Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.

Chapter
1.1:
Vacuum Nomenclature and Definitions
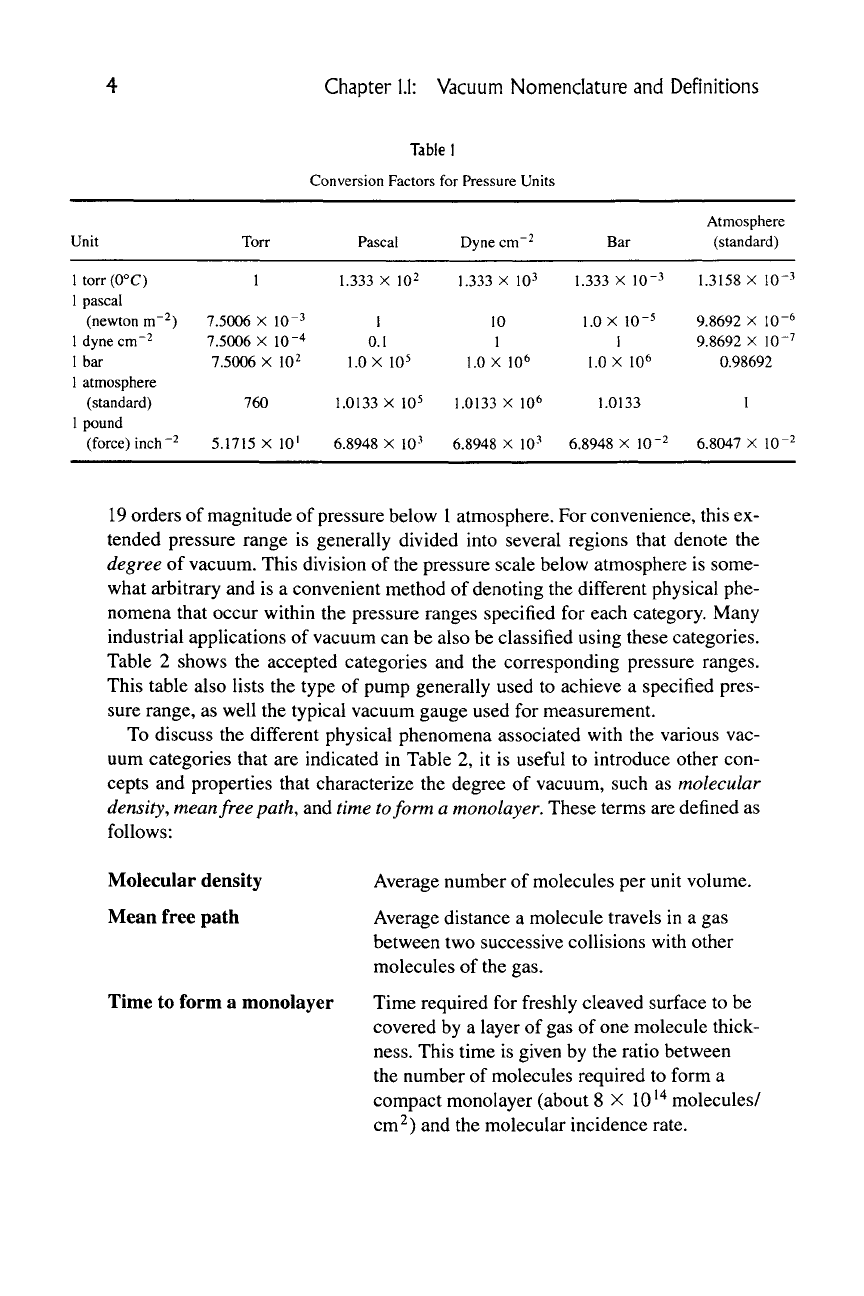
Table I
Conversion Factors for Pressure Units
Unit
Torr
Pascal Dyne cm
Bar
Atmosphere
(standard)
1.333X102
1.333 XIO^ 1.333 X 10"^
1.3158
X 10"^
1 pascal
(newton m~2) 7.5006 X 10"^
dyne
Ibar
1 torr (0°C)
1
^^IICWIUU 111 ) /..
