Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

Tin is added in amounts up to 1.0% in order to improve corrosion-
resistance, particularly in naval brass and Admiralty brass for condenser
tubes.
Such small quantities of tin are retained in solid solution. Alterna-
tively, small amounts of arsenic (0.01-0.05%) may be added to 70-30
brass destined for the manufacture of condenser tubes, as it is said to
improve corrosion-resistance and inhibit dezincification.
Lead,
in amounts
of the order of 2.0%, is added to improve machinability. It is insoluble
in brass, and exists as small globules, which cause local fractures during
machining (6.66). Aluminium is sometimes added, in amounts up to 2.0%,
to brass for the manufacture of naval condenser tubes, since it imparts
excellent corrosion-resistance, particularly to impingement' attack. Nickel
is retained in solid solution, and small amounts may be added to brass to
improve corrosion-resistance. The now obsolete twelve-sided threepenny
pieces were made from a brass containing 20% zinc, 1% nickel and the
balance copper.
16.35 The Hot-working a + |3' brasses Whilst the a-brasses are
specifically cold-working alloys they are generally hot-worked in the
'breaking-down' stages; but a + (3' alloys, containing not more than 60%
copper are shaped almost entirely by hot work.
The only important 'straight' brass in this group is 60-40 brass, formerly
known as 'Muntz metal'. As already mentioned, the a-phase is entirely
absorbed into the (3-phase when the alloy is heated to about 750
0
C, so that
the best hot-working temperature range is while cooling between 750 and
650
0
C,
during which range the a-phase is being deposited. The mechanical-
working process breaks down the a-phase into small particles as it is
deposited, and prevents the reintroduction of the coarse Widmanstatten
structure.
16.36 Free-cutting Brasses of the 60-40 type contain about 2.0%
lead, whilst a similar alloy of higher purity is used extensively for hot-
forging where a machining operation is to follow. Up to 0.15% arsenic
may be added to those brasses destined for the manufacture of water
fittings as it is known to improve corrosion-resistance and inhibit dezincifi-
cation.
16.37 High-tensile Brasses are misleadingly called Manganese
Bronze, possibly because the manganese they often contain produces an
oxidised-bronze effect on the surface of extruded rod. These brasses con-
tain 54-62% copper, up to 7.0% other elements and the balance zinc. In
addition to being hot-working alloys, they are also used in the cast form
for such applications as marine propellers, water-turbine runners, rudders,
gun mountings and sights, and locomotive axle boxes. The wrought sec-
tions are used for pump rods, and for stampings and pressings for auto-
mobile fittings and switch gear. The tensile strength is increased, by the
addition of these other elements, to as much as 700 N/mm
2
in the chill-cast
or forged condition. The additions usually in amounts up to 2.0% each,
include manganese, iron and aluminium; whilst up to 1.0% tin may also
be included to improve corrosion-resistance.
Details of some of the more important wrought brasses are given in
Table 16.1.
The Tin Bronzes
16.40 Bronzes containing approximately 10% tin were probably the first
alloys to be used by Man. In Britain bronze articles almost four thousand
years old have been found, and during the Roman occupation of Britain
the copper-mines of Cumberland and Wales were in a state of rapidly
increasing production. Centuries before the Roman invasion, however,
Phoenician traders from Tyre and Sidon brought their ships to Cornwall
in search of tin.
One of the significant factors in the early Roman conquests was
undoubtedly the bronze sword, and it is thought that in even earlier times
metal-workers realised that a high tin content, in the region of 10%, pro-
duced a hard bronze whilst less tin gave a softer alloy. The relatively high
cost of copper—and particularly tin—coupled with competition from other
new and improved materials has led to a decline in the wide use of bronzes
in the modern world.
