Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

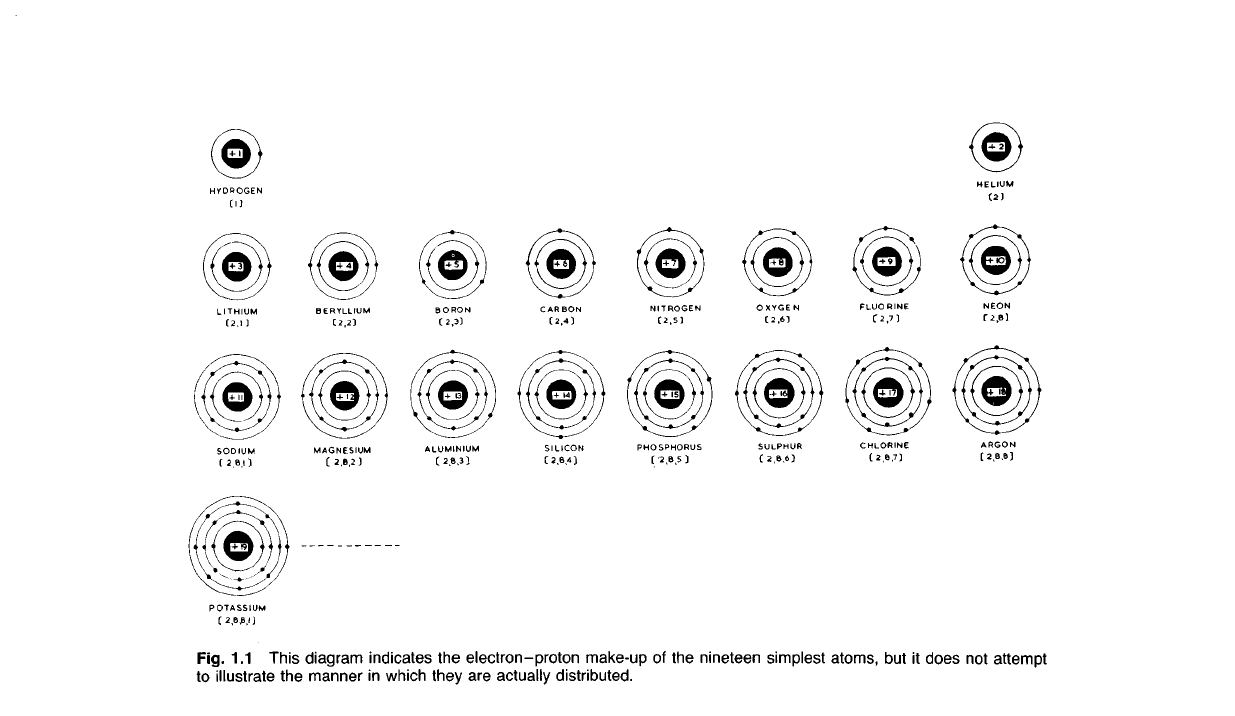
POTASSIUM
C
2,80,1
J
Fig. 1.1 This diagram indicates the
electron-proton
make-up of the nineteen simplest atoms, but it does not attempt
to
illustrate the manner in which they are actually distributed.
ARGON
[2 8.B]
CHLORINE
C
2,8,7)
SULPHUR
C
2,8,6)
NEON
C
2,8)
HELIUM
(2)
FLUORINE
C2.7)
O
XYGE
N
C
2.61
NITROGEN
C2.5)
PHOSPHORUS
C'2,8,5)
SILICON
C
2,8,4]
CARBON
C
2,4)
BORON
C
2,3)
ALUMINIUM
C
2.8,3)
MAGNESIUM
(2.8,2)
SODIUM
[2,8.I)
LITHIUM
[2,1
]
BERYLLIUM
C2.2)
HYDROGEN
CU
rated',
so
that such
an
atom will have
no
tendency
to
combine with others.