ldynecm-2 7.5006 X 10"^
1
0.1
10
-- u.i 1
7.5006 X 10^ 1.0 X 10^ 1.0 X 10^
1 atmosphere
(standard)
1 pound
1.0 X 10-5 9 8692 X 10-^
9.8692 X 10"^
1.0 X 10^ 0.98692
1
(standard) 760
1.0133
X 10^ 1.0133X10^
1.0133
1
pound
(force)
inch-2 5.1715
X
10' 6.8948
X
10^ 6.8948
X
10^ 6.8948
X
lO'^ 6.8047
X
lO'^
19 orders of magnitude of pressure below
1
atmosphere. For convenience, this ex-
tended pressure range is generally divided into several regions that denote the
degree of vacuum. This division of the pressure scale below atmosphere is some-
what arbitrary and is a convenient method of denoting the different physical phe-
nomena that occur within the pressure ranges specified for each category. Many
industrial applications of vacuum can be also be classified using these categories.
Table 2 shows the accepted categories and the corresponding pressure ranges.
This table also lists the type of pump generally used to achieve a specified pres-
sure range, as well the typical vacuum gauge used for measurement.
To discuss the different physical phenomena associated with the various vac-
uum categories that are indicated in Table 2, it is useful to introduce other con-
cepts and properties that characterize the degree of vacuum, such as molecular
density, mean free path, and time to form a monolayer. These terms are defined as
follows:
Molecular density
Mean free path
Time to form a monolayer
Average number of molecules per unit volume.
Average distance a molecule travels in a gas
between two successive collisions with other
molecules of the gas.
Time required for freshly cleaved surface to be
covered by a layer of gas of one molecule thick-
ness.
This time is given by the ratio between
the number of molecules required to form a
compact monolayer (about 8 X
10
^"^
molecules/
cm^) and the molecular incidence rate.
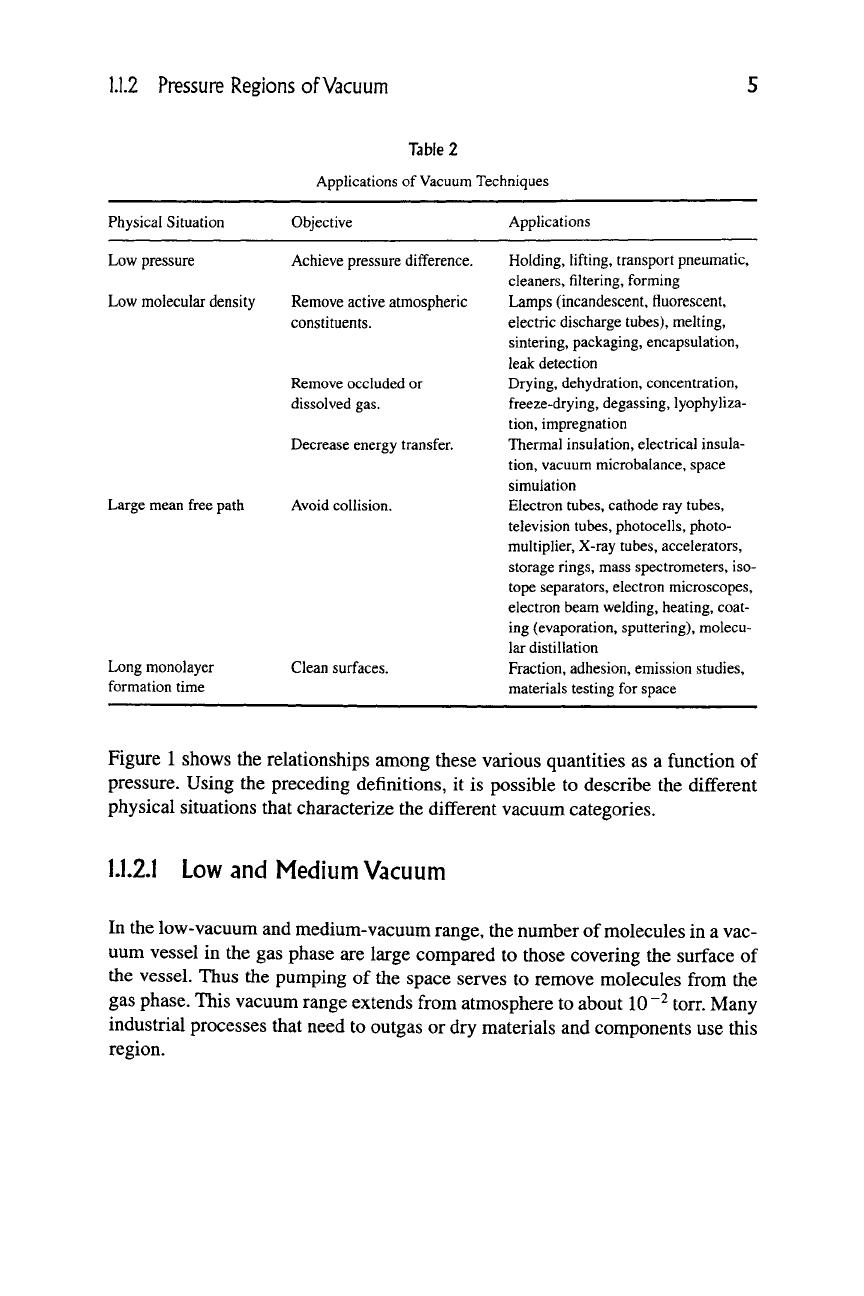
1.1.2 Pressure Regions of Vacuum
Table
2
Applications of Vacuum Techniques
Physical Situation Objective
Applications
Low pressure
Achieve pressure difference.
