Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.


44
Chapter 1.8: Surface Physics and Its Relation to Vacuum Science
methods do not sample only the top surface monolayer of a material. This phe-
nomenon will be discussed later in this book.
1.8.4
SURFACE AREA
The surface of a material is generally thought of as simply the physical surface
area due to the physical size of an object, that is, the integral over x and y (in this
coordinate system). On the atomic level, surfaces are not flat. In fact, even single-
crystal surfaces contain numerous defects such as screw dislocations at the sur-
face,
giving rise to atomic steps as shown in Figure 5.
Polishing a crystal surface may result in multiple steps as shown in Figure 5.
The result of this is to increase the total surface area. Other steps are formed
by the presence of chemically attached atoms caused by, for example, oxidation
of the surface. These nonuniform regions may be many atom layers thick.
Machining a surface also produces macroscopic defects in the material surface.
Lathes produce grooves, due to the tool size, as do many other
procedures.
Some of
the defects can have a "high-aspect ratio"; that is, narrow, deep cuts in the surface
can be produced. Many of the grooves or machining marks can act as capillaries.
The surface area of irregular surfaces or powders can be determined using
models such as the BET (Brunauer-Emmett-Teller) isotherm [12]. This provides
a measure of the heat of adsorption and the volume adsorbed. This is discussed
more fully in the next section.
Fig.
5.
A small portion of
a
surface showing shelves and steps that have a profound effect on the total
surface area.
1.8.5 Surface Adsorption Isotherms
45
1.8.5
SURFACE ADSORPTION ISOTHERMS
Adsorption isotherms describe macroscopic effects of absorption of gases on a
solid surface, that
is,
the effect of relative pressure on an adsorbed surface volume.
Isotherms can be used to measure surface area as well as thermodynamic proper-
ties of the gas-surface interaction. Absorption isotherms include the effects of
surface roughness or porosity. In the following, condensation will be discussed
first and then the effects of the material on which condensation is occurring.
A metallic surface consists of an interfacial layer between the gas phase of the
