Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


Aluminium and its Alloys
17.10 The properties of aluminium which chiefly dictate its use as an
engineering material are its low relative density coupled with a reasonably
high tensile strength when used in one of its alloyed forms. Since its relative
density is only about one third that of steel its alloys are widely used
in aero-, automobile and constructional engineering. A combination of
alloying and heat-treatment can produce alloys which, weight-for-weight,
are in the same class as many steels.
17.11 The high affinity of aluminium for oxygen is both disadvan-
tageous and useful. It is disadvantageous in so far as it increases the cost
of extraction of the metal by making necessary a relatively expensive elec-
trolytic extraction process. Usually a metal is extracted by heating its oxide
ore with a cheap reducing agent, such as carbon (in the form of coke), and
the resulting crude metal is refined by allowing the bulk of the impurities
present in it to be oxidised. This is the basis for the production of pig iron
and its subsequent conversion to steel. The very high chemical affinity of
aluminium for oxygen means that aluminium cannot be reduced by carbon
by ordinary chemical means. Obviously any other reducing agents able
to separate aluminium from oxygen will be thermodynamically far too
expensive, so that aluminium can only be produced economically by elec-
trolytic means.
It was using an expensive reducing agent in the form of metallic potass-
ium which enabled the Danish physicist and chemist H. C. Oersted to
produce the first samples of aluminium in 1825. Consequently aluminium
was worth about £250 per kg in those days, and, even later, it is said that
the more illustrious foreign guests to the Court of Napoleon III were
privileged to use forks and spoons made from aluminium, whilst the French
nobility had to be content with mere gold plate and silver cutlery. In the
Gay Nineties aluminium was still regarded as being in the nature of a
curious precious metal, though it was in 1886 that C. M. Hall, a twenty-two-
year-old student, had discovered a relatively cheap method for producing
aluminium by electrolysing a fused mixture of aluminium oxide and the
mineral cryolite.
17

Aluminium cannot be purified by blowing air through it, as in the case
of iron. This treatment would oxidise the aluminium and leave behind the
impurities. Therefore the ore must be purified before being electrolysed,
and this involves an expensive chemical process. The ore of aluminium is
bauxite, which is named after Les Baux, in the south of France, where it
was discovered in 1821. Although France is still an important producer of
bauxite, Jamaica and the Republic of Guinea mine the largest quantities of
the ore followed by the CIS, Surinam and Guyana. In Europe Hungary,
Greece and Jugoslavia, in addition to France, all produce considerable
quantities of the ore. Britain is wholly dependent upon imported ore to
maintain a token small-scale production at the Kinlochleven and Lochaber
works, both of which are sited with access to water transport and relatively
cheap hydroelectric power in the Scottish Highlands; but most aluminium
is imported as the metal.
17.12 Although aluminium has a great affinity for oxygen, its corrosion-
resistance is relatively high. This is due to the dense impervious film of
oxide which forms on the surface of the metal and protects it from further
oxidation. The corrosion-resistance can be further improved by anodising
(21.91),
a treatment which artificially thickens the natural oxide film. Since
aluminium oxide is extremely hard, wear-resistance is also increased by
the oxide layer; and the slightly porous nature of the surface of the film
allows it to be coloured with either organic or inorganic dyes. In this
respect high oxygen-affinity is an asset.
The high affinity of aluminium for oxygen also makes it useful as a
deoxidant in steels and also in the Thermit process of welding (20.52).
17.13 The fact that aluminium has over 50% of the specific conduc-
tivity of copper means that, weight for weight, it is a better conductor of
electricity than is copper. Hence it is now widely used, generally twisted
round a steel core for strength, as a current carrier in the electric 'grid'
system. Large increases in the price of copper a few years ago led to the
use of aluminium as a domestic current carrier, though this type of wire is
often copper coated to increase the conductivity of the surface skin.
17.14 Unlike copper which is normally available as a high-purity metal
because of the ease of its electrolytic refinement, commercial grades of
'pure'
aluminium may contain no more than 99.0% of the metal. This is
due to the difficulty in refining crude aluminium already mentioned.
Although electrolytic refining methods are now practised producing alu-
minium of 99.99% purity this is obviously costly. Available commercial
qualities covered by BS specifications contain 99.99%,
99.8%,
99.5%,
99.0%
aluminium respectively. The lower commercial grades are used
fairly widely for drawing, pressing and spinning large numbers of cooking
and other kitchen utensils to which non-stick coatings of PTFE (polyte-
trafluoroethene) can be applied where necessary. Aluminium is suitable
for both hot- and cold-working processes but unlike copper, another FCC
metal, it does not exhibit annealing twins following recrystallisation.