Low molecular density Remove active atmospheric
constituents.
Remove occluded or
dissolved gas.
Large mean free path
Long monolayer
formation time
Decrease energy transfer.
Avoid collision.
Clean surfaces.
Holding, lifting, transport pneumatic,
cleaners,
filtering,
forming
Lamps (incandescent, fluorescent,
electric discharge tubes), melting,
sintering, packaging, encapsulation,
leak detection
Drying, dehydration, concentration,
freeze-drying, degassing, lyophyliza-
tion,
impregnation
Thermal insulation, electrical insula-
tion,
vacuum microbalance, space
simulation
Electron tubes, cathode ray tubes,
television tubes, photocells, photo-
multiplier. X-ray tubes, accelerators,
storage rings, mass spectrometers, iso-
tope separators, electron microscopes,
electron beam welding, heating, coat-
ing (evaporation, sputtering), molecu-
lar distillation
Fraction, adhesion, emission studies,
materials testing for space
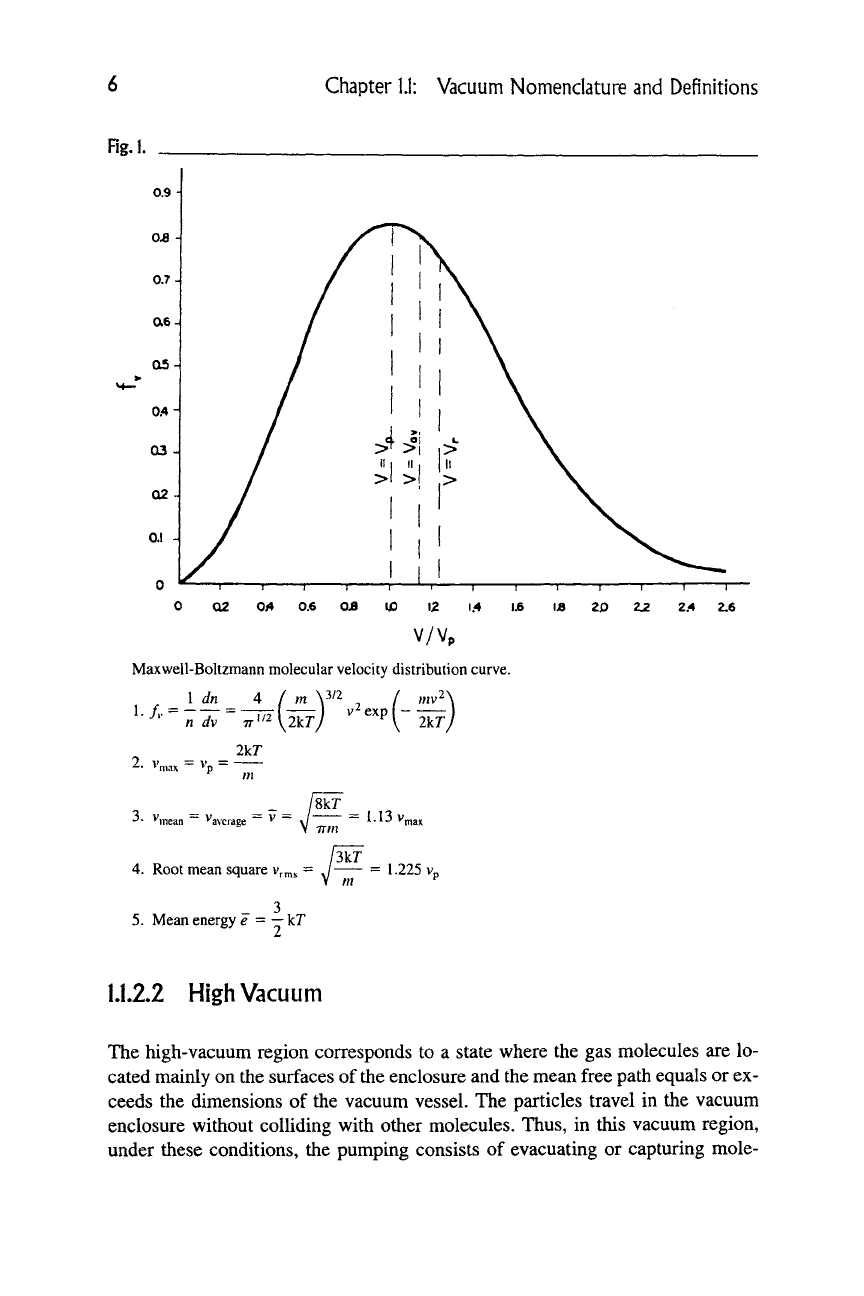
Figure 1 shows the relationships among these various quantities as a function of
pressure. Using the preceding definitions, it is possible to describe the different
physical situations that characterize the different vacuum categories.
1.1.2.1
Low and Medium Vacuum
In the low-vacuum and medium-vacuum range, the number of molecules in a vac-
uum vessel in the gas phase are large compared to those covering the surface of