1.69
The
periodicity
of
properties described above
was
noticed
by
chemists quite early
in the
nineteenth century
and led to the
advent
of
order
in
inorganic chemistry with
the
celebrated 'Periodic Classification
of
the Elements'
by the
Russian chemist Demitri Mendeleef
in
1864.
In
more
recent years this periodicity
in
chemical properties
of the
elements
has
been explained
in
terms
of the
electronic structure
of
the atom
as
outlined
very briefly above.
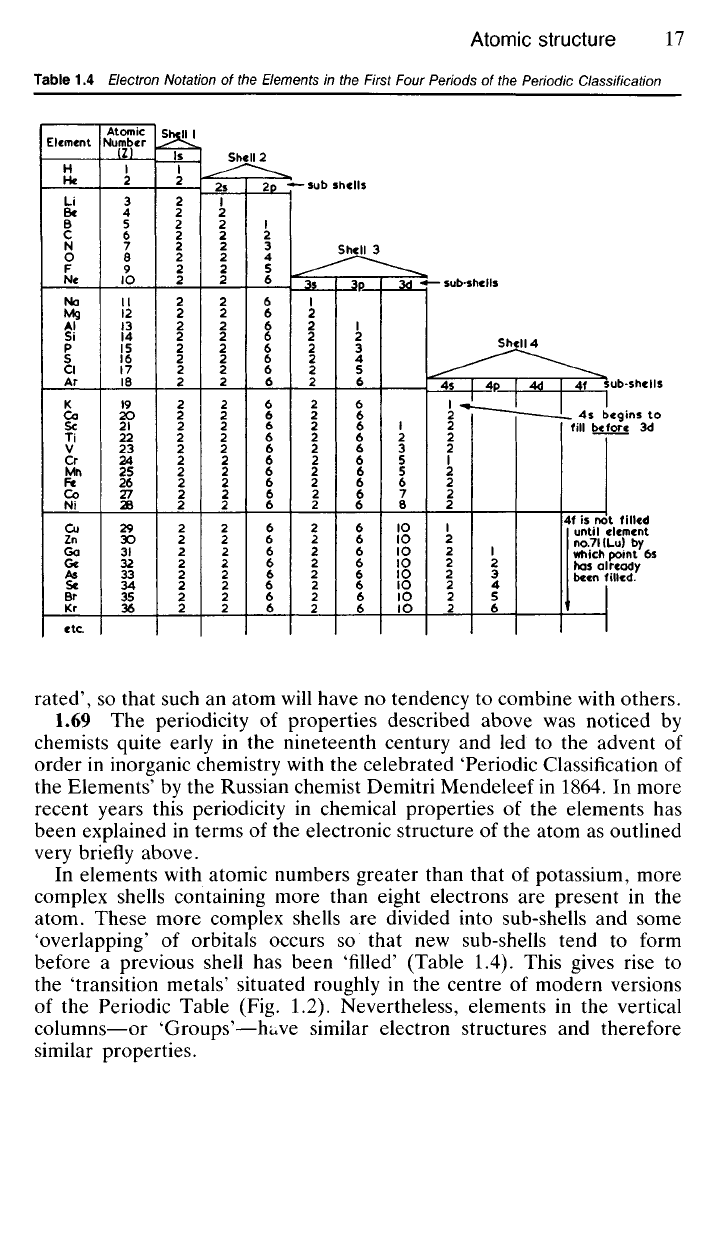
In elements with atomic numbers greater than that
of
potassium, more
complex shells containing more than eight electrons
are
present
in the
atom. These more complex shells
are
divided into sub-shells
and
some
'overlapping'
of
orbitals occurs
so
that
new
sub-shells tend
to
form
before
a
previous shell
has
been 'filled' (Table
1.4).
This gives rise
to
the 'transition metals' situated roughly
in the
centre
of
modern versions
of
the
Periodic Table
(Fig. 1.2).
