Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

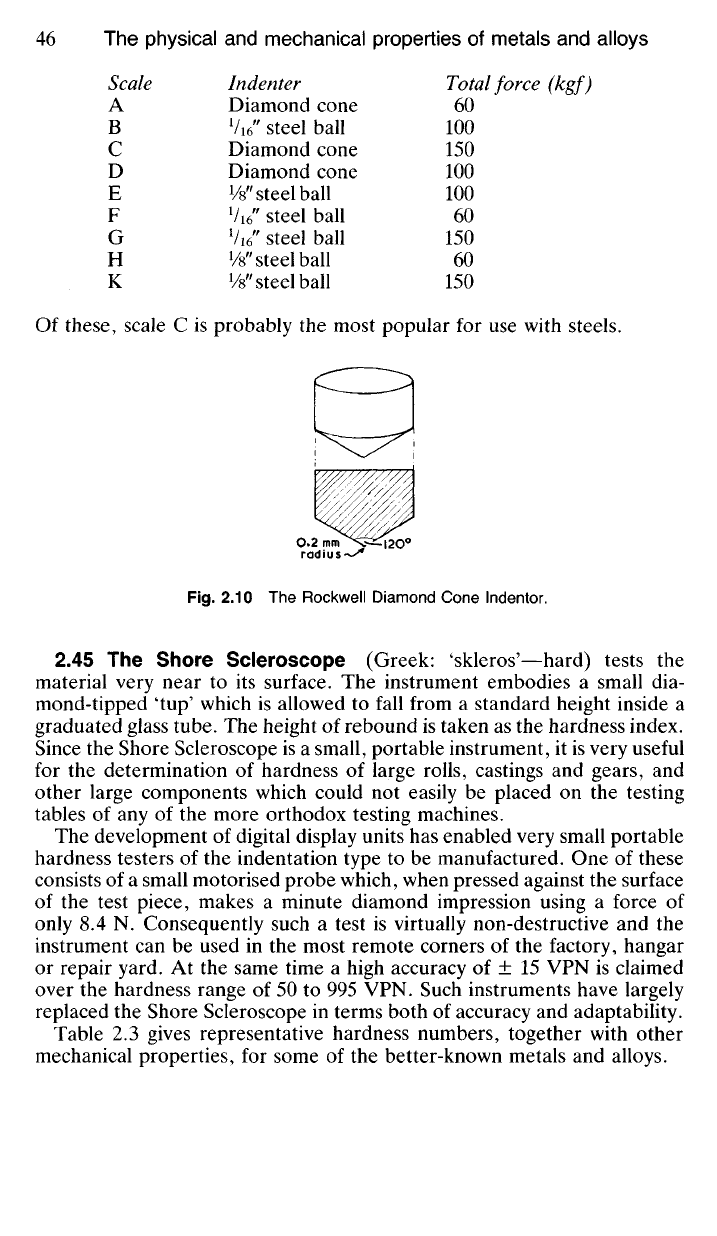
Scale Indenter Total force (kgf)
A Diamond cone 60
B
1
V steel ball 100
C Diamond cone 150
D Diamond cone 100
E
Vs"
steel ball 100
F Vi
6
" steel ball 60
G V
16
" steel ball 150
H
Vs"
steel ball 60
K W steel ball 150
Of these, scale C is probably the most popular for use with steels.
Fig.
2.10 The Rockwell Diamond Cone lndentor.
2.45 The Shore Scleroscope (Greek: 'skleros'—hard) tests the
material very near to its surface. The instrument embodies a small dia-
mond-tipped 'tup' which is allowed to fall from a standard height inside a
graduated glass tube. The height of rebound is taken as the hardness index.
Since the Shore Scleroscope is a small, portable instrument, it is very useful
for the determination of hardness of large rolls, castings and gears, and
other large components which could not easily be placed on the testing
tables of any of the more orthodox testing machines.
The development of digital display units has enabled very small portable
hardness testers of the indentation type to be manufactured. One of these
consists of a small motorised probe which, when pressed against the surface
of the test piece, makes a minute diamond impression using a force of
only 8.4 N. Consequently such a test is virtually non-destructive and the
instrument can be used in the most remote corners of the factory, hangar
or repair yard. At the same time a high accuracy of ± 15 VPN is claimed
over the hardness range of 50 to 995 VPN. Such instruments have largely
replaced the Shore Scleroscope in terms both of accuracy and adaptability.
Table 2.3 gives representative hardness numbers, together with other
mechanical properties, for some of the better-known metals and alloys.