residual gases in the vacuum system. Portions of the gas phase consist of conden-
sible gases such as water vapor. Water vapor is only one of the condensible mole-
cules in
air.
When water vapor condenses on the surface of the metal, from
a
macro-
scopic point of view, a thin layer of water is captured as part of the gas-metal
"interfacial" region. The water volume reaches an equilibrium state with the resid-
ual gas partial pressure of
water.
When the system is further pumped, the water on
the surface tends to return to the gaseous state. As the temperature is changed, the
relationship between the water vapor pressure and the amount of water on the sur-
face of the metal also changes.
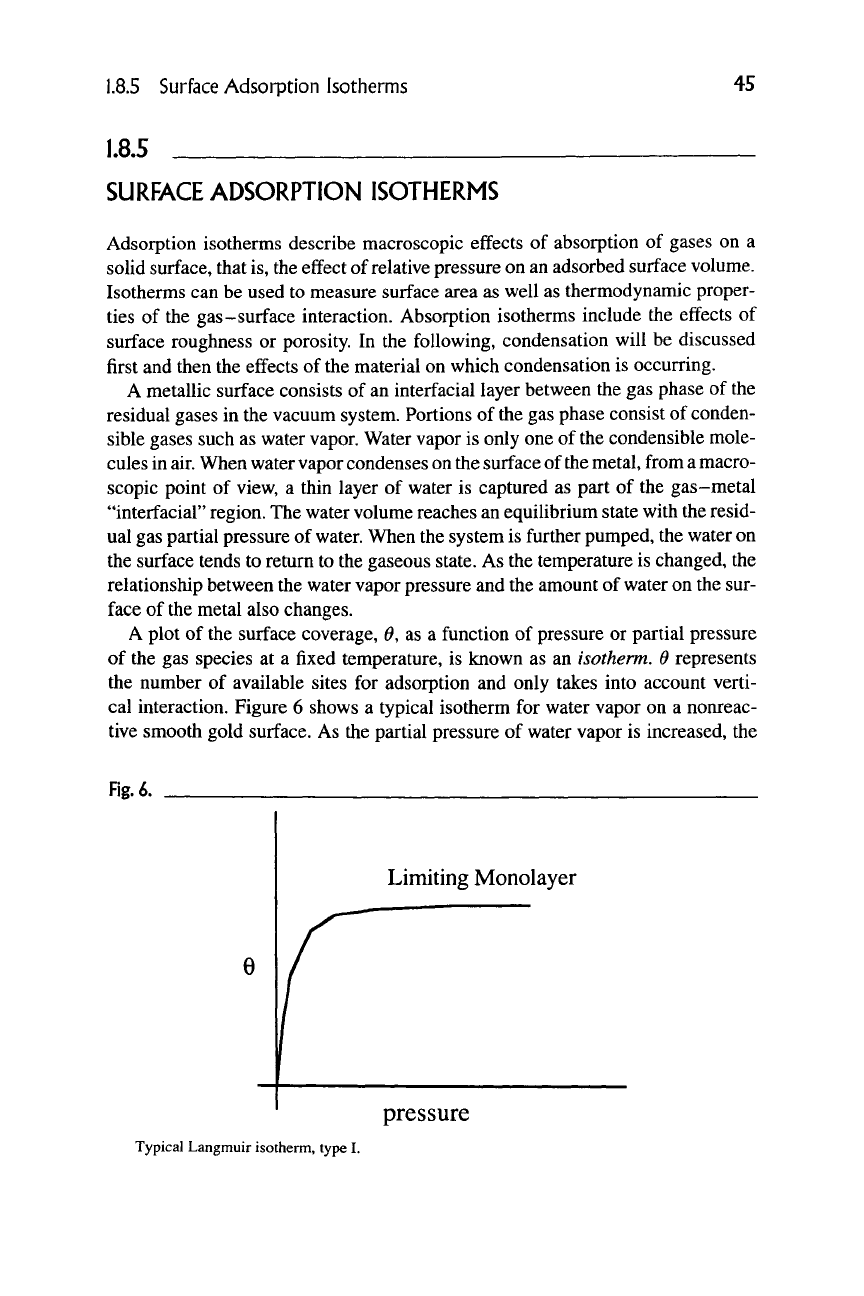
A plot of the surface coverage, 0, as a function of pressure or partial pressure
of the gas species at a fixed temperature, is known as an isotherm. 6 represents
the number of available sites for adsorption and only takes into account verti-
cal interaction. Figure 6 shows a typical isotherm for water vapor on a nonreac-
tive smooth gold surface. As the partial pressure of water vapor is increased, the
Fig.
6.
Limiting Monolayer
pressure
Typical Langmuir isotherm, type I.
46 Chapter 1.8: Surface Physics and Its Relation to
Vacuum
Science
surface coverage increases until a plateau level is reached. This plateau level is
known as a monolayer coverage of the surface and is descriptive of the Langmuir
isotherm [6]. This is rarely found in nature but is used in plotting experimental
data. Continuing to increase pressure eventually permits a second, third, etc. layer
to condense.
The Langmuir isotherm is represented mathematically by
e = bPI{\ + hP) (78)
where P is the pressure
6 is the fractional surface coverage
b is a constant = k\lk2 where k\ = (I/TQ) exp
(—Q/kT)
and
k2 = NaoKlTrmkT)
^'^
where T = temperature °K
Q = activation energy eV
k = Boltzmann constant
A^
= number of sites per unit area
(TQ
= site area
P = pressure (MKS units)
m = weight of a molecule
To = absorption time
More realistic models have been developed that include multilayers on a sur-
face.
The Brunauer-Emmett-Teller or BET isotherm is one of these more sophis-
ticated models [12]. This takes into account vertical atomic interactions but ne-
glects horizontal ones.
