Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.


xiv Contents
This page has been reformatted by Knovel to provide easier navigation.
9.5 Case IV – Two Metals Mutually Soluble in All
Proportions in the Liquid State But Only Partially
Soluble in the Solid State .............................................. 196
9.6 Case V – a System in Which a Peritectic
Transformation Is Involved ............................................ 200
9.7 Case VI – Systems Containing One or More
Intermediate Phase ....................................................... 202
9.8 Precipitation from a Solid Solution ................................ 206
9.9 Alloys Containing More Than Two Metals .................... 210
9.10 Rapid Solidification Processes (RSP) ........................... 211
9.11 Exercises ....................................................................... 212
9.12 Bibliography ................................................................... 217
10. Practical Metallography ............................................. 218
10.1 The Preparation of Specimens for Microscopical
Examination ................................................................... 219
10.2 The Metallurgical Microscope ........................................ 228
10.3 Macro-examination ........................................................ 234
10.4 Sulphur Printing ............................................................. 237
10.5 Exercises ....................................................................... 237
10.6 Bibliography ................................................................... 238
11. The Heat-treatment of Plain Carbon Steels – (I) ....... 239
11.1 Impurities in Steel .......................................................... 248
11.2 The Heat-treatment of Steel .......................................... 251
11.3 Annealing ....................................................................... 252
11.4 Normalising .................................................................... 257
11.5 Exercises ....................................................................... 257
11.6 Bibliography ................................................................... 258
12. The Heat-treatment of Plain Carbon Steels – (II) ...... 259
12.1 Hardening ...................................................................... 260
12.2 Tempering ...................................................................... 266

Contents xv
This page has been reformatted by Knovel to provide easier navigation.
12.3 Isothermal Transformations ........................................... 270
12.4 Hardenability and Ruling Section .................................. 279
12.5 British Standard Specifications for Carbon
Steels ............................................................................. 281
12.6 Exercises ....................................................................... 283
12.7 Bibliography ................................................................... 284
13. Alloy Steels ................................................................. 285
13.1 Nickel Steels .................................................................. 294
13.2 Chromium Steels ........................................................... 297
13.3 Nickel-Chromium Steels ................................................ 302
13.4 Steels Containing Molybdenum .................................... 309
13.5 Steels Containing Vanadium ......................................... 309
13.6 Heat-resisting Steels ..................................................... 312
13.7 Manganese Steels ......................................................... 317
13.8 Steels Containing Tungsten .......................................... 320
13.9 Steels Containing Cobalt ............................................... 324
13.10 Steels Containing Boron ................................................ 326
13.11 Steels Containing Silicon ............................................... 328
13.12 Steels Containing Copper ............................................. 328
13.13 HSLA and Other 'Micro-alloyed' Steels ......................... 330
13.14 Exercises ....................................................................... 331
13.15 Bibliography ................................................................... 332
14. Complex Ferrous Alloys ............................................ 333
14.1 High-speed Steels ......................................................... 333
14.2 Cemented Carbide and Other 'Cermet' Cutting
Materials ........................................................................ 340
14.3 Magnetic Properties and Materials ................................ 341
14.4 Exercises ....................................................................... 351
14.5 Bibliography ................................................................... 352

xvi Contents
This page has been reformatted by Knovel to provide easier navigation.
15. Cast Irons and Alloy Cast Irons ................................. 353
15.1 The Effects of Composition on the Structure of
Cast Iron ........................................................................ 354
15.2 The Effect of Rate of Cooling on the Structure of
Cast Iron ........................................................................ 357
15.3 The Microstructure of Cast Iron ..................................... 360
15.4 'Growth' in Cast Irons .................................................... 360
15.5 Varieties and Uses of Ordinary Cast Iron ..................... 361
15.6 High-strength Cast Irons ............................................... 362
15.7 Alloy Cast Irons .............................................................. 371
15.8 Exercises ....................................................................... 372
15.9 Bibliography ................................................................... 373
16. Copper and the Copper-base Alloys ......................... 374
16.1 Properties and Uses of Copper ..................................... 375
16.2 The Copper-base Alloys ................................................ 378
16.3 The Brasses ................................................................... 378
16.4 The Tin Bronzes ............................................................ 387
16.5 Aluminium Bronze ......................................................... 392
16.6 Copper-Nickel Alloys ..................................................... 397
16.7 Copper Alloys Which Can Be Precipitation
Hardened ....................................................................... 399
16.8 'Shape Memory' Alloys .................................................. 402
16.9 Exercises ....................................................................... 404
16.10 Bibliography ................................................................... 404
17. Aluminium and Its Alloys ........................................... 406
17.1 Alloys of Aluminium ....................................................... 408
17.2 Wrought Alloys Which Are Not Heat-treated ................ 412
17.3 Cast Alloys Which Are Not Heat-treated ....................... 413
17.4 Wrought Alloys Which Are Heat-treated ....................... 415
17.5 Cast Alloys Which Are Heat-treated .............................. 424

Contents xvii
This page has been reformatted by Knovel to provide easier navigation.
