Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.

34 Chapter 1.6: Conductance
But
APT
= APi + AP2 (55)
Furthermore, the mass flow rate must be same throughout the system, other-
wise gas would accumulate in the system. Hence,
Gi
= Q2 = QT (56)
Thus
^ = ^ + ^ (57)
Cx Ci C2
QT _ QT_ QT^
Cj Ci C2
Hence, for conductances in series,
1111 (58)
7;-
= TT + TT + TT etc.
Cj Ci C2 C3
For a pump of speed 5 Ls
~ ^
connected to a chamber by means of a component
of conductance C Ls ~\ the effective speed at the chamber, 5e, is given by
-L
=
i.i (59)
S^ S C
CHAPTER
1.7
Flow Calculations
The conductance of a duct is a measure of its ability to transport gas and is ex-
pressed in units of volume transported per unit time. The quantitative expressions
used to calculate conductance of an element under different circumstances are
fairly complex and depend on the type of flow as well as on geometrical and
surface-related factors. Calculations of conductance and the corresponding gas
flow rate for turbulent flow are difficult to treat analytically. Viscous flow is also
somewhat difficult to treat quantitatively, because it depends not only on the
shape of
the
duct but also on the gas pressure. Fortunately, at most pressures of in-
terest to high-vacuum situations, the flow is molecular rather than viscous. Con-
siderable effort has been expended for developing analytical techniques for deter-
mining flow under viscous and molecular regimes.
1.7.1
EQUATIONS
FOR
VISCOUS FLOW
Generally, quantitative expressions that have been developed for calculating con-
ductance and corresponding flow rate under viscous flow conditions are those for
fairly simple geometrical configurations, such as circular tubes or rectangular
ducts.
These expressions are used, for example, to calculate the time required to
evacuate a vessel or volume of some sort, through a pipe that is usually circular or
rectangular in cross section.
1.7.1.1
Circular Tube
The mass flow rate through a straight tube of circular cross section under viscous
flow conditions, is determined by Poiseuille's equation, namely,
ISBN 0-12-325065-7 Copyright © 1998 by Academic Press
•^^ ^^ Ail rights of reproduction in any form reserved.
35
36 Chapter 1.7: Flow Calculations
=
K-— (60)
Pi
- Pi VL
where
d
is the diameter
of
the tube
L
is the
length
of
the tube
7]
is
the viscosity
of
the
gas
P
is
the average
of
Pj and
Pj,
the pressure
at
the opposite ends
of the tube
For dry
air at
20°C, this equation becomes
750
J^P
Q
= 1
iPi-Pi)
(61)
where
Q is the
mass flow rate
in
torr-L/sec,
d is the
tube diameter
in
inches,
L is
the tube length
in
centimeters, and
P is
the pressure
in
torn
The expression
for
conductance
for a
circular pipe
for air at
20°C
is
2.94Prf^
C
= ;
L/sec
(62)
17.12 Rectangular Duct
For
the
rectangular duct,
let a =
long side
and b =
short side.
The
Poiseuille
equation
for
the rectangular duct
for air is at
20°C
is
30a2b2KP
C
=
L/sec
(63)
JLi
where
^
is
a
shape factor whose value depends
on b/a. As can be
seen,
the
con-
ductance
of the
rectangular slit increases rapidly
as the
cross section changes
from slit to square.
As in the case
of
round pipe, the expression
for
C leads
to a
relation
for
the vol-
ume flow
in
terms
of
the pressure drop along the duct.
P^
/^ A\
AP
where
Thus
CP
F
= -^ (64)
AP
30a^b^K
^
K
= AP
L/sec
(65)

1.7.3 Knudsen's Formulation
37
1.7.2
EQUATIONS FOR MOLECULAR FLOW
At low pressures, intermolecular collisions are less frequent than wall collisions,
so the latter determine the gaseous flow characteristics through the channel. Spe-
cifically, two aspects determine the conductance of a duct during molecular flow:
1.
Rate at which molecules enter the duct
2.
Probability that the molecules are transited through the system
The first depends on the entrance area of the system, while the latter is deter-
mined by the subsequent series of reflections from the walls, which result in the
molecule eventually being transmitted through the duct or reflected back into
original volume.
Consider first the case of very thin aperture plate, for which the area A is more
important in determining conductance than the wall area or wall conditions.
The volume of gas traveling from one side of the aperture to the other side per
unit
time
—
the
aperture conductance — is
Q = \AV,, (66)
when the molecules have a Maxwell-Boltzmann velocity distribution. Conduc-
tance values depend on the molecular mass and kinetic temperature. The case
where wall collisions are more important than the conductance of an aperture is
considered next.