Mathematically, the BET isotherm is
P 1 1 C - I P
+ -^—^.— (79)
(Po-P) e ce^ ce^ p^
where
PQ
is the saturation or condensation pressure, 6 is the surface coverage, ^^
is the monolayer surface coverage, C ^ exp{Q/kT), and Q is the heat of adsorp-
tion. A typical BET plot is where
' ' (80)
plots linearly as a function of
P
(81)
From the slope and intercept, the heat of adsorption and 6^, the monolayer ca-
pacity in /tg/cm^ can be computed. With the heat of adsorption, going back to the

1.8.6 Capillary Action 47
first subsection of this section, it is now possible to compute the residence time
of molecules on a surface and is useful in obtaining a fundamental understand-
ing of the surface on which adsorption occurs. The reader is referred to the litera-
ture for a more detailed discussion of isotherms and their use in surface physics
[6-iai2].
1.8.6
CAPILLARY ACTION
Surface porosity can occur in many forms as shown in Figure 7. Exposure of the
pores to atmosphere or other gaseous environments may result in the condensa-
tion of some species in the mechanical defect structure of a surface. Under the
proper conditions, internal voids, pores, and surface sites can be filled with the
condensate through capillary action. Capillary action occurs when the condensate
wets the substrate. The equation of Young and Laplace predicts a pressure differ-
ential across the gas-to-liquid interface. Therefore, pores will tend to fill with
condensate as a result of the pressure differential. A good review of this theory is
presented in reference [6].
As pumping is performed to evacuate a system, at a fixed temperature, the pres-
sure in the vacuum vessel will lag the theoretical pumping capacity as a result of
trapped condensates, mainly water vapor, evolving from the various surface fea-
tures.
This is shown in Figure 8 on the decreasing pressure isotherm.
Figure 8 shows a isotherm where the volume of adsorption is plotted as a func-
tion of
pressure.
As the pressure is decreased, the curve maintains a high adsorbed
volume until, at a much lower pressure, the adsorbed volume drops. During in-
creasing the pressure, the adsorbed volume is lower than the adsorbed volume at
the same pressure. This hysteresis is indicative of the presence of pores in a sur-
face.
The pores during the decreasing pressure cycle tend to retain a higher ad-
sorbed volume of adsorbate than during the increasing pressure cycle. The differ-
ence can be used to compute the total pore volume.
Fig.
7.
\J
Some typical features that can act as pores on a material surface.
48
Chapter 1.8: Surface Physics and Its Relation to Vacuum Science
Fig.
8.
v/v,
m
Isotherm of
a
typical porous surface shows hysteresis between pumpdown and up to air show-
ing capillarity.
Pores can result from poor welds in metal vacuum systems and act as virtual
leaks.
This results in a hysteresis in the isotherm. Using the BET model for iso-
therms, total porosity can be determined from the area enclosed in the hysteresis
loop.
Ultimately, the total surface area, which is significantly larger than the geo-
metrical area of the surface, can be determined (see Parts 4 and 5 of this book).
In addition to pores at the surface, nonreactive gases can diffuse into the material
surface. This occurs with "Fickian" kinetics [13]. With decreasing pressure, the
gas will tend to diffuse out of the surface but lag the theoretical pumping curve.
In Part 4, the details of surface treatment and how it affects surface morphology
are considered in detail. Perhaps the largest source of gas in a vacuum system af-
ter the initial pumpdown to the region of molecular flow (10 "^ torr and below) is
from the system
itself.
Metal surfaces are not planar but consist of machine
marks, welds, and many other defects that may increase the effective surface
area of the system. If a surface profilometer is drawn across a metal surface
and a three-dimensional map made of the topography, the area of the sample is
1X1 mm^. It is clear that the actual area must be computed from the peaks and
valleys from machining marks, etc. This may increase the effective surface area
by a factor of more than 3. However, this is only a small change in the total sys-
tem volume. It is important, then, to discuss why this surface affects vacuum
system performance.
