An
electron multiplier (continuous dynode, box and grid, Venetian blind, multichan-
nel plate, etc.) can provide a gain (G) from
1
X 10-^ to 1 X 10^ depending on the
number of electron collision stages and on the secondary emission coefficient of
the dynode or collision materials. Most often the gain is adjusted to a modest
value, e.g., G~lX10^or less in order to ensure long-term stability. Further, the
gain is very sensitive to ion mass, chemical reactivity, kinetic energy, and total
charge. Some multipliers have been observed to have a very high gain for low
masses and decrease as a function of m~^^^ with increasing mass
[27]
—
see
Fig-
ure
9(c).
In addition, the secondary emission coefficient from impacting ions varies
as the cos^ 6, where 6 is the angle of incidence of the ion. A calculation of the
partial pressure for a given species can be determined from
^
=
T?
GiTi
(20)
Considering an actual case where the partial pressure of H2O has been measured
one can compare the calculated value. The primary peaks are m/e =18 (H2O''")
and the fragmentation peak is m/e = 17 (OH^). If the peak height currents are
measured to be
1
X
10 ~*
and 2.3 X
10
"^ A, respectively, and the ion source sen-
sitivity is 1.5 X 10~' A/torr and the electron multiplier gain for N2 is 1 X 10^
then one can calculate a value for the partial pressure for H2O. The G for H2O is
G[H20] = G[N2]
m(N2)
mCHjO)
1/2
(21)
= 1 X loM—- =1.25X105
and for the fragment OH is
''28\"^
G[OH] = 1 X loM— = 1.28 X 105
^17/
Since 7-1 for <20 amu, then r[H20]~l, r[OH]~l

3.3.4 Techniques for Analysis 355
Employing Equation (20), the calculated partial pressure is
/?[H20] =
1X10-^
2.3X10"^
4-
1.25 X 10^(1) 1.28 X 10^(1)
(1.5 X 10--^)0.83
/7[H20] = 1.9 X 10-^4oiT
The actual measurement from the calibrated mass spectrometer was
/7[H20] = 1.5 X 10-^^torr
Although the approximate answer from the calculation is instructive and useful,
there is still significant error. Clearly, the calculation does not contain every cor-
rection necessary to make an accurate assessment of the partial pressure! In gen-
eral, the ion source limitations, the filter and detector errors, and anomaUes make
quantitative measurements quite difficult. Calibrations in the selected gases of in-
terest are absolutely essential to obtain meaningful data.
3.3.4.2 Gas Identification
Two primary techniques enable the user to unambiguously analyze an unknown
gas species or mixture of gas species to clearly quantify the individual fractions
of
each.
They are the isotope analysis [28] and the fragmentation analysis [29]. In
most general instances, a combination of both methods will be employed. Al-
though isotopic analysis is a useful technique, it cannot supplant the use of frag-
mentation analysis. Fragmentation analysis typically works from a much greater
number of larger-amplitude mass peaks and is thus likely to give a more complete
rendition of the species present. Obviously, to get an accurate species identifica-
tion, a calibration of peak height for parent and fragments (using the instrument
making the analysis) is required. Even without the calibration the spectral stan-
dards are useful as guides for determining species that may be present in the sys-
tem being analyzed. When analyzing for species, one should always attempt iso-
topic analysis first, followed by fragmentation analysis for confirmation.
ISOTOPE ANALYSIS
The main advantage of using the isotope method is that normally no calibration of
the analyzer is required for identification of a species, although it would be re-
quired for quantification of
results.
The isotopic abundances have been accurately
measured for the elements of interest. In using isotopic identification, it is impor-
tant to keep in mind several factors. The analyzer detector system must be capable
of linear output over three to four orders of magnitude for best results because the

356 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
less abundant isotopes of most of the elements of interest are at the 0.1 to 1.0%
level. Modem amplifiers are linear and stable over five-seven orders of magni-
tude or better, so this factor should not be an issue. It is also important that the an-
alyzer sensitivity not be strongly dependent on the mass number. Most quadru-
pole instruments have a mass sensitivity that decreases at
1
-2% per amu beyond
12 amu. Most of the isotopic species of interest appear above this range. Species
appearing below the linear range are uniquely identified on other bases. Finally,
due to the low peak height of less abundant isotopic peaks, it is important that
they not interfere with other fragments. Ordinarily this is not a problem because
isotopes of species of interest do not overlap and with few exceptions, the less
abundant isotopes appear at higher mass numbers. One exception is that due to
argon. Natural Ar at 40 amu is the most prevalent isotope and is the decay prod-
uct of an abundant element, thus "^^Ai/^^Ai = 300. The abundances of the iso-
topes of all naturally occurring elements are given in the American Institute of
Physics Handbook, Table 8.6.1 [30]. Most analyses in vacuum systems and on
vacuum processes involve only the lightest 18 elements in the periodic table.