the vessel. Thus the pumping of the space serves to remove molecules from the
gas phase. This vacuum range extends from atmosphere to about
10
"^ torr. Many
industrial processes that need to outgas or dry materials and components use this
region.
Chapter
1.1:
Vacuum Nomenclature and Definitions
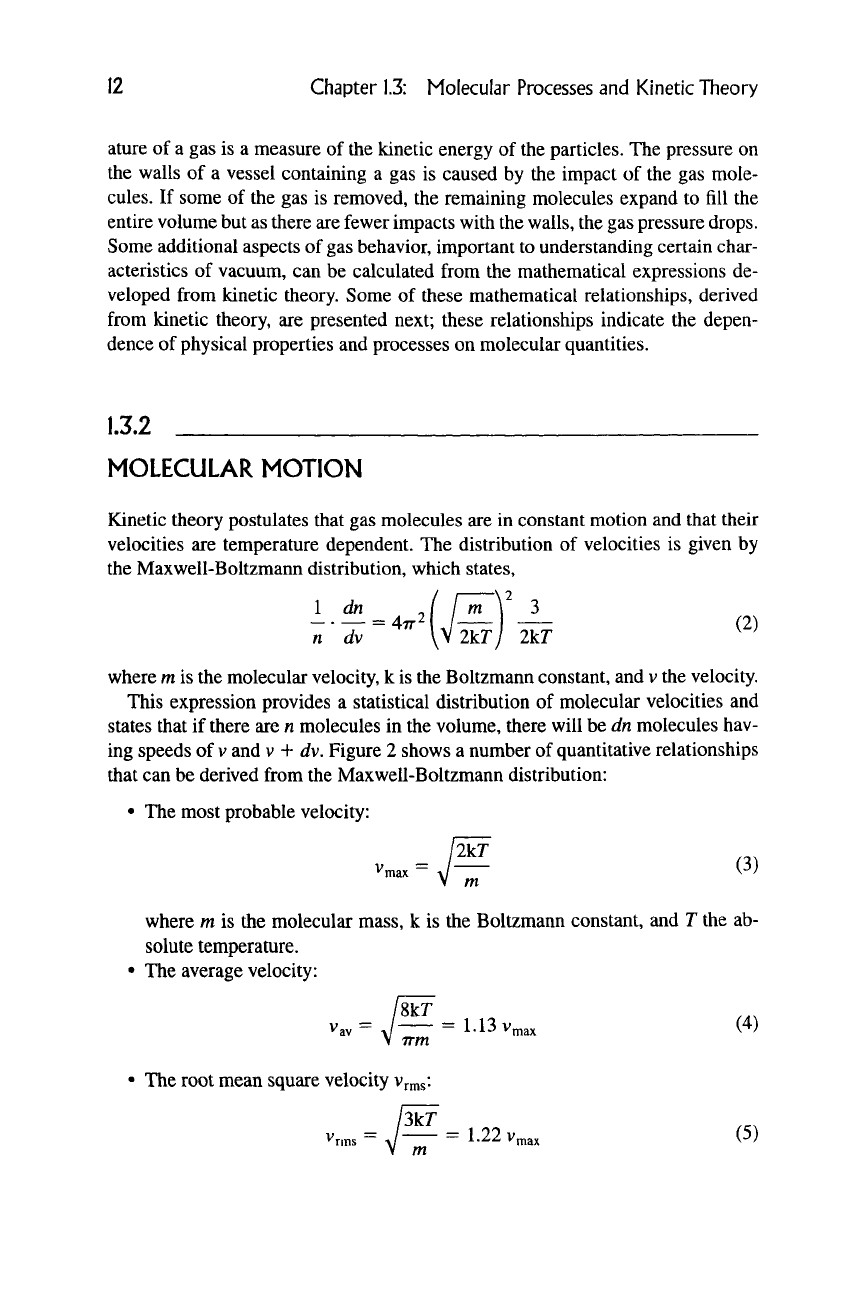
Rg.l.
V/Vp
Maxwell-Boltzmann molecular velocity distribution curve.
I dn A ( m \3/2 / ,„v2\
2.
v„
lYT
3.
V,
mean "average
/8kT
7= J =
1.13v„
\ irm
3kr
4.
Root mean square
Vr^s
- J = 1-225 v,
5. Mean energy e =
—
kT
1.1.2.2
High Vacuum
The high-vacuum region corresponds to a state where the gas molecules are lo-
cated mainly on the surfaces of
the
enclosure and the mean free path equals or ex-
ceeds the dimensions of the vacuum vessel. The particles travel in the vacuum
enclosure without colliding with other molecules. Thus, in this vacuum region,
under these conditions, the pumping consists of evacuating or capturing mole-

1.1.2 Pressure Regions of Vacuum
7
cules.
The molecules leave the surface and individually reach the pump. This re-
gion is extensively used in the preparation and application of vacuum coatings,
surface treatment, and modification. This region extends from
10 ~'^
to
10 ~^
torr.
1.1.2.3
Ultra-High
Vacuum
(UHV)
Under ultra-high-vacuum (UHV) conditions, time to form a monolayer is equal to
or longer than the usual time for most laboratory measurements. Thus clean sur-