1.8.7
CONDENSATION
Gas interaction with a surface is a material, thermal, and morphological phenom-
enon. Condensation implies that the gaseous species develops a multilayer thick

1.8.8 Desorption Phenomenon
49
film that has undergone a phase transition from the gaseous state to either liquid
or solid state on a substrate [18]. A condensation coefficient can be associated
with this phenomenon. This is defined as the probability that a gas molecule will
stick to the condensate on a surface. Experimentally, condensation can readily oc-
cur on a substrate that is held at a temperature well below the boiling point of the
condensate, such as during the evaporative condensation of hot metal on a cold
substrate. The hot metal freezes on impact, forming a film. This phenomenon
forms the physical basis for thin-film deposition and will be discussed later in this
book. The condensed phase will increase in thickness if the vapor pressure of the
condensate is above an equilibrium pressure or evaporate if the condensate is be-
low this equilibrium pressure.
When the substrate temperature is above the boiling point of the condensate,
the sticking coefficient can be small. This is of importance in pumping vacuum
systems and reaching ultimate ultra-high-vacuum limits. Surface condensation of
gases on surfaces is also of importance in pumping where the pumps are dry pumps
based on surface adsorption, UHV pumps, and cryogenic pumps (see later dis-
cussions in this book).
Perhaps the phenomenon best known and studied in the vacuum technique field
is condensation of water vapor on surfaces as it relates to system evacuation. Wa-
ter condensation initially occurs by hydrating surface oxides on the system metal
components. After the surface is hydrated, water adsorption can be macroscopi-
cally defined and measured using the BET isotherm where
PQ
=
a.
pressure of
—20 torr (2.67 kPa). Many layers of water molecules can form a film on the sur-
face.
When this occurs, capillarity can causes water to fill pores on metal sur-
faces,
thereby loading the metal surface with water. Desorption from a saturated
system requires considerably longer pumpdown times than a system in which wa-
ter had not condensed and the metal surfaces are smooth. During pumpdown, the
system pressure may reach a plateau at
PQ.
This is due to the desorption of water
into the vapor state and subsequent pumpout of this vapor. After pumping the va-
