Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.


340 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
other condensables, but is not sufficient to remove all the water because surface
long range forces—see Figure 2(b). The predominant peak is now hydrogen.
Some small peaks like CI {mie = 35, 37), CH4 (parent peak mie = 16) and CO
{m/e = 28) are also present. A high-temperature bakeout (T > 400°C) of a stain-
less steel system for four days is sufficient to desorb almost all the water and it
is no longer significant at room temperature —
see
Figure 2(c). As long as one is
using hot filaments for ion gauges, ion sources in mass spectrometers or for other
instrumentation, the carbon and oxygen-bearing peaks will be clearly apparent.
Hot filaments also serve as heating sources that keep the surrounding stainless
steel envelope hot (~200°C for a
1.5-inch
I.D. [inner diameter] tube) so the out-
gassing peaks will be significant as well. Systems that have elastomer seals will,
in fact, have an additional concern in that the 0-rings are permeable to helium,
hydrogen, carbon dioxide, and other gases that are not permeated by all-metal
seal systems
[7,8].
Finally, some systems will have or acquire a leak from faulty materials, welds,
seals,
bakeouts, or some inadvertent blow to a system component like an electri-
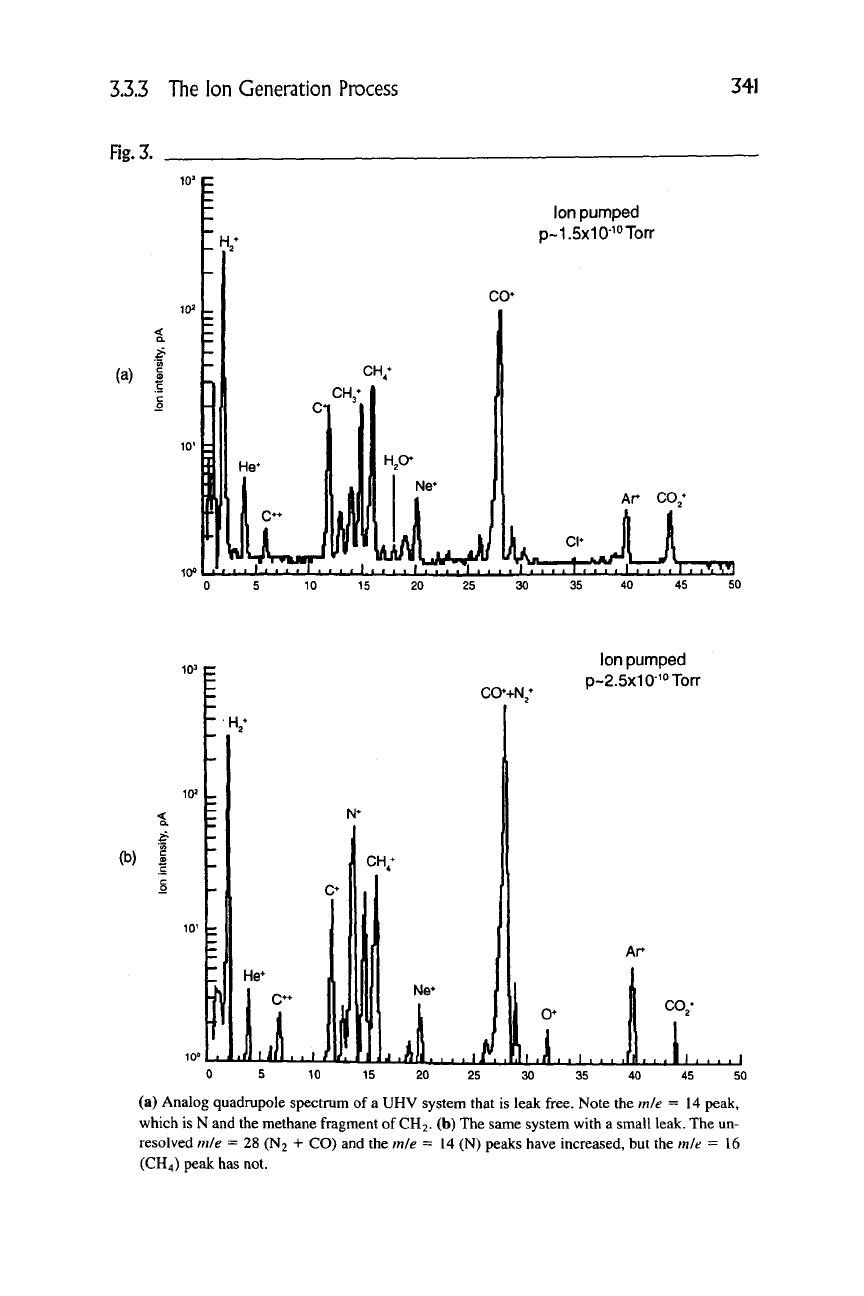
cal feedthrough or a flange seal. Figure 3(a) shows a UHV system that has a resid-
ual pressure of 1.5 X
10 ~^^
torr that is leak free, and Figure 3(b) shows this same
system with a small leak that increased the pressure to 2.5 X
10 ~ ^^
torr. Note that
the peak at m/e
—
14 is much higher than that observed in Figure 3(a). Normally,
when there is no leak, no admission of nitrogen into the system, and the applica-
tion does not generate nitrogen, then no contributions to mie = 28 or mie =14
peaks should occur (CO"^"^ contributes slightly to mie =14)! When there is no
leak, there is only the residual CH2 at mie = 14 due to the fragmentation of
methane, which is only 15% of the parent peak at mie =16. Thus, for a small
residual methane peak, the CH2 is, in general, quite small. However, when there
is a leak, the atomic nitrogen fragmentation peak at mie =14 becomes quite sig-
nificant and is usually larger than the 16 series peaks due to methane. One cannot
separate the N2 and CO2 signals at mie = 28 with most vacuum system mass
spectrometers so the existence of the nitrogen is not immediately clear. The frag-
mentation to atomic nitrogen is, however, tell-tale and serves as an excellent way
of quickly examining the leak-tight integrity of the system.
3.3.3
THE ION GENERATION PROCESS
The generation of ions from solids, liquids, and gases for mass spectral analysis
can proceed by a variety of different methods. For example, Knudsen cells raise
the temperature of the solids and liquids to create a vapor pressure sufficiently
high for electron impact ionization of the gas phase; laser or a spark sources di-
3.3.3 The Ion Generation Process
341
Rg.l
10^
(a)
^
s
10°
••^2*
He*
OH;
CHJ
H,a
Ion pumped
p-1.5x10-^0
Torr
CO*
0 5 10 15 20 25 30
Ar CO;
iT7T/r "t.i.¥|
40 45 50
10^
P