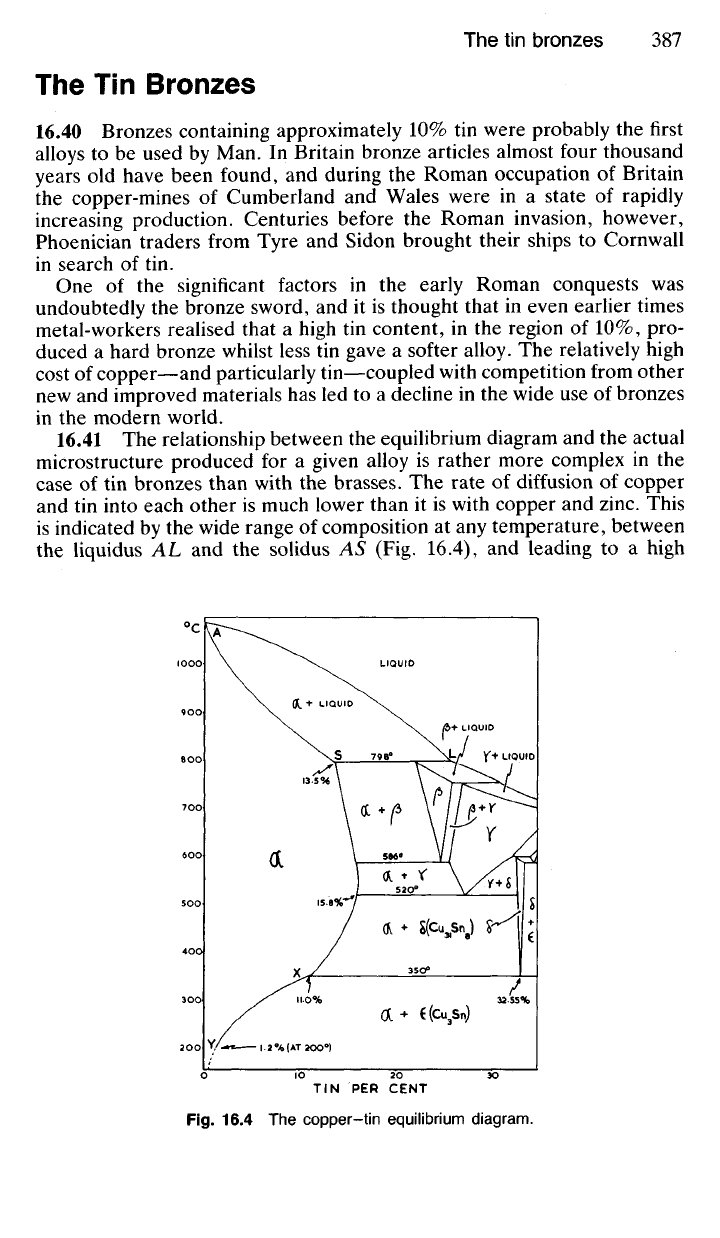
16.41 The relationship between the equilibrium diagram and the actual
microstructure produced for a given alloy is rather more complex in the
case of tin bronzes than with the brasses. The rate of diffusion of copper
and tin into each other is much lower than it is with copper and zinc. This
is indicated by the wide range of composition at any temperature, between
the liquidus AL and the solidus AS (Fig. 16.4), and leading to a high
°c
LIQUID
(K. + LIQUID
LIQUID
LIQUID
TIN PER CENT
Fig.
16.4 The copper-tin equilibrium diagram.

degree of coring during the actual solidification process. Moreover, struc-
tural changes below approximately 400
0
C take place in copper-tin alloys
with extreme sluggishness. Both of these factors mean that a cast bronze,
cooled to ambient temperature under normal industrial conditions, will
rarely exhibit the structure indicated by the equilibrium diagram.
In short, whilst the peritectic reaction (a —»(3) at 789°C and the eutectoid
transformations (|3 -» a + y) at 586°C and (y -> a + 6) at 520
0
C will take
place as indicated by the diagram during ordinary rates of cooling, the
eutectoid transformation (6 —> a + e) at 350
0
C would occur only under
conditions of extremely slow cooling, such as would never be encountered
industrially. Hence the phase 8 (Cu
3
Sn) is never seen in the structure of a
cast bronze containing more than 11.0% tin. Further, due to the slow rate
of diffusion of copper and tin atoms below 350
0
C, the precipitation of 8
from a in alloys containing less than 11.0% tin, in accordance with the
phase boundary XY, will not occur. For practical purposes the reader can
therefore ignore that part of the equilibrium diagram below 400
0
C and
assume that whatever structure has been attained at 400
0
C will persist to
room temperature under normal industrial rates of cooling.
16.42 As with brasses, the a-phase, being a solid solution, is tough and
ductile, so that a-phase alloys can be cold-worked successfully. The
6-phase, however, is an intermetallic compound of composition equivalent
to Cu
3
iSn
8
, and is a hard, brittle, blue substance, whose presence renders
the a + 5 bronzes rather brittle. The 6-phase must, therefore, be absent
from alloys destined for any degree of cold-work.
Due to heavy coring arising from slow rates of diffusion, cast alloys with
as little as 6.0% tin will show particles of 6 at the boundaries of the cored
a crystals. The skeletons of the a-phase crystals will be much richer in
copper than the nominal 94%, thus making the outer fringes correspond-
ingly richer in tin to such an extent that the 6-phase is formed. In order to
make such an alloy amenable for cold-work, the 6-phase can be absorbed
by prolonged annealing (say six hours at 700
0
C), which will promote dif-
fusion so that equilibrium is attained in accordance with the equilibrium
diagram and a uniform a-phase structure produced. Subsequent air-cooling
—or even furnace-cooling at the usual industrial rates—will be too rapid
to permit precipitation of any of the s-phase when the phase boundary
XY is reached; and so the uniform a structure will be retained at room
temperature. By using such initial heat-treatment to produce a uniform a
structure it is possible to cold-work bronzes containing as much as 14%
tin, though in general industrial practice only alloys with up to 7% tin are
produced in wrought form.