por from the system volume from the saturated system walls, the remaining resid-
ual gases are pumped away. A detailed review of the effects of water vapor in vac-
uum systems is presented in reference [18].
1.8.8
DESORPTION PHENOMENON
The long-range bonds found in physical absorption can be overcome by adding
energy to the adsorbed molecule. The net effect may be to lower the total rate of
adsorption or to fully remove the adsorbed species from the surface. As in the
case of adsorption, desorption can be described in terms of rate kinetics and has
50
Chapter 1.8: Surface Physics and Its Relation to Vacuum Science
been measured for a number of material systems [17]. In practical terms, desorp-
tion from material surfaces may limit the pumpdown rate of a vacuum system and
limit its ultimate pressure.
1.8.9
THERMAL DESORPTION
If a molecule is physically adsorbed (if a chemical bond has not been formed be-
tween the surface and the absorbate), then the molecule maintains its gas-phase
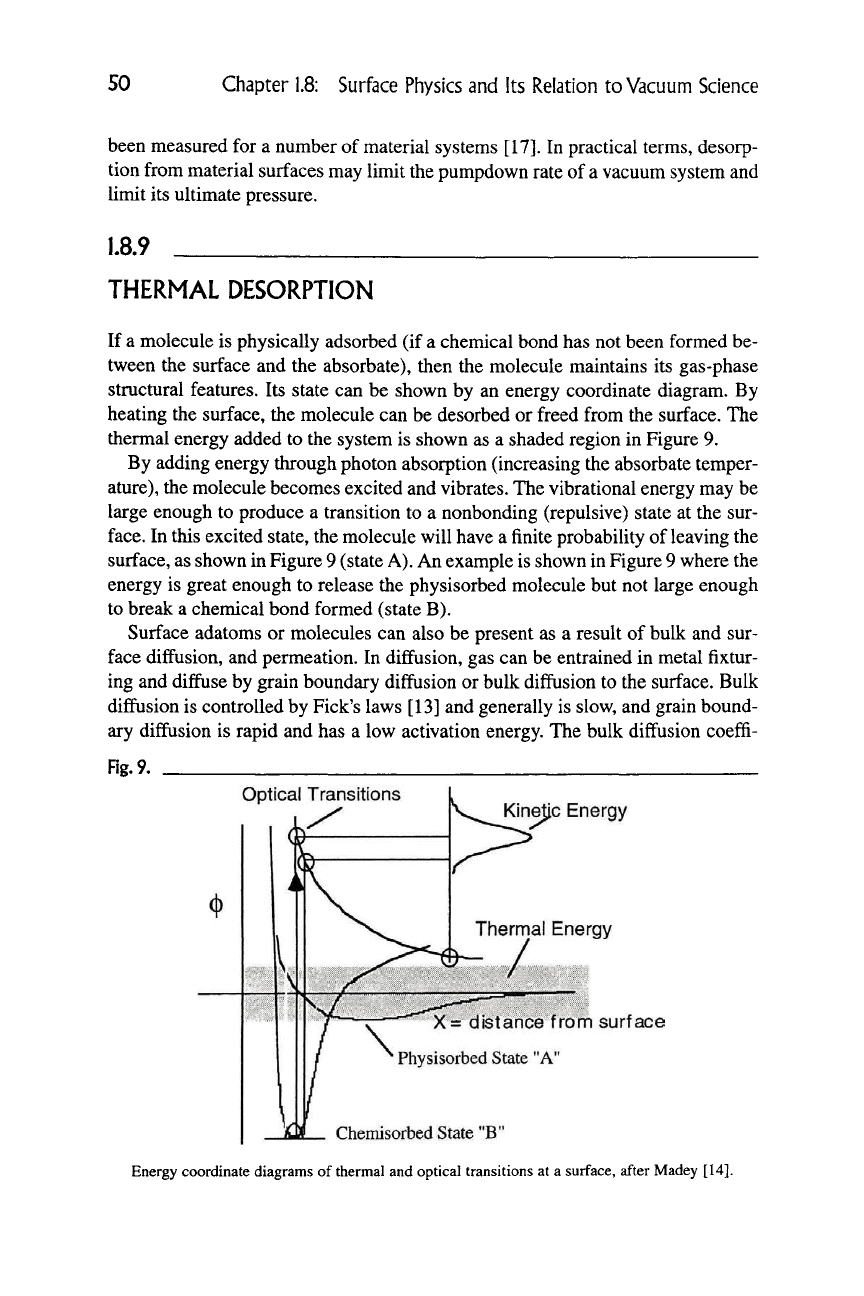
structural features. Its state can be shown by an energy coordinate diagram. By
heating the surface, the molecule can be desorbed or freed from the surface. The
thermal energy added to the system is shown as a shaded region in Figure 9.
By adding energy through photon absorption (increasing the absorbate temper-
ature),
the molecule becomes excited and vibrates. The vibrational energy may be
large enough to produce a transition to a nonbonding (repulsive) state at the sur-
face.
In this excited state, the molecule will have a finite probability of leaving the
surface, as shown in Figure 9 (state
A).
An example is shown in Figure 9 where the
energy is great enough to release the physisorbed molecule but not large enough
to break a chemical bond formed (state B).
Surface adatoms or molecules can also be present as a result of bulk and sur-
face diffusion, and permeation. In diffusion, gas can be entrained in metal fixtur-
ing and diffuse by grain boundary diffusion or bulk diffusion to the surface. Bulk
diffusion is controlled by Fick's laws [13] and generally is slow, and grain bound-
ary diffusion is rapid and has a low activation energy. The bulk diffusion coeffi-
Rg.9.
Optical Transitions
Kinejic Energy
Thermal Energy
X= distance from surface
Physisorbed State "A"
Chemisorbed State "B"
Energy coordinate diagrams of thermal and optical transitions at a surface, after Madey [14].