17.6 Exercises ....................................................................... 428
17.7 Bibliography ................................................................... 429
18. Other Non-ferrous Metals and Alloys ....................... 430
18.1 Magnesium-base Alloys ................................................ 430
18.2 Zinc-base Die-casting Alloys ......................................... 431
18.3 Nickel-Chromium High-temperature Alloys ................... 437
18.4 Bearing Metals ............................................................... 441
18.5 Fusible Alloys ................................................................. 447
18.6 Titanium and Its Alloys .................................................. 447
18.7 Uranium ......................................................................... 452
18.8 Some Uncommon Metals .............................................. 455
18.9 Exercises ....................................................................... 460
18.10 Bibliography ................................................................... 461
19. The Surface Hardening of Steels ............................... 462
19.1 Case-hardening ............................................................. 462
19.2 Case-hardening Steels .................................................. 470
19.3 Nitriding .......................................................................... 472
19.4 Surface Hardening by Localised Heat-treatment .......... 477
19.5 Friction Surfacing ........................................................... 478
19.6 Exercises ....................................................................... 479
19.7 Bibliography ................................................................... 480
20. Metallurgical Principles of the Joining of
Metals .......................................................................... 481
20.1 Soldering ........................................................................ 482
20.2 Brazing ........................................................................... 486
20.3 Welding .......................................................................... 488
20.4 Fusion Welding Processes ............................................ 489
20.5 Solid-phase Welding ...................................................... 494
20.6 The Microstructure of Welds ......................................... 496
20.7 The Inspection and Testing of Welds ............................ 497

xviii Contents
This page has been reformatted by Knovel to provide easier navigation.
20.8 The Weldability of Metals and Alloys ............................ 499
20.9 Exercises ....................................................................... 504
20.10 Bibliography ................................................................... 504
21. Metallic Corrosion and Its Prevention ...................... 506
21.1 The Mechanism of Corrosion ........................................ 507
21.2 Electrolytic Action or Wet Corrosion Involving
Mechanical Stress ......................................................... 518
21.3 Electrolytic Action or Wet Corrosion Involving
Electrolytes of Non-uniform Composition ...................... 520
21.4 The Prevention of Corrosion ......................................... 523
21.5 The Use of a Metal or Alloy Which Is Inherently
Corrosion-resistant ........................................................ 524
21.6 Protection by Metallic Coatings ..................................... 525
21.7 Protection by Oxide Coatings ........................................ 530
21.8 Protection by Other Non-metallic Coatings ................... 532
21.9 Cathodic Protection ....................................................... 535
21.10 Exercises ....................................................................... 536
21.11 Bibliography ................................................................... 537
Answers to Numerical Problems ..................................... 538
Index ................................................................................... 541

1
Some Fundamental
Chemistry
1.10 Towards the end of the fifteenth century the technology of shipbuild-
ing was sufficiently advanced in Europe to allow Columbus to sail west
into the unknown in a search for a new route to distant Cathay. Earlier
that century far to the east in Samarkand in the empire of Tamerlane,
the astronomer Ulug Beg was constructing his great sextant—the massive
quadrant of which we can still see to-day—to measure the period of our
terrestrial year. He succeeded in this enterprise with an error of only 58
seconds, a fact which the locals will tell you with pride. Yet at that time
only seven metals were known to man—copper, silver, gold, mercury,
iron, tin and lead; though some of them had been mixed to produce alloys
like bronze (copper and tin), pewter (tin and lead) and steel (iron and
carbon).
