Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

Exercises
1.
Differentiate between:
(i) Malleability and ductility;
(ii) Toughness and hardness;
(iii) Yield strength and tensile strength. (2.20)
2.
An alloy steel rod of diameter 15 mm is subjected to a tensile force of 150 kN.
What is the tensile stress acting in the rod? (2.30)
3.
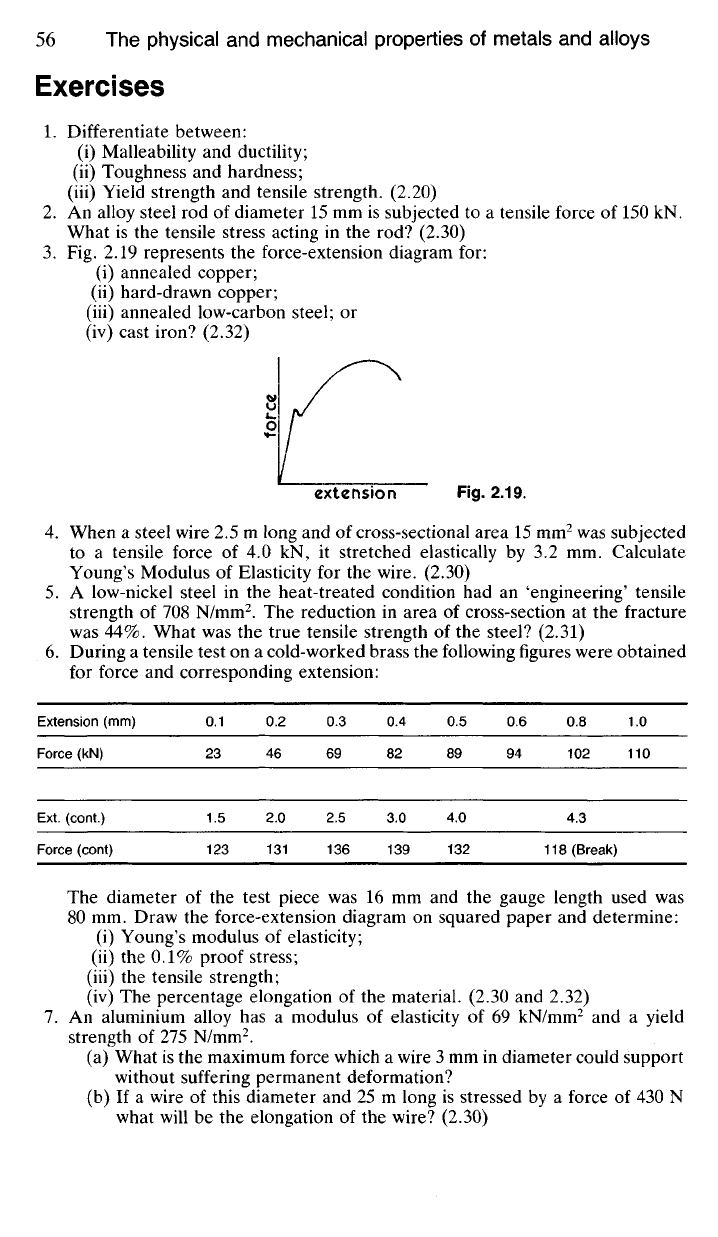
Fig. 2.19 represents the force-extension diagram for:
(i) annealed copper;
(ii) hard-drawn copper;
(iii) annealed low-carbon steel; or
(iv) cast iron? (2.32)
4.
When a steel wire 2.5 m long and of cross-sectional area 15 mm
2
was subjected
to a tensile force of 4.0 kN, it stretched elastically by 3.2 mm. Calculate
Young's Modulus of Elasticity for the wire. (2.30)
5.
A low-nickel steel in the heat-treated condition had an 'engineering' tensile
strength of 708 N/mm
2
. The reduction in area of cross-section at the fracture
was 44%. What was the true tensile strength of the steel? (2.31)
6. During a tensile test on a cold-worked brass the following figures were obtained
for force and corresponding extension:
Extension (mm) 0.1 0.2 0.3 0.4 0.5 0.6 0.8 1.0
Force (kN) 23 46 69 82 89 94 102 110
Ext. (cont.) 1.5 2.0 2.5 3.0 4.0 4.3
Force (cont) 123 131 136 139 132 118 (Break)
The diameter of the test piece was 16 mm and the gauge length used was
80 mm. Draw the force-extension diagram on squared paper and determine:
(i) Young's modulus of elasticity;
(ii) the 0.1% proof stress;
(iii) the tensile strength;
(iv) The percentage elongation of the material. (2.30 and 2.32)
7.
An aluminium alloy has a modulus of elasticity of 69 kN/mm
2
and a yield
strength of 275 N/mm
2
.
(a) What is the maximum force which a wire 3 mm in diameter could support
without suffering permanent deformation?
(b) If a wire of this diameter and 25 m long is stressed by a force of 430 N
what will be the elongation of the wire? (2.30)
force
extension
Fig.
2.19.