1.8.9 Thermal Desorption
51
cient, Z), is typically in the range of
-lO"^"^
-
10 ~^^
cm^/sec. From Pick's first
law, the concentration C ^ erfc (4 X Dt)
"^-^
where D = DoOxp(Eo/kTX that is,
D is thermally activated with an activation energy ofEo, where t is time, D is the
diffusion coefficient, k is the Boltzmann constant, and x is distance. From this,
estimates of
the
time required to form a monolayer on a surface can be obtained. If
the species diffusing from the bulk is volatile — that is, has a high vapor pres-
sure—
then this species will contribute to the pump's gas load.
O'Hanlon describes permeation as gas diffusion from the outer wall to the in-
ner wall of a vacuum system. This process is slow and occurs at a steady rate that
is a function of temperature. This process is similar to and is described by Fick's
laws [13].
Thermally stimulated desorption can be used as a measure of the desorption
energy assuming that the process is a thermally activated process, where the de-
sorption rate is /?
oc
expi-E^/kT) and E^ is the activation energy due to desorp-
tion. The energy levels of the desorbed species are an indication of the physical
binding energy of
the
species to the surface. Experiments have been performed by
measuring the pressure in a sealed vessel or a vessel pumped at a constant
rate.
In
the later case p
°^
Po exp {—E^/kT), where p is the pressure measured with a
gauge. This can be analyzed in terms of the residence time, r. Details of this the-
ory have been published by King [16].
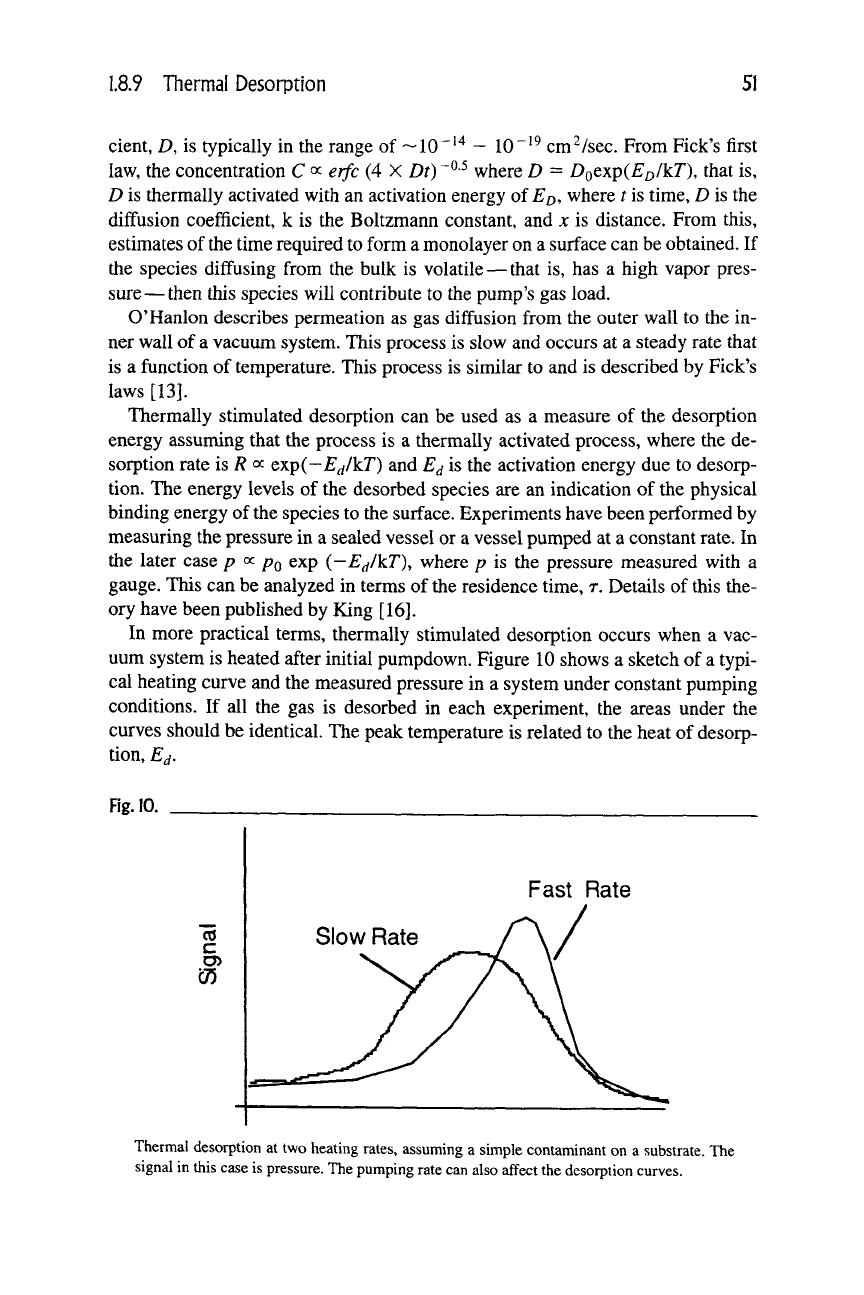
In more practical terms, thermally stimulated desorption occurs when a vac-
uum system is heated after initial pumpdown. Figure 10 shows a sketch of a typi-
cal heating curve and the measured pressure in a system under constant pumping