By 1800 the number of known metals had risen to 23 and by the begin-
ning of the twentieth century to 65. Now, all 70 naturally occurring metallic
elements are known to science and an extra dozen or so have been created
by man from the naturally occurring radioactive elements by various pro-
cesses of 'nuclear engineering'. Nevertheless metallurgy, though a modern
science, has its roots in the ancient crafts of smelting, shaping and treat-
ment of metals. For several hundreds of years smiths had been hardening
steel using heat-treatment processes established painstakingly by trial and
error, yet it is only during this century that metallurgists discovered how
the hardening process worked. Likewise during the First World War the
author's father, then in the Royal Flying Corps, was working with fighter
aeroplanes the engines of which relied on 'age-hardening' aluminium
alloys; but it was quite late in the author's life before a plausible expla-
nation of age-hardening was forthcoming.
Since the days of the Great Victorians there has been an upsurge in
metallurgical research and development, based on the fundamental
sciences of physics and chemistry. To-day a vast reservoir of metallurgical
knowledge exists and the metallurgist is able to design materials to meet
the ever exacting demands of the engineer. Sometimes these demands are
over optimistic and it is hoped that this book may help the engineer to
appreciate the limitations, as well as the expanding range of properties, of
modern alloys.
1.11 Whilst steel is likely to remain the most important metallurgical
material available to the engineer we must not forget the wide range of
relatively sophisticated alloys which have been developed during this cen-
tury. As a result of such development an almost bewildering list of alloy
compositions confronts the engineer in his search for an alloy which will
be both technically and economically suitable for his needs. Fortunately
most of the useful alloys have been classified and rigid specifications laid
down for them by such official bodies as the British Standards Institution
(BSI) and in the USA, the American Society for Testing Materials
(ASTM). Now that we are in Europe' such bodies as Association Frangaise
de Normalisation (AFNOR) and Deutscher Normenausschuss (DNA) also
become increasingly involved.
Sadly, it may be that like many of our public libraries here in the Midlands,
your local library contains proportionally fewer books on technological mat-
ters than it did fifty years ago, and that meagre funds have been expended
on works dealing with the private life of Gazza—or the purple passion publi-
cations of Mills and Boon. Nevertheless at least one library in your region
should contain, by national agreement, a complete set of British Standards
Institution Specifications. In addition to their obvious use, these are a valu-
able mine of information on the compositions and properties of all of our
commercial alloys and engineering materials. A catalogue of all Specifi-
cation Numbers will be available at the information desk. Hence, forearmed
with the necessary metallurgical knowledge, the engineer is able to select an
alloy suitable to his needs and to quote its relevant specification index when
the time comes to convert design into reality.
Atoms,
Elements and Compounds
1.20 It would be difficult to study metallurgy meaningfully without relat-
ing mechanical properties to the elementary forces acting between the
atoms of which a metal is composed. We shall study the structures of atoms
later in the chapter but it suffices at this stage to regard these atoms as tiny
spheres held close to one another by forces of attraction.
1.21 If in a substance all of these atoms are of the same type then the
substance is a chemical element. Thus the salient property of a chemical
element is that it cannot be split up into simpler substances whether by
mechanical or chemical means. Most of the elements are chemically reac-
tive,
so that we find very few of them in their elemental state in the Earth's
crust—oxygen and nitrogen mixed together in the atmosphere are the most
common, whilst a few metals such as copper, gold and silver, also occur
uncombined. Typical substances occurring naturally contain atoms of two
or more kinds.

1.22
Most of the substances we encounter are either chemical com-
pounds or mixtures. The difference between the two is that a compound
is formed when there is a chemical join at the surfaces of two or more
different atoms, whilst in a mixture only mechanical 'entangling' occurs
between discrete particles of the two substances. For example, the pow-
dered element sulphur can be mixed with iron filings and easily separated
again by means of a magnet, but if the mixture is gently heated a vigorous
chemical reaction proceeds and a compound called iron sulphide is formed.
This is different in appearance from either of the parent elements and its
decomposition into the parent elements, sulphur and iron, is now more
difficult and can be accomplished only be chemical means.
1.23 Chemical elements can be represented by a symbol which is usu-
ally an abbreviation of either the English or Latin name, eg O stands
for oxygen whilst Fe stands for 'ferrum', the Latin equivalent of iron'.