8. What method of hardness determination would be suitable for each of the
following components:
(i) a small iron casting;
(ii) a large steel roll in situ;
(iii) small mass-produced finished components;
(iv) a small hardened steel die.
Justify your choice of method in each case. (2.40)
9. What important information is obtained from impact tests? (2.50)
10.
What inspection techniques would be appropriate for detecting the following
defects in cast products:
(i) internal cavities in a large steel casting;
(ii) surface cracks in grey iron castings;
(iii) surface cracks in aluminium alloy castings;
(iv) internal cavities in aluminium alloy casting?
Give reasons for your choice of method in each case. (2.80-2.90)
11.
What non-destructive testing methods would be applied to reveal the presence
of:
(i) subcutaneous slag inclusions in a thick steel plate;
(ii) quench-cracks in a heat-treated carbon steel axle;
(iii) surface cracks near to a welded joint in mild-steel plate?
Give reasons for your choice of method in each case and outline the principles
of the method involved. (2.80-2.90)
Bibliography
Bateson, R. G. and Hyde, J. H., Mechanical Testing, Chapman & Hall.
O'Neill, H., Hardness of Metals and Its Measurement, Chapman & Hall, 1967.
BS 18: 1987 Methods for Tensile Testing of
Metals
(including
aerospace
materials).
BS 240: 1986 Methods for Brinell Hardness Test.
BS 427: 1981 Methods for Vickers Hardness Test.
BS 891: 1989 Methods for Rockwell Hardness Test.
BS 4175: 1989 Methods for Rockwell Superficial Hardness Test (N and T Scales).
BS 131: 1989 Methods for Notched-bar Tests (Part
1-Izod;
Part 2-Charpy).
BS 3855: 1983 Method for Modified Erichsen Cupping Test for Sheet and Metal
Strip.
BS 1639: 1983 Methods for Bend Testing of Metals.
BS 3889: 1983 and 1987 Methods for Non-destructive Testing of Pipes and Tubes.
BS 5996: 1980 Methods for Ultrasonic Testing and Specifying Quality Grades of
Ferritic
Steel Plate.
BS 4080: 1966 Methods for Non-destructive Testing of Steel Castings.
BS 4124: 1987 Methods for Non-destructive Testing of Steel Forgings.
BS
3923:
1972 and 1986 Methods for
Ultrasonic
Examination of Welds.
BS
6443:
1984 Method for Penetrant Flaw Detection.
BS 2600: 1973 and 1983 Methods for Radiographic Examination of Fusion Welded
Butt Joints in Steel.
BS 3451: 1983 Methods for Testing Fusion Welds in Aluminium and Aluminium
Alloys.
BS 709: 1981 Methods for Testing Fusion Welded Joints and Weld Metal in Steel.
BS 6072: 1986 Methods for Magnetic Particle Flaw Detection
BS 4331: 1987 and 1989 Methods for Assessing the Performance
Characteristics
of
Ultrasonic
Flaw Detection Equipment.
3
The Crystalline
Structure of Metals
3.10 All chemical elements can exist as either solids, liquids or gases
depending upon the prevailing conditions of temperature and pressure.
Thus,
at atmospheric pressure, oxygen liquifies at
—
183°C and solidifies at
—219°C. Similarly, at atmospheric pressure, the metal zinc melts at 419°C
and boils at 907
0
C. In the gaseous state particles are in a state of constant
motion and the pressure exerted by the gas is due to the impact of these
particles with the walls of the container. As the temperature of the gas is
increased, the velocity of the particles is increased and so the pressure
exerted by the gas increases, assuming that the container does not allow
the gas to expand. If, however, the gas is allowed to expand the distance
apart of the particles increases and so the potential energy increases.
Engineers will understand the term 'potential energy' as being that
energy possessed by a body by virtue of its ability to do work. Similarly,
matter possesses potential energy by virtue of its state. As the distance
apart of particles increases, so the potential energy increases. In fact this
is a simple application of the First Law of Thermodynamics which states
that if a quantity of heat bQ is supplied to a system, part of that heat
energy may be used to increase the internal energy of the system by an
amount bU and part to perform external work by an amount bW. Thus:
SQ = SU+ SW
In this case bW is the work done by the gas as it expands against some
external pressure. Whatever changes occur, energy is conserved.
In a gas such as oxygen the 'particles' referred to are molecules, each of
which consists of two covalently-bonded atoms but in a metallic gas these
particles consist of single atoms since insufficient valence electrons are
available for metallic atoms to be covalently bonded. Each atom has its
own complement of electrons and in the gaseous state the metallic bond
does not exist.