Pure aluminium is relatively soft and weak with a tensile strength of no
more than 90 N/mm
2
in the annealed condition, so that for most other
engineering purposes it is used in the alloyed form.

Alloys of Aluminium
17.20 The addition of alloying elements is made principally to improve
mechanical properties, such as tensile strength, hardness, rigidity and
machinability, and sometimes to improve fluidity and other casting pro-
perties.
17.21 One of the chief defects to which aluminium alloys are prone is
porosity due to gases dissolved during the melting process. Molten alu-
minium will dissolve considerable amounts of hydrogen if, for any reason,
this is present in the furnace atmosphere. When the metal is cast and
begins to solidify the solubility of hydrogen diminishes almost to zero, so
that tiny bubbles of gas are formed in the partly solid metal. These cannot
escape, and give rise to pinhole porosity. The defect is eliminated by
treating the molten metal before casting with a suitable flux, or by bubbling
nitrogen or chlorine through the melt. A more practical method is to use
specially prepared tablets of hexachloroethane which decompose liberating
small quantities of chlorine when the tablet is plunged beneath the surface
of the melt.
17.22 Aluminium alloys are used in both the cast and wrought con-
ditions. Whilst the mechanical properties of many of them, both cast and
wrought, can be improved by precipitation hardening, a number are used
without any such treatment being applied. Forty years ago there was a
bewildering multitude of aluminium alloys in the manufacturers' lists. For-
tunately obsolescence and rationalisation has reduced the number con-
siderably, though some new compositions have been introduced as a result
of expansion of the market for aluminium alloys outside the field of
aerospace.
17.23 Most of the aluminium alloys available commercially are sup-
plied according to British Standards Specifications and are covered by
General Engineering Specifications BS 1470/1475 (wrought materials) and
BS 1490 (cast alloys). Specialised applications are covered under a number
of supplementary specifications, whilst a few manufacturers produce alloys
not covered by a BS number. For wrought alloys the general specification
number, ie BS 1470/1475, is followed by a four-digit designation identifying
a specific alloy. The first of the four digits indicates the alloy group as
follows:
Aluminium (99.0% min. and
greater) - lxxx
Aluminium alloy groups (by
major alloying elements): Copper - 2xxx
Manganese - 3xxx
Silicon - 4xxx
Magnesium - 5xxx
Mg + Si - 6xxx
Zinc - 7xxx
Other elements - 8xxx
Unused series - 9xxx

In the first group (aluminium containing more than 99.0% Al) the last
two digits are the same as the two digits to the right of the decimal point
in the 'minimum % aluminium' when expressed to the nearest
0.01%.
The
second digit in the designation indicates modifications in impurity limits or
alloying elements. When the second digit is zero it indicates unalloyed
aluminium having natural impurity limits; integers 1 to 9, assigned consecu-
tively as required, indicate special control of one or more impurities or
alloying elements.
In Groups 2xxx to 8xxx the last two digits in the designation have no
special significance and are used only to identify different alloys in the
group. The second digit in the designation indicates modifications. Thus,
if the second digit is zero it indicates the original alloy; integers 1 to 9
assigned consecutively indicate modifications. National variations of
wrought aluminium alloys registered by another country are identified by
a serial letter after the four-digit designation.
The condition of the material may be denoted by suffix letters as follows:
M - material 'as manufactured'.
O - fully annealed to give lowest strength.
Hl
to
H8 - work-hardened. Material cold-worked after annealing.
Designations are in ascending order of strength and
hardness.
TB - solution-treated and 'aged' naturally. (Material
receives no cold-work after solution treatment.)
TE - cooled from an elevated-temperature shaping process
and precipitation hardened.
TF - solution treated and precipitation hardened.
TH - solution treated, cold-worked and then precipitation
hardened.
TD - solution treated, cold-worked and 'aged' naturally
(tubes).
The cast aluminium alloys are covered by BS 1490 and here the suffix
letters indicate the condition of the material as follows:
M - 'as cast' with no further treatment
TS - castings 'stress relieved' only
TE - precipitation treatment after casting
TB - solution treated only
TB7 - solution treated and 'stabilised'
TF - solution treated and precipitation hardened
TF7 - solution treated, precipitation hardened and then
'stabilised'.
It is convenient to classify aluminium alloys into the following four main
groups according to the condition in which they are employed.
