Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.

330
Chapter
3.2:
Mass Analysis
and
Partial Pressure Measurement
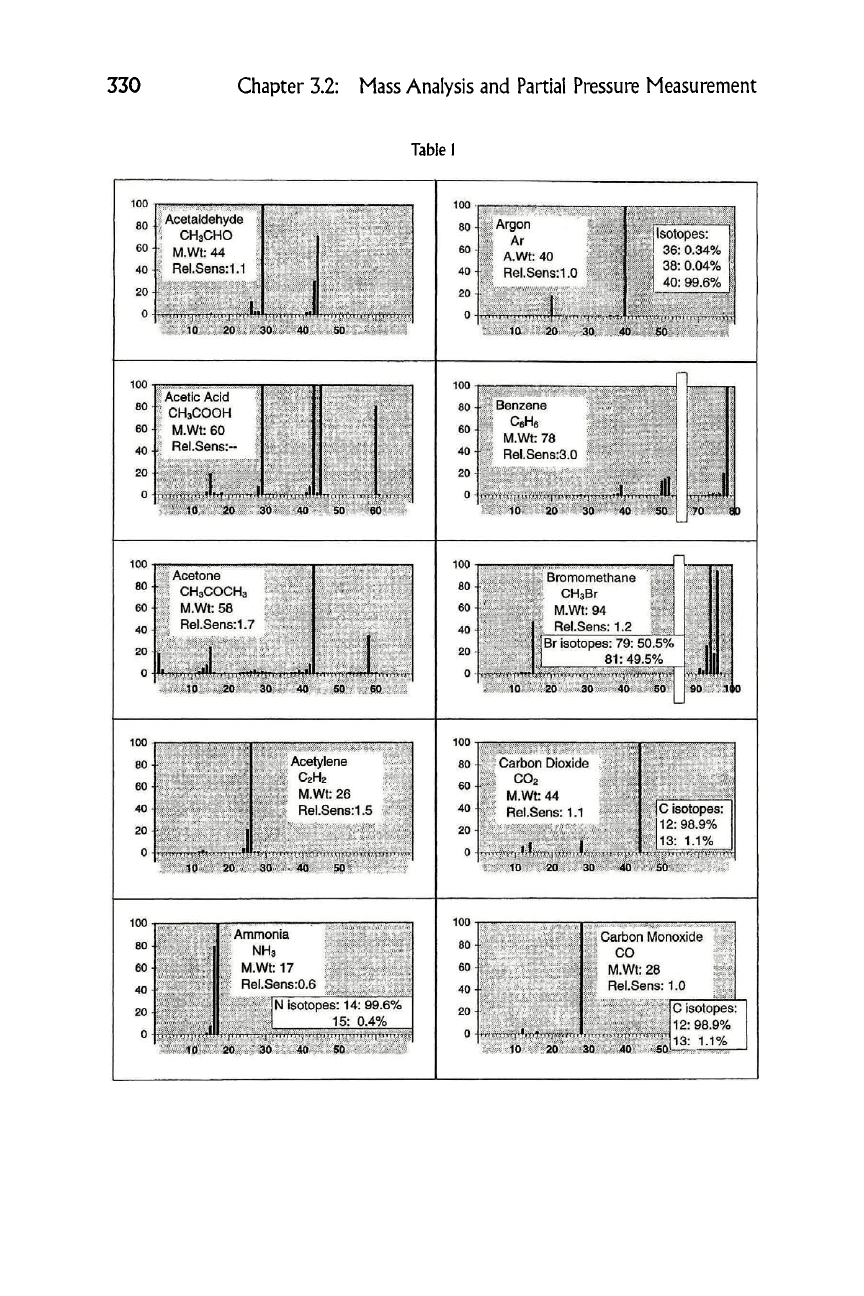
Table
1
100
80
60
40
20
0
Acetaldehyde I
CH3CHO
M.Wt: 44
Rel.Sens:1.1
10
;*^^'»t^•<^l•l;(>»Vy^f^<1rMlVM•;^
J
100
80
60
40
20 +
Argon
Ar
A.Wt: 40
Rel.Sens:1.0
30
isotopes:
36:
0.34%
38:
0.04%
40:
99.6%
40.