O»2 mm
radius
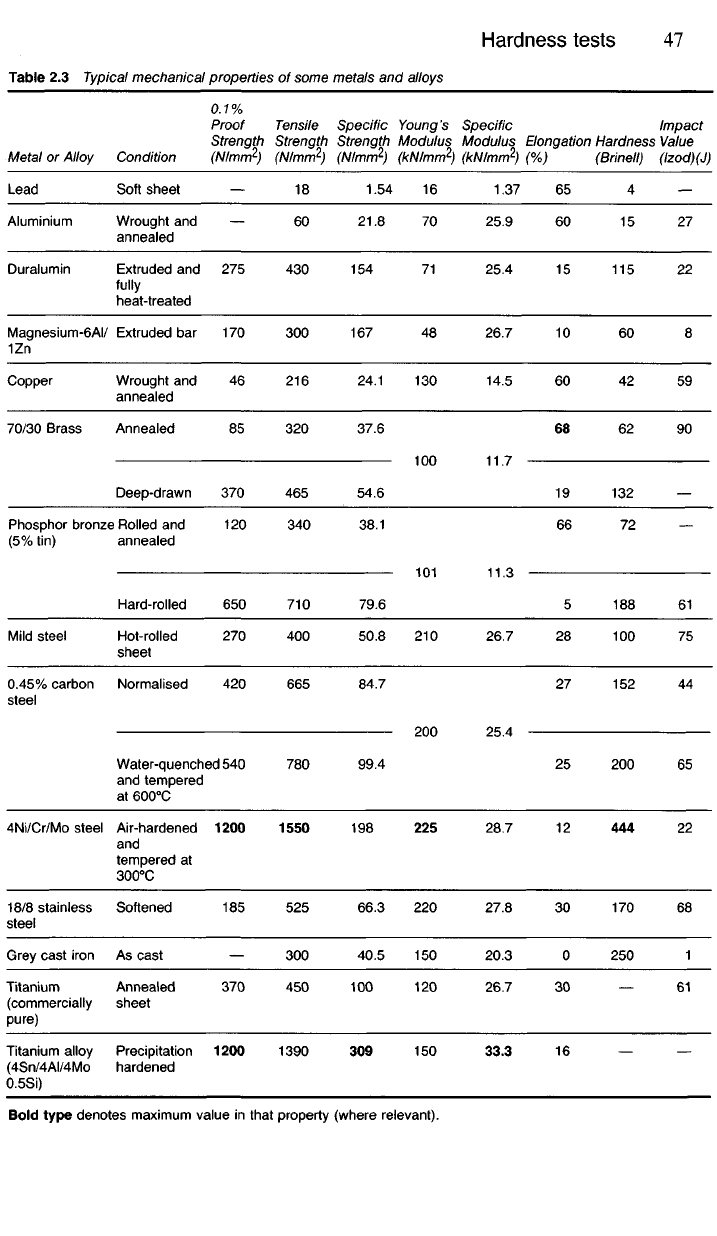
Table 2.3 Typical mechanical properties of some metals and alloys
Metal or Alloy
Lead
Aluminium
Duralumin
Magnesium-6AI/
1Zn
Copper
70/30 Brass
Phosphor bronze
(5%
tin)
Mild steel
0.45% carbon
steel
4Ni/Cr/Mo steel
18/8 stainless
steel
Grey cast iron
Titanium
(commercially
pure)
Titanium alloy
(4Sn/4AI/4Mo
0.5Si)
Condition
Soft sheet
Wrought and
annealed
Extruded and
fully
heat-treated
Extruded bar
Wrought and
annealed
Annealed
Deep-drawn
j Rolled and
annealed
Hard-rolled
Hot-rolled
sheet
Normalised
0.7%
Proof
Strength
(N/mm
2
)
275
170
46
85
370
120
650
270
420
Water-quenched
and tempered
at 600
0
C
Air-hardened
and
tempered at
300
0
C
Softened
As cast
Annealed
sheet
Precipitation
hardened
540
1200
185
370
1200
Tensile
Strength
(N/mm
2
)
18
60
430
300
216
320
465
340
710
400
665
780
1550
525
300
450
1390
Specific
Strength
(N/mm
2
)
1.54
21.8
154
167
24.1
37.6
54.6
38.1
79.6
50.8
84.7
99.4
198
66.3
40.5
100
309
Young's
Modulus
(kN/mm
2
)
16
70
71
48
130
100
101
210
oc\c\
225
220
150
120
150
Specific
Modulus
(kN/mm
2
)
1.37
25.9
25.4
26.7
14.5
11.7
11.3
26.7
QC A
28.7
27.8
20.3
26.7
33.3
Elongation
(%)
65
60
15
10
60
68
19
66
5
28
27
25
12
30
0
30
16
Hardness
(Brinell)
4
15
115
60
42
62
132
72
188
100
152
200
444
170
250
Impact
Value
(IZOd)(J)
27
22
8
59
90
61
75
44
65
22
68
1
61
Bold type denotes maximum value in that property (where relevant).