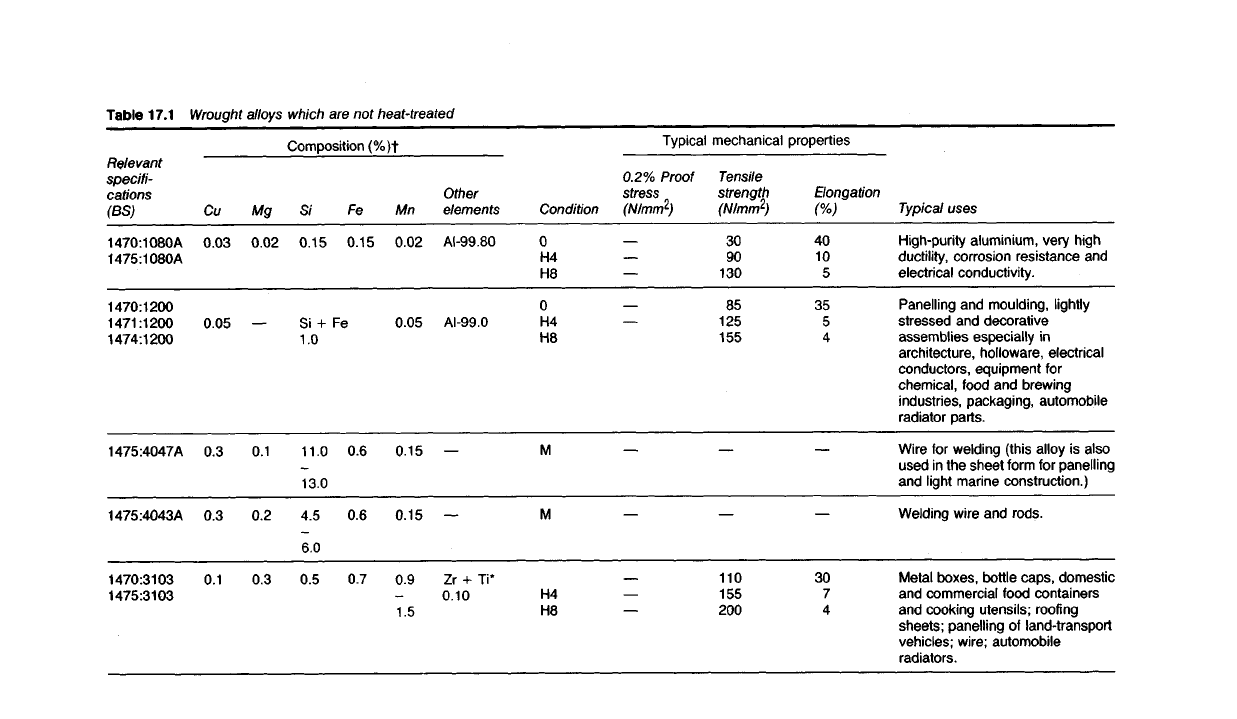
Table
17.1 Wrought alloys
which
are not
heat-treated
Typical
uses
High-purity
aluminium,
very high
ductility, corrosion resistance and
electrical conductivity.
Panelling and moulding, lightly
stressed and decorative
assemblies
especially
in
architecture, holloware, electrical
conductors,
equipment
for
chemical,
food and brewing
industries, packaging, automobile
radiator parts.
Wire for welding (this alloy is also
used in the sheet
form
for panelling
and light marine construction.)
Welding wire and rods.
Metal
boxes, bottle caps, domestic
and commercial food containers
and cooking utensils; roofing
sheets;
panelling
of
land-transport
vehicles; wire;
automobile
radiators.