The results of an isotopic analysis at the 1% level should be within a few per-
cent of
the
level predicted. At the
0.01%
level, the results should be within several
tens of percent of the level predicted from natural abundance. If one arrives at val-
ues outside these ranges, then interference is surely present and an isotopic analy-
sis will need to be supplemented with fragmentation analysis.
When looking at pure elemental gases, such as nitrogen and oxygen, one may
find the heavier isotopes to be slightly depleted. This occurs because these pure
gases are obtained by fractionation. The lower-molecular-weight species boil at
lower temperatures. The rarer higher-boiling-point species may be separated and
sold at a greater price of various isotopic tracer experiments. A slight depletion
can also occur if the gases have been introduced by diffusion or molecular flow,
because the flow is dependent on (m)
~^^^.
Any of these effects typically alter the
ratios by a maximum of
~3%.
When looking at an atomic species, the expected ratio of the isotopes can be
obtained directly from the aforementioned table. It is noteworthy that the rarer
isotopes of the elements of primary interest—i.e., hydrogen, carbon, nitrogen,
oxygen, and chlorine with an atomic number (Z) of 18 or
less
—
are
1 or 2 amu
greater than the major isotopes for the element. This means that the largest rare
isotopic peaks for all molecules will appear at 1 or 2 amu greater mass than the
peak of the molecule in question.
To
calculate the relative abundance of the isotope,
peak height of isotope species ^ ,^^^
Relative abundance (%) = , , . , \ -^---^ — X 100 (22)
peak height of most abundant species
For illustration, consider the spectra of Ne. The spectra for Ne at masses 20, 21,
and 22 amu was observed to have peak heights of
90.92,
0.257, and 8.82 arbitrary
3.3.4 Techniques for Analysis 357
units,
respectively. The relative abundance in percent is, then,
For^ONe-
[^^Ne]
90.92
Fo-^'N. liMx,00 = 5:^X100-0.282
{mie = 21) [20Ne] 90.92
For^^Ne-
[^^Ne]
8.82
imie = 22)
[^''Ne]
90.92
The resulting spectrum of Ne and its isotopes should therefore appear in these
proportions. A second example, CO, will show peaks at masses 28,29,30, and 31
because CO has isotopes of both carbon and oxygen. The relative abundances
should be
For '2C'*0- ["^C] X ['^O] 98.89 X 99.76
^^^ ^ ^- -V-!—\^ X 100 = X 100 = 100
{mIe = 28) ['^C] X ['^O] 98.89 X 99.76
For'-^C'^O + '^C'^O: ['^C] X ['^O]
['-^C]
X ['^O]
imIe = 29)
[
''C] X
[ '^O] [
'^C] X
[
"-0]
1.11X99.76
98.89X0.0374
X 100 + ——-— X 100 = 1.16
98.89 X 99.76 98.89 X 99.76
For'^C'^0+>^C'«0: ['^C] X ['^Q] ["C] X ['«Q]
(m/e = 30) ['2C] X ['^O] ^ ^^ ^ [i2c] x ['^O]
1.11X0.034
98.89X0.204
X
100
+ ————— X 100 =
0.205
98.89 X 99.76 98.89 X 99.76
For
i-^C'^O-
V^C] X ['^O] 1.11 X
0.204
M. =
31)-
[-^C]X[-^0]^^^ = 98.89X99.76^^^ = ^-^^^^
Clearly, in order to observe the full isotope spectrum contributing to the CO
signal, the instrument must be sensitive enough to detect at least five decades of
signal intensity less than that of the parent peak at mIe = 28 amu. If the partial
pressure of CO is ~1 X 10"^ torr, one requires an instrument with a minimum
detectable partial pressure of ~1 X
10 "'"^
torr to fully see the isotope signal at
rule = 31. In addition to sufficient sensitivity, it is also necessary for the instru-
ment to be linear over the full range to accurately compare the relative peak
heights. Armed with this spectrum, one can now unambiguously identify this gas
if it is the only species in this mass range. Unfortunately, the situation is seldom
this simple unless one is backfilling with CO from a pure source. Molecular ni-
trogen and ethylene are also contributors to mIe = 28, thus requiring the decon-