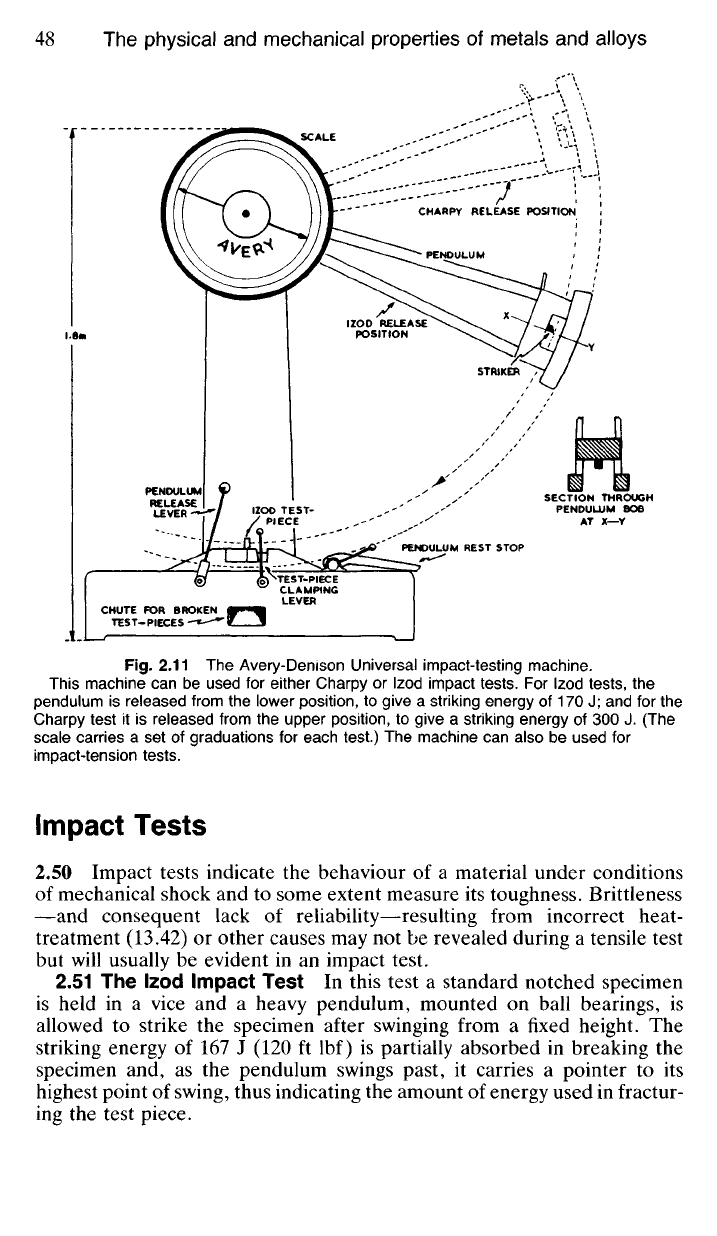
Fig.
2.11 The Avery-Denison Universal impact-testing machine.
This machine can be used for either Charpy or Izod impact tests. For Izod tests, the
pendulum is released from the lower position, to give a striking energy of 170 J; and for the
Charpy test it is released from the upper position, to give a striking energy of 300 J. (The
scale carries a set of graduations for each test.) The machine can also be used for
impact-tension tests.
Impact Tests
2.50 Impact tests indicate the behaviour of a material under conditions
of mechanical shock and to some extent measure its toughness. Brittleness
—and consequent lack of reliability—resulting from incorrect heat-
treatment (13.42) or other causes may not be revealed during a tensile test
but will usually be evident in an impact test.
2.51 The Izod Impact Test In this test a standard notched specimen
is held in a vice and a heavy pendulum, mounted on ball bearings, is
allowed to strike the specimen after swinging from a fixed height. The
striking energy of 167 J (120 ft lbf) is partially absorbed in breaking the
specimen and, as the pendulum swings past, it carries a pointer to its
highest point of swing, thus indicating the amount of energy used in fractur-
ing the test piece.
TEST-PIECE
CLAMPING
LEVER
CHUTE FOR BROKEN
TEST-PIECES
PENDULUM
RELEASE
LEVER
IZOO TEST-
PIECE
PENOULUM REST STOP
SECTION THROUGH
PENDULUM BO8
AT X-Y
IZOD RELEASE
POSITION
PENDULUM
STRIKER
CHARPY RELEASE POSITION
SCALE
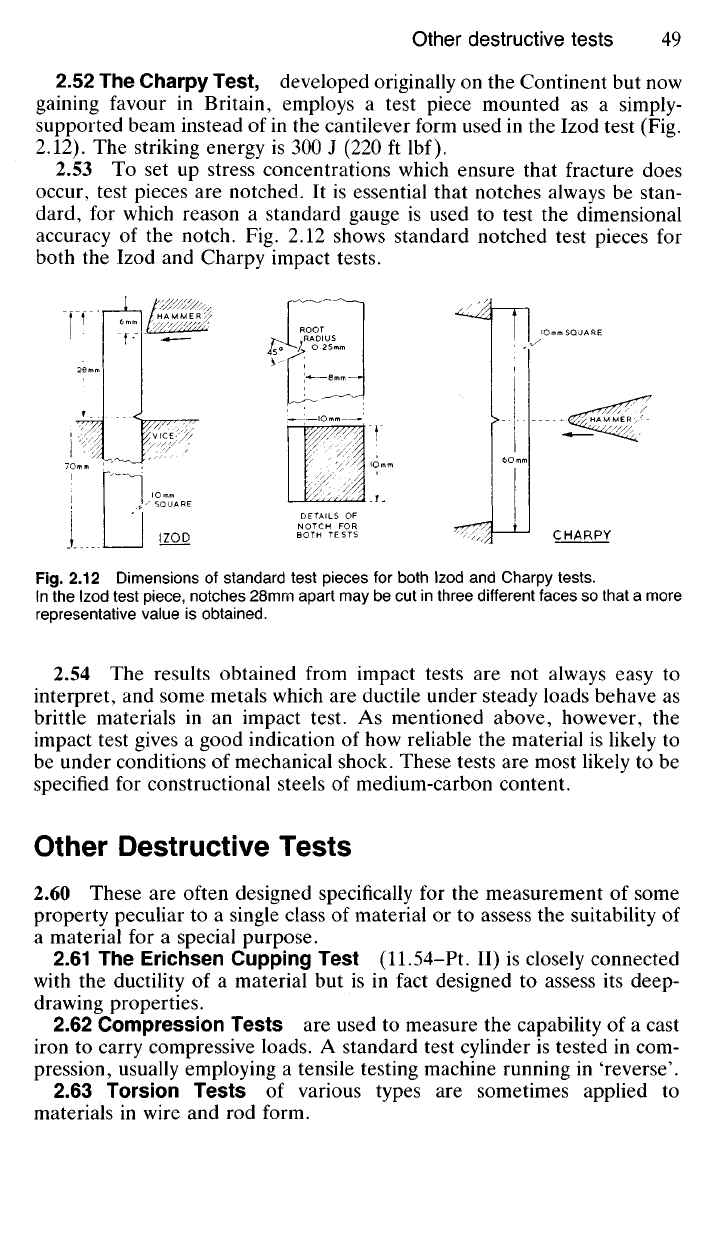
2.52 The Charpy Test, developed originally
on the
Continent
but now
gaining favour
in
Britain, employs
a
test piece mounted
as a
simply-
supported beam instead
of in the
cantilever form used
in the
Izod test
(Fig.