conditions. If all the gas is desorbed in each experiment, the areas under the
curves should be identical. The peak temperature is related to the heat of desorp-
tion, E^,
Rg.lO.
CO
c
O)
(75
Fast Rate
Slow Rate
Thermal desorption at two heating rates, assuming a simple contaminant on a substrate. The
signal in this case is pressure. The pumping rate can also affect the desorption curves.
52
Chapter 1.8: Surface Physics and
Its
Relation to Vacuum Science
Thermal desorption is common used to clean vacuum systems and can be ob-
served when thermally degassing a system prior to operation. Near room temper-
ature the thermal desorption rate is inversely proportional to time, t~^ [1]. This
phenomenon has been extensively studied and is found to be very important in the
application of vacuum technology.
1.8.10
PHOTOACTIVATION
When an atom is physically or chemically adsorbed on a surface, it can be re-
leased by the addition of energy through photon absorption. Referring to Figure 11,
a vertical photon absorption process excites the atom without the addition of heat.
Rg.n.
lE+01
lE-10-
PUMPDOWN
VOLUME
TIME (sec)
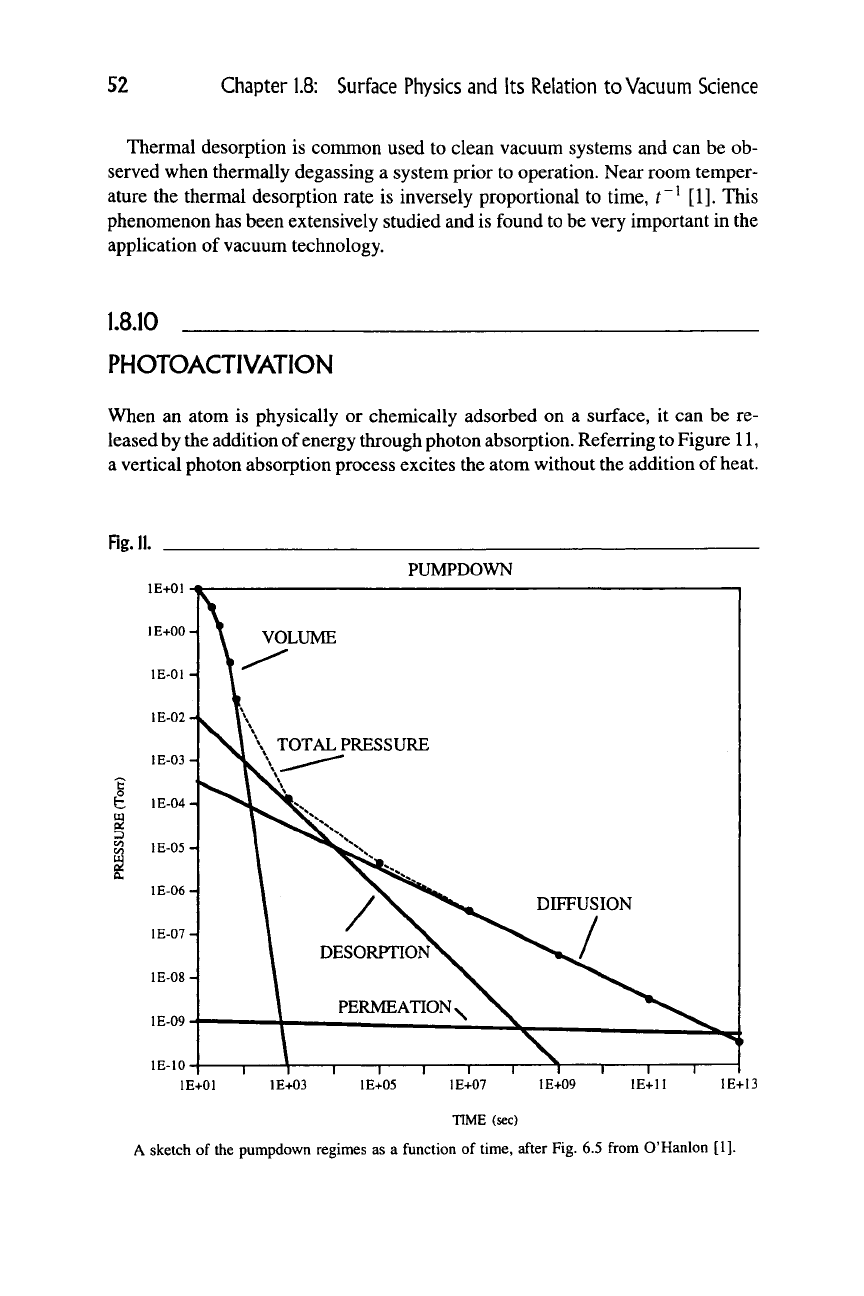
A sketch of the pumpdown regimes as a function of time, after Fig. 6.5 from O'Hanlon [1].
1.8.12
Electron- and Ion-Stimulated Desorption 53
If the energy is large enough, the absorbate will be excited and an ion, excited
atom, or neutral atom will produced. The physical phenomenon is described in
terms of a model that predicts the formation of a repulsive state formation. The
repulsive electronic state converts to motion, kinetic energy, and the molecule or
atom leaves the surface as shown in Figure 11 and is free to move into the vacuum.
Photon-stimulated desorption (PSD) has become a surface science method for