Nevertheless, elements
in the
vertical
columns—or 'Groups'—have similar electron structures
and
therefore
similar properties.
4f
is not
filled
until element
no.7l(Lu)
by
which point
6s
has already
been filled.
4s begins
to
fill before
3d
sub-shells
Shell
4
sub-shells
Shell
3
sub shells
Shell
2
Shell
I
Atomic
Number
Element
Table 1.4 Electron Notation of the Elements in the First Four Periods of the Periodic Classification

Chemical Combination
and
Valence
1.70
It was mentioned above that chemical combination between two
atoms is governed by the number of electrons in the outer electron shell
of each. Moreover, it was pointed out that those elements whose atoms
had eight electrons in the outer shell (the noble gases neon and argon) had
no inclination to combine with other elements and therefore had no chemi-
cal affinity. It is therefore reasonable to suppose that the completion of
the 'octet' of electrons in the outer shell of an atom leads to a valence of
zero.
The noble gas helium, with a completed 'duplet' of electrons in the
single shell, behaves in a similar manner.
As far as the simpler atoms we have been discussing are concerned the
tendency is for them to attempt to attain this noble-gas structure of a stable
octet (or duplet) of electrons in the outer shell. Their chemical properties
are reflected in this tendency. With the more complex atoms the situation
is not quite so simple, since these atoms possess larger outer shells which
are generally sub-divided, to the extent that electrons may begin to fill a
new outer 'sub-shell' before the penultimate sub-shell has been completed.
As mentioned above this would explain the existence of groups of metallic
elements the properties of which are transitional between those of one
well-defined group and those of the next. The broad principles of the
electronic theory of valence mentioned here in connection with the simpler
atoms will apply. On these general lines three main forms of combination
exist.
1.71 Electro-valent Combination In this type of combination a metallic
atom loses the electrons which constitute its outer shell (or sub-shell) and
the number of electrons so lost are equivalent to the numerical valence of
the element. These lost electrons are transferred to the outer electron shells
of the non-metallic atom (or atoms) with which the metal is combining. In
this way a complete shell of electrons is left behind in the metallic particle
whilst a hitherto incomplete shell is filled in the non-metallic particle.
Let us consider the combination which takes place between the metal
sodium and the non-metal chlorine to form sodium chloride (common
salt).
The sodium atom has a single electron in its outer shell and this
transfers to join the seven electrons in the outer shell of the chlorine atom.
When this occurs each resultant particle is left with a complete octet in the
outer shell. (The sodium particle now has the same electron structure as the
noble gas neon, and the chlorine particle has the same electron structure as
the noble gas argon.) The balance of electrical charges which existed
between protons and electrons in the original atoms is, however, upset.
Since the sodium atom has lost a negatively charged particle (an electron),
the remaining sodium particle must now possess a resultant positive charge.
Meanwhile the chlorine atom has gained this electron so the resultant
chlorine particle must carry a negative charge. These charged particles,
derived from atoms in this manner, are called ions. In terms of symbols
the sodium ion is written thus, Na
+
, and the chlorine ion, Cl".