Typical mechanical properties
Elongation
(%)
40
10
5
35
5
4
30
7
4
Tensile
strength
(N/mm
2
)
30
90
130
85
125
155
110
155
200
0.2% Proof
stress
(N/mm
2
)
Condition
0
H4
H8
0
H4
H8
M
M
H4
H8
Composition
(%)t
Other
elements
AI-99.80
AI-99.0
Zr
+ Ti*
0.10
Mn
0.02
0.05
0.15
0.15
0.9
1.5
Fe
0.15
S/
0.15
Si
+ Fe
1.0
0.6
0.6
0.7
11.0
13.0
4.5
6.0
0.5
Mg
0.02
0.1
0.2
0.3
Cu
0.03
0.05
0.3
0.3
0.1
Relevant
specifi-
cations
(BS)
1470:1080A
1475:1080A
1470:1200
1471:1200
1474:1200
1475:4047A
1475.-4043A
1470:3103
1475:3103
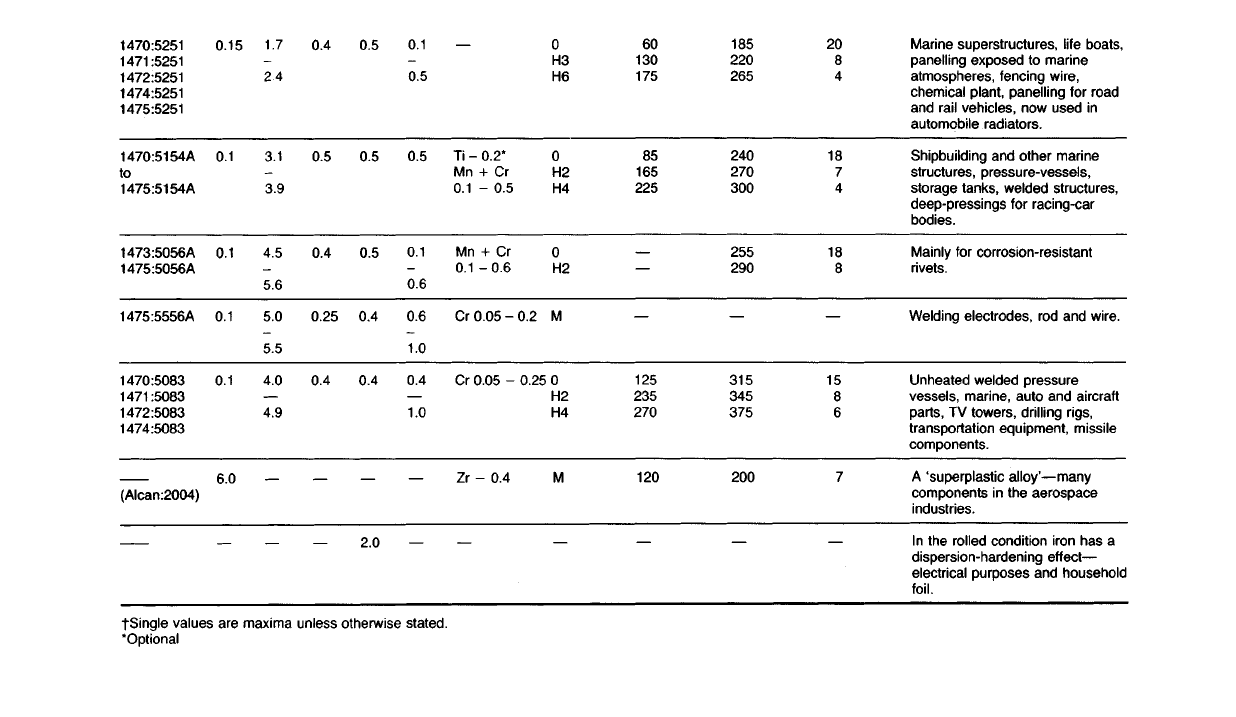
Marine superstructures, life boats,
panelling exposed to marine
atmospheres, fencing wire,
chemical plant, panelling for road
and rail vehicles, now used in
automobile radiators.
Shipbuilding and other marine
structures,
pressure-vessels,
storage tanks, welded structures,
deep-pressings for racing-car
bodies.
Mainly for corrosion-resistant
rivets.
Welding electrodes, rod and wire.
Unheated welded pressure
vessels,
marine, auto and aircraft
parts,
TV towers, drilling rigs,
transportation
equipment, missile
components.
A
'superplastic alloy'—many
components in the aerospace
industries.
In
the rolled condition iron has a
dispersion-hardening effect—
electrical purposes and household
foil.
20
8
4
18
7
4
18
8
15
8
6
7
185
220
265
240
270
300
255
290
315
345
375
200
60
130
175
85
165
225
125
235
270
120
0
H3
H6
0
H2
H4
0
H2
M
0
H2
H4
M
Ti - 0.2*
Mn + Cr
0.1
- 0.5
Mn + Cr
0.1
-0.6
Cr
0.05 - 0.2
Cr
0.05 - 0.25
Zr
- 0.4
0.1
0.5
0.5
0.1
0.6
0.6
1.0
0.4
1.0
0.5
0.5
0.5
0.4
0.4
2.0
0.4
0.5
0.4
0.25
0.4
1.7
24
3.1
3.9
4.5
5.6
5.0
5.5
4.0
4.9
0.15
0.1
0.1
0.1
0.1
6.0
1470:5251
1471:5251
1472:5251
1474:5251
1475:5251
1470:5154A
to
1475:5154A
1473:5056A
1475:5056A
1475:5556A
1470:5083
1471:5083
1472:5083
1474:5083
(Alcan:2004)
tSingle
values are maxima unless otherwise stated.