The tin bronzes can be classified as follows:
16.43 Plain Tin Bronzes These comprise both wrought and cast
alloys, the former usually containing up to 7% tin and the latter as much
as 18% tin. The wrought alloys contain none of the 6-phase, the absence
of which makes them amenable to shaping by cold-working operations.
These alloys are usually supplied as rolled sheet, drawn rod or drawn
turbine blading.
The cast alloys are used mainly for bearings, since the structure fulfils

the requirements for that duty, namely, hard particles of the S-phase,
which will resist wear, embedded in a matrix of the a-phase, which will
resist shock.
16.44 Phosphor-bronzes Most of the tin bronzes mentioned above
contain small amounts of phosphorus (up to 0.05%) residual from deoxi-
dation which is carried out before casting. They are often incorrectly called
phosphor-bronzes. True phosphor-bronze contains phosphorus as a delib-
erate alloying addition, generally present in amounts between 0.1 and
1.0%.
Wrought phosphor-bronzes contain up to 8.0% tin and up to 0.3% phos-
phorus, and, like the plain tin bronzes, are supplied in the form of rod,
wire and turbine blading. The phosphorus not only increases the tensile
strength but also, it is claimed, improves the corrosion-resistance.
Cast phosphor-bronzes contain up to 13.0% tin and up to 1.0% phos-
phorus, and are used mainly for bearings and other components where a
low friction coefficient is desirable, coupled with high strength and tough-
ness.
The phosphorus is usually present in these alloys as copper phos-
phide, C113P, a hard compound which forms a ternary eutectoid with the
a- and 6-phases.
16.45 Bronzes Containing Zinc These, again, comprise both wrought
and cast alloys. The wrought alloys are used chiefly for the manufacture
of coinage and contain up to 3.0% tin and up to 2.5% zinc. In recent years
the tin content of British 'copper' coinage has been reduced from the
pre-war figure of 3.0% to as low as 0.5%. This began as a war-time measure
when the Japanese occupation of Malaya cut off our main supplies of tin;
and has continued since because of the high prices which have prevailed
for the metal. The replacement of tin by some zinc cheapens the alloy,
zinc being only about a tenth the price of tin. A subsidiary function of zinc
is that, like phosphorus, it acts as a deoxidiser and forms zinc oxide, ZnO,
which floats to the top of the melt. The wrought bronzes containing zinc
are all a-phase alloys with similar structures to those of straight tin bronzes
of like compositions.
The best known of the cast alloys is 'Admiralty gunmetal', or 88-10-2
gunmetal, containing 10% tin and 2% zinc. It is no longer used in naval
ordnance, but is still widely employed where a strong, corrosion-resistant
casting is required. Its structure is similar to that of a straight tin bronze
containing 11% tin, so that, due to considerable coring, a lot of a + 5
eutectoid will be present. The zinc not only cheapens the alloy and acts as
a deoxidiser but also improves casting fluidity.