173
KNUDSEN'S FORMULATION
The conductance Cj of a length of long tube of length L with uniform cross-
sectional area A and perimeter //, was calculated by Knudsen to be:
C. = |^v„ (67,
The assumptions for obtaining the general result are
1.
Length of tube is much greater than the diameter.
2.
Direction of reflected molecules is independent of the incident direction.
3.
Reflected molecules are distributed equally per unit angle (cosine law for re-
flection from a Lambertian surface).

38 Chapter 1.7: Flow Calculations
Relationships derived from the general equation are given in Table 5 for simple
geometries. Assumption
1
indicates that the effect of aperture is insignificant, and
the conductance value is given by the preceding equation is for molecules that are
well within the tube and are sufficiently removed from the aperture so that it is of
no consequence. A rough attempt to correct this deficiency is to include a series
conductance of the entrance aperture. Weissler and Carlson [5] gives a formula
for a tube of perimeter //, area
A,
and length L:
-(-^•^r^.
(68)
1.7.4
CLAUSING FACTORS
The conductance of a long tube is approximately related to the conductance of the
entrance aperture through the factor [1 + (3/16)(L//M)]"' C^. This factor can be
interpreted as the probability that a molecule incident on the aperature will be
transmitted through the tube and leave at the other end.
It is convenient to discuss conductance in terms of the aperture conductance
and corresponding probability of passage, ^1^2'—^he Clausing factor—so that
C=C,' Pi^2 = (l/4)VavAiPi^2 (69)
Because conductance is independent of direction,
A,P,^2 = A2P2^, (70)
Examples: The throughput in pressure-volume units per unit time (torr
•
cm^ •
s~^) through a long tube is given approximately by
e=i^y^'^
- ^^) ^''^
where d is the tube diameter in cm
L is the tube length in cm
/la is the average velocity of a molecule in cm s~^
Pi and
P2
are the pressures (in torr) at the opposite ends of the tube

1.7.4 Clausing Factors 39
Approximate values for some probabilities of passage are accurate to within
±10%.
A variety of techniques, which include analytical methods [6], Monte
Carlo calculations, and variation methods [16]. Carlson lists different geometries
that have been investigated and cites the corresponding references. Numerical ex-
amples can be found in Carlson [22].
CHAPTER 1.8
Surface Physics and Its
Relation to Vacuum Science
The interaction of gas molecules with a solid surface is the basis for vacuum tech-
nology
[6-10].
Vacuum pumps and pumping, gas transport through a physical
system, system pumpdown, and material behavior under rarefied gas conditions
all involve the interaction of gas molecules with a solid or liquid surface. The sur-
face of a material is generally considered the topmost atomic layer. This may be
the terminating layer of a single crystal material or a more complex structure of
another material. At atmospheric pressure, gas-solid interfacial interactions are
characterized by chemical reaction kinetics, wettability through condensation,
diffusion, and other well-known mechanisms. As the pressure is decreased, the
number of molecules available to interact with a surface is decreased until pres-
sures are so low that the number of molecules colliding with a surface is best de-
scribed by statistics. This is where the conventional gas laws break down. Some
of the phenomena mentioned earlier are surveyed in this section.
1.8.1
PHYSICAL ADSORPTION OR "ADSORPTION"
When the surface of a material is exposed to an ultra-high-vacuum ambient, gas
molecules tend to "stick" to clean surfaces. Equilibrium is rapid, and the phenom-
enon is entirely reversible. When a molecule reacts at the surface to form a strong
bond, this is known as chemisorption and is discussed briefly in this section. For
a given pressure and temperature, the kinetic theory of
gases,
assuming Brownian
ISBN 0-12-325065-7 Copyright © 1998 by Academic Press
$25.00 All rights of reproduction in any form reserved.
40

1.8.1
Physical
Adsorption or
"Adsorption"
41
motion of the molecules, can be used to compute the number of molecules hitting
the surface. More simply, from PV (unit volume) = MRT, the basic gas law, the
flux striking a unit area of a surface is defined as
F = N^^PilTrMRT)-^^^ (#/cm2/s) (73)
where
A^av
is Avogadro's number, M is the molecular weight, P is pressure in torr,
ris the temperature in Kelvin, and R is the universal gas constant. This can be re-
duced to
F = 3.51 X 10^2 P(torr) [M(g/mole) TCK)]'^^^ (74)
The flux of gas molecules, F, that come in contact with a surface is roughly
0.5 X 10^2 P (torr) for air. At 10"^ torr, F « 10^^ molecules/cm^ sec impacting
a surface.