r HJ
10*
(b) I
P
He*
I ifI
.
. t /
Ne*
CO*+N*
Ion pumped
p~2.5x10-^°Torr
Ar
CO;
20 25
111
I III I I I I 1 I ilH t • f I I 1
30 35 40 45
J
(a) Analog quadrupole spectrum of
a
UHV system that is leak free. Note the m/e = 14 peak,
which is N and the methane fragment of
CH2.
(b) The same system with a small leak. The un-
resolved m/e = 28 (N2 + CO) and the m/e = 14 (N) peaks have increased, but the m/e = 16
(CH4) peak has not.

342 Chapter 3.3: Practical Aspects of Vacuurn System Mass Spectrometers
rectly generate ions by localized vaporization at the point of photon/electron and
solid impact; glow discharges create ions that bombard the surface and sputter
the material of interest into the gas phase. However, for almost all gas-phase
measurements in vacuum systems, the ions are created by electron impact from
electrons that are generated by thermionic emission. The myriad of different
filament emitters has been addressed in the preceding section (see Chapter 3.2,
Lieszkovszky).
3.3.3.1 Ionization Efficiency
The efficiency of ionization is a strict function of the ion source design. An axial
type of ion source (similar to an ionization gauge) is shown in Figure 4(a). The
axial design is similar to a total pressure ionization gauge with the exception there
is no axial collector and the ions are extracted through an aperture plate at the
base of the grid. The electrons are accelerated toward the grid and normal to the
axis of the grid where they make an orbital trajectory that passes through the grid
about five times on the average--see Figure 4(b). The longer the path length, the
higher the probability of an ionization event and therefore the higher the sensitiv-
ity. The yield of ions (J0) exiting the aperture to the flux of atoms and molecules
(Ji) entering the ion source can be roughly estimated from,
Jo SkBT
-- = (1)
Ji Aevi
where S is the ion source sensitivity, k B is Boltzmann's constant, T is the gas tem-
perature, A is the extraction aperture, e is the electronic charge and vi is the mo-
lecular velocity [9]. As an example, the number of ions formed per molecule for
molecular nitrogen at room temperature and the ion source sensitivity for the
source configuration in Figure 4 (S --~ 1 X 10 -4 amperes torr- 1 ), is approximately
4 X 10 -6. Or said another way, one ion is formed for approximately 4 X 105 mol-
ecules that move through the ion source. Figure 5 shows the probability of ioniza-
tion for positive ions of several gases as a function of electron energy. Negative
ions are also formed, but in far less abundance. The probability of ionization for
all the gases is a maximum between 50 and 200 eV and the usually followed cus-
tom is to operate ion sources at 70 eV. The ions that are formed within the grid
shown in Figure 4 experience a negative extraction potential leaking through the
aperture (along the axis), which causes the ions to accelerate through the aperture
into the analyzer with typically about 10-30 eV kinetic energy (depending, of
course, on the applied potentials). The angle of entry into the mass analyzers is, of
course, critical to the resulting resolution and is a function of the type of analyzer.