;$o
100
80
60
40
Acetic Acid
CH3COOH
M.Wt: 60
Rel.Sens:-
0-tM.jM'^i{M.!>y^.tuV'jfl»rrrv.>)>v}»HWTi.v|t>
10 20 iO 40 SO
100
80
60
40
20
0
1 , <~ "4
i Benzene
1 CeHe
T M.Wt: 78
T Rel.Sens:3.0
f
;V
10 20 30 40
~
,'.r
so
100
80
60
40
Acetone
CH3COCH3
M.Wt: 58
Rel.Sens:1.7
O
30
100
80
60
40 +
20 j
0
Bromomethane
CHaBr
M.Wt: 94
Rel.Sens: 1.2
Br isotopes: 79: 50.5%
81:49.5%
100
80
60
40
20
0
irnHI
Acetylene
C2H2
~ M.Wt: 26
Rel.Sens: 1.5
40
100
80
60
40
20
0
+ Carbon Dioxide
i CO2 ,t
T M.Wt: 44
r Rel.Sens: 1.1
]
*i i
^
to 20 30 40
C isotopes: I
12:98.9%
13:
1.1% 1
SO
100
80
60
40
20
0
Ammonia
NH3
M.Wt: 17
Rel.Sens:0.6
10V' .^20-
N Isotopes: 14:99.6%
15:
0.4%
50
100
80
60
40
20
0
Carbon Monoxide
CO
M.Wt: 28
Rel.Sens: 1.0
IC isotopes:
i.__jl2:98.9%
T'^ilS:
1.1%
Tables
1-5
331
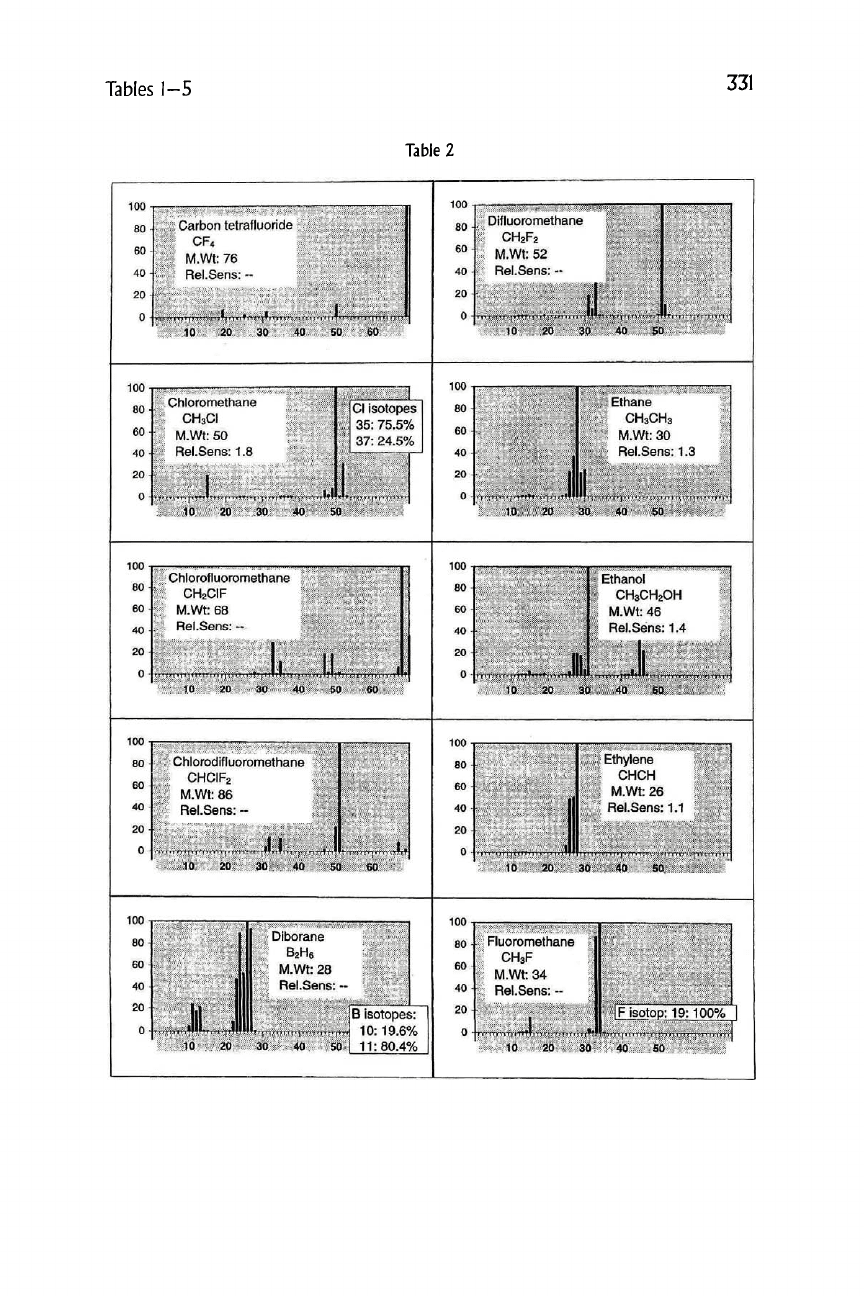
Table
2
100
80
60
40
20
0
Carbon tetrafluoride
CF4
M.Wt:
76
Rel.Sens:
~
100
80
60
40
Chloromethane
CH3CI
M.Wt:
50
Rel.Sens:
1.8
pr^irt-
0 4+tTTTx»Trpr^'rt-f»j'-
10'
20
100
80
Difluoromethane
CH2F2
M.Wt:
52
Rel.Sens:
--
100-]
80
J
60
J
40]
20 j
0 -1
T
"
L..rt^^,t~^
10
""^^J
i^:M
20
\
-
Ethane
CH3CH3
1
M.Wt:
30 1
;,.. Rel.Sens:
1.3 i
ll
lln
>f'ty''¥*1j'»Trrf fyyif^rrt
n
rrrjrt^Ttr*)'^
3D
40 60 -
100
80
60
40
Chlorofluoromethane
CH2CIF
M.Wt:
68
Rel.Sens:
~
i'>y'{rtTTfTn'p>'n'>)^f*i»r'>'-!<''
100
80
60
40
20
0
Ethanol
CH3CH2OH
M.Wt:
46
Rel.Sens:
1.4
80
60
40
20
0
Chlorodlfluoromethane
CHCIF2
M.Wt:
86
Rel.Sens:-
1
1
T
»•! 1!
1
r'l
1
[It
I'l'x'i'i'i]!
f
>•>•»!)