2.12).
The
striking energy
is 300 J (220 ft lbf).
2.53
To set up
stress concentrations which ensure that fracture does
occur, test pieces
are
notched.
It is
essential that notches always
be
stan-
dard,
for
which reason
a
standard gauge
is
used
to
test
the
dimensional
accuracy
of the
notch.
Fig. 2.12
shows standard notched test pieces
for
both
the
Izod
and
Charpy impact tests.
Fig.
2.12
Dimensions
of
standard test pieces
for
both Izod
and
Charpy tests.
In
the
Izod test piece, notches 28mm apart
may be cut in
three different faces
so
that
a
more
representative value
is
obtained.
2.54
The
results obtained from impact tests
are not
always easy
to
interpret,
and
some metals which
are
ductile under steady loads behave
as
brittle materials
in an
impact test.
As
mentioned above, however,
the
impact test gives
a
good indication
of
how reliable
the
material
is
likely
to
be under conditions
of
mechanical shock. These tests
are
most likely
to be
specified
for
constructional steels
of
medium-carbon content.
Other Destructive Tests
2.60 These
are
often designed specifically
for the
measurement
of
some
property peculiar
to a
single class
of
material
or to
assess
the
suitability
of
a material
for a
special purpose.
2.61
The
Erichsen Cupping Test (11.54-Pt.
II) is
closely connected
with
the
ductility
of a
material
but is in
fact designed
to
assess
its
deep-
drawing properties.
2.62 Compression Tests
are
used
to
measure
the
capability
of a
cast
iron
to
carry compressive loads.
A
standard test cylinder
is
tested
in com-
pression, usually employing
a
tensile testing machine running
in
'reverse'.
2.63 Torsion Tests
of
various types
are
sometimes applied
to
materials
in
wire
and rod
form.
IOmm SQUARE
HAMMER
ROOT
RADIUS
HAMMER
VICE
SQUARE
IZOD
DETAILS
OF
NOTCH
FOR
BOTH TESTS
CHARPY

Non-destructive Tests
2.70 The mechanical tests already mentioned are of a destructive nature
and are subject to the availability of separate test pieces which are reason-
ably representative of the production material. Thus, wrought products
such as rolled strip, extruded rod and drawn wire are generally uniform in
mechanical properties throughout a large batch and can be sampled with
confidence. Parts which are produced individually, however, such as cast-
ings and welded joints, may vary widely in quality purely because they are
made individually and under the influence of many variable factors. If the
quality of such components is important and the expense justified—as in
the case of aircraft castings—it may be necessary to test each component
individually by some form of non-destructive test. Such tests seek to detect
faults and flaws either at the surface or below it, and a number of suitable
methods is available in each case.
The Detection of Surface Faults
2.80 It is often possible to detect and evaluate surface faults by simple
visual examination with or without the use of a hand magnifier. The pres-
ence of fine hair-line cracks is less easy to detect by visual means and some
aid is generally necessary. Such surface cracks may be associated with
the heat-treatment of steel or, in a welded joint, with contraction during
cooling.
2.81 Penetrant Methods In these methods the surface to be examined
is cleaned and then dried. A penetrant fluid is then sprayed or swabbed
on the surface which should be warmed to about 90
0
C. After sufficient
time has elapsed for the penetrant to fill any fissures which may be present
the excess is flushed from the surface with warm water (the surface tension
of the water is too high to allow it to enter the narrow fissure). The test
surface is then carefully dried, coated with fine powdered chalk and set
aside for some time. As the coated surface cools, it contracts and penetrant
tends to be squeezed out of any cracks, so that the chalk layer becomes
stained, thus revealing the presence of the cracks. Most penetrants of this
type contain a scarlet dye which renders the stain immediately noticeable.
Aluminium alloy castings are often examined in this way.
Penetrants containing a compound which fluoresces under the action of
ultra-violet light may also be used. This renders the use of messy chalk
unnecessary. When the prepared surface is illuminated by ultra-violet light,
the cracks containing the penetrant are revealed as bright lines on a dark
background. Penetrant methods are particularly useful for the examination
of non-ferrous metals and austenitic (non-magnetic) steels.