faces can be prepared and their properties determined before an adsorbed gas
layer is formed. This vacuum range extends from about 10"^ to 10"^^ torr.
An indication of the extensive application of vacuum technology in many key
industrial processes in a diverse range of industries is illustrated in Table 2, where
common vacuum industrial processes are classified according to the degree of
vacuum used.

CHAPTER
1.2
Gas Properties
1.2.1
DESCRIPTION
OF
VACUUM AS THE CHARACTERISTICS
OF A LOW-PRESSURE GAS
The behavior and characteristics of gases are fundamental to vacuum systems.
Even at the extremely low pressures typically encountered in vacuum technology,
gases essentially still behave as gases. The necessity for creating a vacuum is usu-
ally related to the need to reduce the number density of gaseous molecules, or their
surface collision rates. Behavior of gases in vacuum systems can be generally dis-
cussed in terms of the ideal gas laws; and some aspects of overall behavior of
vac-
uum systems can
be
described by the static and dynamic properties of
gases.
The be-
havior of
gases
at low pressure and various aspects of gas flow are considered next.
1.2.2
CHARACTERISTICS OF A GAS—BASIC DEFINITIONS
A low-pressure sample of gas can be completely described if at least three of the
four quantities that relate to it are known. These quantities are its pressure,
vol-
ume, temperature, and the amount of gas in the sample.