*Optional
Wrought Alloys Which Are Not Heat-treated
17.30 The main requirements of alloys in this group are sufficient strength
and rigidity in the work-hardened state, coupled with good corrosion-
resistance. These properties are typical of the alloys shown in Table 17.1.
As will be seen, these alloys are widely used in the manufacture of panels
for land-transport vehicles. Here the high corrosion-resistance of the alu-
minium-magnesium alloys is utilised, those with a higher magnesium value
having an excellent resistance to sea-water and marine atmospheres, so
that they are used extensively for marine superstructures. The desired
mechanical properties are produced by the degree of cold-work applied in
the final cold-working operation, and these alloys are commonly supplied
as soft (0), or having undergone varying degrees of work-hardening as
denoted by the BS Specification suffixes Hl, H2, H3, etc. up to H8 (full
hard).
The main disadvantage is that, once the material has been finished
to size, no further variation can be made in mechanical properties (other
than softening by annealing), whereas, with the precipitation-hardening
alloys, the properties can be varied, within limits, by heat-treatment.
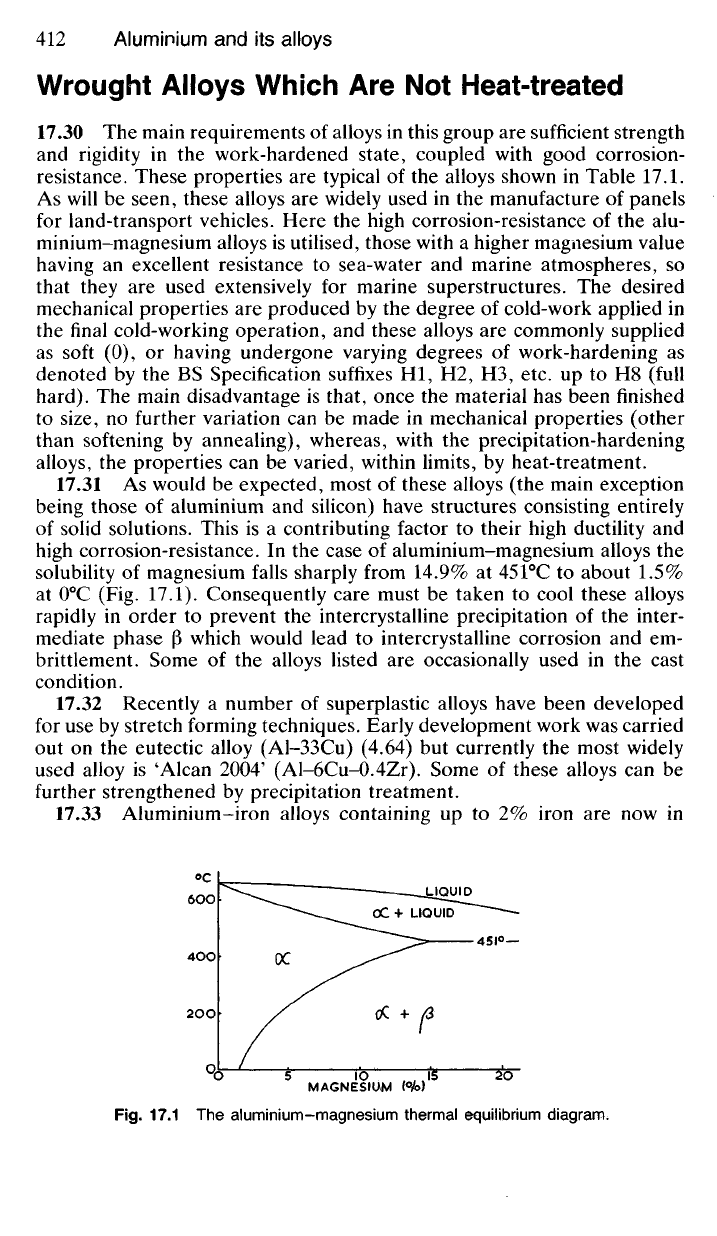
17.31 As would be expected, most of these alloys (the main exception
being those of aluminium and silicon) have structures consisting entirely
of solid solutions. This is a contributing factor to their high ductility and
high corrosion-resistance. In the case of aluminium-magnesium alloys the
solubility of magnesium falls sharply from 14.9% at 451°C to about 1.5%
at 0
0
C (Fig. 17.1). Consequently care must be taken to cool these alloys
rapidly in order to prevent the intercrystalline precipitation of the inter-
mediate phase (3 which would lead to intercrystalline corrosion and em-
brittlement. Some of the alloys listed are occasionally used in the cast
condition.