Ordinarily, a symbol written thus refers to a single atom of the element,
whilst two atoms (constituting what in this instance we call a molecule)
would be indicated so: O2.
1.24 Table 1.1 includes some of the more important elements we are
likely to encounter in a study of metallurgy. The term 'relative atomic
mass',
(formerly 'atomic weight'), mentioned in this table must not be con-
fused with the relative density of the element. The latter value will depend
upon how closely the atoms, whether small or large, are packed together.
Since atoms are very small particles (the mass of the hydrogen atom is
1.673 x 10~
27
kg), it would be inconvenient to use such small values in
everyday chemical calculations. Consequently, since the hydrogen atom
was known to be the smallest, its relative mass was taken as unity and the
relative masses of the atoms of other elements calculated as multiples of
this.
Thus relative atomic mass became
mass of one atom of the element
mass of one atom of hydrogen
Later it was found more useful to adjust the relative atomic mass of oxygen
(by far the most common element) to exactly 16.0000. On this basis the
relative atomic mass of hydrogen became 1.008 instead of
1.0000.
More
recently chemists and physicists have agreed to relate atomic masses to
that of the carbon isotope (C = 12.0000). (See paragraph
1.90.)
1.25 The most common metallic element in the Earth's crust is alu-
minium (Table 1.2) but as a commercially usable metal it is not the cheap-
est. This is because clay, the most abundant mineral containing aluminium,
is very difficult—and therefore costly—to decompose chemically. There-
fore our aluminium supply comes from the mineral bauxite (originally
mined near the village of Les Baux, in France), which is a relatively scarce
ore.
It will be seen from the table that apart from iron most of the useful
metallic elements account for only a very small proportion of the Earth's
crust. Fortunately they occur in relatively concentrated deposits which
makes their mining and extraction economically possible.
In passing it is interesting to note that in the Universe as a whole hydro-
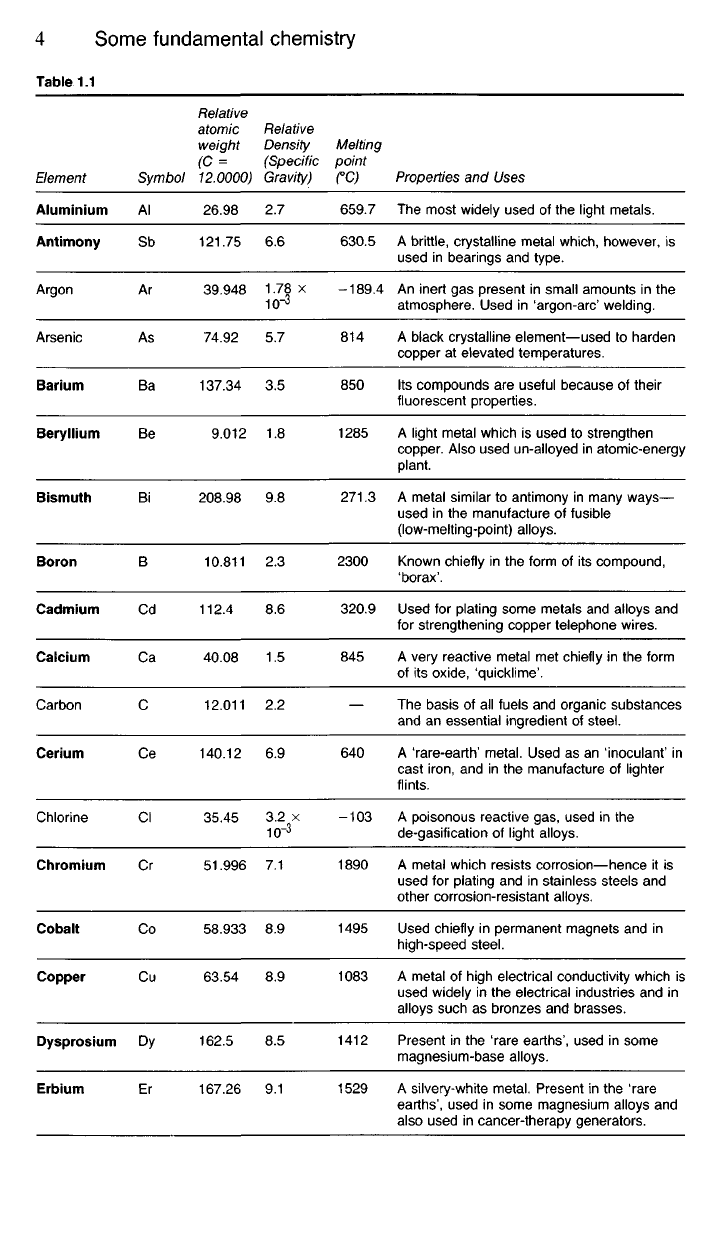