2.82 Magnetic Dust Methods consist in laying the steel component
across the arms of a magnetising machine and then sprinkling it with a
special magnetic powder. The excess powder is blown away, and any cracks
or defects are then revealed by a bunch of powder sticking to the area on
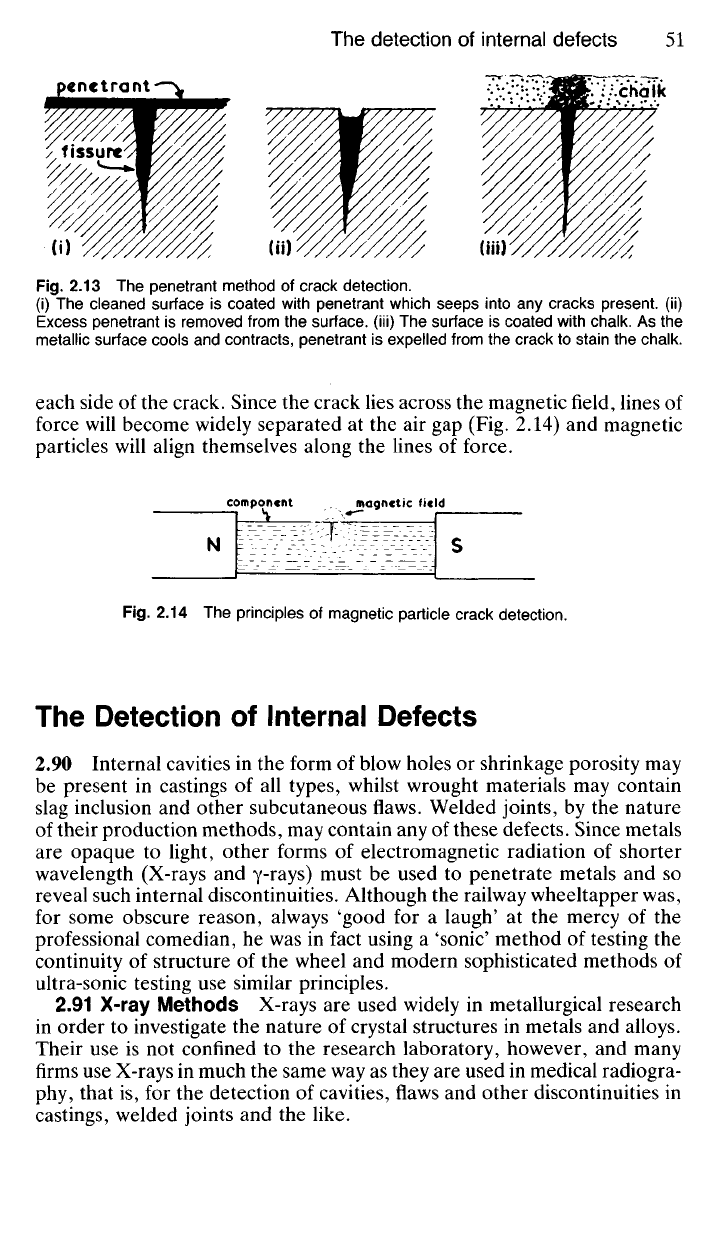
Fig.
2.13 The penetrant method of crack detection.
(i) The cleaned surface is coated with penetrant which seeps into any cracks present, (ii)
Excess penetrant is removed from the surface, (iii) The surface is coated with chalk. As the
metallic surface cools and contracts, penetrant is expelled from the crack to stain the chalk.
each side of the crack. Since the crack lies across the magnetic field, lines of
force will become widely separated at the air gap (Fig. 2.14) and magnetic
particles will align themselves along the lines of force.
Fig.
2.14 The principles of magnetic particle crack detection.
The Detection of Internal Defects
2.90 Internal cavities in the form of blow holes or shrinkage porosity may
be present in castings of all types, whilst wrought materials may contain
slag inclusion and other subcutaneous flaws. Welded joints, by the nature
of their production methods, may contain any of these defects. Since metals
are opaque to light, other forms of electromagnetic radiation of shorter
wavelength (X-rays and y-rays) must be used to penetrate metals and so
reveal such internal discontinuities. Although the railway wheeltapper was,
for some obscure reason, always 'good for a laugh' at the mercy of the
professional comedian, he was in fact using a 'sonic' method of testing the
continuity of structure of the wheel and modern sophisticated methods of
ultra-sonic testing use similar principles.
2.91 X-ray Methods X-rays are used widely in metallurgical research
in order to investigate the nature of crystal structures in metals and alloys.
Their use is not confined to the research laboratory, however, and many
firms use X-rays in much the same way as they are used in medical radiogra-
phy, that is, for the detection of cavities, flaws and other discontinuities in
castings, welded joints and the like.