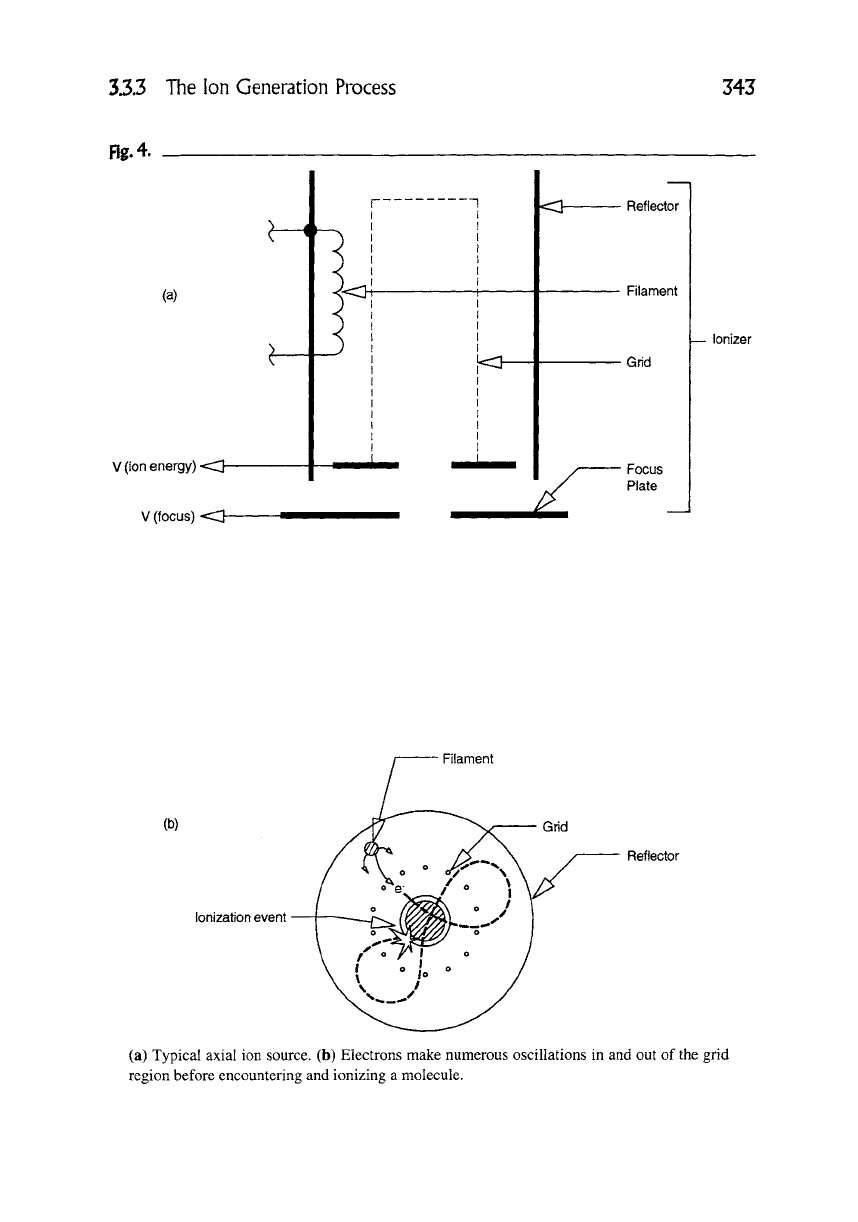
Fig. 4.
343
(a)
(b)
V (ion energy) "=::S]
V (focus) <:S]
3.3.3 The lon Generation Process
m
Reflector
Filament
Grid
Focus
Plate
--Ionizer
,~ent
Ionization event
Reflector
(a) Typical axial ion source. (b) Electrons make numerous oscillations in and out of the grid
region before encountering and ionizing a molecule.