Pressure: Pressure is defined as the force per unit area that a gas exerts on the
walls of
its
container. In the MKS system of
units,
pressure is expressed as newton
per square meter, or newton/m^ or newton m~^. The MKS unit is the pascal,
where
1
torr =133 pascal and 1 pascal = 7.5 torr.
Volume: The volume is simply a measure of the space a gas takes up; it is usu-
ally set by the dimensions of the enclosure. The MKS unit of volume is the m-^
but liters are extensively employed to refer to pumping rates, gas flow measure-
ISBN 0-12-325065-7 Copyright © 1998 by Academic Press
$25.00 All rights of reproduction in any form reserved.

1.2.3 Gas Laws 9
ments, etc. The pumping speed of mechanical pumps is often expressed in cfm
(cubic feet per minute).
Temperature: The temperature of a gas at pressure below
1
torr is determined
mainly by the temperature of the surfaces with which the gas comes in contact.
Typically the gas is at room temperature. In deriving the equations that describe
the behavior of gases, the unit of temperature is K or Kelvin.
Amount of
gas:
The amount or mass of gas in a given sample is measured in
molar gram units or moles.
Gram molecule or mole: Defined as that quantity of gas (or any substance)
having a mass equal to its molecular weight in grams. A gram molecule contains
6 X
10^-^
molecules. One mole of any gas at 0°C and a pressure of 760 torr,
occupies 22.4 liters of
volume.
The mass of
1
mole of gas is exactly equivalent
to its molecular weight in grams.
Gram molecular volume: The volume occupied by a gram molecule of gas is
a universal constant; it is found experimentally to be 22.414 liters at 760 torr and
0°C.
As 1 mole of any gas, at a temperature of 0°C and a pressure of 760 torr
occupies a volume of 22.4 liters, it is possible from this relationship to calculate
the molecular density of any volume of gas if
its
temperature and pressure are
known. For example,
1
cubic centimeter of air at 760 torr and 0°C contains
2.7 X
10 ^^
molecules; whereas at a pressure of
1
torr and a temperature of 0°C,
1 cubic centimeter of air contains 3.54 X
10 ^^
molecules.
1.2.3
GAS LAWS
The gas laws (Boyle's, Charles's, Gay-Lussac's) lead to relationships of the bulk
physical quantities of the gas, such as pressure, volume, temperature, and the
amount of gas to one another. These relationships describe the behavior of
a
given
quantity of an "ideal" gas; an ideal gas is one where the volume of all the mole-
cules is negligible compared to the volume of the gas, and the energy of attraction
between the molecules is negligible compared to their mean thermal energy. This
means that the sample of gas is dilute and is at a temperature that is not low
enough to condense it. Gases that are ideal at room temperature include O2, Ne,
Ar, CO, H2, N2, and NO. A summary of the relationships that result from apph-
cations of the gas laws to an ideal gas is provided here:
Boyle's law: States that the product of pressure and volume,
pW,
is constant for
a given mass of gas at constant temperature.
Charles's law: States that
WIT is
constant for a given mass of gas at a constant
pressure, where 1/is the gas volume and T = the absolute temperature.
Avogadro's law: States that equal volumes of gas at any gas at the same tem-

10 Chapter 1.2: Gas Properties
perature and pressure contain the same number of molecules. From this law can
be obtained an important relationship between the number of moles in a sample
and the pressure the gas exerts.
General gas law: The general gas law relates all four quantities needed to
describe the state of a gas. The general law states that
PV=nRT (1)
for a given mass of gas, where R = universal gas constant (constant of propor-
tionality) with a value of 62.4 torr-liter/mole°K, and n is the number of moles in
volume V.
This law is known as the "ideal" gas law, because it is exactly true for ideal
gases;
most gases at reduced pressures behave as ideal gases.
Dalton's law of partial pressures: The total pressure exerted by a mixture
of gases is equal to the sum of the partial pressures exerted by the individual
components.
Partial pressure: The partial pressure exerted by any one component of a
mixture of gases is the pressure exerted by that component if it occupied that
volume alone.
Avogadro's law: Equal volumes of all ideal gases measured at the same tem-
perature and pressure contain the same number of molecules.
Avogadro's number: The number of molecules in a gram molecule of gas or
any substance is a universal constant and is
6.023
X
10^-^.
Loschmidt's number: The number of molecules per
cm"^
of gas at 760 torr
and 0°C is a universal constant equal to 2.637 X
10 ^^.
