Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

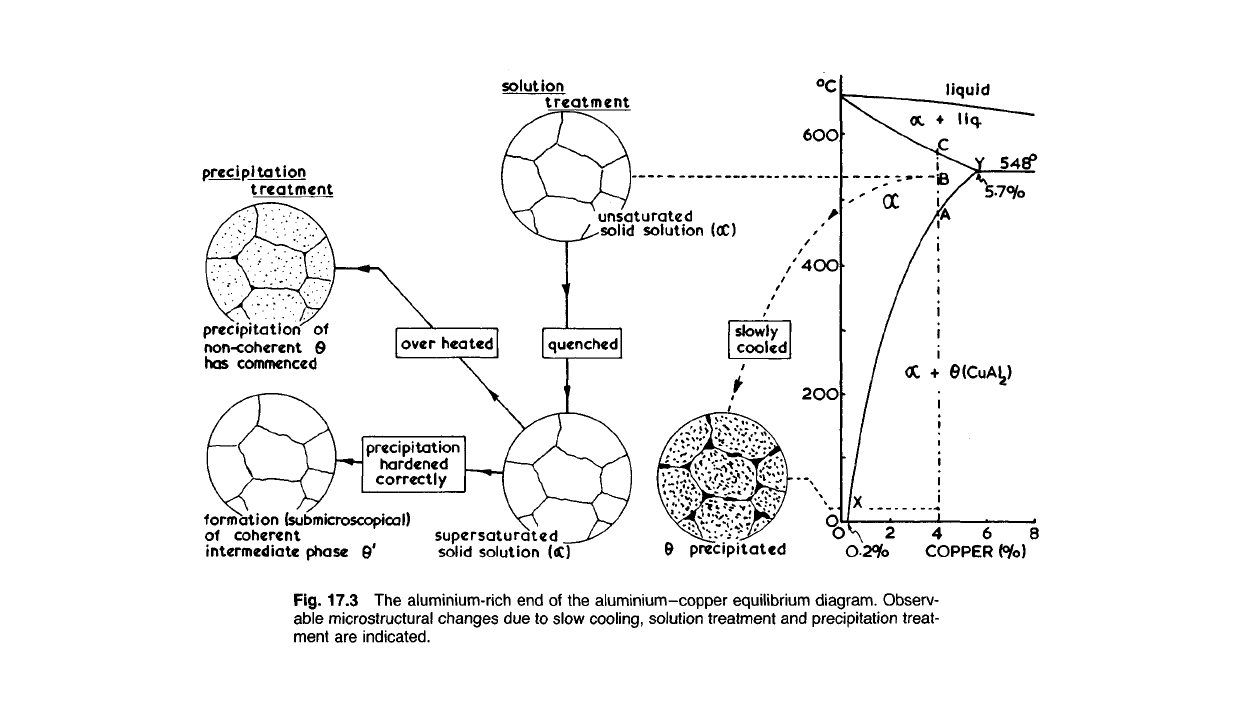
Fig. 17.3 The
aluminium-rich
end of the
aluminium-copper
equilibrium
diagram.
Observ-
able
microstructural changes due to slow
cooling,
solution
treatment and
precipitation
treat-
ment are
indicated.
COPPER Wo)
liquid
°C
solution
treatment
unsaturated
solid
solution
(OC)
slowly
cooled
quenched
9 precipitated
supersaturated
solid
solution
((JC)
over
heated
precipitation
hardened
correctly
formation (submicroscopical)
of
coherent
intermediate phase 9'
precipitation of
non-coherent 9
has
commenced
precipitation
treatment

in the solvent metal takes place with variation in temperature (9.90) it is
more widely used in suitable aluminium-base alloys than in any others.
The phenomenon was first observed by a German research metallurgist,
Dr. Alfred WiIm who, in 1906, noticed that an aluminium-copper alloy
which had been water-quenched from a temperature of about 500
0
C, sub-
sequently hardened unassisted at ambient temperature over a period of
several days. The strength increased in this way, reaching a maximum
value in just under a week, and the effect was subsequently known as
age-hardening. About four years later WiIm transferred the sole rights of
his patent to the Diirener Metal Works in Germany, and the alloy pro-
duced was named Duralumin. Although this name is often used to describe
any wrought aluminium alloy of this type, it is, strictly speaking the pro-
prietary name given to a series of alloys produced by certain companies
both here and abroad. The first significant use of duralumin was during
the First World War, when it found application in the structural members
of the airships bearing the name of Graf von Zeppelin.
17.51 The 'age-hardening' process can be accelerated and higher
strengths obtained if the quenched alloy is heated at temperatures up to
180
0
C.