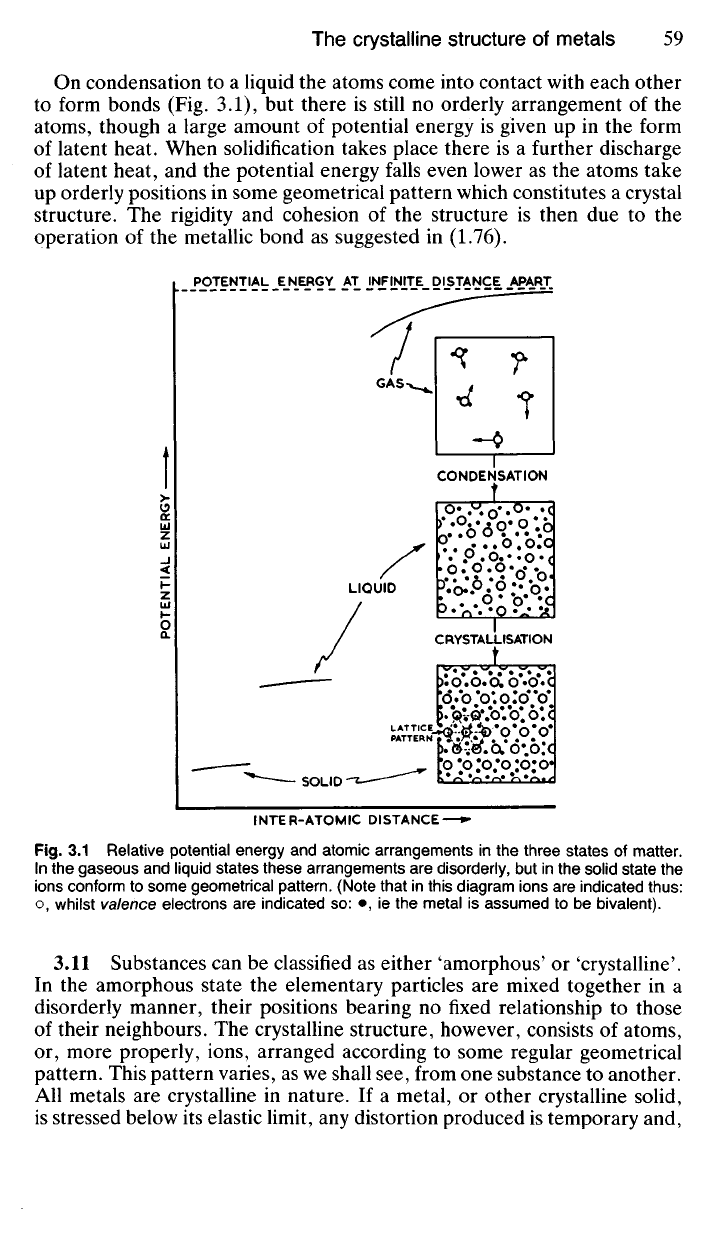
On condensation to a liquid the atoms come into contact with each other
to form bonds (Fig. 3.1), but there is still no orderly arrangement of the
atoms, though a large amount of potential energy is given up in the form
of latent heat. When solidification takes place there is a further discharge
of latent heat, and the potential energy falls even lower as the atoms take
up orderly positions in some geometrical pattern which constitutes a crystal
structure. The rigidity and cohesion of the structure is then due to the
operation of the metallic bond as suggested in (1.76).
Fig.
3.1 Relative potential energy and atomic arrangements in the three states of matter.
In the gaseous and liquid states these arrangements are disorderly, but in the solid state the
ions conform to some geometrical pattern. (Note that in this diagram ions are indicated thus:
o, whilst valence electrons are indicated so: •, ie the metal is assumed to be bivalent).
3.11 Substances can be classified as either 'amorphous' or 'crystalline'.
In the amorphous state the elementary particles are mixed together in a
disorderly manner, their positions bearing no fixed relationship to those
of their neighbours. The crystalline structure, however, consists of atoms,
or, more properly, ions, arranged according to some regular geometrical
pattern. This pattern varies, as we shall see, from one substance to another.
All metals are crystalline in nature. If a metal, or other crystalline solid,
is stressed below its elastic limit, any distortion produced is temporary and,
POTENTIAL
ENERGY
POTENTIAL ENERGY AT INFINITE DISTANCE APART
CONDENSATION
GAS
LIQUID
CRYSTALLISATION
LATTICE
PATTERN
SOLID
INTER-ATOMIC DISTANCE

when the stress is removed, the solid will return to its original shape. Thus,
removal of stress leads to removal of strain and we say that the substance
is elastic.
The amorphous structure is typical of all liquids in that the atoms or
molecules of which they are composed can be moved easily with respect
to each other, since they do not conform to any fixed pattern. In the case
of liquids of simple chemical formulae in which the molecules are small,
the forces of attraction between these molecules are not sufficient to pre-
vent the liquid from flowing under its own weight; that is, it possesses
high 'mobility'. Many substances, generally regarded as being solids, are
amorphous in nature and rely on the existence of 'long-chain' molecules,
in which all atoms are co-valently bonded, to give them strength as in
certain super polymers (1.75). In these substances the large thread-like
molecules, often containing many thousands of atoms each, are not able
to slide over each other as is the case with relatively simple molecules in
a liquid. The sum of all separate van der Waals' forces of attraction acting
between these large molecules are much greater and, since there will be
considerable mutual entanglement by virtue of their fibrous nature, the
resultant amorphous mass will lack mobility and will be extremely viscous,
or 'plastic'. An amorphous structure, therefore, does not generally possess
elasticity but only plasticity. A notable exception to this statement is pro-
vided by rubber, in which the 'folded' nature of the long-chain molecules
gives rise to elasticity (1.75).