1.72 Since sodium ions and chlorine ions are oppositely charged they
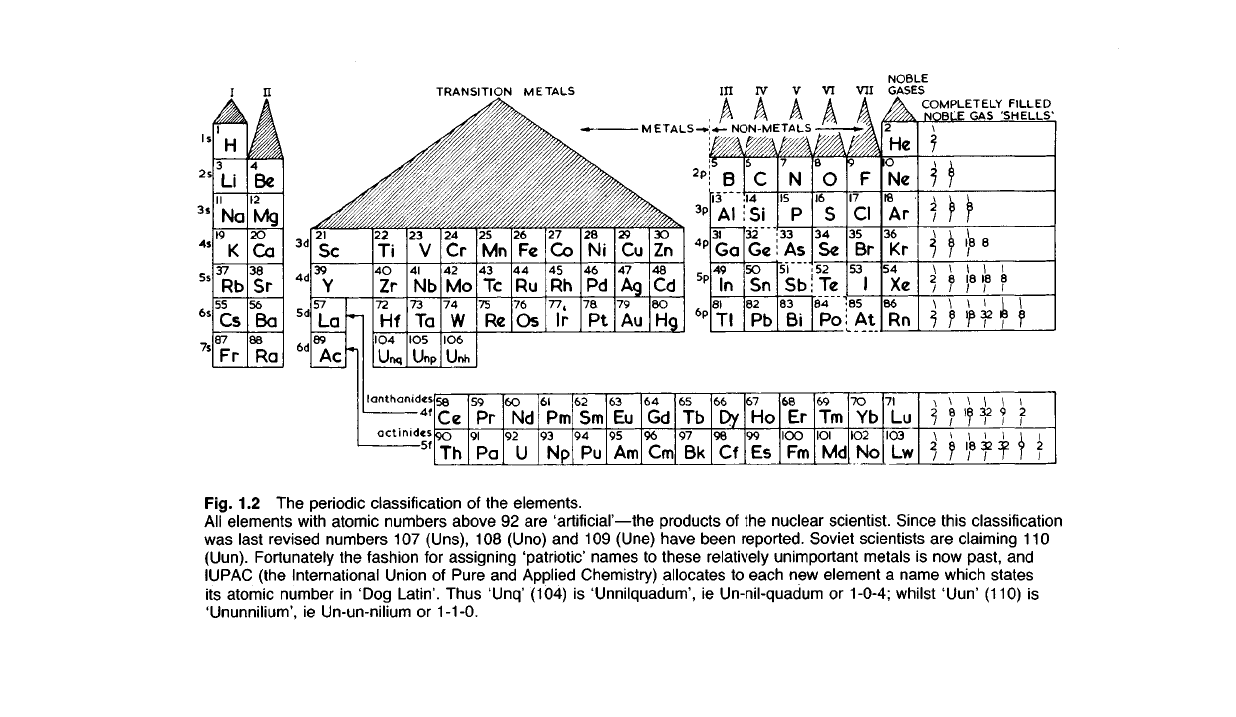
Fig. 1.2 The periodic classification of the elements.
All
elements with atomic numbers above 92 are 'artificial'—the products of the nuclear scientist. Since this classification
was
last revised numbers 107 (Uns), 108 (Uno) and 109 (Une) have been reported. Soviet scientists are
claiming
110
(Uun). Fortunately the fashion for assigning 'patriotic' names to these relatively unimportant metals is now past, and
IUPAC
(the International Union of Pure and Applied Chemistry) allocates to each new element a name which states
its
atomic number in 'Dog Latin'. Thus 'Unq' (104) is 'Unnilquadum', ie Un-nil-quadum or 1-0-4; whilst 'Uun' (110) is
'Ununnilium',
ie Un-un-nilium or 1-1-0.
NOBLE
GASES
COMPLETELY
FILLED
NOBLE
GAS
'SHELLS'
NON-METALS
METALS
TRANSITION
METALS
lanthanides
actinides

Fig.
1.3 The
formation
of the
electro-valent bond
in
sodium chloride,
by the
transfer
of
an electron from
the
sodium atom
to the
chlorine atom.
will attract each other
and the
salt sodium chloride crystallises
in a
simple
cubic form
in
which sodium ions
and
chlorine ions arrange themselves
in
the manner indicated
in
Fig.
1.4.
Except
for the
force
of
attraction which
exists between oppositely charged particles,
no
other 'bond' exists between
sodium ions
and
chlorine ions,
and
when
a
crystal
of
sodium chloride
is
dissolved
in
water separate sodium
and
chlorine ions
are
released
and can
move
as
separate particles
in
solution. Such
a
solution
is
known
as an
electrolyte because
it
will conduct electricity.