Such treatment was originally known as artificial age-hardening but
both of these descriptions have been replaced in metallurgical nomencla-
ture by the general term precipitation hardening. Much speculation took
place over the years as to the fundamental nature of this phenomenon but
metallurgists now generally agree that the increases in strength and hard-
ness produced are directly connected with the formation of coherent pre-
cipitates (9.92) within the lattice of the parent solid solution. We will
consider the application of this principle to the precipitation-hardening of
aluminium-copper alloys.
17.52 Let us assume that we have an aluminium-copper alloy contain-
ing 4.0% copper. At temperatures above 550
0
C this will consist entirely of
a solid solution as indicated by the equilibrium diagram (Fig. 17.3). If we
now allow the alloy to cool very slowly to room temperature, equilibrium
will be reached at each stage and particles of the intermetallic compound
CuAl
2
(0) will form as a non-coherent precipitate. This precipitation will
commence at A and continue until at room temperature only 0.2% (X)
copper remains in solution in the aluminium. The resulting structure will
lack strength because only 0.2% copper is left in solution, and it will be
brittle because of the presence of coarse particles of CuAl
2
.
If the alloy is now slowly reheated, the particles of CuAl
2
will be gradu-
ally absorbed, until at A we once more have a complete solid solution a
(in industrial practice a slightly higher temperature, B, will be used to
ensure complete solution of the CuAl
2
). On quenching the alloy we retain
the copper in solution, and, in fact, produce a supersaturated solution of
copper in aluminium at room temperature. In this condition the alloy is
somewhat stronger and harder because there is more copper actually in
solid solution in the aluminium, and it is also much more ductile, because
the brittle particles of CuAl
2
are now absent. So far these phenomena
permit of a straightforward explanation, forthcoming from a simple study
of the microstructure. What happens subsequently is of a sub-microscopical
nature, that is, its observation is beyond the range of an ordinary optical
microscope.
17.53 If the quenched alloy is allowed to remain at room temperature,
it will be found that strength and hardness gradually increase (with a corre-
sponding reduction in ductility) and reach a maximum in about six days.
After this time has elapsed no further appreciable changes occur in the
properties. The completely a-phase structure obtained by quenching is not
the equilibrium structure at room temperature. It is in fact super-saturated
with copper so that there is a strong urge for copper to be rejected from
the solid solution a as particles of the non-coherent precipitate CuAb (0).
This stage is never actually reached at room temperature because of the
sluggishness of diffusion of the copper atoms within the aluminium lattice.
However, some movement does occur and the copper atoms take up pos-
itions within the aluminium lattice so that nuclei of the intermediate phase
(0') are formed. These nuclei are present as a coherent precipitate continu-
ous with the original a lattice, and in this form, cause distortion within the
a lattice. This effectively hinders the movement of dislocations (9.92) and
so the yield strength is increased. The sub-microscopical change within the
structure can be represented so:
Cu + 2Al -• &
[Al lattice] [Al lattice] [Intermediate
coherent
precipitate]
An improvement in properties over those obtained by ordinary natural
age-hardening can be attained by tempering the quenched alloy at tempera-
tures up to nearly 200
0
C for short periods. This treatment—called precipi-
tation treatment—increases the amount of the intermediate coherent
precipitate 0' by accelerating the rate of diffusion, and so strength and
hardness of the alloy rise still further. If the alloy is heated to a higher
temperature, a stage is reached where the structure begins to revert rapidly
to one of equilibrium and the coherent intermediate phase 0' precipitates
fully as non-coherent particles of 0 (CuAh):
0' - 0
[Intermediate [Non-coherent
coherent precipitate
precipitate] CuAl
2
]
When this occurs both strength and hardness begin to fall. Further
increases in temperature will cause the 0 particles to grow to a size making
them easily visible with an ordinary optical microscope, and this will be
accompanied by a progressive deterioration in mechanical properties. Fig.