A piece of metal consists of a mass of separate crystals irregular in shape
but interlocking with each other rather like a three-dimensional jig-saw
puzzle. Within each crystal the atoms are regularly spaced with respect to
one another. The state of affairs at the crystal boundaries has long been a
subject for conjecture, but it is now widely held that in these regions there
exists a film of metal, some three atoms thick, in which the atoms do not
conform to any pattern (Fig. 3.2). This crystal boundary film is in fact of
an amorphous nature. The metallic bond acts within and across this crystal
boundary. Consequently, the crystal boundary is not necessarily an area
of weakness except at high temperatures when inter-atomic distances
increase, and so bond strength decreases. Thus at high temperatures metals
are more likely to fail by fracture following the crystal boundaries, whilst
at low temperatures failure by transcrystalline fracture is common.
3.12 When a pure liquid solidifies into a crystalline solid, it does so at
a fixed temperature called the freezing point. During the crystallisation
process the atoms assume positions according to some geometrical pattern
(Fig. 3.1), and whilst this is taking place, heat (the latent heat of solidifi-
cation) is given out in accordance with the laws of thermodynamics, with-
out any fall in temperature taking place. A typical cooling curve for a pure
metal is shown in Fig. 3.3.
3.13 Atoms are very small entities indeed, and it has been calculated
that approximately 85 000 000 000 000 000 000 atoms are contained in
1 mm
3
of copper. Thus an individual copper atom measures approximately
2.552 x 10~
10
m in diameter, putting it far beyond the range of an ordinary
optical microscope with its maximum magnification of only 2000. However,
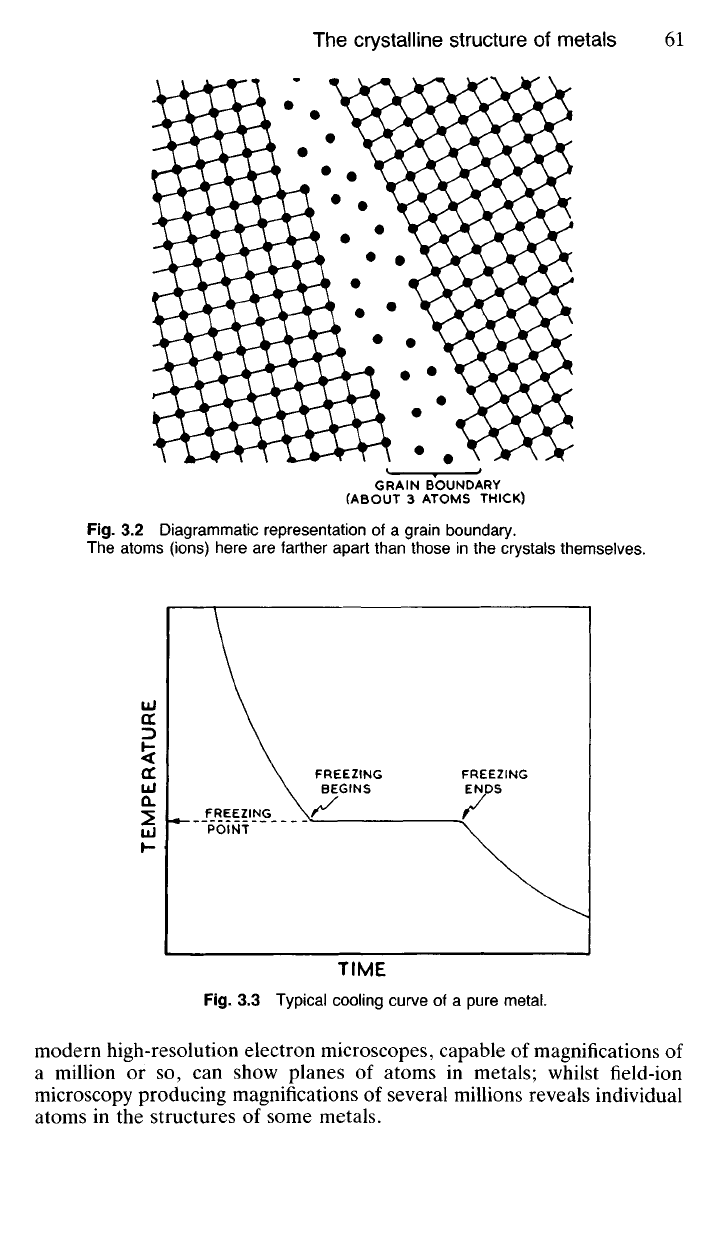
TIME
Fig.
3.3 Typical cooling curve of a pure metal.
modern high-resolution electron microscopes, capable of magnifications of
a million or so, can show planes of atoms in metals; whilst field-ion
microscopy producing magnifications of several millions reveals individual
atoms in the structures of some metals.