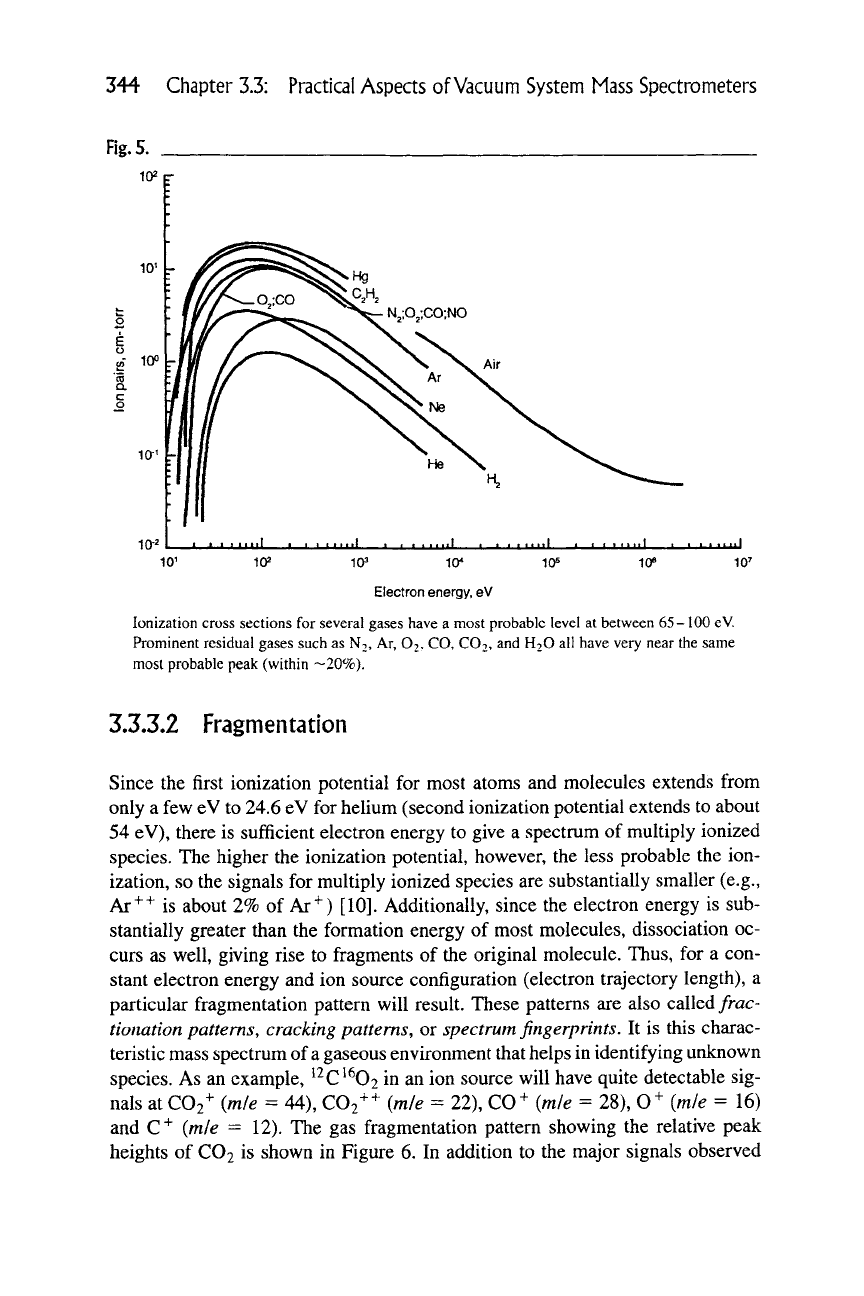
344 Chapter 3.3: Practical Aspects of
Vacuum
System Mass Spectrometers
Fig.
5.
102 r
Electron energy, eV
Ionization cross sections for several gases have a most probable level at between 65-100 eV.
Prominent residual gases such as
NT,
Ar, O2, CO, CO2, and H2O all have very near the same
most probable peak (within —20%).