17.4 illustrates the general effects of variations in time and temperature
during post-quenching treatment for a typical alloy of the precipitation-
hardening variety. At room temperature (20
0
C) the tensile strength
increases slowly and reaches a maximum of about 390 N/mm
2
after approxi-
mately 100 hours. Precipitation-treatment at temperatures above 100
0
C
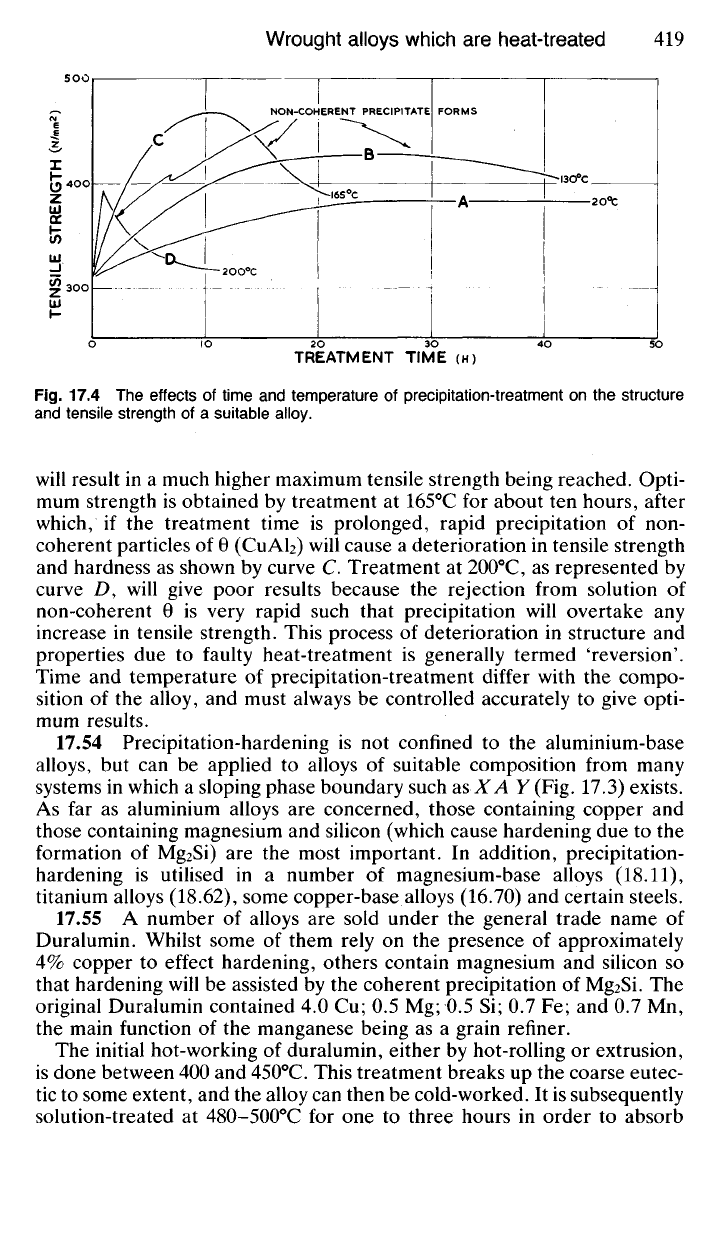
TREATMENT TIME (H)
Fig.
17.4 The effects of time and temperature of precipitation-treatment on the structure
and tensile strength of a suitable alloy.
will result in a much higher maximum tensile strength being reached. Opti-
mum strength is obtained by treatment at 165°C for about ten hours, after
which, if the treatment time is prolonged, rapid precipitation of non-
coherent particles of 6 (CuAl
2
) will cause a deterioration in tensile strength
and hardness as shown by curve C. Treatment at 200
0
C, as represented by
curve Z), will give poor results because the rejection from solution of
non-coherent 9 is very rapid such that precipitation will overtake any
increase in tensile strength. This process of deterioration in structure and
properties due to faulty heat-treatment is generally termed 'reversion'.
Time and temperature of precipitation-treatment differ with the compo-
sition of the alloy, and must always be controlled accurately to give opti-
mum results.
17.54 Precipitation-hardening is not confined to the aluminium-base
alloys, but can be applied to alloys of suitable composition from many
systems in which a sloping phase boundary such as X A Y (Fig. 17.3) exists.
As far as aluminium alloys are concerned, those containing copper and
those containing magnesium and silicon (which cause hardening due to the
formation of Mg
2
Si) are the most important. In addition, precipitation-
hardening is utilised in a number of magnesium-base alloys (18.11),
titanium alloys (18.62), some copper-base alloys (16.70) and certain steels.
17.55 A number of alloys are sold under the general trade name of
Duralumin. Whilst some of them rely on the presence of approximately
4%
copper to effect hardening, others contain magnesium and silicon so
that hardening will be assisted by the coherent precipitation of Mg
2
Si. The
original Duralumin contained 4.0 Cu; 0.5 Mg; 0.5 Si; 0.7 Fe; and 0.7 Mn,
the main function of the manganese being as a grain refiner.
The initial hot-working of duralumin, either by hot-rolling or extrusion,
is done between 400 and 450
0
C. This treatment breaks up the coarse eutec-
tic to some extent, and the alloy can then be cold-worked. It is subsequently
solution-treated at 480-500
0
C for one to three hours in order to absorb
TENSILE STRENGTH (NW)
NON-COHERENT PRECIPITATE FORMS

the CuAl
2
and Mg2Si and is then water-quenched. Accurate temperature
control is essential, as the temperature of treatment must be maintained
between A and C (Fig. 17.3). After quenching, cold-working operations
can be carried out on the resulting solid-solution structure if desired. The
alloy is subsequently hardened, either by allowing it to remain at room
temperature for about five days or by heating it to some temperature
between 100 and 160
0
C for up to ten hours, according to the properties
required. Heating at above 160
0
C may cause visible particles of CuAl
2
to appear in the microstructure, with consequent losses in strength and
hardness.