TEMPERATURE
GRAIN BOUNDARY
(ABOUT 3 ATOMS THICK)
Fig.
3.2 Diagrammatic representation of a grain boundary.
The atoms (ions) here are farther apart than those in the crystals themselves.
FREEZING
POINT
FREEZING
BEGINS
FREEZING
ENDS
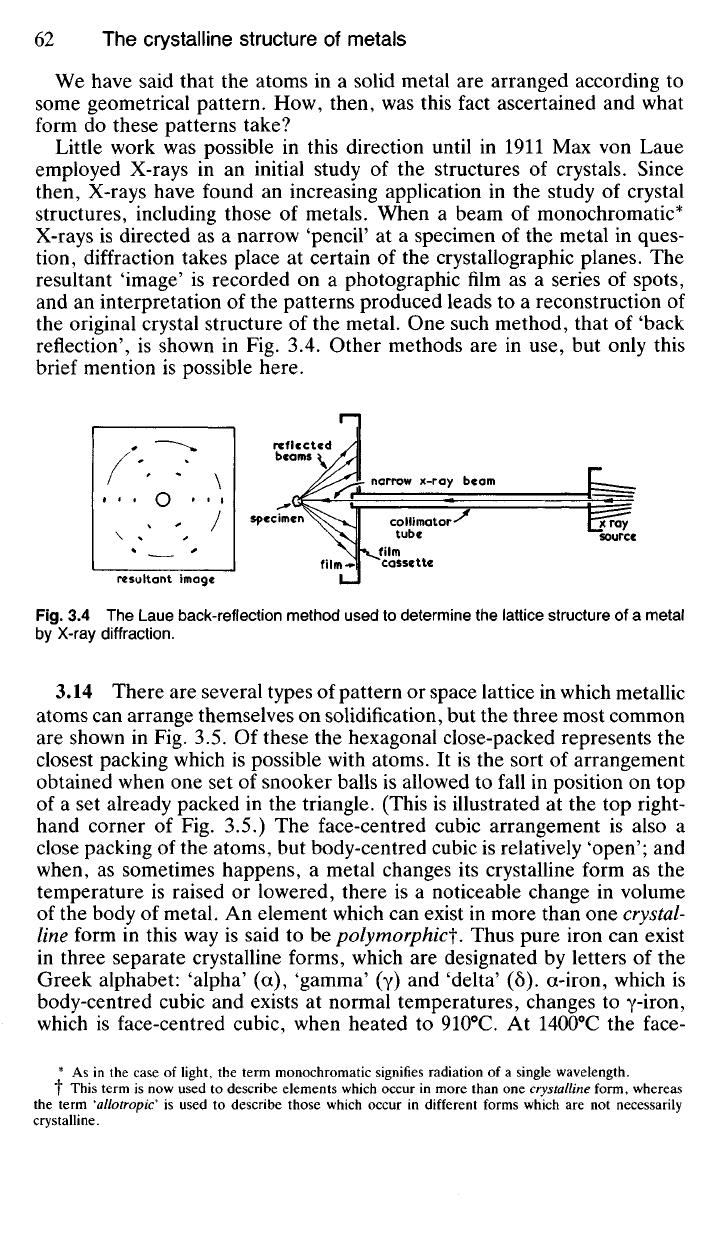
We have said that the atoms in a solid metal are arranged according to
some geometrical pattern. How, then, was this fact ascertained and what
form do these patterns take?
Little work was possible in this direction until in 1911 Max von Laue
employed X-rays in an initial study of the structures of crystals. Since
then, X-rays have found an increasing application in the study of crystal
structures, including those of metals. When a beam of monochromatic*
X-rays is directed as a narrow 'pencil' at a specimen of the metal in ques-
tion, diffraction takes place at certain of the crystallographic planes. The
resultant 'image' is recorded on a photographic film as a series of spots,
and an interpretation of the patterns produced leads to a reconstruction of
the original crystal structure of the metal. One such method, that of 'back
reflection', is shown in Fig. 3.4. Other methods are in use, but only this
brief mention is possible here.
Fig.
3.4 The Laue back-reflection method used to determine the lattice structure of a metal
by X-ray diffraction.
3.14 There are several types of pattern or space lattice in which metallic
atoms can arrange themselves on solidification, but the three most common
are shown in Fig. 3.5. Of these the hexagonal close-packed represents the
closest packing which is possible with atoms. It is the sort of arrangement
obtained when one set of snooker balls is allowed to fall in position on top
of a set already packed in the triangle. (This is illustrated at the top right-
hand corner of Fig. 3.5.) The face-centred cubic arrangement is also a
close packing of the atoms, but body-centred cubic is relatively 'open'; and
when, as sometimes happens, a metal changes its crystalline form as the
temperature is raised or lowered, there is a noticeable change in volume
of the body of metal. An element which can exist in more than one crystal-
line form in this way is said to be polymorphic^. Thus pure iron can exist
in three separate crystalline forms, which are designated by letters of the
Greek alphabet: 'alpha' (a), 'gamma' (y) and 'delta' (8). a-iron, which is
body-centred cubic and exists at normal temperatures, changes to y-iron,
which is face-centred cubic, when heated to 910
0
C. At 1400
0
C the face-
* As in the case of light, the term monochromatic signifies radiation of a single wavelength.