(•'
}W>
>Tm»|rr
nrr^^
10
20 ao 40 SI
\^,,^„,^^J^
)
€0
Ethylene
CHCH
M.Wt:
26
Rel.Sens:
1.1
10
20 30 40 SO
100
80
60
40
Fluoromethane
CH3F
M.Wt:
34
Rel.Sens:
-
JFlsotop: 19:100%
10
20 30 40 60
332
Chapter 3.2: Mass Analysis and Partial Pressure Measurement
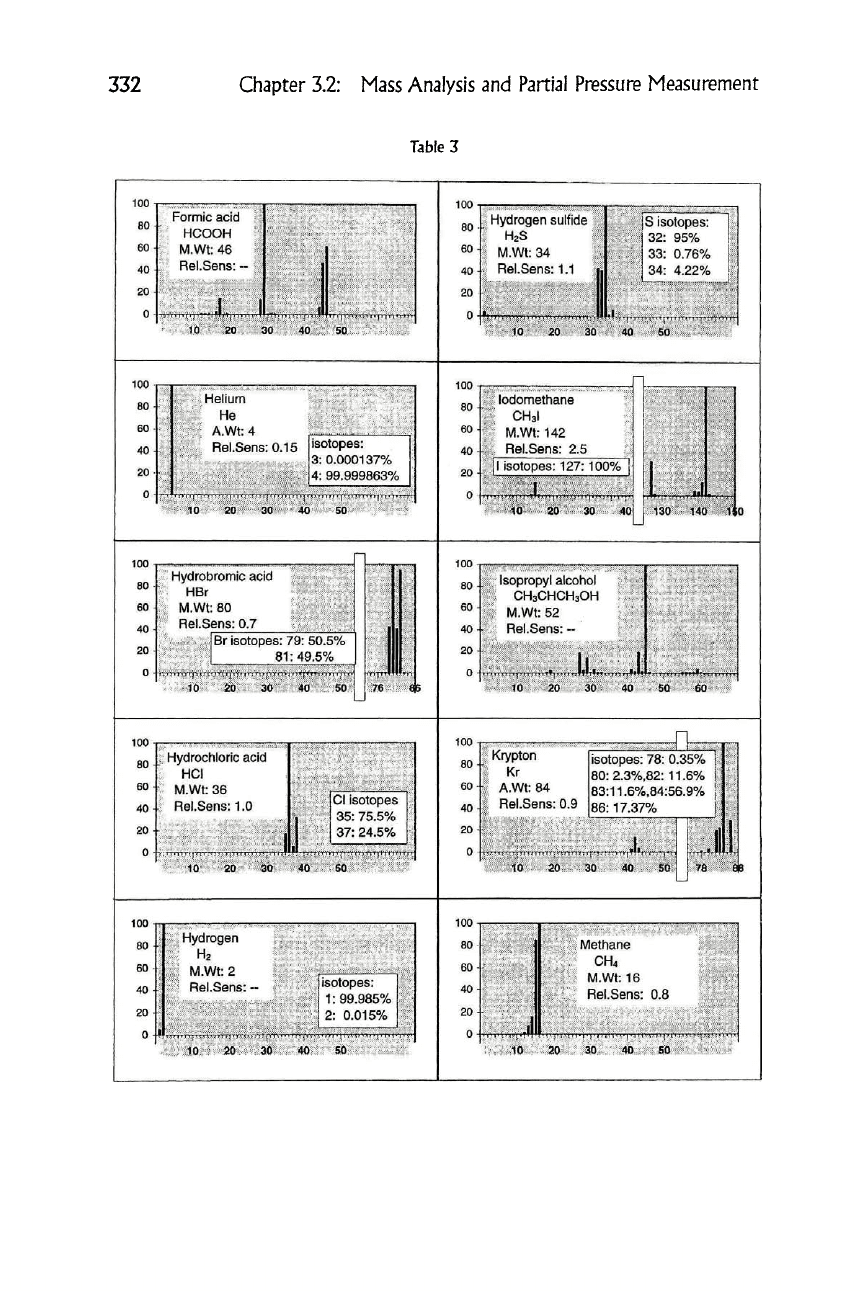
Table 3
1
Helium
He
A.Wt: 4
Rel.Sens:0.15
^
isotopes: 1
3:0.000137%
4:99.999863% |
' ' •
1 «
*^' '•!
<
'
<••'•
100
80
Hydrogen sulfide
HgS
M.Wt: 34
Rel.Sens: 1.1
10^
20
S isotopes:
32:
33:
34:
95%
0.76%
4.22%
Ito
40
100
80
60
40
20
0
lodomethane
CH3I
M.Wt: 142
Rel.Sens: 2.5
I
I
{isotopes:
127:100% 1
130
100
1
80
60
40
20
Hydrobromic acid
HBr
M.Wt: 80
Rel.Sens: 0.7 j
10
Br isotopes: 79: 50.5%
81:49.5%
20 30 40 50
IM
100
80
60
40
20
0
Isopropyl alcohol
CH3CHCH3OH
M.Wt: 52
Rel.Sens: -
,il i
100
80
60
40
20
0
I Hydrochloric acid