17.32 Recently a number of superplastic alloys have been developed
for use by stretch forming techniques. Early development work was carried
out on the eutectic alloy (Al-33Cu) (4.64) but currently the most widely
used alloy is 'Alcan 2004' (Al-6Cu-0.4Zr). Some of these alloys can be
further strengthened by precipitation treatment.
17.33 Aluminium-iron alloys containing up to 2% iron are now in
MAGNESIUM (%>)
Fig.
17.1 The aluminium-magnesium thermal equilibrium diagram.
oc
LIQUID
OC + LIQUID
OC
OC
+ /3
production. These alloys can be rolled to foil which has higher strength
and good electrical conductivity, the rolling technique being such that
nearly all of the iron remains out of solid solution. In this condition it has
a considerable dispersion hardening effect whilst at the same time, since
little iron is in solution, electrical conductivity remains high.
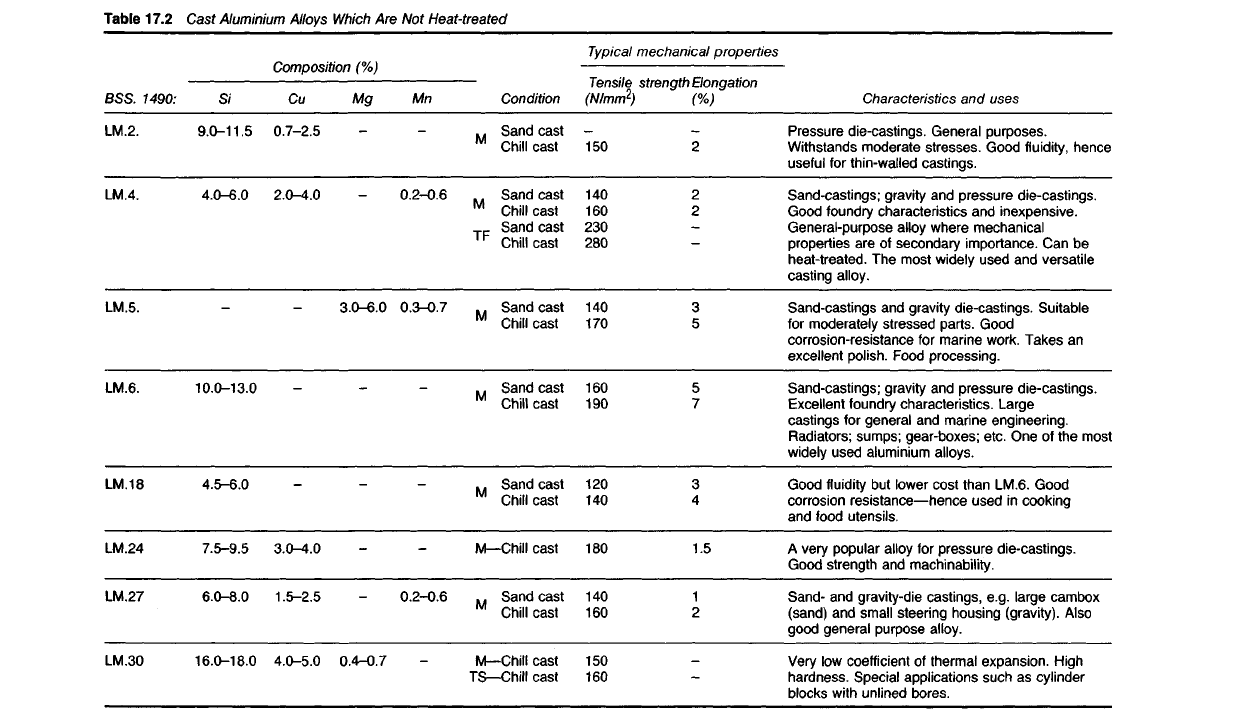
Cast Alloys which are not Heat-treated
17.40 Alloys in this group are widely used in the form of general-purpose
sand castings and die castings. They are used where rigidity, good corrosion
resistance and fluidity in casting are of greater importance than strength.