16.46 Bronzes Containing Lead Up to 2.0% lead is sometimes added
to bronzes as well as to brasses in order to improve machinability. Larger
amounts are added for some special bearings; and such bronzes permit 20%
higher loading than do lead-base or tin-base white metals. The thermal
conductivity of these bronzes is also higher, so that they can be used at
higher speeds, since heat is dissipated more quickly. With normal lubrica-
tion they have excellent wear resistance but, equally important, the seizure
resistance is high since lead will function temporarily as a lubricant should
normal lubrication fail. Leaded bearing bronzes contain up to 20% lead
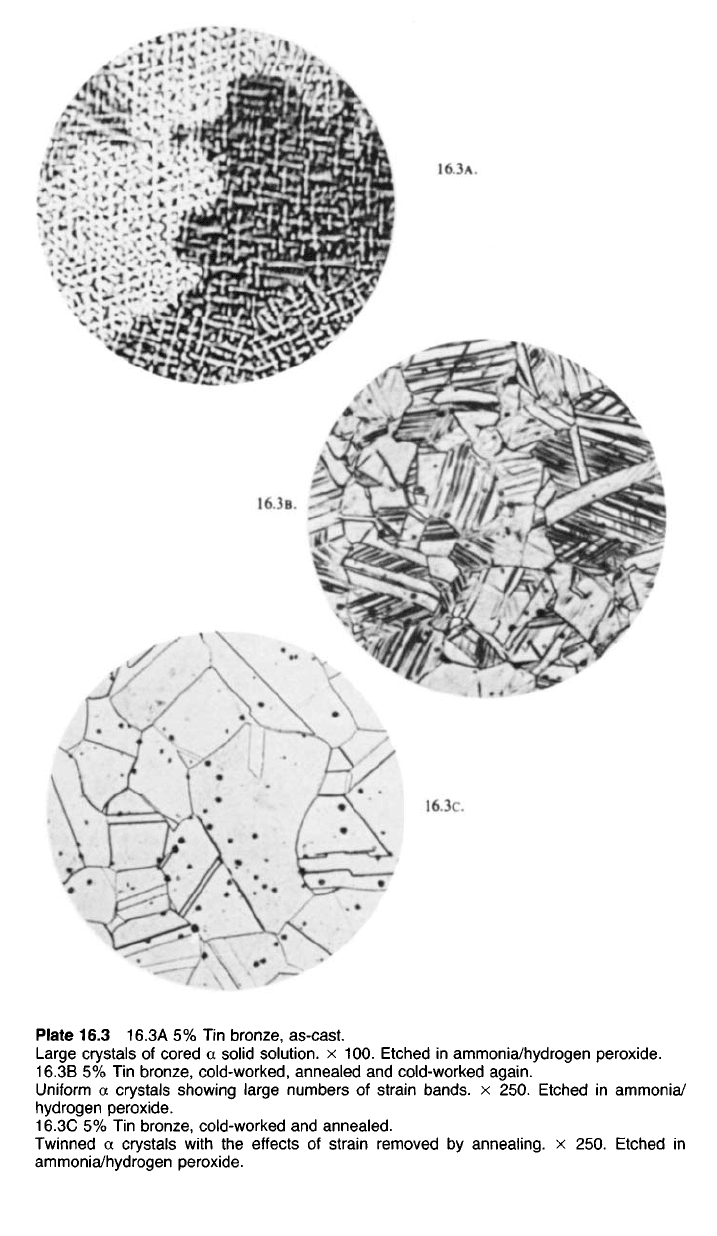
Plate 16.3 16.3A 5% Tin bronze, as-cast.
Large crystals of cored a solid solution, x 100. Etched in ammonia/hydrogen peroxide.
16.3B 5% Tin bronze, cold-worked, annealed and cold-worked again.
Uniform a crystals showing large numbers of strain bands, x 250. Etched in ammonia/
hydrogen peroxide.
16.3C 5% Tin bronze, cold-worked and annealed.
Twinned a crystals with the effects of strain removed by annealing, x 250. Etched in
ammonia/hydrogen peroxide.
16.3c.
16.3B.
16.3A.
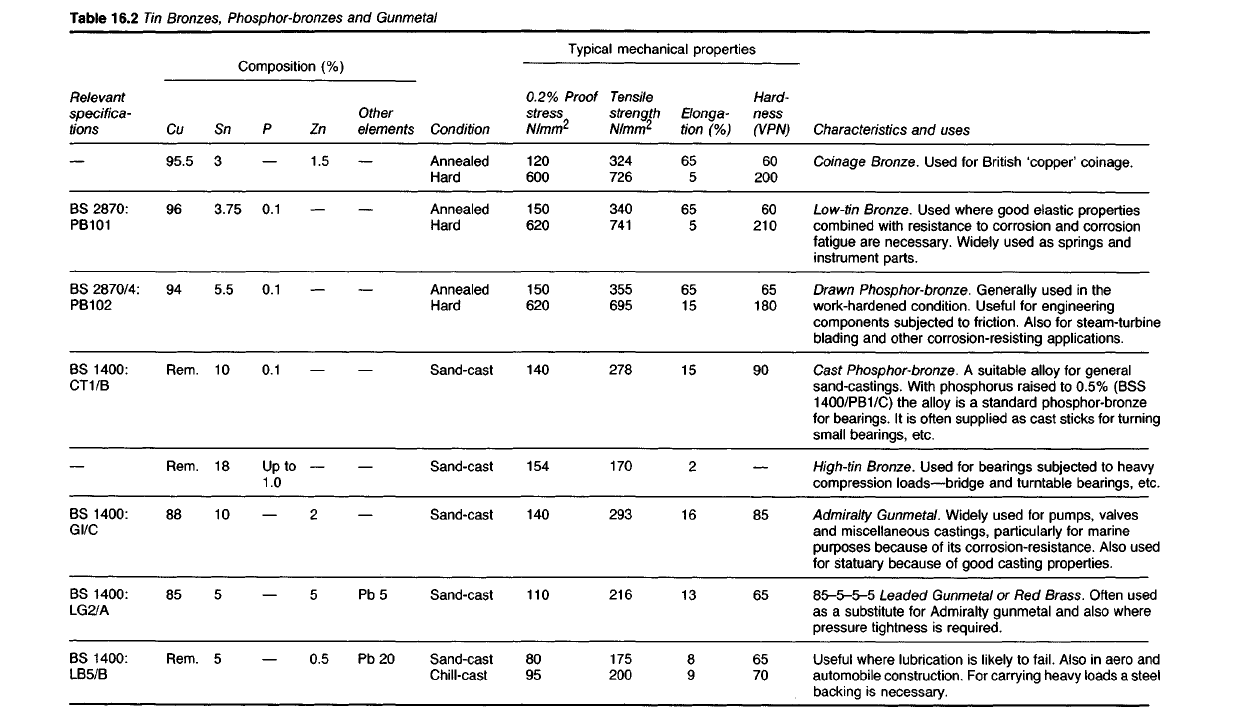
Table
16.2 Tin
Bronzes,
Phosphor-bronzes
and Gunmetal
Characteristics and uses
Coinage
Bronze. Used for
British
'copper' coinage.