penetrant
fissure
(i)
(H)
(Hi)
chalk
component
magnetic field
N
S
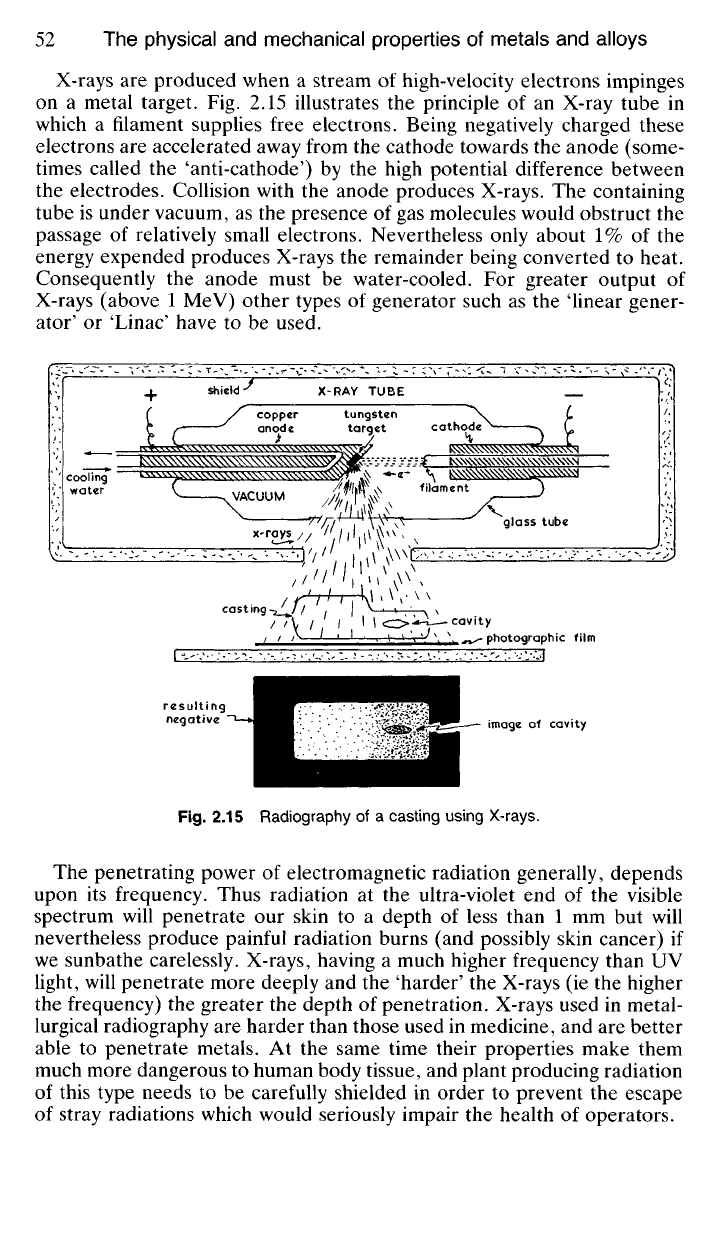
X-rays
are
produced when
a
stream
of
high-velocity electrons impinges
on
a
metal target.
Fig. 2.15
illustrates
the
principle
of an
X-ray tube
in
which
a
filament supplies free electrons. Being negatively charged these
electrons
are
accelerated away from
the
cathode towards
the
anode (some-
times called
the
'anti-cathode')
by the
high potential difference between
the electrodes. Collision with
the
anode produces X-rays.
The
containing
tube
is
under vacuum,
as the
presence
of gas
molecules would obstruct
the
passage
of
relatively small electrons. Nevertheless only about
1% of the
energy expended produces X-rays
the
remainder being converted
to
heat.
Consequently
the
anode must
be
water-cooled.
For
greater output
of
X-rays (above
1 MeV)
other types
of
generator such
as the
'linear gener-
ator'
or
'Linac' have
to be
used.
Fig.
2.15
Radiography
of a
casting using X-rays.
The penetrating power
of
electromagnetic radiation generally, depends
upon
its
frequency. Thus radiation
at the
ultra-violet
end of the
visible
spectrum will penetrate
our
skin
to a
depth
of
less than
1 mm but
will
nevertheless produce painful radiation burns
(and
possibly skin cancer)
if
we sunbathe carelessly. X-rays, having
a
much higher frequency than
UV
light, will penetrate more deeply
and the
'harder'
the
X-rays
(ie the
higher
the frequency)
the
greater
the
depth
of
penetration. X-rays used
in
metal-
lurgical radiography
are
harder than those used
in
medicine,
and are
better
able
to
penetrate metals.
At the
same time their properties make them
much more dangerous
to
human body tissue,
and
plant producing radiation
of this type needs
to be
carefully shielded
in
order
to
prevent
the
escape
of stray radiations which would seriously impair
the
health
of
operators.
cooling
water
VACUUM
cathode
tungsten
target
X-RAY
TUBE
shield
copper
anode
filament
glass tube
x-rays
casting
cavity
photographic film
resulting
negative
image
of
cavity

Like light, X-rays travel in straight lines, but whilst metals are opaque
to light they are moderately transparent to X-rays, particularly those of
high frequency. Fig. 2.15 illustrates the principle of radiography. A casting
is interposed between a shielded source of X-rays and a photographic film.
Some of the radiation will be absorbed by the metal so that the density of
the photographic image will vary with the thickness of the metal through
which the rays have passed.
2.92 X-rays are absorbed logarithmically—
/ =
I
o
e-*
Where I
0
and / are the intensities of incident and emergent radiation
respectively, d the thickness and pi the linear coefficient of absorption of
radiation, (i is lower for radiation of higher frequency.
A cavity in the casting will result in those X-rays which pass through the
cavity being less effectively absorbed than those rays which travel through
the full thickness of metal. Consequently the cavity will show as a dark
patch on the resultant photographic negative in the same way that a greater
intensity of light affects an ordinary photographic negative.
A fluorescent screen may be substituted for the photographic film so
that the resultant radiograph may be viewed instantaneously. This type of
fluoroscopy is obviously much cheaper and quicker but is less sensitive
than photography and its use is generally limited to the less-dense metals
and alloys.
2.93 y-ray Methods can also be used in the radiography of metals. Since
they are of shorter wavelength than are X-rays, they are able to penetrate
more effectively a greater thickness of metal. Hence they are particularly
useful in the radiography of steel, which absorbs radiation more readily
than do light alloys.
2.94 y-rays constitute a major proportion of the dangerous radiation
emanating from 'nuclear waste' and from the fall-out of nuclear explosions.