If we
place
two
electrodes
into such
a
solution
and
connect them
to a
direct-current supply,
the
posi-
tively charged sodium ions will travel
to the
negative electrode
and the
negatively charged chlorine ions will travel
to the
positive electrode.
The
applied EMF does
not
'split
up' the
sodium chloride—the latter ionises
as
soon
as it
dissolves
in
water.
1.73 Thus,
the
unit
in
solid sodium chloride
is the
crystal, whilst
in
solution separate ions
of
sodium
and
chlorine exist.
In
reality there
is no
sodium chloride molecule
and it is
therefore incorrect
to
express
the
salt
as 'NaCl'. Busy chemists
are,
however,
in the
habit
of
using symbols
in
this manner
as a
type
of
chemical shorthand.
The
author
has in
fact been
guilty
of
this indiscretion earlier
in
this chapter when discussing formulae
and equations
in
which electro-valent compounds
are
involved.
For
example,
the
equation representing
the
reaction between hydrochloric acid
and caustic soda (1.51) would more correctly
be
written:
BEFORE
REACTION
17
PROTONS
Il
PROTONS
Il PROTONS
Il ELECTRONSU,8,IJ
CHLORINE ATOM
17 PROTONS
17 ELECTRONS (2,8,7^
AFTER
REACTION
CHLORINE ION
17 PROTONS
IB ELECTRONS(2,8,8J
-HENCE A RESULTANT
NEGATIVE CHARGE.
Il P ROTONS
IO ELECTRONS (2,8)
-HENCE A RESULTANT
POSITIVE CHARGE.
17
PROTONS
I I
PROTONS
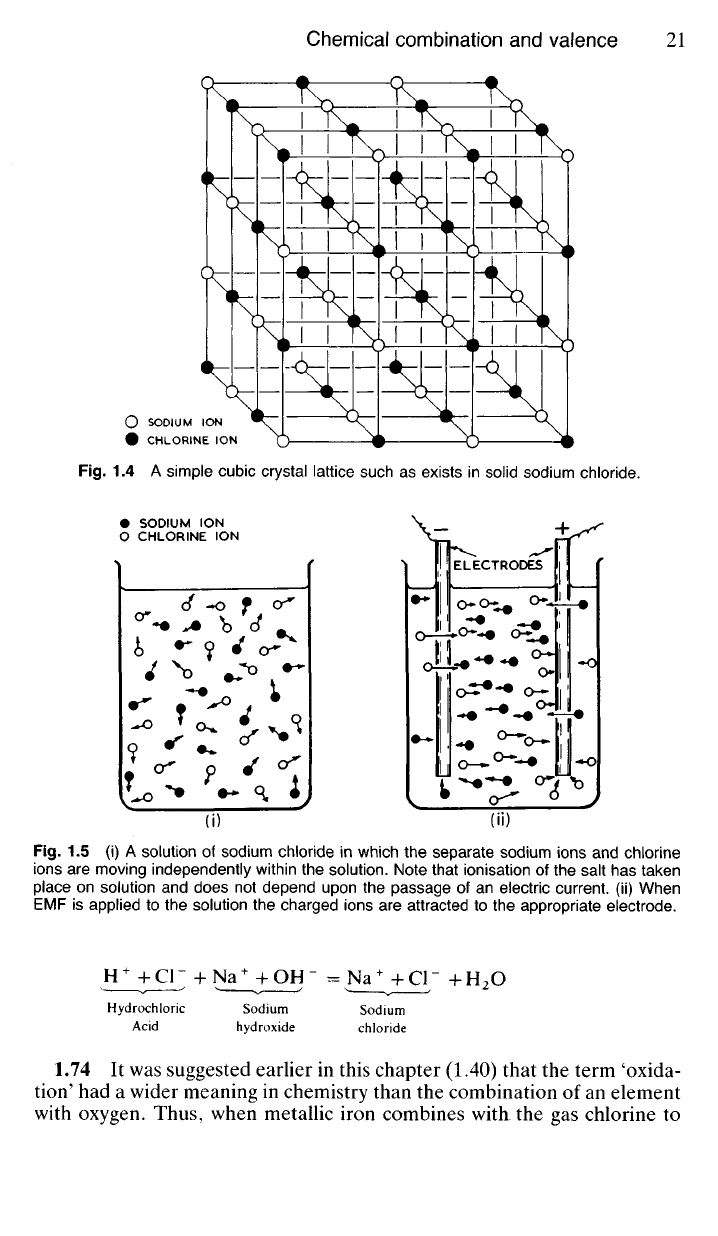
Fig.