T HCI
1 M.Wt: 36
1 Rel.Sens: 1.0
t
^
1
1
|*,.Min.;•«..•,•.'» .,*}).u.):.riMii»
'
'»
CI isotopes 1
35:
75.5% 1
37:24.5% |
,.'i'.r<r,'>«vp'i^''ffr»r|
100
80
60
40
20
0
10 20 3b 40
Krypton
Kr
A.Wt: 84
Rel.Sens: 0.9
dl
isotopes: 78: 0.35%
80:2.3%,82:11.6%
83:11.6%,84:56.9%
86:17.37%
nrrrpr
30
J
80
i
60 4
40
4
20
+
0
-1
1 Hydrogen
1 H2
1 M.Wt: 2
j Rel.Sens: -
10 20 30 40
isotopes:
1:99.985%
2:
0.015%
50
100
80
60
40
20
0
[
- 1
^^
10
20
Methane
CH4
M.Wt: 16
Rel.Sens: 0.8
30 40 so
Tables
1-5
333
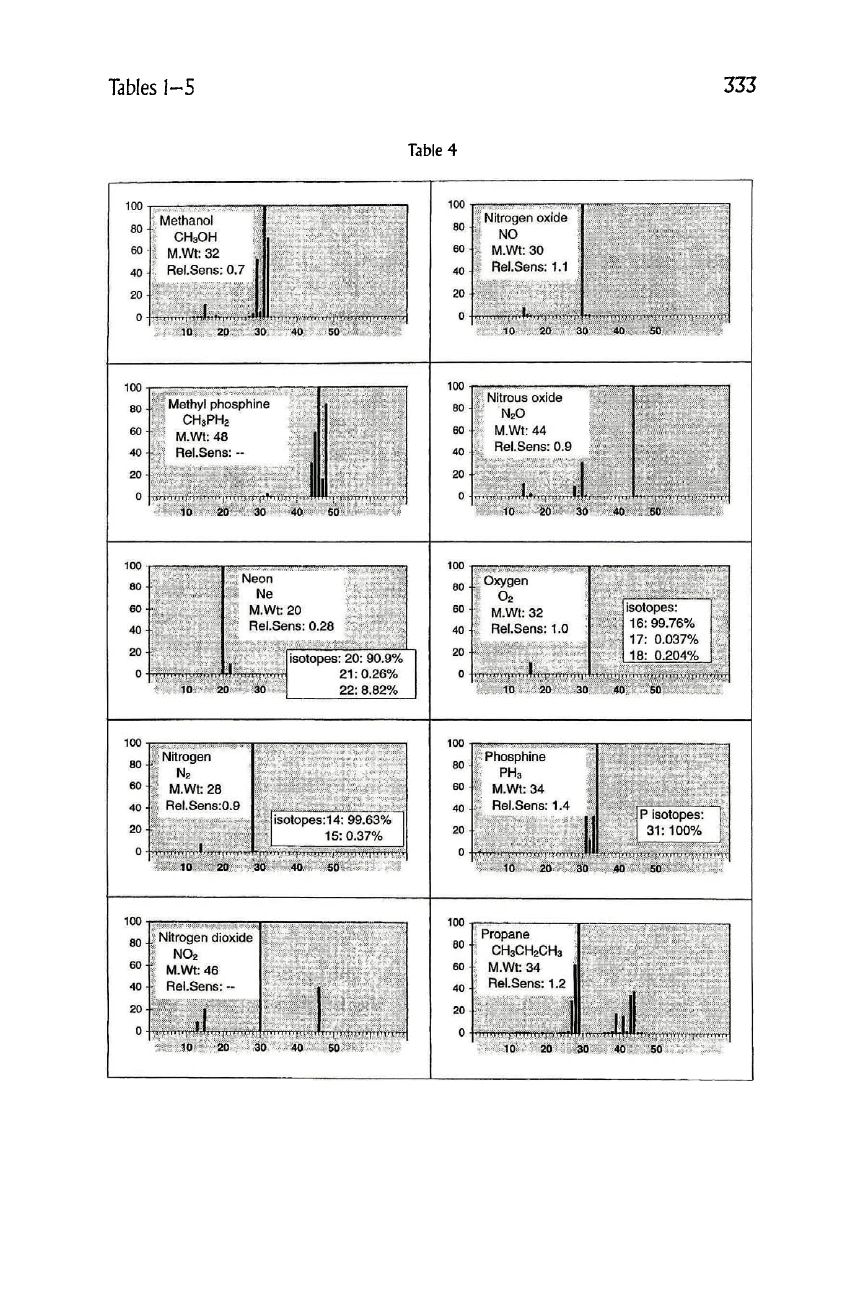
Table
4
100
80
Methanol
CH3OH
M.Wt: 32
Rel.Sens: 0.7
„t>,rr...>>»l|ll,
80
60
40-
20
Nitrogen oxide
NO
M.Wt: 30
Rel.Sens: 1.1
• ?T.
10 26 30 40
50
100
80
60
40
20
0
Methyl phosphine
CH3PH2
M.Wt: 48
Rel.Sens: -
(
100
80
60
40
20
0
10 20 30 40
Nitrous oxide
N2O
M.Wt: 44
Rel.Sens: 0.9
J.