t This term is now used to describe elements which occur in more than one
crystalline
form, whereas
the term 'allotropic' is used to describe those which occur in different forms which are not necessarily
crystalline.
resultant image
reflected
beams
specimen
narrow x-ray beam
collimator
tube
film
cassette
film
x ray
source
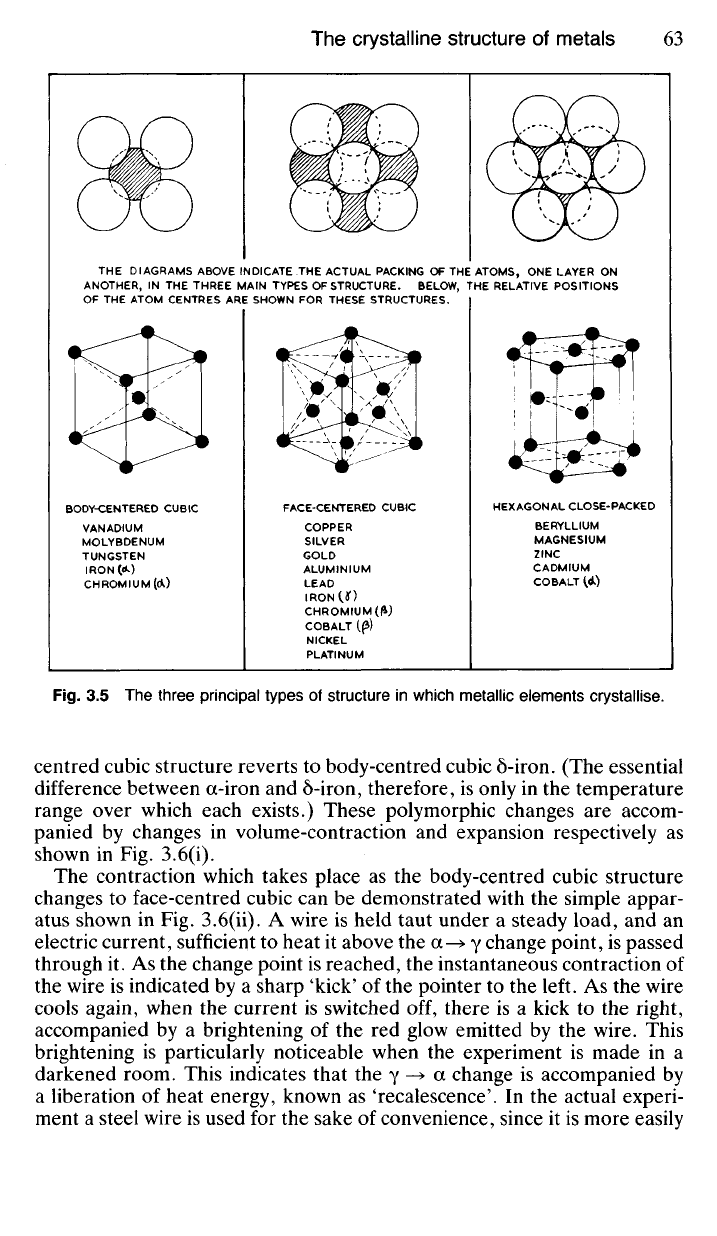
Fig.
3.5 The three principal types of structure in which metallic elements crystallise.
centred cubic structure reverts to body-centred cubic 6-iron. (The essential
difference between a-iron and 6-iron, therefore, is only in the temperature
range over which each exists.) These polymorphic changes are accom-
panied by changes in volume-contraction and expansion respectively as
shown in Fig. 3.6(i).
The contraction which takes place as the body-centred cubic structure
changes to face-centred cubic can be demonstrated with the simple appar-
atus shown in Fig. 3.6(ii). A wire is held taut under a steady load, and an
electric current, sufficient to heat it above the a—» y change point, is passed
through it. As the change point is reached, the instantaneous contraction of
the wire is indicated by a sharp 'kick' of the pointer to the left. As the wire
cools again, when the current is switched off, there is a kick to the right,
accompanied by a brightening of the red glow emitted by the wire. This
brightening is particularly noticeable when the experiment is made in a
darkened room. This indicates that the y
—»
a change is accompanied by
a liberation of heat energy, known as 'recalescence'. In the actual experi-
ment a steel wire is used for the sake of convenience, since it is more easily
THE
DIAGRAMS ABOVE INDICATE THE ACTUAL PACKING OF THE
ATOMS,
ONE LAYER ON
ANOTHER,
IN THE THREE MAIN TYPES OF
STRUCTURE.
BELOW, THE RELATIVE POSITIONS
OF
THE ATOM CENTRES ARE SHOWN FOR THESE STRUCTURES.