1.5 (i) A solution of sodium chloride in which the separate sodium ions and chlorine
ions are moving independently within the solution. Note that ionisation of the salt has taken
place on solution and does not depend upon the passage of an electric current, (ii) When
EMF is applied to the solution the charged ions are attracted to the appropriate electrode.
H
+
+Cl" +NaN
1
OH" - Na
+
+Cl" +H
2
O
Hydrochloric Sodium Sodium
Acid hydroxide chloride
1.74 It was suggested earlier in this chapter (1.40) that the term 'oxida-
tion' had a wider meaning in chemistry than the combination of an element
with oxygen. Thus, when metallic iron combines with the gas chlorine to
SODIUM ION
CHLORINE ION
Fig.
1.4 A simple cubic crystal lattice such as exists in solid sodium chloride.
SODIUM ION
CHLORINE ION
ELECTRODES
(i)
(ii)
form iron(//) chloride, FeCl
2
, the iron is said to have been oxidised, whilst
when the iron(II) chloride so produced combines with still more chlorine
to form iron(///) chloride, FeCl
3
, the iron(II) chloride in turn has been
oxidised. At each stage the 'electronegative portion' of the substance has
increased. We can now relate this process of oxidation to a transfer of
electrons. In being oxidised, an atom of iron has lost electrons to become
an ion—first the iron(//) ion, Fe
++
, at which stage it has lost two electrons
and then the iron(///) ion, Fe
+++
, when it has lost three electrons to the
chlorine atoms:
Fe + Cl
2
-+Fe
++
+2Cr-^>Fe
+ + +
+3Cl"
iron(II) chloride iron(III) chloride
Thus oxidation of a substance, in this case iron, involves a loss of electrons
by atoms of that substance.
Conversely, reduction involves a gain of electrons. For example, when
iron(III) oxide, Fe
2
O
3