3S8 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
volution of the overlapping data. As previously discussed, a leak-tight vacuum
system will not have nitrogen as part of the residual spectrum, but there are in-
stances where a leak may exist or some operation involving N2 may occur. Thus,
in this case, mie = 28 is obviously not pure CO or pure N2. A similar analysis to
that just conducted for CO shows that pure N2 has normalized mass peaks of 100
at mIe = 28,0.75 at mIe = 29, and 1.4 X
10 ~^
at mIe = 30. Assume that the mea-
sured spectrum has 100 units of CO and N2 at mIe = 28, 1.0 unit at mIe = 29,
0.14 units at m/e = 30 and 1.7 X 10"^ units atm/e = 31. The following analysis
can be applied:
Let
X
= CO fraction and y = N2 fraction;
For m/e = 28: x + y = 100
For m/e = 29: 0J5y +
1.16A:
= 1.0
X
= 0.61 andy = 0.39
(61%
CO and 39% N2)
To check if
this
is reasonable, the m/e = 30 peak can be examined to see if the ex-
pected relative fraction correctly corresponds to 61% CO. Since for comparable
amounts of CO and N2, the value of the N2 isotope contribution at m/e = 30 is
so small (1.4 X
10
~^),
the m/e = 30 signal must be predominantly CO. If we di-
vide the observed peak height of 0.14 at m/e = 30 by 61 at m/e = 28, one obtains
0.23 X 10"^, which is in reasonable agreement with the 0.21% for m/e = 30 for
CO.
If
C2H4
had also been expected as a contribution to m/e = 28, the m/e = 30
peak would have to be used to determine the fraction of CO in the three-way mix-
ture.
Although the fractional contributions in this example give us an idea as to
the relative amounts of each species, this does not mean that the partial pressures
are related in the exact same ratio. The partial pressures would have to be evalu-
ated by calibrating the instrument for these gases. The complexity of calculating
the expected values for isotopic peaks obviously increases as the number of atoms
in a molecule increases. For more detailed analysis of this procedure, please see
the notes of
L.
C. Beavis [28].
FRAGMENTATION ANALYSIS
In general, a clean, dry-pumped vacuum system will have mostly inorganic resid-
ual gases (usually with the exception of CH4). If the system is unbaked, the pri-
mary residual gas will be H2O and perhaps some solvent residues like acetone
and/or ethanol. The inorganic gases are, in most cases, 2- or 3-atom molecules
such as H2, H2O, CO, O2 and CO2 with even mass numbers. Further, ionization
is more probable than is molecular dissociation, so the fragmentation patterns are
dominated by the parent peak. The inert gases are stable, nonreactive atoms. If the
vacuum system is pumped by an untrapped rotary vane and/or a diffusion pump,
the residual gases will be dominated by organic vapors and gases. If the pumps
are well trapped, organics will be present, but dominated by inorganic gases. Or-
3.3.4 Techniques
for
Analysis
359
ganics contain many more atoms than an inorganic molecule and are more likely
to dissociate before ionization, so the parent peak will, very often, not be pre-
dominant. Dissociation usually occurs at the carbon-carbon single bonds, which
means that a CH2 (Am = 14) or a
CH3
(Am = 15) group is the first to break away.
The resulting fragment molecules are usually an odd mass number. For example,
ethanol (C2H6O) is an often-used solvent cleaner in vacuum systems and usually
leaves a residual vapor. Under electron impact, the methyl group in this molecule
is the the most probable dissociation fragment leaving the CH2OH {mie = 31) as
the dominant peak. Subsequent to that, the single bonds associated with hydrogen
are the next most likely to dissociate, so the subsequent fragmentation proceeds
in descending steps of one mass unit.