3.3.3.2 Fragmentation
Since the first ionization potential for most atoms and molecules extends from
only a few eV to 24.6 eV for helium (second ionization potential extends to about
54 eV), there is sufficient electron energy to give a spectrum of multiply ionized
species. The higher the ionization potential, however, the less probable the ion-
ization, so the signals for multiply ionized species are substantially smaller (e.g.,
Ar"^"^ is about 2% of Ar"*") [10]. Additionally, since the electron energy is sub-
stantially greater than the formation energy of most molecules, dissociation oc-
curs as well, giving rise to fragments of the original molecule. Thus, for a con-
stant electron energy and ion source configuration (electron trajectory length), a
particular fragmentation pattern will result. These patterns are also called frac-
tionation patterns, cracking patterns, or spectrum fingerprints. It is this charac-
teristic mass spectrum of a gaseous environment that helps in identifying unknown
species. As an example,
^^C ^^Oi
in an ion source will have quite detectable sig-
nals at CO2'' {mie = 44), CO2'-' {mie = 22), CO"^ {mie = 28), O^ {m/e = 16)
and C"^ {m/e = 12). The gas fragmentation pattern showing the relative peak
heights of CO2 is shown in Figure 6. In addition to the major signals observed
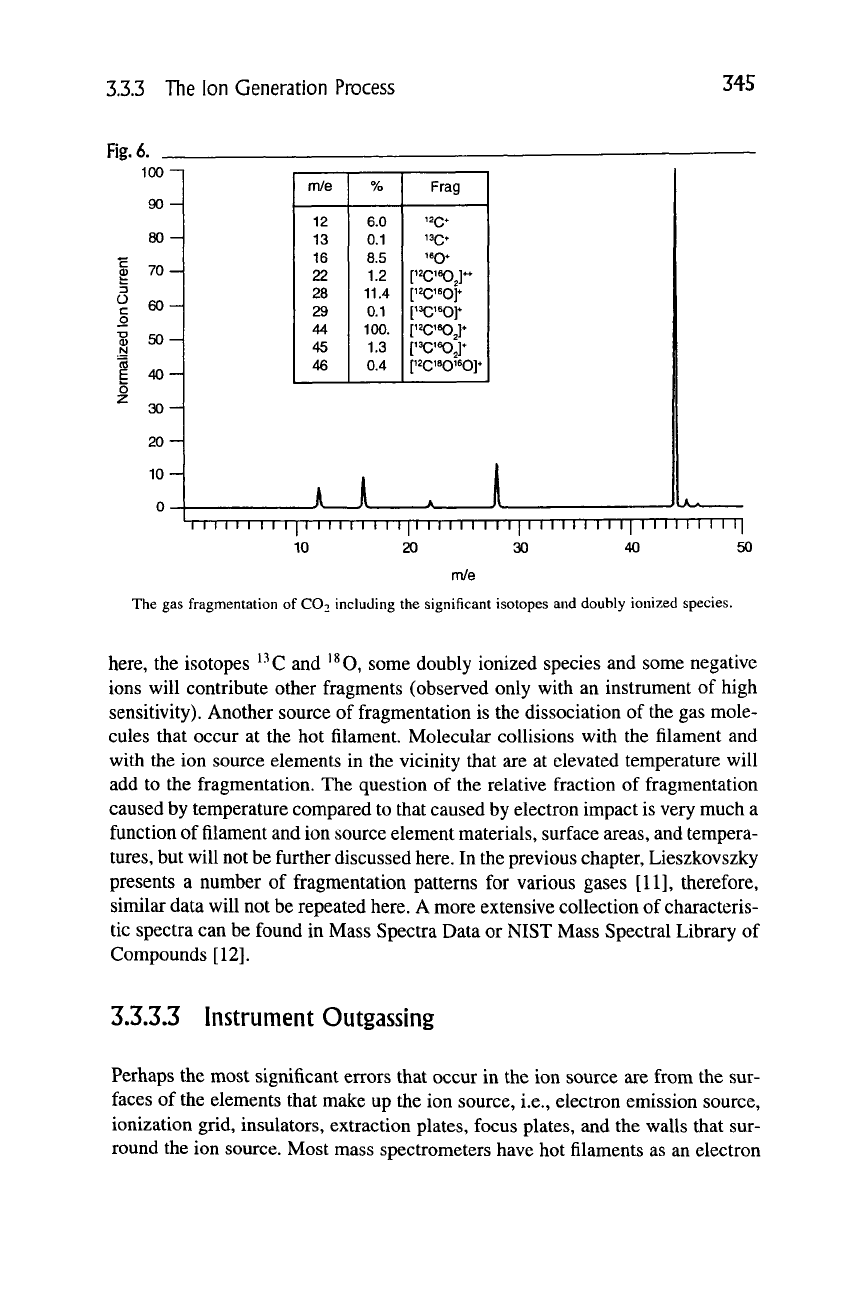
3.3.3 The Ion Generation Process
345
Fig.
6. _
100-
90
80
H
70-
o
c
o
B
.y
CO
£
o
z
60
50
40
30
20
10
0
m/e
12
13
16
22
28
29
44
45
46
%
6.0
0.1
8.5
1.2
11.4
0.1
100.