Low-tin
Bronze. Used where good elastic properties
combined with resistance to corrosion and corrosion
fatigue are necessary. Widely used as springs and
instrument
parts.
Drawn
Phosphor-bronze.
Generally
used
in the
work-hardened condition. Useful for engineering
components subjected to friction. Also for steam-turbine
blading and other corrosion-resisting applications.
Cast
Phosphor-bronze. A suitable alloy for general
sand-castings. With phosphorus raised to 0.5% (BSS
1400/PB1/C)
the alloy is a standard phosphor-bronze
for
bearings. It is often supplied as cast sticks for turning
small bearings, etc.
High-tin
Bronze.
Used
for bearings subjected to heavy
compression
loads—bridge and turntable bearings, etc.
Admiralty
Gunmetal. Widely used for pumps, valves
and miscellaneous castings, particularly for marine
purposes because of its corrosion-resistance.
Also
used
for
statuary because of good casting properties.
85-5-5-5
Leaded
Gunmetal or Red
Brass.
Often
used
as a substitute for Admiralty gunmetal and also where
pressure
tightness is required.
Useful where lubrication is likely to
fail.
Also
in aero and
automobile
construction. For carrying heavy loads a steel
backing is necessary.
Typical mechanical properties
Hard-
ness
(VPN)
60
200
60
210
65
180
90
85
65
65
70
Elonga-
tion (%)
65
5
65
5
65
15
15
2
16
13
8
9
Tensile
strength
N/mm
2
324
726
340
741
355
695
278
170
293
216
175
200
0.2% Proof
stress
N/mm
2