When a gas molecule hits a surface and sticks, it is said to be adsorbed if it
does not return to the gaseous state; that
is,
the molecule is trapped on the surface.
The surface can be viewed classically, as a number of "billiard ball-type mole-
cules"
on the surface (Figure 3).
For this to occur, a long-range force must be acting on gas molecules, causing
them to group together on the surface through a mutual attractive force called the
van der Waals force. These forces, known as dispersion forces, are electrical in
nature
[7].
In this case, atoms tend to group together through the mutual attractive
force (the van der Waals force, /) and was shown by de Boer to have a depen-
dence:/oc l/j3, where d is the distance between interacting molecules and a sur-
face.
This comes about through the interaction of distributed charges and dipole
moment interaction. The force is always attractive and is described by Adamson
in some detail [6].
Due to thermal vibration, adsorbed molecules will reside on the surface for a
finite time depending on the energy of the thermal oscillation (temperature). This
Fig.
3.
available surface site(s)
for adsorption
QSl
i
t
^: '.• ^: ^: ^: ^: ^: ^: '-• ^: ^: ^: ^: ••-• ^: ^: ^: ^: ^: ^: ^: ^: ^: ^: ^: ^: ^: ^: <^:
u ^ • ^ • ^ • ^ • ^ • ^.
1^
• ^. ^ • ^. ^ • ^ • ji - ,r • j» • ^ • ^ • •• • ^ • ^ •
J"
•
d"
•
J"
• w • ^ • iT • ^ • ^ • ^j
A billiard ball diagram of molecules adsorbing on a
flat
surface.

42 Chapter 1.8: Surface Physics and
Its
Relation
to
Vacuum Science
is known as the heat of adsorption,
H^^^.
The residence time, r, of a molecule is
where
T = Toe^^^^^/^^ (75)
To
is typically in the range of 10"^^ to
10 ~^^
sec. The fractional surface occupa-
tion of the molecules is 6:
^(number/cm^) ^ FT
=
A^av
PilTTMRT)
-1/2
(molecules/cm2/sec)Toe
^"^'^'^'^
(sec) (76)
This assumes that all the molecules that hit an available site stick to it.
Multilayer physical adsorption occurs in many material systems. It was shown
to exist at cryogenic temperatures by Gomer and his co-workers [10]. Visual evi-
dence was observed using the field ion microscope. Molecules absorbed at very
low temperatures (<70°K) were mobile and could laterally diffuse. A surface
dif-
fusion coefficient is defined from the motion of the "boundary" and given by
Ds = a2^exp(-E/kT) (77)
where a = jump length (~3A), v is the jump frequency (~
10
^^/sec),
and E is the
activation energy for surface diffusion.
1.8.2
CHEMISORPTION
Physisorption phenomena are reversible phenomena; that is, with the addition of
some stimulus such as thermal energy, the gas species or molecule will desorb
from the surface. Using Somorjai's notation [7], this can be written chemically as
A ^ A* where A* is a nondissociated adsorbed molecule. When chemisorption
occurs, A* undergoes a one-directional reaction to form a new species on the sur-
face.
This requires a specific energy, and has a specific rate constant associated
with the process. When the bonding energy between the absorbed species and the
surface is large, the molecule or atom is considered to be chemisorbed to the sur-
face.