10 20 30 40^^ 50
Neon
Ne
M.Wt: 20
Rel.Sens: 0.28
isotopes: 20: 90.9%
21:0.26%
22:
8.82%
100
80
1 Oxygen
T O2
]
M.Wt:
32
1:
Rel.Sens: 1.0
isotopes:
16:99.76%
17:
0.037%
18:
0.204%
rrm^trirfrm-rrtprvr
100
80
60
40
20
0
Nitrogen
N2
M.Wt: 28
Rel.Sens:0.9
20
isotopes:
14:
99.63%
15:0.37%
100
80
60
40
20
1 Phosphine
1 PH3
1 M.Wt: 34
j Rel.Sens: 1.4
1
il
|,r,r,,rni
P isotopes: f
31:100%
1
'"'«''
" "*i
80
60
40
20
0
. Nitrogen dioxide
NO2
M.Wt: 46
Rel.Sens: -
".^' 4 "
10 20 3
1
1
a 40 50
100
80
60
40
20
0
Propane
CH3CH2CH3
M.Wt: 34
Rel.Sens: 1.2
rrrprm,
10
UP
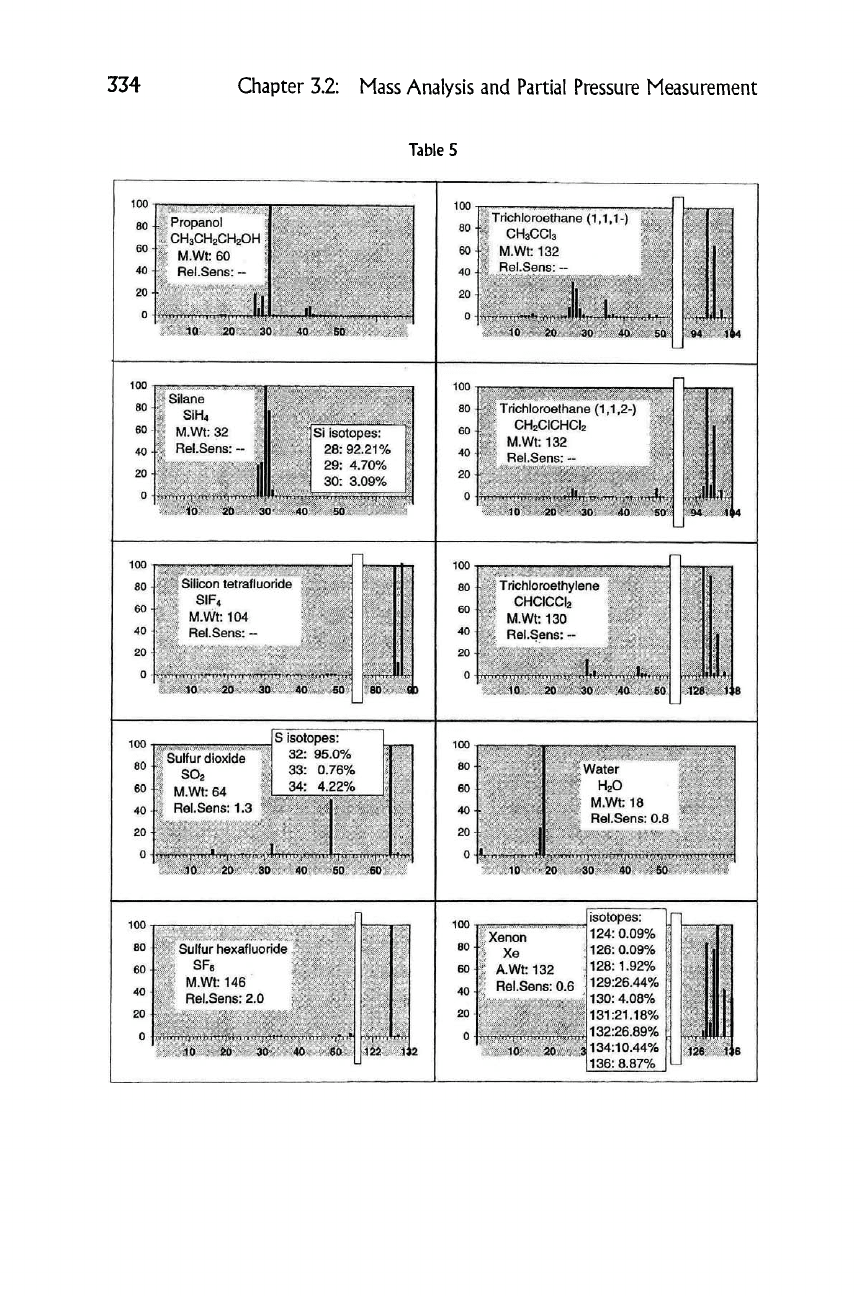
334
Chapter 3.2: Mass Analysis and Partial Pressure Measurement
Table
5
100
80
60
40
20
0
Propanol
CH3CH2CH2OH
M.Wt:60
Rel.Sens:
~
\m^i^fiyi
}irm<»'?}lbftTA^'M.^^i«u|.^uMn^]
.30.
40^
iBOJ
^
^A
100
80
60
40
20
0
;
Trichloroethane (1,1,1-)
t CH3CCI3
:
M.Wt:132
•
"<•'*.
>.'^6'^
~^.'^20?f
31|:*>;
x;<^
^*i?frSii
;vi3l^>
m
100
80
60
40
20
0
1
*
Silane
T; siH4
|;
M.Wt:32
K Rel.Sens:-
":^'^^^?%-
^ ^
ri*i;\^>'T "J
Vt''j||Lt>;>.K
'^^f^f'K>r''^^
Si isotopes:
H
28:92.21%
f
29:
4.70%
30:
3.09%
1
80
f <
Trichloroethane (1,1,2-)
,
go
1 r
CH2CICHCI2
'-^ •
t)] M.Wt:132
>,
^^'T.'
Rel.Sens:-
V'\:
0
l>
.>:.^,..^:'*^^«^^::.-'^V.^
-.^i/.;
[v^
iM
mtn
100
80
60
40
20
0
^
Silicon tetrafluoride
; \';
5: >
:
SIF4
;r'''^'
;
M.Wt:104
' %^^
Rel.Sens:
- V . ]
t<tM
M <
j
•
I;»»I'.
<r}M.
I
•
aiCfu^j
MiltliVti
viVf.fttJ
100
80
60
40
20
0
' Trichloroethylene
^^:l;
- -'
: CHCICCI2 r s/^~
M.Wt:130 w^f Y
. Rel.Sens:-
KXV^
^V-
iltoUvlW
100
80
60
40
20
0
S isotopes:
32:
95.0%
33:
0.76%
34:
4.22%
Sulfur dioxide
; SO2
'
M.Wt:64
.^,^,.,,,,.;.