17.56 Just as the application of heat will accelerate precipitation-
hardening so will refrigeration impede the process. This fact was utilised
extensively in aircraft production during the Second World War. An alloy
rivet must be of such a composition that it will 'age' at ambient tempera-
ture,
since it is inappropriate to precipitation-harden a completed airframe
structure by any sort of furnace treatment. Unfortunately once solution-
treated such a rivet will soon begin to harden at ambient temperature and
if this hardening had begun any attempt to drive the rivet in this hard
condition would cause it to split. Hence, immediately after quenching from
the solution-treatment temperature, rivets were transferred to a refriger-
ator at about
—
20
0
C.
By this means age-hardening was considerably slowed
down and rivets could be stored at sub-zero temperatures until required.
17.57 Although the addition of copper forms the basis of many of the
precipitation-hardening aluminium alloys, copper is absent from a number
of them which rely instead on the presence of magnesium and silicon.
Such alloys have a high electrical conductivity approaching that of pure
aluminium, so that they can be used for the manufacture of overhead
conductors of electricity. Most commercial grades of aluminium contain
iron as an impurity, but in some of these alloys it is utilised in greater
amounts in order to increase strength by promoting the formation of FeALi,
which assists in precipitation-hardening. Titanium finds application as a
grain refiner, whilst other alloys containing zinc and chromium produce
tensile strengths in excess of 620 N/mm
2
in the heat-treated condition.
17.58 In the 1920s aluminium-lithium alloys were investigated as
being potentially useful in aircraft construction, since the low relative den-
sity of lithium (0.51) could mean reductions of 10% in the density of alloys
containing the metal. Subsequently interest was revived in lithium as an
alloying element and the US Navy's 'Vigilante' contains the alloy '2020',
though here lithium was used to increase the temperature range over which
existing aluminium-copper alloys could be used rather than to reduce
weight. More recent research has been based on alloys containing 2.5%
lithium along with small amounts of magnesium, copper and—as a grain
refiner—zirconium. The development of RSP (9.110) has led to experiments
with alloys containing 4% lithium since under these conditions more
lithium is retained in solution.
Since lithium is one of the chemically reactive 'alkali metals' in Group
I of the Periodic Classification (Fig. 1.2) there are difficulties encountered
in melting and casting these alloys. Moreover, early aluminium-lithium
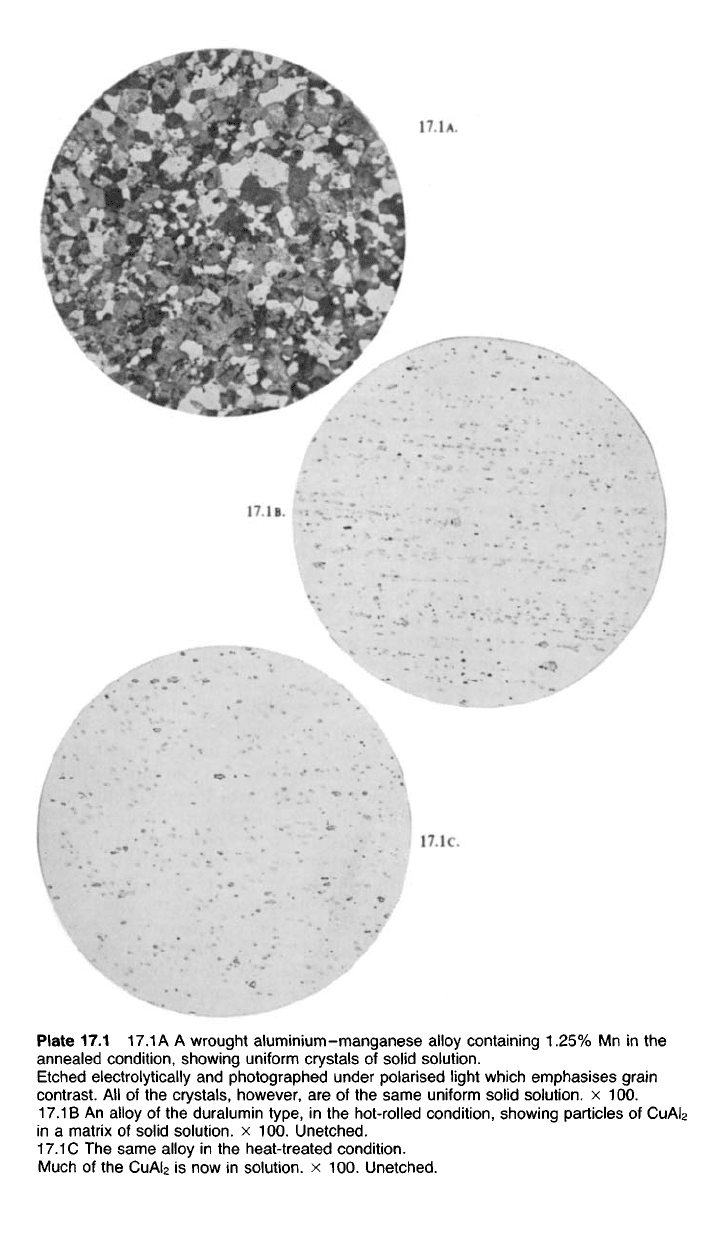
Plate 17.1
17.1
A A wrought aluminium-manganese alloy containing 1.25% Mn in the
annealed condition, showing uniform crystals of solid solution.