BODY-CENTERED
CUBIC
VANADIUM
MOLYBDENUM
TUNGSTEN
IRON(^)
CHROMIUM(Ci)
FACE-CENTERED
CUBIC
COPPER
SILVER
GOLD
ALUMINIUM
LEAD
IRONU)
CHROMIUM (£)
COBALT
((3)
NICKEL
PLATINUM
HEXAGONAL
CLOSE-PACKED
BERYLLIUM
MAGNESIUM
ZINC
CADMIUM
COBALT
^d)
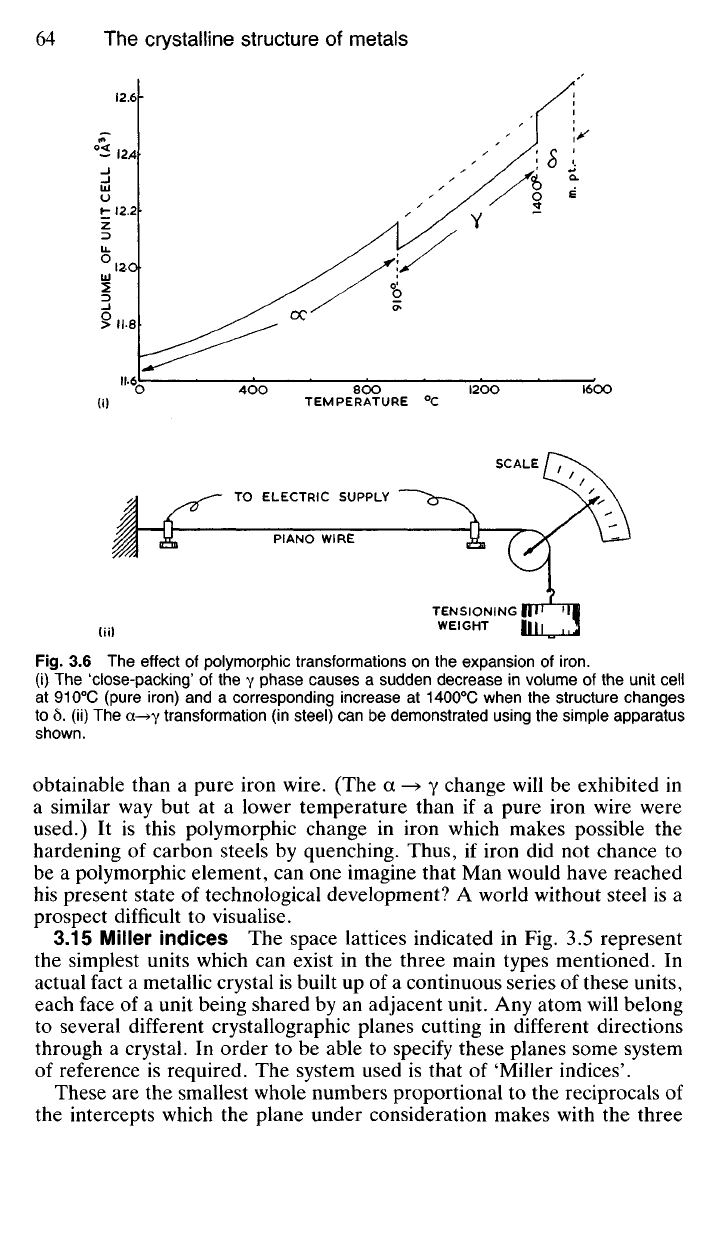
Fig.
3.6 The effect of polymorphic transformations on the expansion of
iron.
(i) The 'close-packing' of the y phase causes a sudden decrease in volume of the unit cell
at 910
0
C (pure iron) and a corresponding increase at 1400
0
C when the structure changes
to 6. (ii) The a-^y transformation (in steel) can be demonstrated using the simple apparatus
shown.
obtainable than a pure iron wire. (The a
—»
y change will be exhibited in
a similar way but at a lower temperature than if a pure iron wire were
used.) It is this polymorphic change in iron which makes possible the
hardening of carbon steels by quenching. Thus, if iron did not chance to
be a polymorphic element, can one imagine that Man would have reached
his present state of technological development? A world without steel is a
prospect difficult to visualise.
3.15 Miller indices The space lattices indicated in Fig. 3.5 represent
the simplest units which can exist in the three main types mentioned. In
actual fact a metallic crystal is built up of a continuous series of these units,
each face of a unit being shared by an adjacent unit. Any atom will belong
to several different crystallographic planes cutting in different directions
through a crystal. In order to be able to specify these planes some system
of reference is required. The system used is that of 'Miller indices'.