ReLSens:
1.3
5V€ vi<^;;y|-:^r\<
^.i'^
ww^^^^^-
fefrr^
r^arrr
Xynm-jy
100
80
60
40
20
0
--m
" Water
[(f^?^::^
H2O i'fv:
M.Wt: 18
ik^i^.',,.
M-^
40^ 50
:60
^
\
^jV6^'>;rJa,:'
^.M"-
'.^^''40>
'
"i-i^J^^^^'
*^
^'"
-^
100
80
f
60
40
20
0
Sulfur hexafluoride
SFB
M.Wt: 146
'
Rel.Sens:
2.0
;^M-^^iw>hKTj|$^1i^»^<p1f«%xy*i'>'t
^•ifrM
'10 \{^'<^^'^~'--^4(i
-^«^;
1^
nz
100
^
80
60
40
20
0
Xenon
Xe
!:
A.Wt:132
Rel.Sens:
0.6
' '-'}
^^
llu
^
'-^ll
12iS
j
J
1
lie
CHAPTER 3.3
Practical Aspects of Vacuum
System Mass Spectrometers
R.
A. Outlaw
Teledyne Brown
Engineering:
IHastings Instruments
The most severe deficiency of total pressure instruments is that they provide vir-
tually no chemical composition information concerning the gas environment in a
vacuum system. The lack of identification of
the
partial pressures that make up the
total pressure is such an enormous disadvantage to system diagnostics that any
good vacuum system should have a mass spectrometer (also known as residual gas
analyzer, partial pressure analyzer, or simply mass spec). It provides, first of
all,
a
built-in leak detector, which is necessary to confirm the vacuum integrity of a sys-
tem. Second, it measures the state of cleanliness of a system by indicating the par-
tial pressure and type of residual gas. Third, it is also a research tool that provides
numerous diagnostic capabilities such as determining gas purity, gas desorption
from elements of interest, and gas changes that occur during various processes.
3.3.1
HISTORICAL INSIGHT
Historical insight into the still developing utility of vacuum system mass spec-
trometers is of some interest
[1-3].
Before 1950, a good vacuum or a low total
pressure was the essential environment required for conducting various processes
such as minimizing the slag in a vacuum furnace or minimizing the oxidation rate
ISBN 0-12-325065-7 Copyright © 1998 by Academic Press
*25.00 All rights of reproduction in any form reserved.
335

336 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
of the filament in an early incandescent light bulb or a vacuum tube. Other pro-
cesses, such as charge particle spectroscopy, require a low total pressure to ensure
a sufficiently long mean free path to allow the collection of charged species for
analysis. Measurement of the composition and partial pressure of the gases that
comprise the total pressure was initially of some interest, but not achievable.
Eventually, because of progressing scientific objectives, engineers and scientists
began to actively pursue methods to determine the identity of
the
gas species. One
needed to know, for example, the actual composition of a total pressure to under-
stand what the limiting factors were to achieving even lower
pressures.
Mass spec-
trometers developed in the beginning of this century had emphasis primarily on
the physics of the mechanism. J. J. Thompson's positive ray analyzer (1910) and
Dempster's 180-degree magnetic sector (1918) were the first of this kind [4].
However, in the latter part of the 1930s, A. O. C. Nier began applied studies in
atmospheric research with the primary interest in understanding the variation in
composition and magnitude of the gas species as a function of altitude [5]. Nier
made many advances in the technology of mass spectrometry, especially with a
unique ion source that provided high resolution. He used both magnetic sector
and quadrupole-type instruments. Concurrent with the work of Nier, interest in
understanding the chemical composition of complex organic molecules, the study
of isotopes, and the pursuit of equipment to provide lower total pressures drove
the technology. In the latter part of the 1950s, the well-funded space program
gave another boost to the continuing development. The National Aeronautics and
Space Administration's (NASA) research in examining the composition profile of
the atmosphere with altitude, the residual gases in space, the determination of the
atmospheres of planetary bodies, and space simulation propelled new and inno-
vative laboratory and flight experiments
[6].
The Apollo missions used mass spec-
trometers to measure the hydrogen atmosphere on the moon and the Viking mis-
sion to measure the CO2 atmosphere of
Mars.
These are only a few examples of
the wealth of information mass spectrometers have provided to the understanding
of space. The funding pumped into the study of vacuum technology waned
significantly after the moon landings, but mass spectrometers still continued to be
slowly developed and the uses for these instruments rapidly increased. In the
middle 1970s, the semiconductor industry became the primary driver for vacuum
equipment and the development of mass spectrometers got another boost. This
continues today—the sensitivity, resolution, and performance of these instru-
ments as well as the software have all dramatically improved.
3.3.2
EXPECTED GASES IN A VACUUM SYSTEM
The operational history of a vacuum system determines what residual gases exist
in that vacuum system. For example, of what sort of materials is the system made

33.2 Expected Gases in
a
Vacuum System 337
and what materials have been added to the system? What treatment did the mate-
rials receive? Have the materials been chemically cleaned (what chemical pro-
cess?),
electropolished (what process?), vacuum fired (at what temperature, time,
and vacuum level?), baked out (at what temperature, time, and vacuum level?), or
given other processes that will leave a tell-tale spectrum? Exposure to various
gases and environments in normal operation usually always leaves a characteris-
tic adsorbed residue and associated vapor. It may not be an exact representation
of the previous gas exposure because of surface chemistry, but may be some re-
action product. The longer the exposure to a particular high pressure, the more
the residual effect due to that exposure. Water vapor {mie =18 amu) is the domi-
nant residual gas after exposure to an atmosphere of air because of the strong,
multilayer, physical adsorption that occurs on the oxide surfaces of the system
walls and components contained therein. If it is a rainy day and the laboratory air
handling system does not substantially reduce the partial pressure of water vapor
in the laboratory, one will see a much larger residual water peak in the vacuum
system simply because of the longer time for accumulation. If a vacuum system
is exposed to the vapors of someone having an aromatic lunch, or exposed to a
strong aftershave, perfumes, or even cleaning solvents, the organic molecules will
certainly end up adsorbed on the surfaces of the vacuum system and a residual or-
ganic partial pressure will be observed after pumpdown. A newly painted room is
another obvious disaster for open vacuum chambers! The usual thumb rule is that
if you can smell it, then the system is already very contaminated.