Etched electrolytically and photographed under polarised light which emphasises grain
contrast. All of the crystals, however, are of the same uniform solid solution, x 100.
17.1 B An alloy of the duralumin type, in the hot-rolled condition, showing particles of
CuAI
2
in a matrix of solid solution, x 100. Unetched.
17.1
C The same alloy in the heat-treated condition.
Much of the
CuAI
2
is now in solution, x 100. Unetched.
17.1c.
17.1B.
17.1A.
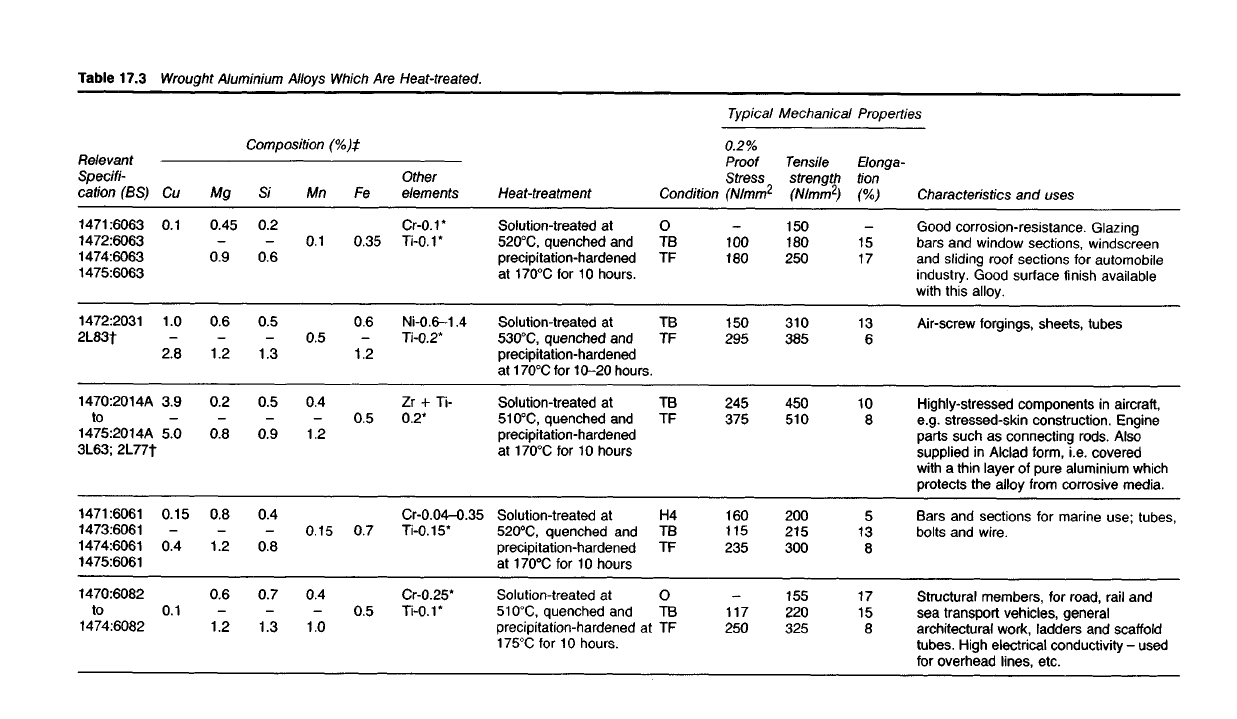
Table 17.3
Wrought
Aluminium Alloys
Which
Are Heat-treated.
Typical
Mechanical
Properties
Characteristics
and
uses
Good
corrosion-resistance. Glazing
bars and window sections, windscreen
and sliding roof sections for automobile
industry.
Good surface finish available
with
this alloy.