120
600
150
620
150
620
140
154
140
110
80
95
Condition
Annealed
Hard
Annealed
Hard
Annealed
Hard
Sand-cast
Sand-cast
Sand-cast
Sand-cast
Sand-cast
Chill-cast
Composition (%)
Other
elements
Pb 5
Pb
20
Zn
1.5
2
5
0.5
P
0.1
0.1
0.1
Up to
1.0
Sn
3
3.75
5.5
10
18
10
5
5
Cu
95.5
96
94
Rem.
Rem.
88
85
Rem.
Relevant
specifica-
tions
BS
2870:
PB101
BS
2870/4:
PB102
BS 1400:
CT1/B
BS 1400:
Gl/C
BS 1400:
LG2/A
BS 1400:
LB5/B
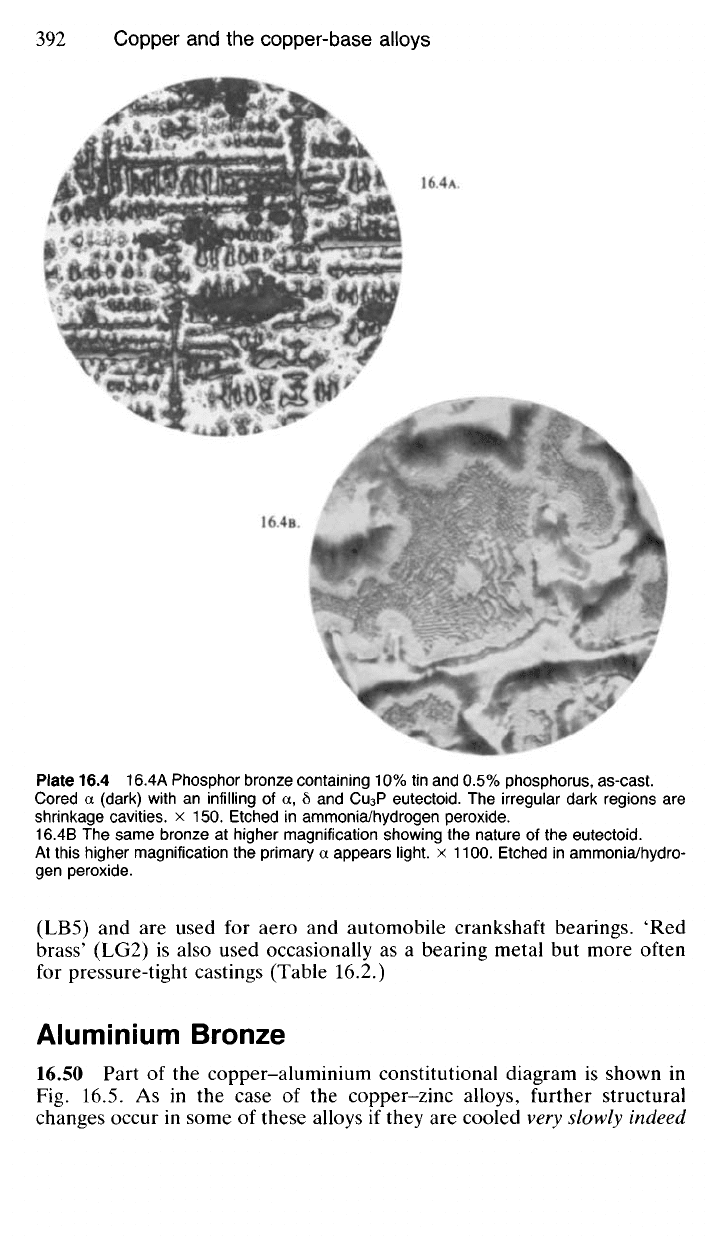
Plate 16.4 16.4A Phosphor bronze containing 10% tin and 0.5% phosphorus, as-cast.
Cored a (dark) with an infilling of a, 6 and Cu
3
P eutectoid. The irregular dark regions are
shrinkage cavities, x 150. Etched in ammonia/hydrogen peroxide.
16.4B The same bronze at higher magnification showing the nature of the eutectoid.
At this higher magnification the primary a appears light, x 1100. Etched in ammonia/hydro-
gen peroxide.
(LB5) and are used for aero and automobile crankshaft bearings. 'Red
brass'
(LG2) is also used occasionally as a bearing metal but more often
for pressure-tight castings (Table 16.2.)
Aluminium Bronze
16.50 Part of the copper-aluminium constitutional diagram is shown in
Fig. 16.5. As in the case of the copper-zinc alloys, further structural
changes occur in some of these alloys if they are cooled very slowly indeed
16.4A.
16.4B.
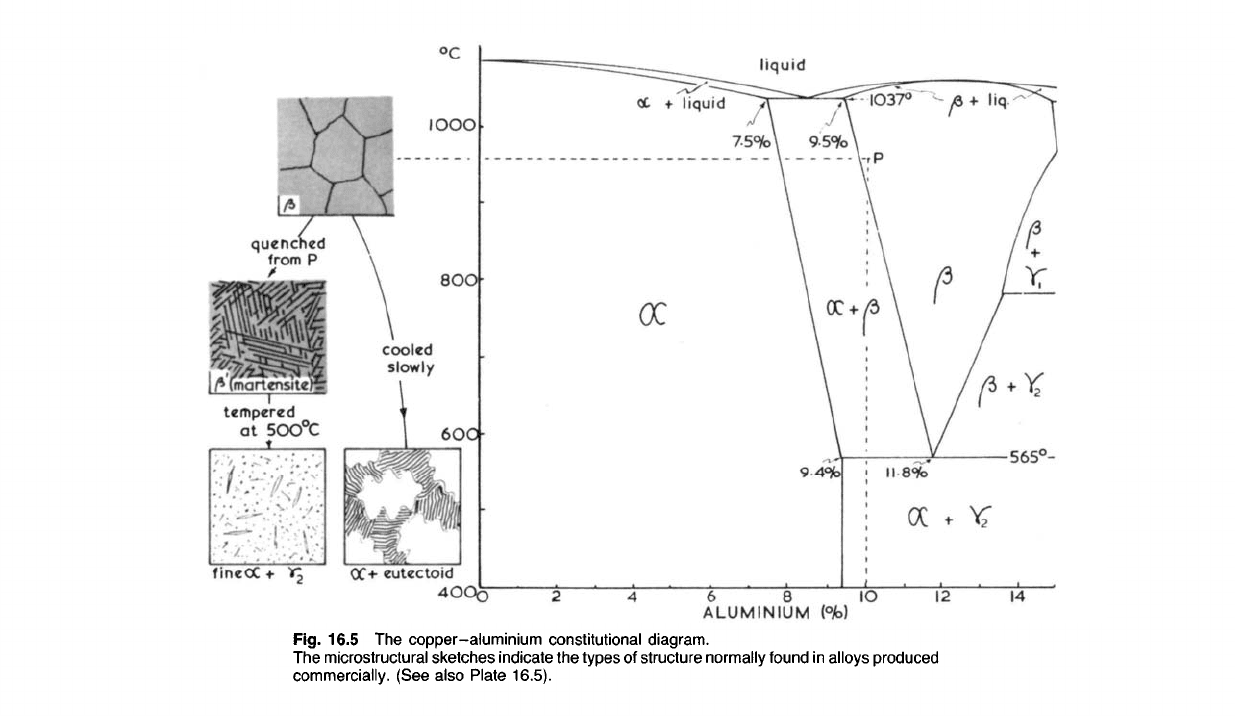
ALUMINIUM
(°h)
Fig. 16.5 The
copper-aluminium
constitutional diagram.