, is reduced to metallic iron in the blast furnace the
Fe
+++
ion receives electrons and becomes an atom of iron:
Fe
+
+ +
+3e->Fe
1.75 Covalent Combination In this type of chemical combination there
is no loss' of electrons from one atom to another. Instead a certain number
of electrons are 'shared' between two or more atoms to produce a stable
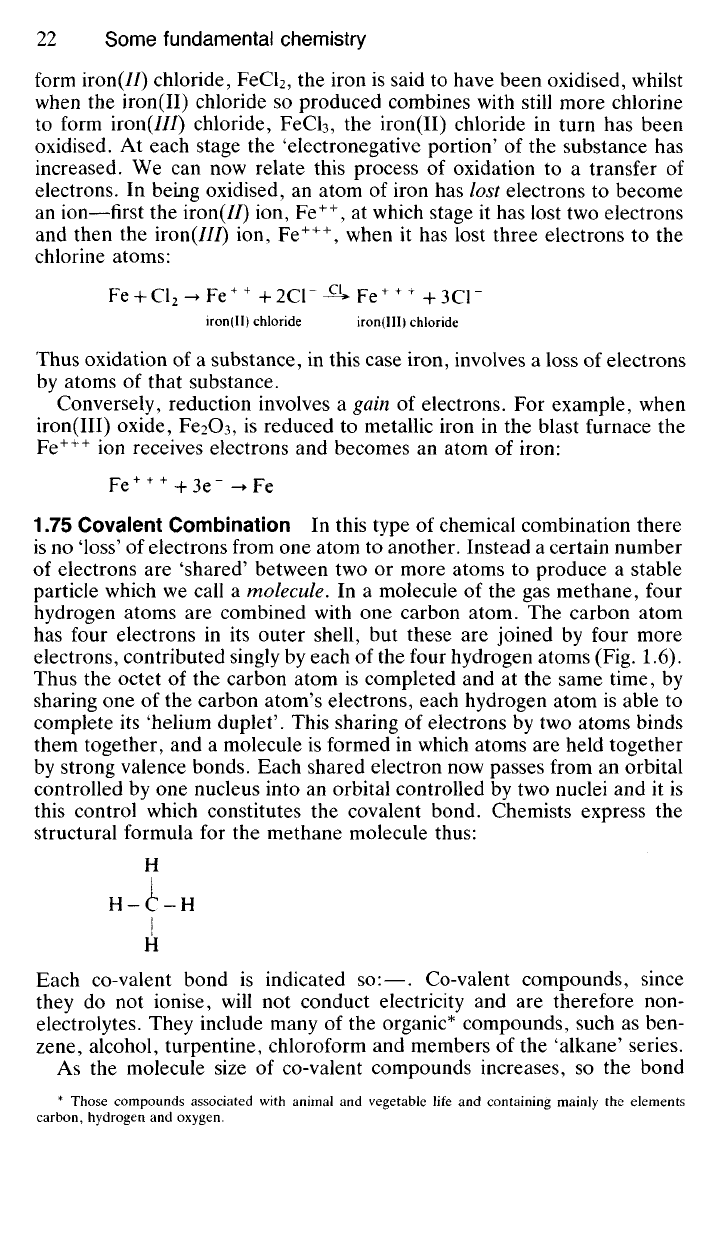
particle which we call a molecule. In a molecule of the gas methane, four
hydrogen atoms are combined with one carbon atom. The carbon atom
has four electrons in its outer shell, but these are joined by four more
electrons, contributed singly by each of the four hydrogen atoms (Fig. 1.6).
Thus the octet of the carbon atom is completed and at the same time, by
sharing one of the carbon atom's electrons, each hydrogen atom is able to
complete its 'helium duplet'. This sharing of electrons by two atoms binds
them together, and a molecule is formed in which atoms are held together
by strong valence bonds. Each shared electron now passes from an orbital
controlled by one nucleus into an orbital controlled by two nuclei and it is
this control which constitutes the covalent bond. Chemists express the
structural formula for the methane molecule thus:
H
H-C-H
I
H
Each co-valent bond is indicated so: —. Co-valent compounds, since
they do not ionise, will not conduct electricity and are therefore non-
electrolytes. They include many of the organic* compounds, such as ben-
zene,
alcohol, turpentine, chloroform and members of the 'alkane' series.
As the molecule size of co-valent compounds increases, so the bond
* Those compounds associated with animal and vegetable life and containing mainly the elements
carbon, hydrogen and oxygen.