Air-screw
forgings, sheets, tubes
Highly-stressed components in aircraft,
e.g. stressed-skin construction. Engine
parts such as
connecting
rods. Also
supplied
in Alclad form, i.e. covered
with a thin layer of pure aluminium which
protects the alloy from corrosive media.
Bars
and sections for marine use; tubes,
bolts and wire.
Structural members, for road,
rail
and
sea transport vehicles, general
architectural
work,
ladders
and scaffold
tubes. High electrical conductivity - used
for
overhead
lines,
etc.
Elonga-
tion
(%)
15
17
13
6
10
8
5
13
8
17
15
8
Tensile
strength
(N/mm
2
)
150
180
250
310
385
450
510
200
215
300
155
220
325
0.2%
Proof
Stress
(N/mm
2
100
180
150
295
245
375
160
115
235
117
250
Condition
O
TB
TF
TB
TF
TB
TF
H4
TB
TF
O
TB
TF
Heat-treatment
Solution-treated at
520
0
C, quenched and
precipitation-hardened
at
170
0
C for 10 hours.
Solution-treated at
530
0
C,
quenched
and
precipitation-hardened
at
170°Cfor 10-20 hours.
Solution-treated at
510°C, quenched and
precipitation-hardened
at
170°Cfor 10 hours
Solution-treated at
520
0
C, quenched and
precipitation-hardened
at
170
0
C for 10 hours
Solution-treated
at
510
0
C,
quenched and
precipitation-hardened at
175°Cfor
10 hours.
Composition
(%)t
Other
elements
Cr-0.1*
Ti-0.1*
Ni-0.6-1.4
Ti-0.2*
Zr
+ Ti-
0.2*
Cr-0.04-0.35
Ti-0.15*
Cr-0.25*
Ti-0.1*
Fe
0.35
0.6
1.2
0.5
0.7
0.5
Mn
0.1
0.5
0.4
1.2
0.15
0.4
1.0
S/
0.2
0.6
0.5
1.3
0.5
0.9
0.4
0.8
0.7
1.3
Mg
0.45
0.9
0.6
1.2
0.2
0.8
0.8
1.2
0.6
1.2
Cu
0.1
1.0
2.8
3.9
5.0
0.15
0.4
0.1
Relevant
Specifi-
cation
(BS)
1471:6063
1472:6063
1474:6063
1475:6063
1472:2031
2L83|
1470:2014A
to
1475:2014A
3L63;
2L77f
1471:6061
1473:6061
1474:6061
1475:6061
1470:6082
to
1474:6082
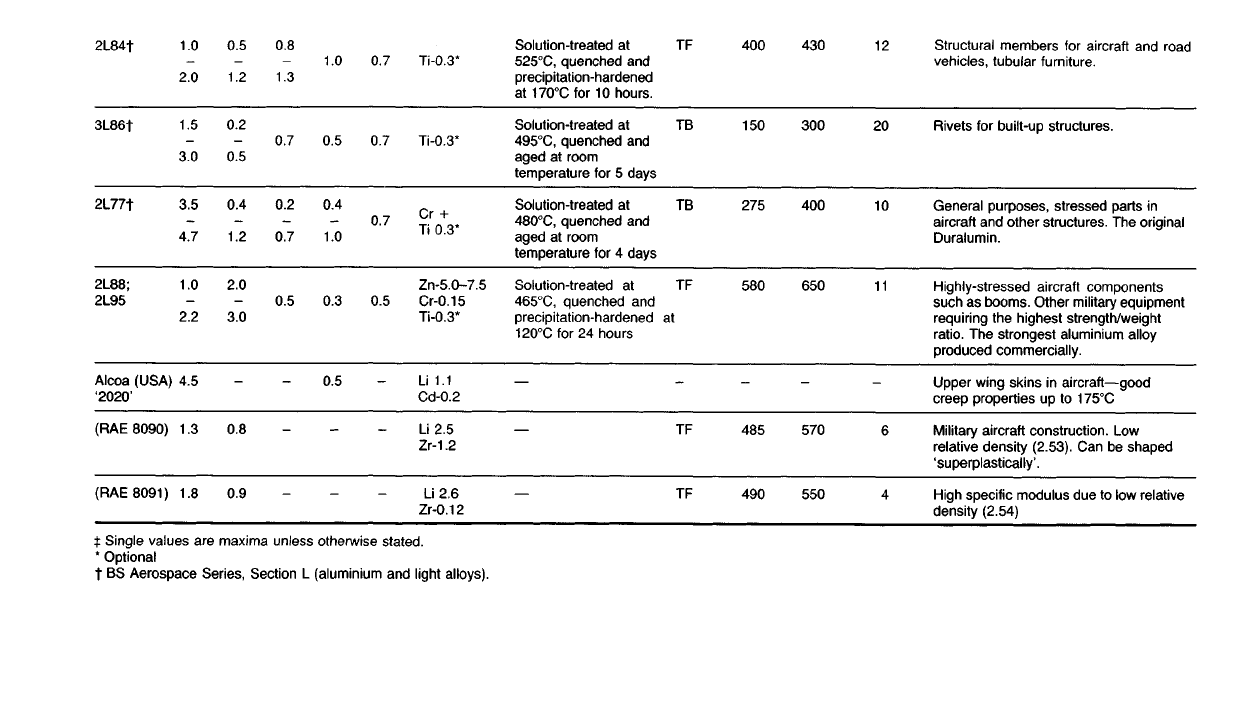
Structural
members
for
aircraft
and
road
vehicles, tubular
furniture.