For 1 mole at standard temperature and pressure (STP), P = 760 torr =
1,013,250
dynes/cm^, V = 22.414 liters, and T = 273.2°K, whence R = 8.31 X
10^
ergs per gram molecule or in thermal units R/J = 1.99 cal per °K (J = mechan-
ical equivalent of heat = 4.182 joules cal
^).
In more tangible terms, therefore, 1.99
cal will raise the temperature of
1
mole of any ideal gas 1°K. Alternatively, hav-
ing raised the temperature of
1
mole of any ideal gas by 1°K, the increase in en-
ergy of the gas amounts to 8.31 joules.
1.2.3.1
Nonideal Gases
Examples of some common nonideal gases are ammonia, ethane, benzene, CO2,
mercury vapor, NOSob, SO, and SO2. The gas laws for any gas have to account
for behavior of a gas at low temperature. Below a certain temperature, called the
critical temperature,
TQ,
the gas begins to condense. Below this critical tempera-
ture,
there is a vapor pressure of gas over the liquid condensate, called the vapor
pressure. If the gas is condensed (V is decreased), the pressure will not change,
but more gas will condense into the liquid phase. As the temperature is lowered,
fewer molecules are present over the liquid and the vapor pressure is lower.
CHAPTER
1.3
Molecular Processes
and Kinetic Theory
1.3.1
GENERAL DESCRIPTION
The gas laws describe the bulk behavior of gases but provide no insight as to why
gases should behave in this fashion. Kinetic theory is an attempt to explain the be-
havior of gases in terms of the behavior of the individual molecules that make
up the gas. The results of kinetic theory are in close agreement with the gas laws.
The theory is based on the following assumptions:
1.
A gas is composed of a large number of molecules. All the molecules of a
given chemical substance are exactly alike.
2.
The molecules are separated by distances that are large in comparison with
their own dimensions.
3.
The molecules are in constant state of random and chaotic motion. This mo-
tion is related to the temperature of the gas.
4.
The molecules exert no force on each other or on the walls of their container
except when they collide. The volume occupied by the molecules is negli-
gible compared with the volume occupied by the gas.
5.
The molecules behave as perfect elastic spheres.
These assumptions are clearly idealized, and no known gas behaves exactly in
accordance with this set of assumptions. However, the theory based on these as-
sumptions explains the behavior of real gases in very satisfactory manner.
The theory describes the motion of molecules that are in constant motion. The
molecules are free to wander throughout any space available to them. The temper-
ISBN 0-12-325065-7 Copyright © 1998 by Academic Pi ess
^•^-^•^ All rights of reproduction in any form reserved.
11
12 Chapter 1.3: Molecular Processes and Kinetic Theory
ature of a gas is a measure of the kinetic energy of the particles. The pressure on
the walls of a vessel containing a gas is caused by the impact of the gas mole-
cules.
If some of the gas is removed, the remaining molecules expand to fill the
entire volume but as there are fewer impacts with the walls, the gas pressure drops.
Some additional aspects of gas behavior, important to understanding certain char-
acteristics of vacuum, can be calculated from the mathematical expressions de-
veloped from kinetic theory. Some of these mathematical relationships, derived
from kinetic theory, are presented next; these relationships indicate the depen-
dence of physical properties and processes on molecular quantities.
1.3.2
MOLECULAR MOTION
Kinetic theory postulates that gas molecules are in constant motion and that their
velocities are temperature dependent. The distribution of velocities is given by
the Maxwell-Boltzmann distribution, which states,
/ I \2
I dn ^l m \
(2)
where m is the molecular velocity, k is the Boltzmann constant, and v the velocity.
This expression provides a statistical distribution of molecular velocities and
states that if there are n molecules in the volume, there will be dn molecules hav-
ing speeds of
v
and v + dv. Figure 2 shows a number of quantitative relationships
that can be derived from the Maxwell-Boltzmann distribution:
• The most probable velocity:
"^ (3)
m
where m is the molecular mass, k is the Boltzmann constant, and T the ab-
solute temperature.