The
microstructural sketches
indicate
the types of structure normally found in alloys produced
commercially. (See also Plate 16.5).
liquid
QC
+
liquid
0
C
cooled
slowly
quenched
from
P
tempered
at 5OO°C
OC+
eutectoid
f
ineOC
+• Y^

under laboratory conditions below 400
0
C. However, such very slow cooling
rates are never encountered in industrial production methods and we can
assume that the a + 72 structure persists in alloys containing between 9.4
and 16.2% aluminium which are cooled reasonably slowly ('furnace
cooled') to ambient temperatures. Consequently that part of the consti-
tutional diagram below 500
0
C can be ignored for our purposes and is not
shown here.
Like the brasses the aluminium bronzes can be divided into two main
groups; the cold-working and the hot-working or casting alloys respect-
ively. The constitutional diagram indicates that a solid solution (a) contain-
ing up to 9.4% aluminium at room temperature, is formed. Like the other
a solid solutions based on copper, it is quite ductile. With more than 9.4%
aluminium the phase y
2
is formed. This is an intermetallic compound of
the formula Cu
9
Al
4
and, in common with compounds of this type, is very
hard and brittle, resulting in an overall brittleness of alloys containing the
y
2
-phase.
16.51 Further inspection of the constitutional diagram reveals simi-
larities between it and the iron-carbon diagram. The two a-phases are
analogous; the (3-phase solid solution of the copper-aluminium diagram
corresponds to the y (austenite) phase of the iron-carbon diagram; and
the a +
Y2
eutectoid is similar to the ferrite + cementite eutectoid (pearlite)
of the steels. As a result of these similarities in the positions of the phase
fields in the respective diagrams, a 10% aluminium bronze can be heat
treated in a manner parallel to that of steel so that a martensite-type
transformation occurs. Nevertheless it should be realised that the crystal-
lography of the aluminium bronzes is different from that of the correspond-
ing steels.
Consider a 10% aluminium bronze; this will consist entirely of the phases
a and y
2
if it is allowed to cool slowly in a furnace to ambient temperature.
If it is reheated the a + y
2
eutectoid is transformed to the solid solution (3
when the eutectoid temperature (565°C) is reached, and as the temperature
rises further, the a-phase is absorbed until at about 900
0
C the structure
consists entirely of the solid solution (3. Water-quenching from this tem-
perature produces a structure consisting of the phase p'. This is not shown
in the equilibrium diagram, since, like martensite in steels, it is not an
equilibrium phase. The P'-phase is hard and brittle like martensite, and is
in fact very similar in microstructural appearance. Tempering this P'-phase
at 500
0
C causes the precipitation of a fine agglomerate of the phases a and
y
2
, closely resembling the tempered martensite of a steel treated in a paral-
lel manner.