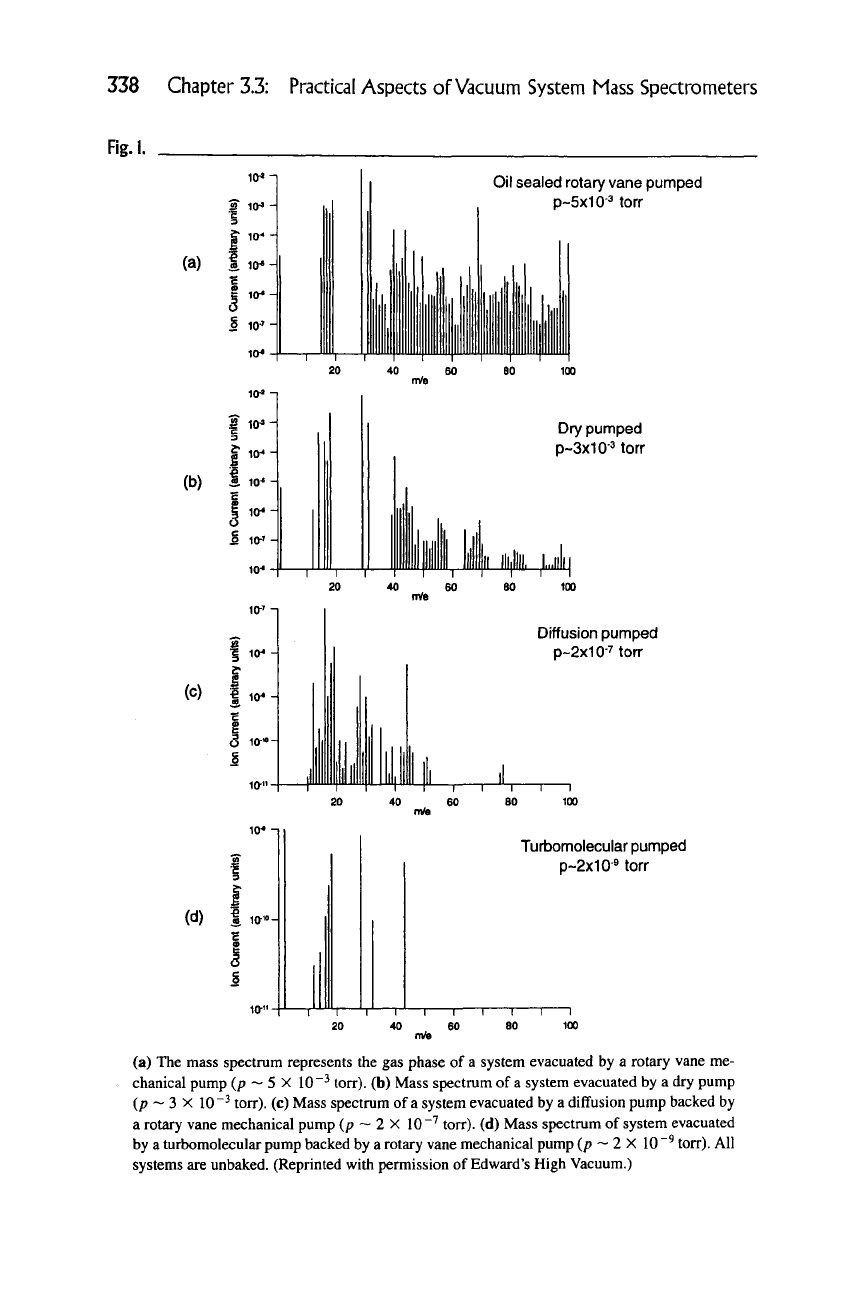
Another very important issue is the type of pumping employed. Consider, for
example, the usual stainless steel system pumped down by different pumping
mechanisms. If the resulting ultimate pressure is acquired by an untrapped rotary
vane mechanical pump (the ultimate pressure ~1 X 10"'^ torr is too high to be
directly sampled by most mass spectrometers, but can be observed indirectly by
differential pumping of the instrument), the spectrum will show a large organic
signature of pump oils and water vapor. A most undesirable level of contaminant
vapors is shown in Figure 1(a). The pump oils will generally have a characteristic
series of fragment peaks at 43 and 57 amu. If the system is dry-pumped, a much
lower level of residual organics will be observed, primarily because the signal
is vapor from the bearing greases of the pump —
see
Figure 1(b). High vacuum
{<p ~ 1 X 10~^ torr) obtained by using an untrapped diffusion pump shows a
substantial reduction in the water vapor but a significant level of organic signature
from the type of diffusion pump oil that is used (Figure Ic). If a turbo pump is
used—Figure
1(d)
—
the
organic peaks are much less prevalent. The dominant
partial pressure is again water vapor, but the second most prevalent signal is now
CO and CO2 at mass 28 and 44 amu respectively. Of course, as the total pressure
is reduced the sum of all the partial pressures is also reduced. The impact of sys-
tem bakeout is shown in the next series of figures. Figure 2(a) is a spectrum of
an unbaked ion pumped system with an ultimate pressure of 1 X 10"^ torr. A
modest bakeout of 150°C will remove a large fraction of the water vapor and
338 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
Fig.l.