Rivets
for
built-up structures.
General purposes, stressed parts
in
aircraft
and
other structures.
The
original
Duralumin.
Highly-stressed aircraft components
such
as
booms. Other military equipment
requiring
the
highest strength/weight
ratio.
The
strongest aluminium alloy
produced commercially.
Upper wing skins
in
aircraft—good
creep properties
up to
175°C
Military aircraft construction.
Low
relative density (2.53).
Can be
shaped
'superplastically'.
High specific modulus
due to low
relative
density
(2.54)
12
20
10
11
6
4
430
300
400
650
570
550
400
150
275
580
485
490
TF
TB
TB
TF
TF
TF
Solution-treated
at
525
0
C,
quenched
and
precipitation-hardened
at
170
0
C
for 10
hours.
Solution-treated
at
495
0
C,
quenched
and
aged
at
room
temperature
for 5
days
Solution-treated
at
48O
0
C,
quenched
and
aged
at
room
temperature for
4
days
Solution-treated
at
465°C, quenched
and
precipitation-hardened
at
120°Cfor
24
hours
Ti-0.3*
Ti-0.3*
Cr
+
Ti
0.3*
Zn-5.0-7.5
Cr-0.15
Ti-0.3*
Li
1.1
Cd-0.2
Li
2.5
Zr-1.2
Li
2.6
Zr-0.12
0.7
0.7
0.7
0.5
1.0
0.5
0.4
1.0
0.3
0.5
0.8
1.3
0.7
0.2
0.7
0.5
0.5
1.2
0.2
0.5
0.4
1.2
2.0
3.0
0.8
0.9
1.0
2.0
1.5
3.0
3.5
4.7
1.0
2.2
4.5
1.3
1.8
2L84f
3L86f
2L77f
2L88;
2L95
Alcoa
(USA)
'2020'
(RAE
8090)
(RAE
8091)
t
Single values
are
maxima unless otherwise stated.
*
Optional
t BS
Aerospace Series, Section
L
(aluminium
and
light alloys).

alloys failed because of poor ductility and impact values. However, in
recent British alloys, these values have been maintained at satisfactory
levels along with a relative density of only 2.54, and hence an increase in
specific modulus (2.36) of up to 25%.
Precipitation hardening is due mainly to the phase S' (AbLi) a precipi-
tate coherent with the matrix. Magnesium is added to reduce density
further and also confer limited solid-solution hardening, but unfortunately
this leads to the formation of detrimental grain-boundary precipitates of
Al
2
MgLi. However the addition of some copper causes the precipitation
of 6' and the phase Ti (Al
2
CuLi) instead. The simultaneous precipitation
of two phases gives rise to an appreciable improvement in impact toughness
when precipitation of the two phases is suitably controlled.
A selection of typical wrought heat-treatable alloys of aluminium is given
in Table 17.3.
Cast Alloys which are Heat-treated
17.60 German air-raids on England during the First World War were
carried out by 'Zeppelins', the airframes of which were constructed from
Duralumin. Britain's defence against these raids was provided by heavier-
than-air flying machines, the airframes of which were largely of non-
metallic construction. Nevertheless light-weight internal combustion
engines were required to power these aircraft and the National Physical
Laboratory carried out much of the development work on new aluminium
alloys in those days.
One of the very successful alloys developed by NPL is still known by
the series letter used to identify it during development—Y Alloy. Like
Duralumin this is of the 4% copper type but contains also about 2% nickel
and 1.5% magnesium. Precipitation-hardening is due to the combined
effects of coherent precipitates based on both CuAl
2
and NiALi, and whilst
fundamentally a casting alloy it can be used in the wrought condition. As
a cast alloy it is useful where reasonable strength at high temperatures is
required, as in high-duty pistons and cylinder heads for internal combustion
engines. Its relatively low coefficient of expansion also makes it useful in
this direction.