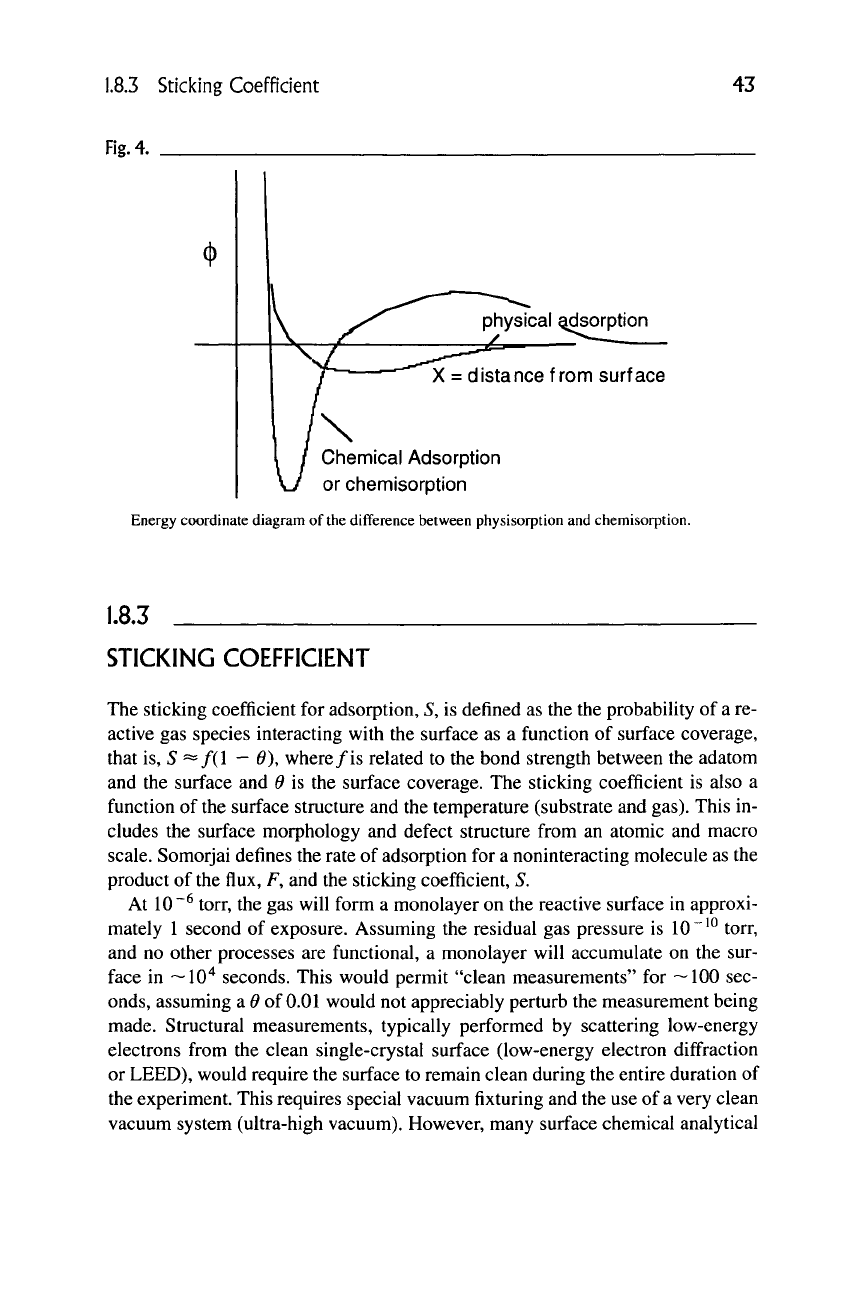
Isotherms are generally not as important in characterizing the surface, be-
cause only the first monolayer absorption is important. Figure 4 shows a typical
energy diagram as a function of the distance from a surface. Molecules or atoms
physically adsorbed are in a shallow potential well, as shown. Chemically ad-
sorbed molecules reside in the deeper potential well. To break this bond, a signifi-
cant amount of excess energy is needed to raise the molecule to an energy state
greater than the barrier energy to free it from the surface.
1.8.3 Sticking Coefficient
43
Fig.
4.
X = distance from surface
\
Chemical Adsorption
or chemisorption
Energy coordinate diagram of
the
difference between physisorption and chemisorption.
1.8.3
STICKING COEFFICIENT
The sticking coefficient for adsorption, 5, is defined as the the probability of a re-
active gas species interacting with the surface as a function of surface coverage,
that is, 5 «/(l
—
9), where/is related to the bond strength between the adatom
and the surface and 6 is the surface coverage. The sticking coefficient is also a
function of the surface structure and the temperature (substrate and gas). This in-
cludes the surface morphology and defect structure from an atomic and macro
scale. Somorjai defines the rate of adsorption for a noninteracting molecule as the
product of the flux, F, and the sticking coefficient, S.
At
10 ~^
torr, the gas will form a monolayer on the reactive surface in approxi-
mately 1 second of exposure. Assuming the residual gas pressure is
10 "^^
torr,
and no other processes are functional, a monolayer will accumulate on the sur-
face in
-^10"*
seconds. This would permit "clean measurements" for ~100 sec-
onds,
assuming a 0 of
0.01
would not appreciably perturb the measurement being
made. Structural measurements, typically performed by scattering low-energy
electrons from the clean single-crystal surface (low-energy electron diffraction
or LEED), would require the surface to remain clean during the entire duration of
the experiment. This requires special vacuum fixturing and the use of a very clean
vacuum system (ultra-high vacuum). However, many surface chemical analytical