In fact the thermal equilibrium characteristics of 10% aluminium bronze
resemble more closely those of an air-hardening alloy steel than those of
a plain carbon steel. For example, if the 10% aluminium bronze is air
cooled from the P-phase field ('normalised') then the resultant structure is
likely to be either P' ('martensitic') or a bainitic type of structure containing
finely precipitated y
2
. The a +
Y2
('pearlitic') structure will only be obtained
by annealing, followed by furnace cooling to ambient temperature. Ex-
tremely slow cooling under controlled laboratory conditions may cause a
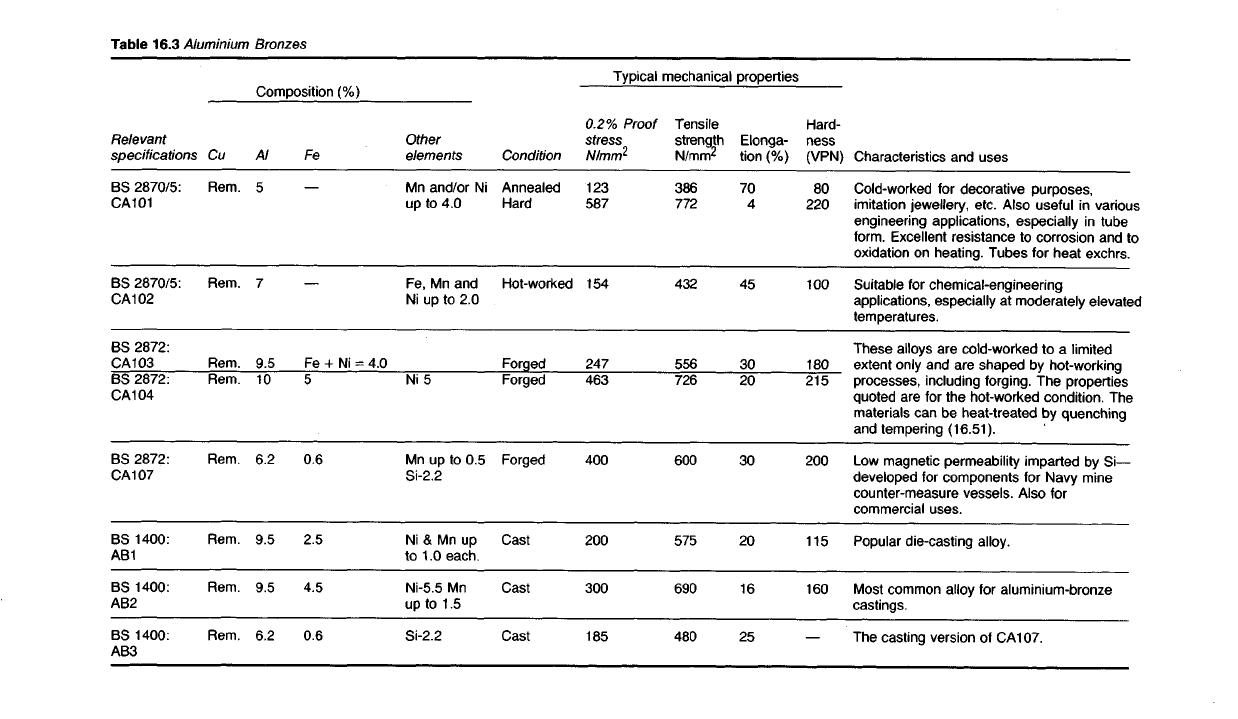
Table
16.3
Aluminium
Bronzes
Characteristics and uses
Cold-worked for decorative purposes,
imitation jewellery, etc. Also
useful
in various
engineering applications, especially in tube
form.
Excellent resistance to corrosion and to
oxidation
on heating. Tubes for heat exchrs.
Suitable
for
chemical-engineering
applications, especially at moderately elevated
temperatures.
These
alloys are cold-worked to a limited
extent only and are
shaped
by hot-working
processes, including forging. The properties
quoted are for the hot-worked condition. The
materials can be heat-treated by quenching
and tempering (16.51).
Low
magnetic permeability imparted by Si—
developed for components for Navy mine
counter-measure vessels. Also for
commercial uses.
Popular die-casting alloy.
Most
common alloy for aluminium-bronze
castings.
The casting version of CA107.
Typical mechanical properties
Hard-
ness
(VPN)
80
220
100
180
215
200
115
160
Elonga-
tion
(%)
70
4
45
30
20
30
20
16
25
Tensile
strength
N/mm
2
386
772
432
556
726
600
575
690
480
0.2% Proof
stress
N/mm
2
123
587
154
247
463
400
200
300
185
Condition
Annealed
Hard
Hot-worked
Forged
Forged
Forged
Cast
Cast
Cast
Composition (%)
Other
elements
Mn and/or Ni
up to 4.0
Fe, Mn and
Ni up to 2.0
Ni
5
Mn up to 0.5
Si-2.2
Ni
& Mn up
to
1.0 each.
Ni-5.5 Mn
up to 1.5
Si-2.2
Fe
Fe + Ni = 4.0
5
0.6
2.5
4.5
0.6
Al
5
7
9.5
10
6.2
9.5
9.5
6.2
Cu
Rem.
Rem.
Rem.
Rem.
Rem.
Rem.
Rem.
Rem.
Relevant
specifications
BS
2870/5:
CA101
BS
2870/5:
CA102
BS
2872:
CA103
BS
2872:
CA104
BS
2872:
CA107
BS 1400:
AB1
BS 1400:
AB2
BS 1400:
AB3