investigating the effect of photons on surfaces. Energy threshold measurements
provide direct information relating to the bond-breaking process. Madey [15] de-
scribes in his review article that the threshold energy for neutral desorption can
be as low as 5 eV (deep UV). At higher energies (—15 eW), one expects ion de-
sorption to occur [19].
.8.11
ULTRASONIC DESORPTION
Ultrasound-Stimulated desorption is for practical purposes used to clean metal
vacuum systems while under vacuum. The ultrasonic power is coupled to the sys-
tem through impedance matching barium titanate crystals to the metal and thick-
ness of the structure. This method of causing desorption is based on energy ab-
sorbtion by the absorbate causing localized heating, thereby releasing molecules
through the addition of what is believed to be thermal energy. Water vapor is the
most conmion species to be desorbed from metal surfaces using this method.
1.8.12
ELECTRON-AND ION-STIMULATED DESORPTION
This phenomenon has also been developed into a surface analytical technique
known as electron-stimulated desorption (BSD) where specific species and/or ions
are studied by low-energy electron diffraction as a surface-sensitive tool or by di-
rectly detecting the desorbed species leaving the surface using, for example, mass
spectrometry. Low-energy electrons (<500
eW)
are used to bombard monolayer-
coated ultra-clean surfaces. Both chemisorbed and physisorbed molecules can be
released from the surface as ions or neutrals. This method is sensitive to the bond
strength and surface structure on an atomic scale. The cross section for the for-
mation of neutrals and ions by electron bombardment is in some cases rather
large (10 "^ molecules/incident electron), consequently the signal expected from
the surface can be large.