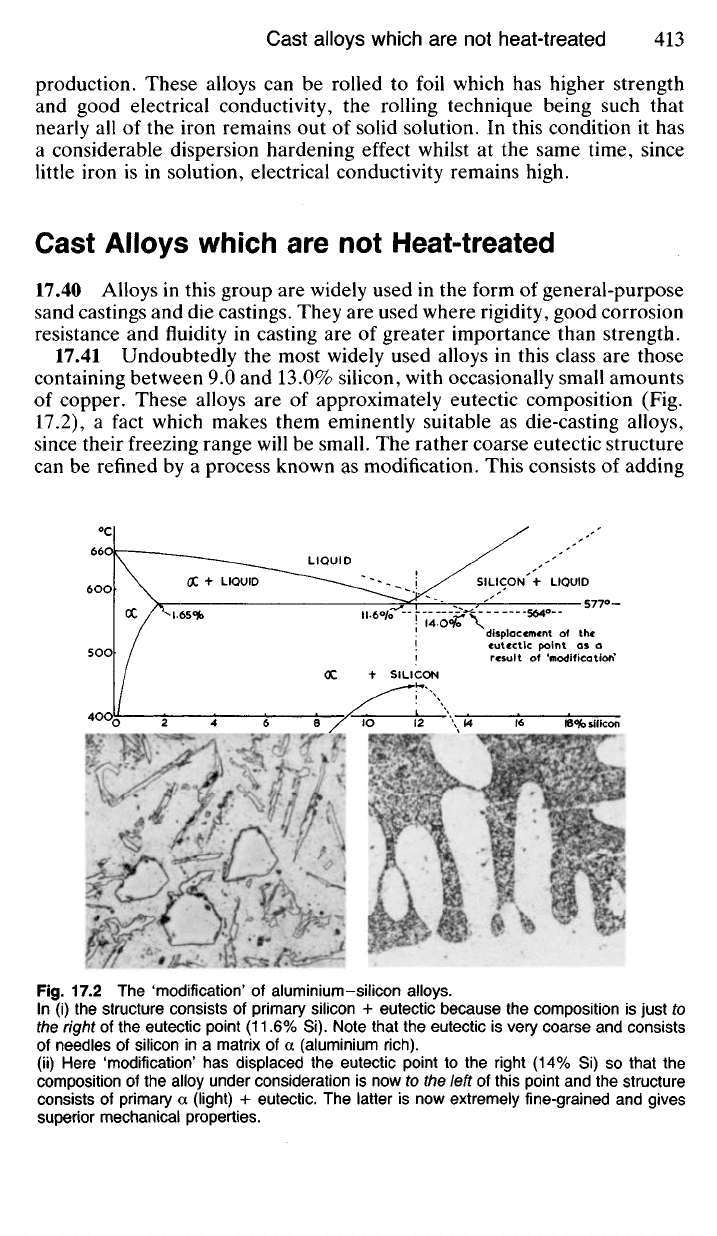
17.41 Undoubtedly the most widely used alloys in this class are those
containing between 9.0 and 13.0% silicon, with occasionally small amounts
of copper. These alloys are of approximately eutectic composition (Fig.
17.2),
a fact which makes them eminently suitable as die-casting alloys,
since their freezing range will be small. The rather coarse eutectic structure
can be refined by a process known as modification. This consists of adding
Fig.
17.2 The 'modification' of aluminium-silicon alloys.
In (i) the structure consists of primary silicon + eutectic because the composition is just to
the right of the eutectic point (11.6% Si). Note that the eutectic is very coarse and consists
of needles of silicon in a matrix of a (aluminium rich).
(ii) Here 'modification' has displaced the eutectic point to the right (14% Si) so that the
composition of the alloy under consideration is now to the left of this point and the structure
consists of primary a (light) + eutectic. The latter is now extremely fine-grained and gives
superior mechanical properties.
°c
LIQUID
OC + LIQUID
SILICON + LIQUID
displacement of the
cuteclic point as a
result of 'modification
1
OC
+
SILICON
I8<fc silicon
Table
17.2
Cast
Aluminium
Alloys
Which
Are Not
Heat-treated
Characteristics
and
uses
Pressure
die-castings. General purposes.
Withstands moderate stresses. Good
fluidity,
hence
useful
for
thin-walled castings.
Sand-castings; gravity
and
pressure die-castings.
Good foundry characteristics
and
inexpensive.
General-purpose
alloy
where mechanical
properties
are of
secondary
importance.
Can be
heat-treated.
The
most
widely used
and
versatile
casting alloy.
Sand-castings
and
gravity die-castings. Suitable
for
moderately stressed parts. Good
corrosion-resistance
for
marine work. Takes
an
excellent
polish.