Table 1.1
Element
Aluminium
Antimony
Argon
Arsenic
Barium
Beryllium
Bismuth
Boron
Cadmium
Calcium
Carbon
Cerium
Chlorine
Chromium
Cobalt
Copper
Dysprosium
Erbium
Symbol
Al
Sb
Ar
As
Ba
Be
Bi
B
Cd
Ca
C
Ce
Cl
Cr
Co
Cu
Dy
Er
Relative
atomic
weight
(C =
12.0000)
26.98
121.75
39.948
74.92
137.34
9.012
208.98
10.811
112.4
40.08
12.011
140.12
35.45
51.996
58.933
63.54
162.5
167.26
Relative
Density
(Specific
Gravity)
2.7
6.6
1.78 x
10~
3
5.7
3.5
1.8
9.8
2.3
8.6
1.5
2.2
6.9
3.2 x
10-
3
7.1
8.9
8.9
8.5
9.1
Melting
point
CC)
659.7
630.5
-189.4
814
850
1285
271.3
2300
320.9
845
640
-103
1890
1495
1083
1412
1529
Properties and Uses
The most widely used of the light metals.
A brittle, crystalline metal which, however, is
used in bearings and type.
An inert gas present in small amounts in the
atmosphere. Used in 'argon-arc' welding.
A black crystalline element—used to harden
copper at elevated temperatures.
Its compounds are useful because of their
fluorescent properties.
A light metal which is used to strengthen
copper. Also used un-alloyed in atomic-energy
plant.
A metal similar to antimony in many ways—
used in the manufacture of fusible
(low-melting-point) alloys.
Known chiefly in the form of its compound,
'borax'.
Used for plating some metals and alloys and
for strengthening copper telephone wires.
A very reactive metal met chiefly in the form
of its oxide, 'quicklime'.
The basis of all fuels and organic substances
and an essential ingredient of steel.
A 'rare-earth' metal. Used as an 'inoculant' in
cast
iron,
and in the manufacture of lighter
flints.
A poisonous reactive gas, used in the
de-gasification of light alloys.
A metal which resists corrosion—hence it is
used for plating and in stainless steels and
other corrosion-resistant alloys.
Used chiefly in permanent magnets and in
high-speed steel.
A metal of high electrical conductivity which is
used widely in the electrical industries and in
alloys such as bronzes and brasses.
Present in the 'rare earths', used in some
magnesium-base alloys.
A silvery-white metal. Present in the 'rare
earths', used in some magnesium alloys and
also used in cancer-therapy generators.