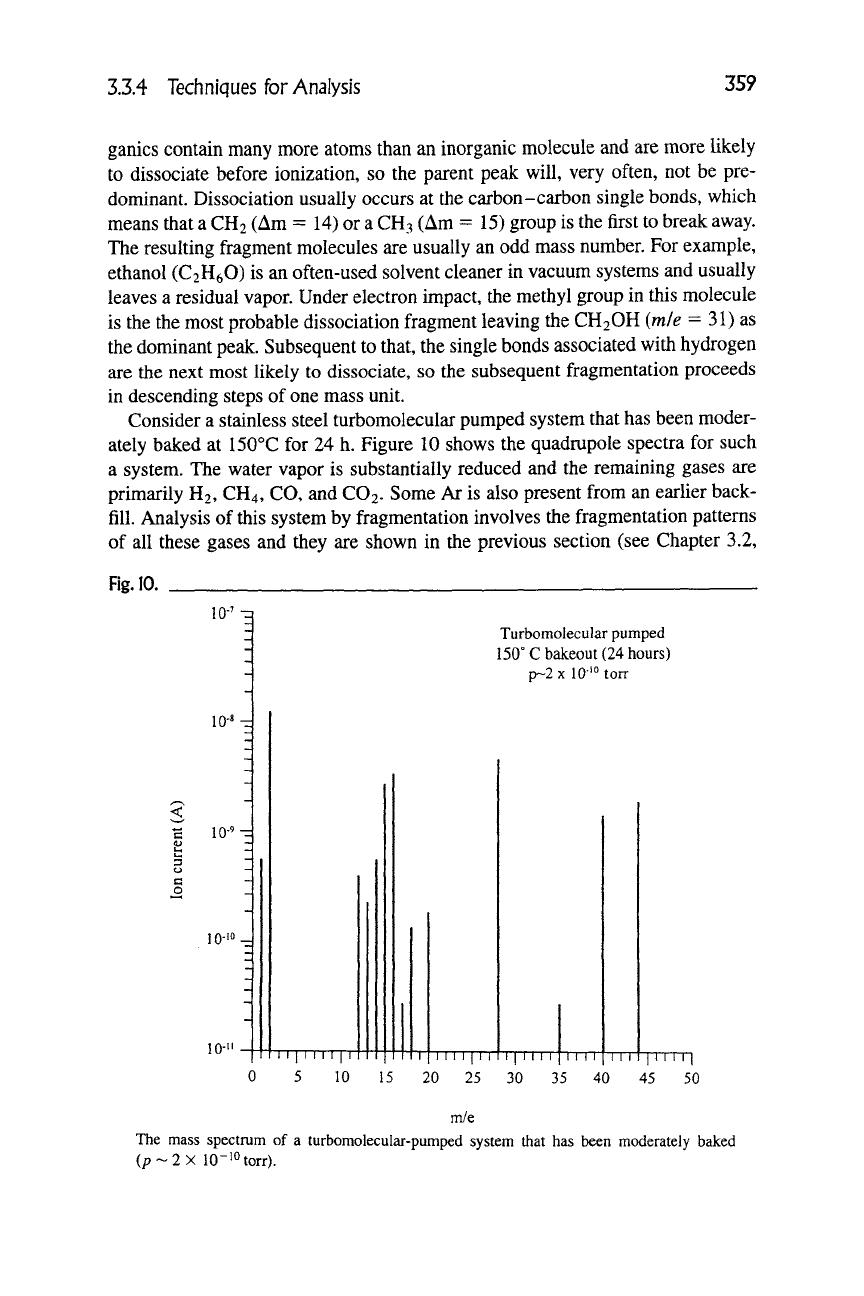
Consider a stainless steel turbomolecular pumped system that has been moder-
ately baked at 150°C for 24 h. Figure 10 shows the quadrupole spectra for such
a system. The water vapor is substantially reduced and the remaining gases are
primarily H2, CH4, CO, and CO2. Some Ar is also present from an earlier back-
fill. Analysis of this system by fragmentation involves the fragmentation patterns
of all these gases and they are shown in the previous section (see Chapter 3.2,
Fig. 10
10-^
io-«-d
10-^
lO-'M
10-'
Turbomolecular pumped
150° Cbakeout (24 hours)
p~2xl0-'°torr
I I I I I I I I I
I I I I I I I
I I I I I I
TTTT TTT
TTTTTl
0 5 10 15 20 25 30 35 40 45 50
m/e
The mass spectrum of a turbomolecular-pumped system that has been moderately baked
(/7~2X 10-10 torr).