Initially naturally-occurring radium and radon (18.70) were used as a
source of y-rays but artificially activated isotopes of other elements are
now generally used. These activated isotopes are prepared by bombarding
the element with neutrons in an atomic pile. A nucleus struck by a neutron
absorbs it and then contains an excess of energy which is subsequently
released as y-rays. Commonly used isotopes are shown in Table 2.4. Of
these iridium-192 and cobalt-60 are the most widely used in industry.
2.95 Manipulation of the isotope as a source of y-radiation in metallur-
gical radiography is in many respects more simple than is the case with
X-rays, though security arrangements are extremely important in view of
the facts that y-radiation is 'harder' than X-radiation and that it takes place
continuously from the source without any outside stimulation. All y-ray
sources are controlled remotely, generally using a manual wind-out system
(Fig. 2.16). When not in use the isotope is stored in a shielded container
of some y-ray absorbent material such as lead. Because they are 'harder'
than X-rays, y-rays can be used to radiograph considerable thicknesses of
steel. Since the radiation source is small and compact and needs no external
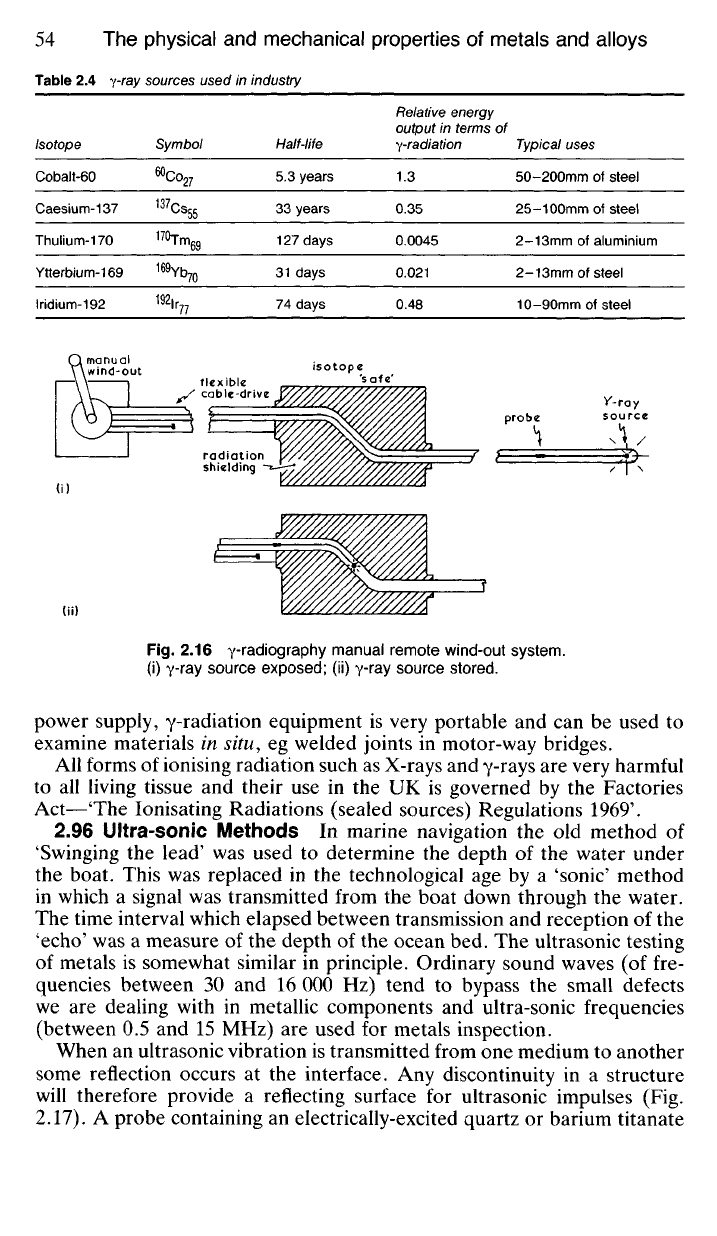
Fig.
2.16 y-radiography manual remote wind-out system,
(i) y-ray source exposed; (ii) y-ray source stored.
power supply, y-radiation equipment
is
very portable
and can be
used
to
examine materials
in
situ,
eg
welded joints
in
motor-way bridges.
All forms
of
ionising radiation such
as
X-rays and y-rays
are
very harmful
to
all
living tissue
and
their
use in the UK is
governed
by the
Factories
Act—The Ionisating Radiations (sealed sources) Regulations 1969'.
2.96 Ultra-sonic Methods
In
marine navigation
the old
method
of
'Swinging
the
lead'
was
used
to
determine
the
depth
of the
water under
the boat. This
was
replaced
in the
technological
age by a
'sonic' method
in which
a
signal
was
transmitted from
the
boat down through
the
water.
The time interval which elapsed between transmission
and
reception
of the
'echo'
was a
measure
of the
depth
of the
ocean bed.
The
ultrasonic testing
of metals
is
somewhat similar
in
principle. Ordinary sound waves
(of fre-
quencies between
30 and 16 000 Hz)
tend
to
bypass
the
small defects
we
are
dealing with
in
metallic components
and
ultra-sonic frequencies
(between
0.5 and 15
MHz)
are
used
for
metals inspection.
When
an
ultrasonic vibration
is
transmitted from one medium
to
another
some reflection occurs
at the
interface.
Any
discontinuity
in a
structure
will therefore provide
a
reflecting surface
for
ultrasonic impulses
(Fig.
2.17).