17.61 There are other casting alloys similar in composition to
4
Y Alloy',
but containing rather less copper. Some of these alloys (eg RR50) can be
precipitation hardened by heating at 155-170
0
C without the usual prelimi-
nary solution-treatment at a high temperature. This is due to sluggishness
in precipitation of phases like CuAl
2
and NiAl
3
as the casting cools, follow-
ing the initial pouring process. The 5.0 and 13.0% types of aluminium-sili-
con alloy are brought into this group by the addition of small amounts of
either copper and nickel or magnesium and manganese, which render the
alloys amenable to precipitation-hardening.
17.62 The use of aluminium-silicon alloys for automobile cylinder
blocks has obvious advantages in terms of weight saved as compared with
the usual iron casting. Other advantages include higher thermal conduc-
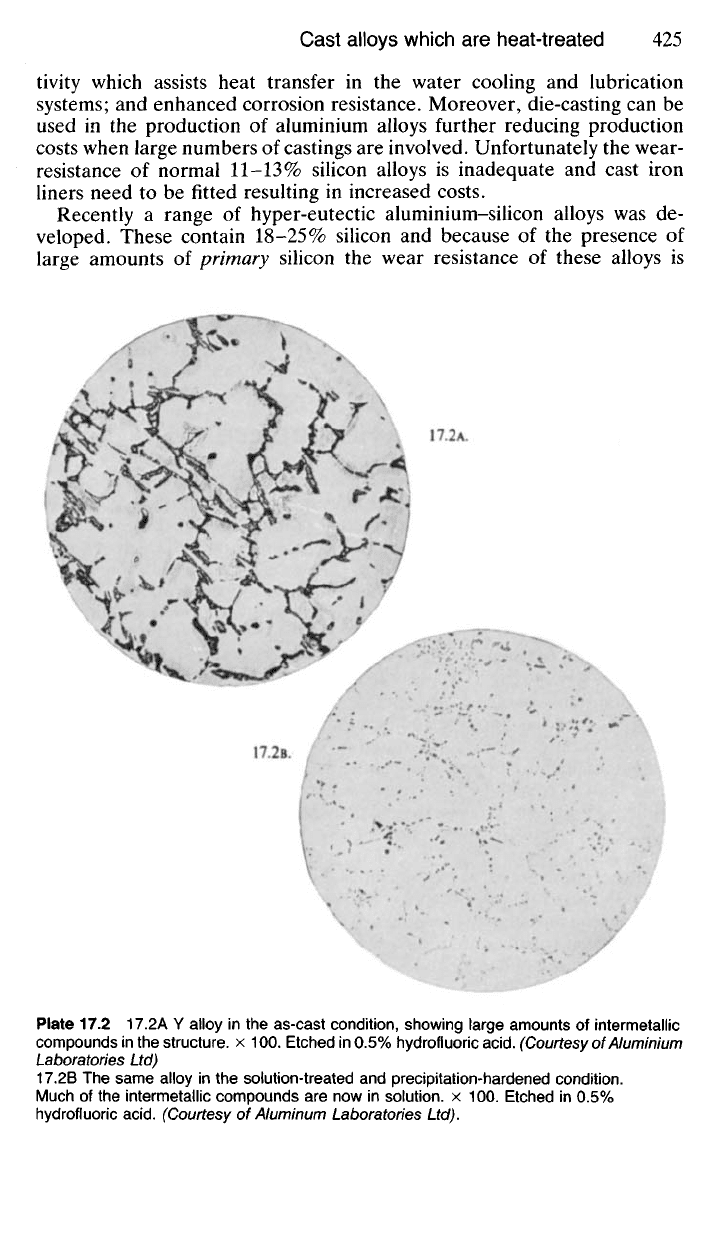
Plate 17.2 17.2A Y alloy in the as-cast condition, showing large amounts of intermetallic
compounds in the structure, x 100. Etched in 0.5% hydrofluoric
acid.
(Courtesy of Aluminium
Laboratories Ltd)
17.2B The same alloy in the solution-treated and precipitation-hardened condition.
Much of the intermetallic compounds are now in solution, x 100. Etched in 0.5%
hydrofluoric
acid.
(Courtesy of Aluminum Laboratories Ltd).
tivity which assists heat transfer in the water cooling and lubrication
systems; and enhanced corrosion resistance. Moreover, die-casting can be
used in the production of aluminium alloys further reducing production
costs when large numbers of castings are involved. Unfortunately the wear-
resistance of normal
11-13%
silicon alloys is inadequate and cast iron
liners need to be fitted resulting in increased costs.
Recently a range of hyper-eutectic aluminium-silicon alloys was de-
veloped. These contain 18-25% silicon and because of the presence of
large amounts of primary silicon the wear resistance of these alloys is
17.2 A.
17.2B.