1.3
0.4
Frag
^20*
^^0*
160*
[12C160J-
['^'^oy
['^'^OY
P^C'SOJ*
r^^^j*
pcisQieo]*
JLJ.
I M I I I I I I I I I I I I I I I I I I I I I I I I I I M I I I I I I I I I I I I l|
10 20 30 40 50
m/e
The gas fragmentation of CO2 including the significant isotopes and doubly ionized species.
here,
the isotopes ^-^C and ^^O, some doubly ionized species and some negative
ions will contribute other fragments (observed only with an instrument of high
sensitivity). Another source of fragmentation is the dissociation of the gas mole-
cules that occur at the hot filament. Molecular collisions with the filament and
with the ion source elements in the vicinity that are at elevated temperature will
add to the fragmentation. The question of the relative fraction of fragmentation
caused by temperature compared to that caused by electron impact is very much a
function of filament and ion source element materials, surface areas, and tempera-
tures,
but will not be further discussed
here.
In the previous chapter, Lieszkovszky
presents a number of fragmentation patterns for various gases [11], therefore,
similar data will not be repeated here. A more extensive collection of characteris-
tic spectra can be found in Mass Spectra Data or NIST Mass Spectral Library of
Compounds [12].
3.3.3.3
Instrument Outgassing
Perhaps the most significant errors that occur in the ion source are from the sur-
faces of the elements that make up the ion source, i.e., electron emission source,
ionization grid, insulators, extraction plates, focus plates, and the walls that sur-
round the ion source. Most mass spectrometers have hot filaments as an electron

346 Chapter 3.3: Practical Aspects of Vacuum System Mass Spectrometers
source, which may operate at a temperature up to 2000°C. The radiant heat to the
surrounding elements can increase their temperature to greater than 200°C, which,
in turn, substantially increases the outgassing from the aforementioned elements
and more often than not contributes more outgassing to the system than from the
cold surfaces from the rest of the system. Thus, at ultimate pressure, the mass
spectrometer may be measuring its own generated pressure and not a true repre-
sentation of the system pressure. Closed ion sources are much worse in providing
nonrepresentative spectra in that they have less conductance available for efficient
cleanup during bakeout or degassing. Although normally at room temperature,
outgassing from the analyzer section and detector section can also be a significant
contributor to the total instrument outgassing flux because most are closed de-
signs (surrounded by shields), which tend to limit efficient degassing. Pumping of
these volumes can only be done through the small conductance of the ion source
extraction aperture back into the ionization volume and into the system. The ob-
vious solution set to these problems is to use instrument materials (especially in
the vicinity of that hot filament) that have been vacuum-fired so that the bulk hy-
drogen and other dissolved species are substantially reduced, to design all the in-
strument components with a very open structure, and to degas the surfaces by ex-
ternal bakeout and/or internal electron bombardment. Some mass spectrometers
do not have internal degas capability so one often depends on external bakeouts.
Usually, the bakeout temperature employed is so low (<250°C, limited by the con-
struction materials in the instrument) and the bakeout time kept so short (<24h),
that the instrument still contributes too much outgassing to the system pressure.
For UHV applications, a bakeout of 400°C for four to five days followed by sev-
eral days at 150°C at full operating emission current is desirable. A simple way to
ensure that the bakeout time and temperature have been sufficient to properly
clean the mass spectrometer is to turn off
the
filament and wait until the total pres-
sure indication of the system ionization gauge has reached steady-state level. If
the relative decrease is more than 10% of the original reading of that gauge, the
mass spectrometer is contributing too much gas load to the system for a repre-
sentative measurement and must be further cleaned. Of course, the use of cold-
cathode sources would substantially reduce these problems and thus is highly de-
sirable, but does not obviate the need for the aforementioned material processing
and degassing.
3.3.3.4 Surface Chemistry
The operation of the hot filament has many unwanted effects from the generation
of gases that are not representative of the true system pressure. The filament tem-
perature may be as high as 2000°C for tungsten (depending on the emission cur-

33.3 The Ion Generation Process 347
rent) and, as previously mentioned, will heat the surrounding materials, including
the walls of the chamber, to much higher than room temperature where, in addi-
tion to higher outgassing rates, a variety of surface reactions occur. In general, the
filament will crack or dissociate most incident molecular gases, leaving a veri-
table plethora of energetic species to promote chemical reaction at the filament
and vicinal surfaces. In addition to the species produced by neutral reaction at hot
surfaces, the chemistry is further complicated by reactions from positive and nega-
tive ions and the range of excited states generated by electron impact. At these
elevated temperatures, the hydrogen, carbon, sulfur, alkalis, and other interstitial
impurities in the bulk of the filament and adjacent surfaces rapidly diffuse to the
surface and are available for chemical combination with existing surface species
or the incident gases. All the surfaces have oxides, so oxygen is readily available.