Food processing.
Sand-castings; gravity
and
pressure die-castings.
Excellent foundry characteristics. Large
castings
for
general
and
marine engineering.
Radiators; sumps; gear-boxes;
etc. One of the
most
widely used aluminium alloys.
Good fluidity
but
lower cost than LM.6. Good
corrosion resistance—hence used
in
cooking
and food utensils.
A
very
popular
alloy
for
pressure die-castings.
Good strength
and
machinability.
Sand-
and
gravity-die castings,
e.g.
large cambox
(sand)
and
small steering housing (gravity).
Also
good general purpose alloy.
Very
low
coefficient
of
thermal expansion. High
hardness. Special
applications
such
as
cylinder
blocks with
unlined
bores.
Typical
mechanical properties
Tensile
strength
Elongation
(%)
2
2
2
3
5
5
7
3
4
1.5
1
2
(N/mm
d
)
150
140
160
230
280
140
170
160
190
120
140
180
140
160
150
160
Condition
M
Sand cast
M
Chill
cast
Sand cast
M
Chill
cast
TP
Sand cast
"~
Chill
cast
M
Sand cast
M
Chill cast
..
Sand cast
Chill
cast
M
Sand cast
M
Chill cast
M—Chill cast
M
Sand cast
M
Chill cast
M—Chill cast
TS-Chill
cast
Composition
(%)
Mn
0.2-0.6
0.3-0.7
0.2-0.6
Mg
3.0-6.0
0.4^-0.7
Cu
0.7-2.5
2.0-4.0
3.0-4.0
1.5-2.5
4.0-5.0
Si
9.0-11.5
4.0-6.0
10.0-13.0
4.5-6.0
7.5-9.5
6.0-8.0
16.0-18.0
BSS.
1490:
LM.2.
LM.4.
LM.5.
LM.6.
LM.18
LM.24
LM.27
LM.30

small amounts of sodium (about 0.01% by weight of the charge) to the
melt just before casting. The effect is to delay the precipitation of silicon
when the normal eutectic temperature is reached, and also cause a shift of
the eutectic composition towards the right of the equilibrium diagram.
Therefore, as much as 14% silicon may be present in a modified alloy
without any primary silicon crystals forming in the structure (Fig. 17.2).
It is thought that sodium collects in the liquid at its interface with the
newly-formed silicon crystals, inhibiting and delaying their growth. Thus,
undercooling occurs and new silicon nuclei are formed in large numbers
resulting in a relatively fine-grained eutectic structure. Remelting tends to
restore the original structure due to a loss of sodium by oxidation. The
modification process raises the tensile strength from 120 to 200 N/mm
2
and
the elongation from 5.0 to over 15.0%. The relatively high ductility of this
cast eutectic alloy is due to the fact that the a-solid-solution phase in the
eutectic constitutes nearly 90% of the total structure. As will be seen (Fig.
17.2) it is therefore continuous in the microstructure and acts as a cushion
against much of the brittleness arising from the hard silicon phase.
These aluminium-silicon alloys can therefore be produced in the
wrought form, though they are better known as materials for die-casting
and other types of casting where intricate sections are required, since high
fluidity and low shrinkage, as well as the narrow freezing range mentioned
above, make them very suitable for such purposes. Their high corrosion-
resistance makes these alloys useful for marine work, and the fact that
they are somewhat lighter than the aluminium-copper alloys makes them
suitable for aero and automobile construction.
17.42 Aluminium-copper alloys containing up to 10% copper were
popular in the early days of the aluminium industry. These contained
considerable amounts of the brittle compound CuAl
2
(see Fig. 17.3) and
were useful when rigidity and good casting properties were required in
components not subjected to shock. They also machined well but since
their corrosion-resistance was poor they have become completely obsolete.
However, small amounts of copper are added to some of the alu-
minium-silicon alloys in order to improve strength and machinability at
the cost of some losses in castability and corrosion resistance. One of these
alloys (LM4) though generally used in the 'as-cast' form is responsive to
heat-treatment.
17.43 The main feature of the aluminium-magnesium-manganese
alloys is good corrosion resistance which enables them to receive a high
polish. Since many of these alloys are also rigid and shock-resistant they
are very suitable for moderately-stressed parts in marine constructions.
Wrought Alloys which are Heat-treated
17.50 Without doubt the most metallurgically-important feature of the
aluminium alloys is the ability of some of them to undergo a change in
properties when suitably heat-treated. Although this phenomenon is
common to many alloys in which a change in solubility of some constituent