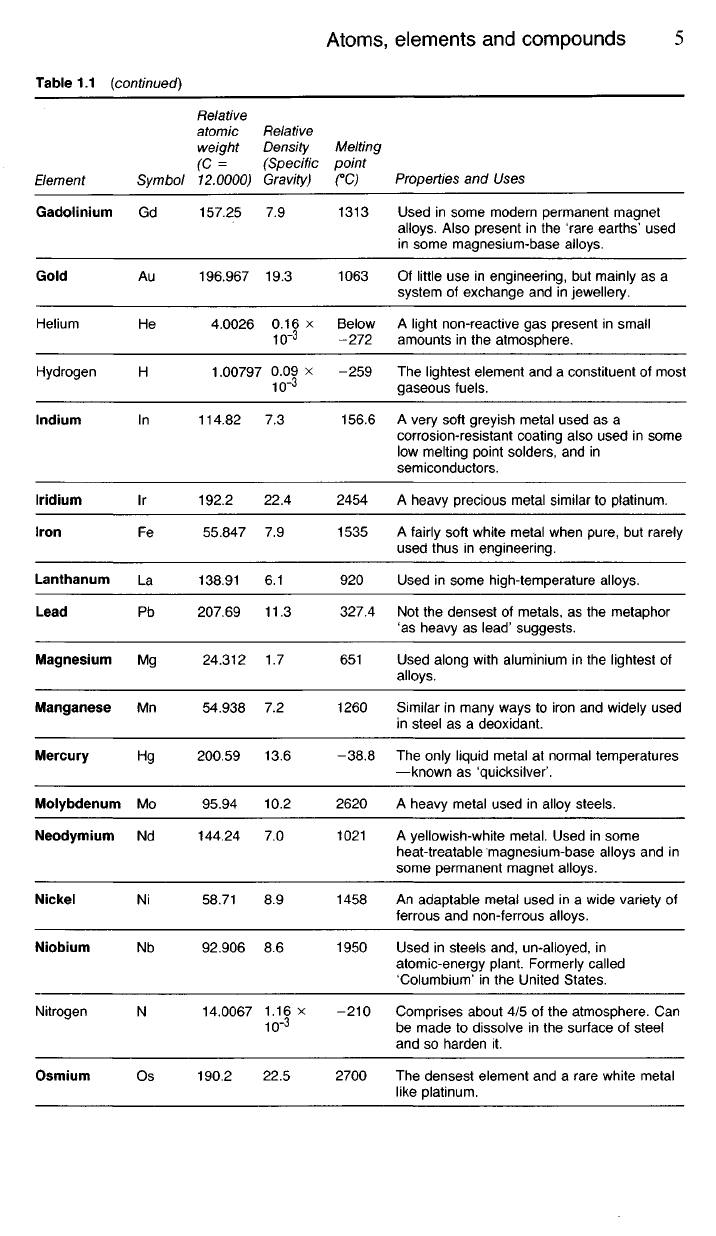
Table 1.1 {continued)
Element
Gadolinium
Gold
Helium
Hydrogen
Indium
lridium
Iron
Lanthanum
Lead
Magnesium
Manganese
Mercury
Molybdenum
Neodymium
Nickel
Niobium
Nitrogen
Osmium
Symbol
Gd
Au
He
H
In
Ir
Fe
La
Pb
Mg
Mn
Hg
Mo
Nd
Ni
Nb
N
Os
Relative
atomic
weight
(C =
12.0000)
157.25
196.967
4.0026
1.00797
114.82
192.2
55.847
138.91
207.69
24.312
54.938
200.59
95.94
144.24
58.71
92.906
14.0067
190.2
Relative
Density
(Specific
Gravity)
7.9
19.3
0.16 x
10~
3
0.09 x
10"
3
7.3
22.4
7.9
6.1
11.3
1.7
7.2
13.6
10.2
7.0
8.9
8.6
1.16 x
10~
3
22.5
Melting
point
CC)
1313
1063
Below
-272
-259
156.6
2454
1535
920
327.4
651
1260
-38.8
2620
1021
1458
1950
-210
2700
Properties and Uses
Used in some modern permanent magnet
alloys. Also present in the 'rare earths' used
in some magnesium-base alloys.
Of little use in engineering, but mainly as a
system of exchange and in jewellery.
A light non-reactive gas present in small
amounts in the atmosphere.
The lightest element and a constituent of most
gaseous fuels.
A very soft greyish metal used as a
corrosion-resistant coating also used in some
low melting point solders, and in
semiconductors.
A heavy precious metal similar to platinum.
A fairly soft white metal when pure, but rarely
used thus in engineering.
Used in some high-temperature alloys.
Not the densest of metals, as the metaphor
'as heavy as
lead'
suggests.
Used along with aluminium in the lightest of
alloys.
Similar in many ways to iron and widely used
in steel as a deoxidant.
The only liquid metal at normal temperatures
—known as 'quicksilver'.
A heavy metal used in alloy steels.
A yellowish-white metal. Used in some
heat-treatable magnesium-base alloys and in
some permanent magnet alloys.
An adaptable metal used in a wide variety of
ferrous and non-ferrous alloys.
Used in steels and, un-alloyed, in
atomic-energy plant. Formerly called
'Columbium' in the United States.
Comprises about 4/5 of the atmosphere. Can
be made to dissolve in the surface of steel
and so harden it.
The densest element and a rare white metal
like platinum.