A
probe containing
an
electrically-excited quartz
or
barium titanate
(i)
(ii)
manual
wind-out
flexible
cable-drive
isotope
'safe'
probe
V-ray
source
radiation
shielding
Table
2.4
y-ray sources used
in
industry
Isotope
Cobalt-60
Caesium-137
Thulium-170
Ytterbium-169
lridium-192
Symbol
60
Co
27
137
Cs
55
170
T-S
9
169
Yb
70
192
Ir
77
Half-life
5.3 years
33 years
127 days
31 days
74 days
Relative energy
output
in
terms
of
y-radiation
1.3
0.35
0.0045
0.021
0.48
Typical uses
50-200mm
of
steel
25-100mm
of
steel
2-13mm
of
aluminium
2-13mm
of
steel
10-90mm
of
steel
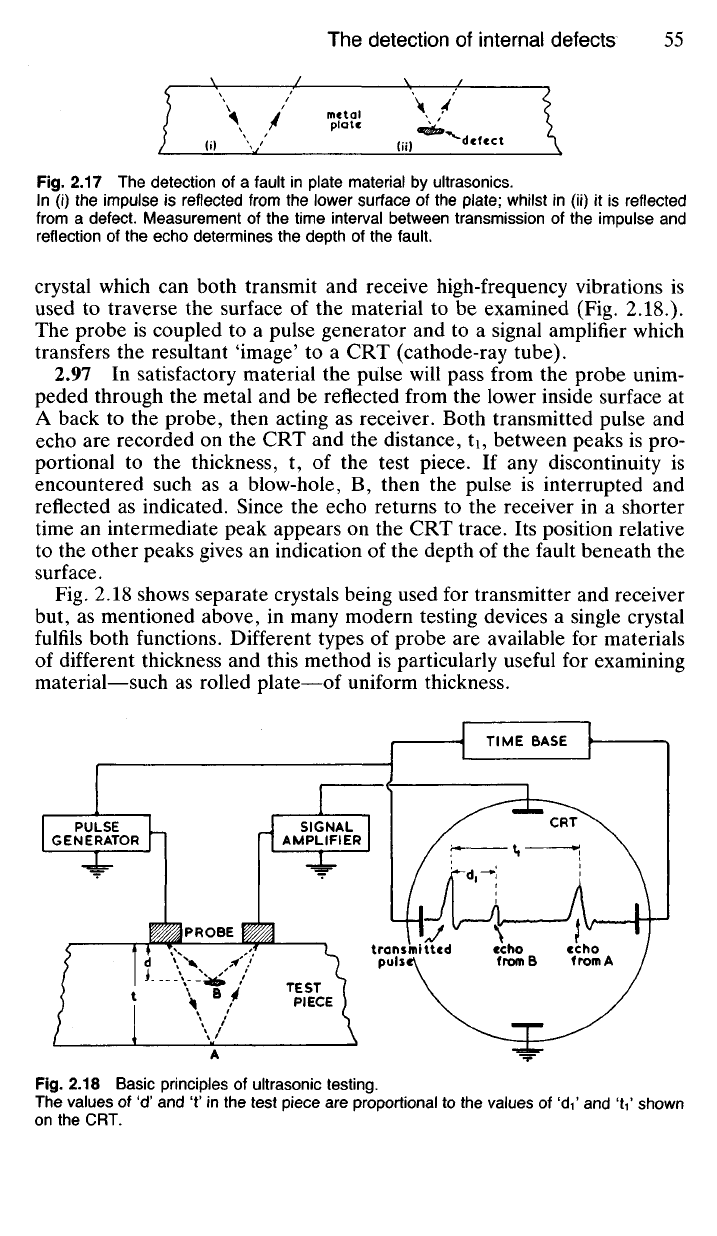
Fig.
2.17 The detection of a fault in plate material by ultrasonics.
In (i) the impulse is reflected from the lower surface of the plate; whilst in (ii) it is reflected
from a defect. Measurement of the time interval between transmission of the impulse and
reflection of the echo determines the depth of the fault.
crystal which can both transmit and receive high-frequency vibrations is
used to traverse the surface of the material to be examined (Fig. 2.18.).
The probe is coupled to a pulse generator and to a signal amplifier which
transfers the resultant 'image' to a CRT (cathode-ray tube).
2.97 In satisfactory material the pulse will pass from the probe unim-
peded through the metal and be reflected from the lower inside surface at
A back to the probe, then acting as receiver. Both transmitted pulse and
echo are recorded on the CRT and the distance, ti, between peaks is pro-
portional to the thickness, t, of the test piece. If any discontinuity is
encountered such as a blow-hole, B, then the pulse is interrupted and
reflected as indicated. Since the echo returns to the receiver in a shorter
time an intermediate peak appears on the CRT trace. Its position relative
to the other peaks gives an indication of the depth of the fault beneath the
surface.
Fig. 2.18 shows separate crystals being used for transmitter and receiver
but, as mentioned above, in many modern testing devices a single crystal
fulfils both functions. Different types of probe are available for materials
of different thickness and this method is particularly useful for examining
material—such as rolled plate—of uniform thickness.
Fig.
2.18 Basic principles of ultrasonic testing.
The values of 'd' and T in the test piece are proportional to the values of 'di
1
and V shown
on the CRT.
metal
plate
defect
(ii)
(i)
PULSE
GENERATOR
SIGNAL
AMPLIFIER
TIME BASE
CRT
PROBE
TEST
PIECE
transmitted
pulse
echo
from B
echo
from A