The average velocity:
=
1.13v,^
(4)
irm
• The root mean square velocity Vf,
3kT
=
1.22v„3x
(5)
m
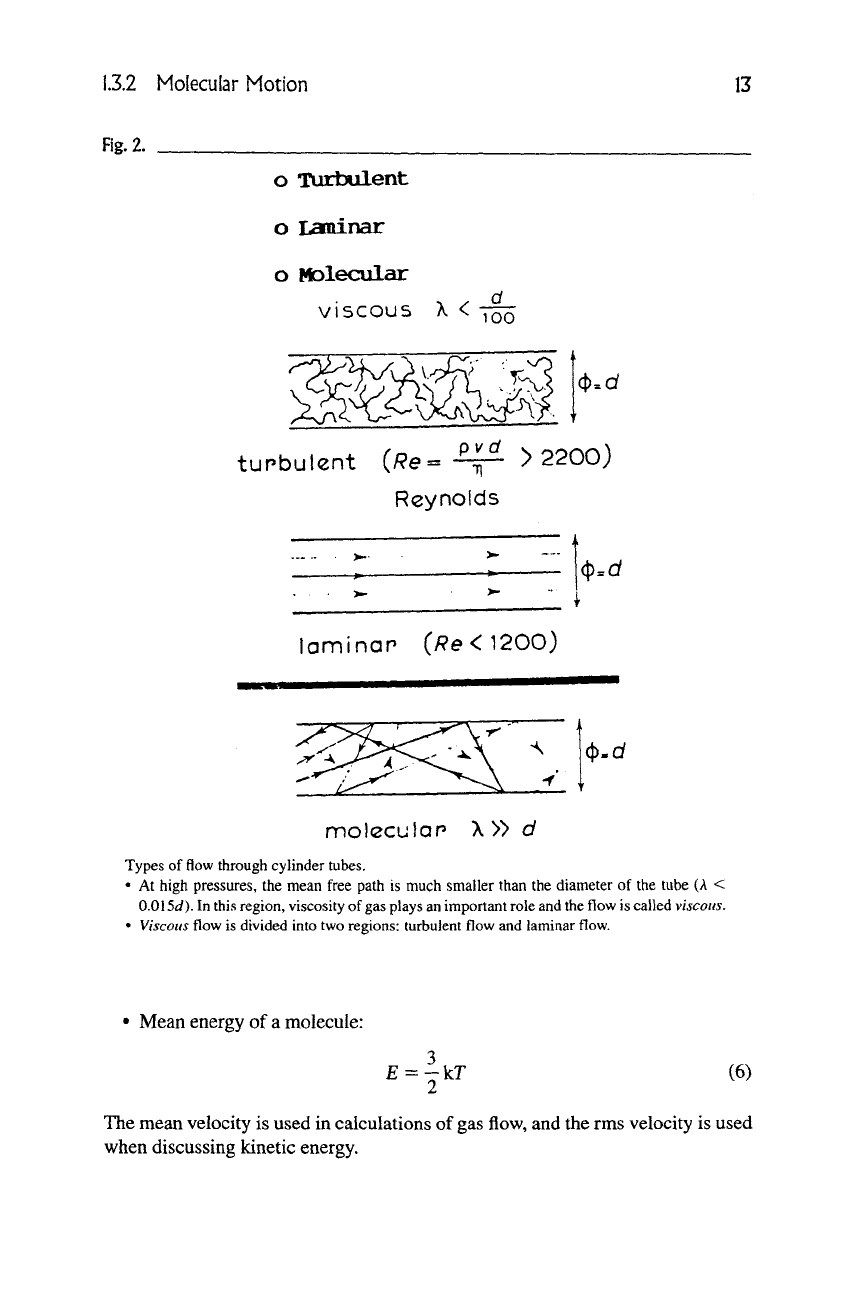
L3.2 Molecular Motion
13
Fig.
2.
o TUrtwlent
o Lanainar
o Molecular
viscous X < 7
00
tupbulent (/?e=-^ > 2200)
Reynolds
laminap (/?e<1200)
(t)=Gf
(t).d
±. I
molcculan
X
» d
Types of flow through cylinder tubes.
• At high pressures, the mean free path is much smaller than the diameter of the tube (A <
0.0\5d). In this region, viscosity of
gas
plays an important role and the flow is called viscous.
•
Viscous
flow is divided into two regions: turbulent flow and laminar flow.
• Mean energy of a molecule:
2
(6)
The mean velocity is used in calculations of gas flow, and the rms velocity is used
when discussing kinetic energy.