These are the smallest whole numbers proportional to the reciprocals of
the intercepts which the plane under consideration makes with the three
VOLUME
OFUNITCELL (A
3
)
TEMPERATURE
0
C
(i)
TO ELECTRIC SUPPLY
PIANO WIRE
(ii)
SCALE
TENSIONING
WEIGHT
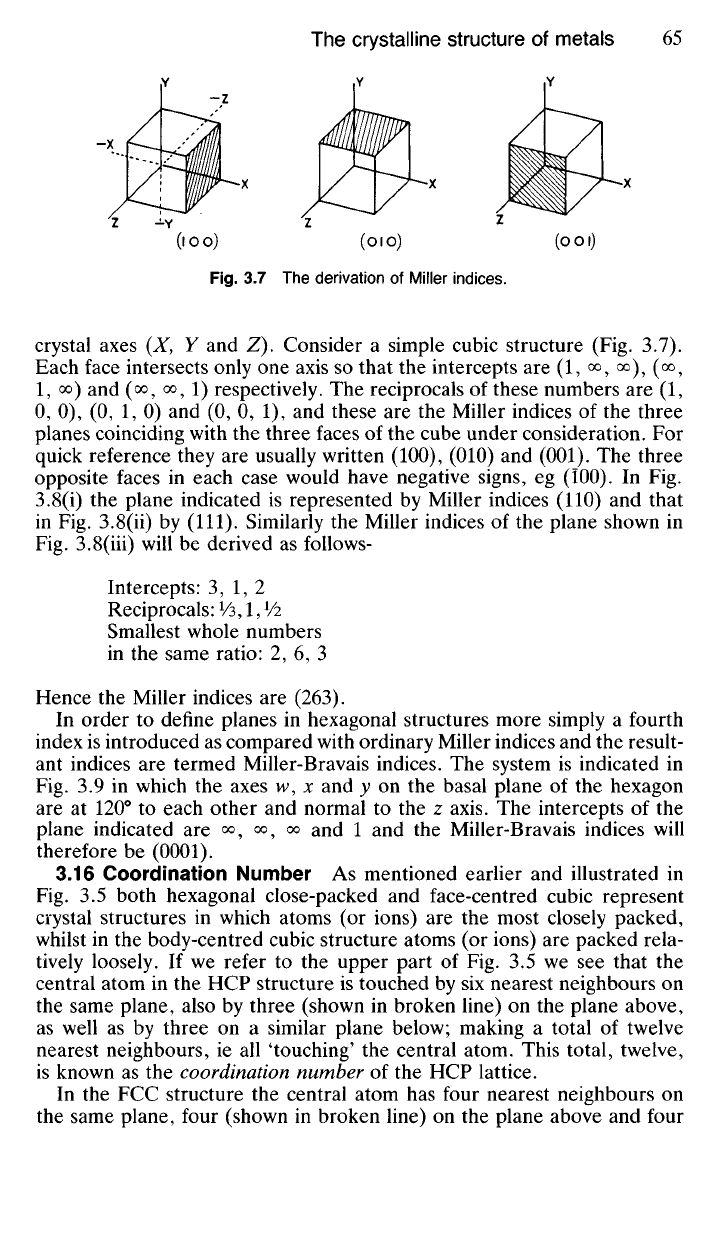
Fig.
3.7 The derivation of Miller indices.
crystal axes (X, Y and Z). Consider a simple cubic structure (Fig. 3.7).
Each face intersects only one axis so that the intercepts are (1, o°, o°), (<*>,
1,
oo) and (oo
?
oo
?
l) respectively. The reciprocals of these numbers are (1,
0, 0), (0, 1, 0) and (0, 0, 1), and these are the Miller indices of the three
planes coinciding with the three faces of the cube under consideration. For
quick reference they are usually written (100), (010) and (001). The three
opposite faces in each case would have negative signs, eg (100). In Fig.
3.8(i) the plane indicated is represented by Miller indices (110) and that
in Fig. 3.8(ii) by (111). Similarly the Miller indices of the plane shown in
Fig. 3.8(iii) will be derived as follows-
Intercepts: 3, 1, 2
Reciprocals:
Vs,
1, Vi
Smallest whole numbers
in the same ratio: 2, 6, 3
Hence the Miller indices are (263).
In order to define planes in hexagonal structures more simply a fourth
index is introduced as compared with ordinary Miller indices and the result-
ant indices are termed Miller-Bravais indices. The system is indicated in
Fig. 3.9 in which the axes w, x and y on the basal plane of the hexagon
are at 120° to each other and normal to the z axis. The intercepts of the
plane indicated are oo, oo
?
oo and 1 and the Miller-Bravais indices will
therefore be (0001).
3.16 Coordination Number As mentioned earlier and illustrated in
Fig. 3.5 both hexagonal close-packed and face-centred cubic represent
crystal structures in which atoms (or ions) are the most closely packed,
whilst in the body-centred cubic structure atoms (or ions) are packed rela-
tively loosely. If we refer to the upper part of Fig. 3.5 we see that the
central atom in the HCP structure is touched by six nearest neighbours on
the same plane, also by three (shown in broken line) on the plane above,
as well as by three on a similar plane below; making a total of twelve
nearest neighbours, ie all 'touching' the central atom. This total, twelve,
is known as the coordination number of the HCP lattice.
In the FCC structure the central atom has four nearest neighbours on
the same plane, four (shown in broken line) on the plane above and four