Carbon has been shown to segregate to the surface of stainless steel at bakeout
temperatures of only 250°C [13]. Ubiquitous hydrogen is continually present at
the surface because it is the most prevalent atom dissolved in metals. Another pri-
mary source of oxygen and hydrogen can be from system gases such as H2O. The
end result is that the filament temperature creates atomic oxygen and hydrogen,
which result in the generation of
CO,
CO2, CH4 and other species as well. Some
probable reactions at a hot W- or Th02-coated W filament are [14,15]:
H2 4- W(s) -> H + H + W(s) (2)
Th02/W(s) -^ Th/W(s) + 0/W(s) (3)
0/W(s) + C/W(s) -^ CO + W(s) (4)
W(s) + 3H2O -^ WO3 (v) + 6H (5)
WO3 + M(s) -> W03/M(s) (6)
O2 -f 2C/W(s) -^ 20/W(s) + 2C/W(s) -^ 2CO + W(s) (7)
O2 -f C/W(s) -^ CO2 + W(s) (8)
O2 + 4H/W(s) -> 2H2O + W(s) (9)
C,Hy + W(s) -^ CAV(s) + H/W(s) (10)
Clearly, however, many other surface contaminants are available, so that in less
clean systems, the generated species are far more complex and far more exten-
sive.
If lower work function filament materials (such as LaB^) or coatings (such
as Th02) are used, then the filament temperature (1200-1400°C) and the vicinal
surface temperatures are reduced, which, in turn, results in reduced surface chem-
istry. Another problem is the admission of reactive gases to the system, which
will further exacerbate the chemistry and the complexity of undesired foreign
species.

348 Chapter 3.3: Practical Aspects of
Vacuum
System Mass Spectrometers
3.33.5 Electron Stimulated Desorption
The ion sources and the electron multipliers of mass spectrometers experience
electron impact collisions with the grid (or collector) and with multiplier surfaces,
respectively. Since all surfaces have oxides, carbon, and other adsorbed species,
there is a probability of desorption of these species into the gas phase. Electron-
stimulated desorption (ESD) has been a major scientific area in surface science
for many years since first studied by Redhead and by Menzel and Gomer [16,17].
Later, different mechanisms for this phenomena were described by Knotek and
Feibleman and by Antoniewitz [18,19]. Several good reviews exist on this subject
and give a comprehensive discussion of the area [20,21]. Essentially, the incident
primary electrons from the filament impact the ion source grid or electron collec-
tor and excite the adsorbed atoms or molecules to anti-bonding states where they
desorb as neutrals or, to a much lesser extent, as ions and become part of the gas
phase —
see
Figure 7(a). The cross sections for the particular mechanism vary
from ~
1
X
10 ~ ^^
to 1 X
10 ~^^
cm^ and the mean kinetic energy for the ions has
been found to be often around 5 eV
[20].
The ESD neutrals are ionized by the pri-
mary electron flux and, along with the ions, are analyzed as if they were part of
the representative gas phase. The adsorbed state of the surface species controls
what is the operative mechanism of ESD. The flux,
JQ,
of desorbed species has
been observed to follow
Jo =
QecToJ,-
(11)
where Q is the ESD cross section for the adsorbed state, 6 is the coverage, a^
is the monolayer concentration, and J^- is the electron flux. For a given cross
section, the ESD flux is directly related to the electron flux and the surface con-
centration of adsorbed species. For example, if the adsorbed species on the grid
of the ionizer has a coverage equal to 1, a conservative ESD cross section of
1 X
10 ~^^
cm^ is employed and the electron emission is 1 mA, then the flux of
molecules into the ionizing region is ~6 X 10^^ molecules cm~^s"^ or 2 X lO"'^
torr liters cm'-^s'^ This flux is the equivalent of >
10
times the outgassing of un-
baked stainless steel, clearly indicating that substantial errors can result!
The origin of atomic H and O (and the recombined molecules H2 and O2),
H2O,
CO and CO2 detected by the mass spectrometer is quite difficult to estab-
lish. The question is simply what is due to ESD and what is truly representative of
the gas phase. Another often observed ESD peak is at m/e = 19, which can be F
^
or H30"^. The source of the peak may less likely be fluorine than hydronium,
which readily forms because there is an ample supply of oxygen and hydrogen.
A dramatic example of ESD-generated oxygen atom neutrals occurs when the
grid of an ion source is covered with a thin film of
Ag.