Oil sealed rotary vane pumped
p~5x10-3
torr
Dry pumped
p~3x10-Morr
Diffusion pumped
p~2x10-7
ton-
Turbomolecular pumped
p~2x10-9
torr
(a) The mass spectrum represents the gas phase of a system evacuated by a rotary vane me-
chanical pump (p ~ 5 X 10"^ torr), (b) Mass spectrum of
a
system evacuated by a dry pump
(p ~ 3 X
10"^
torr). (c) Mass spectrum of
a
system evacuated by a diffusion pump backed by
a rotary vane mechanical pump (p ~ 2 X 10"^ torr). (d) Mass spectrum of system evacuated
by
a
turbomolecular pump backed by
a
rotary vane mechanical pump (p ~ 2 X
10 "^
torr). All
systems are unbaked. (Reprinted with permission of Edward's High Vacuum.)
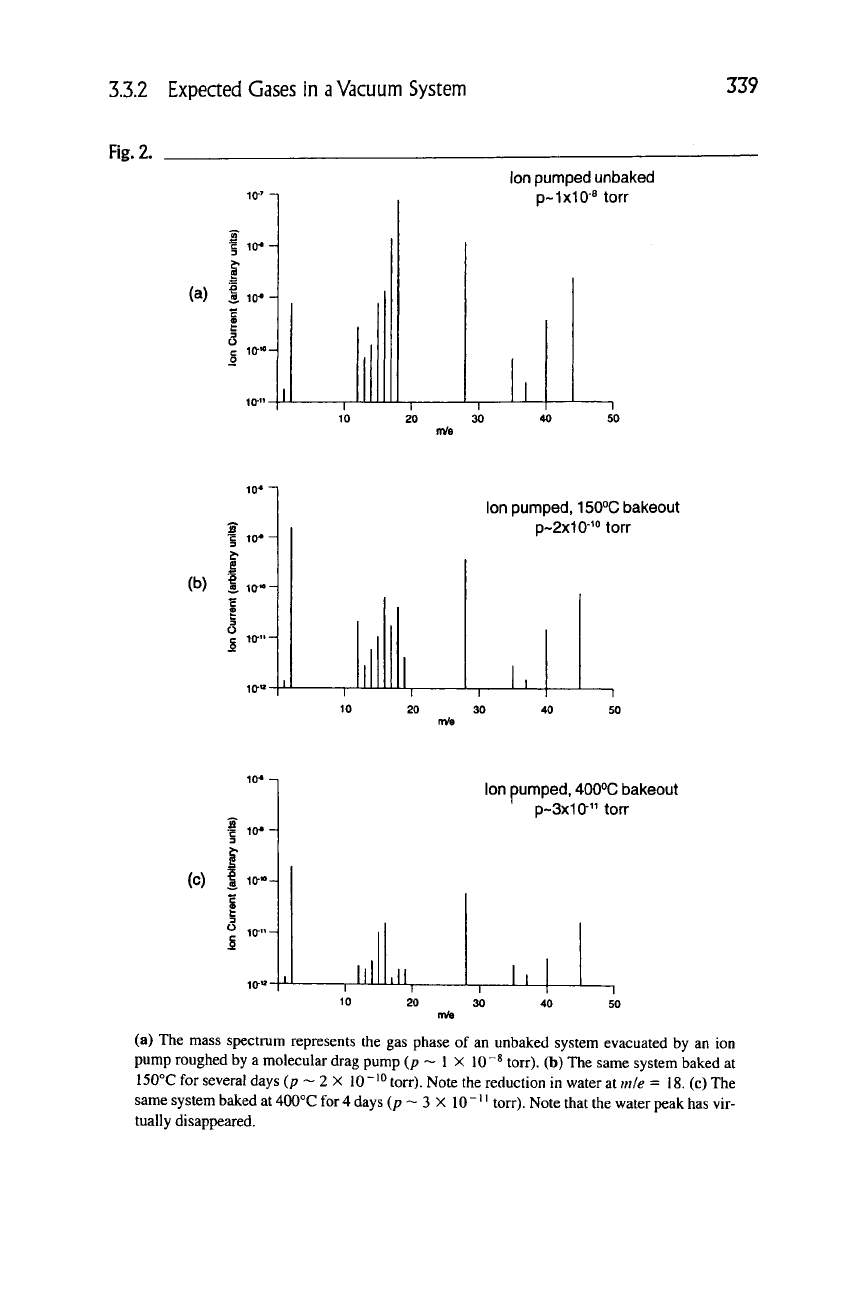
3.3.2 Expected Gases in
a
Vacuum System
339
Fig.
2.
(a) I
Ion pumped unbaked
p~1x10-« torr
J3
1 10*
(b) tia-o
I
Ion pumped, 150°C bakeout
p~2x10-^Morr
50
(c)
I
I
I
3
O
ilillll
Ion pumped, 400°C bakeout
p~3x10" ton-
~i
30
-1
50
(a) The mass spectrum represents the gas phase of an unbaked system evacuated by an ion
pump roughed by a molecular drag pump (p ~ 1 X 10"^ torr). (b) The same system baked at
150°C for several days (/? ~ 2 X 10~'° torr). Note the reduction in water at mie = 18. (c) The
same system baked at 400°C for 4 days (p ~ 3 X
10
" " torr). Note that the water peak has vir-
tually disappeared.