Silver is well known to dis-
sociatively adsorb oxygen at room temperature, so the grid surface is covered
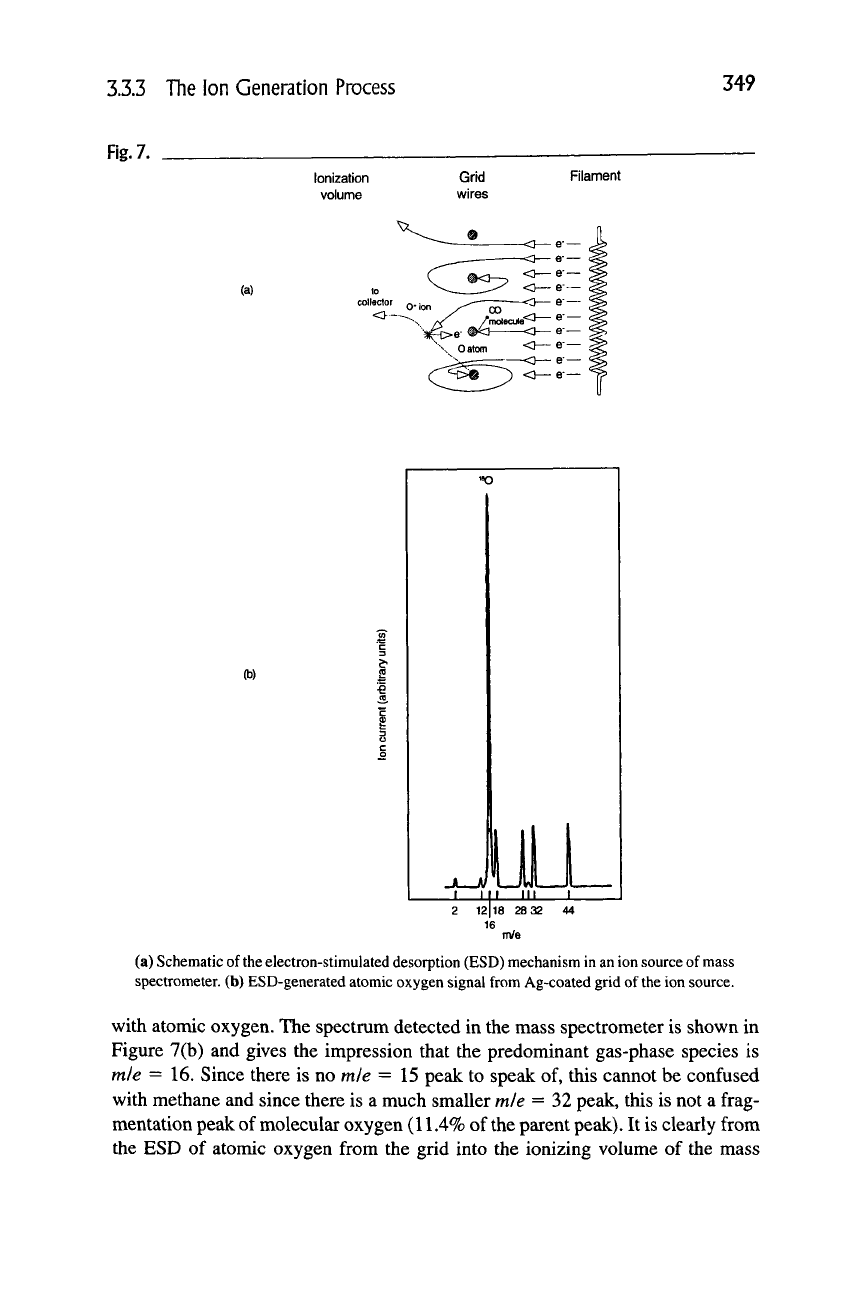
3.3.3 The Ion Generation Process
349
Rg.7.
Ionization
volume
Grid
wires
Filament
(a)
(b)
(a) Schematic of
the
electron-stimulated desorption (ESD) mechanism in an ion source of mass
spectrometer, (b) ESD-generated atomic oxygen signal from Ag-coated grid of
the
ion source.
with atomic oxygen. The spectrum detected in the mass spectrometer is shown in
Figure 7(b) and gives the impression that the predominant gas-phase species is
mie =16. Since there is no mie =15 peak to speak of, this cannot be confused
with methane and since there is a much smaller mie = 32 peak, this is not a frag-
mentation peak of molecular oxygen (11.4% of the parent
peak).
It is clearly from
the ESD of atomic oxygen from the grid into the ionizing volume of the mass