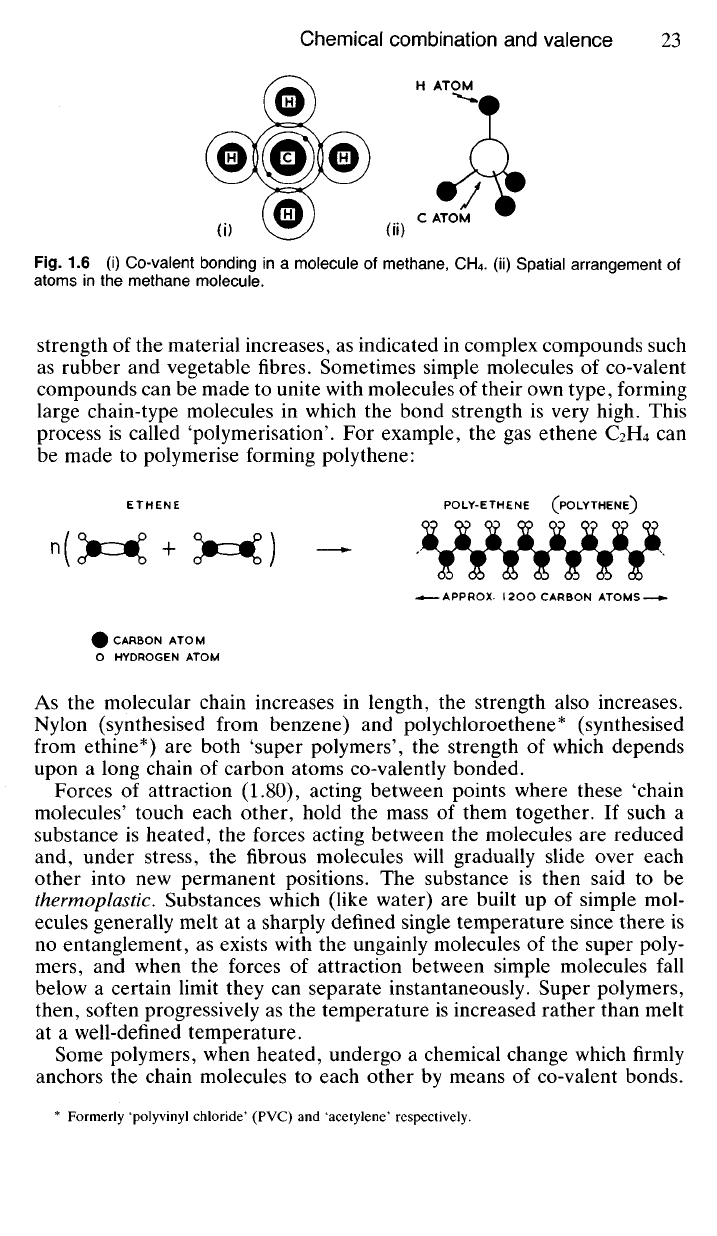
Fig.
1.6 (i) Co-valent bonding in a molecule of methane, CH
4
. (ii) Spatial arrangement of
atoms in the methane molecule.
strength of the material increases, as indicated in complex compounds such
as rubber and vegetable fibres. Sometimes simple molecules of co-valent
compounds can be made to unite with molecules of their own type, forming
large chain-type molecules in which the bond strength is very high. This
process is called 'polymerisation'. For example, the gas ethene C2H4 can
be made to polymerise forming polythene:
ETHENE POLY-ETHENE (POLYTHENE^
——APPROX- I 2OO CARBON ATOMS
CARBON ATOM
HYDROGEN ATOM
As the molecular chain increases in length, the strength also increases.
Nylon (synthesised from benzene) and polychloroethene* (synthesised
from ethine*) are both 'super polymers', the strength of which depends
upon a long chain of carbon atoms co-valently bonded.
Forces of attraction (1.80), acting between points where these 'chain
molecules' touch each other, hold the mass of them together. If such a
substance is heated, the forces acting between the molecules are reduced
and, under stress, the fibrous molecules will gradually slide over each
other into new permanent positions. The substance is then said to be
thermoplastic. Substances which (like water) are built up of simple mol-
ecules generally melt at a sharply defined single temperature since there is
no entanglement, as exists with the ungainly molecules of the super poly-
mers,
and when the forces of attraction between simple molecules fall
below a certain limit they can separate instantaneously. Super polymers,
then, soften progressively as the temperature is increased rather than melt
at a well-defined temperature.
Some polymers, when heated, undergo a chemical change which firmly
anchors the chain molecules to each other by means of co-valent bonds.
* Formerly 'polyvinyl chloride' (PVC) and 'acetylene' respectively.
H ATOM
CATOM
(ii)(i)
On cooling, the material is rigid and is said to be thermosetting. Bakelite
is such a substance.
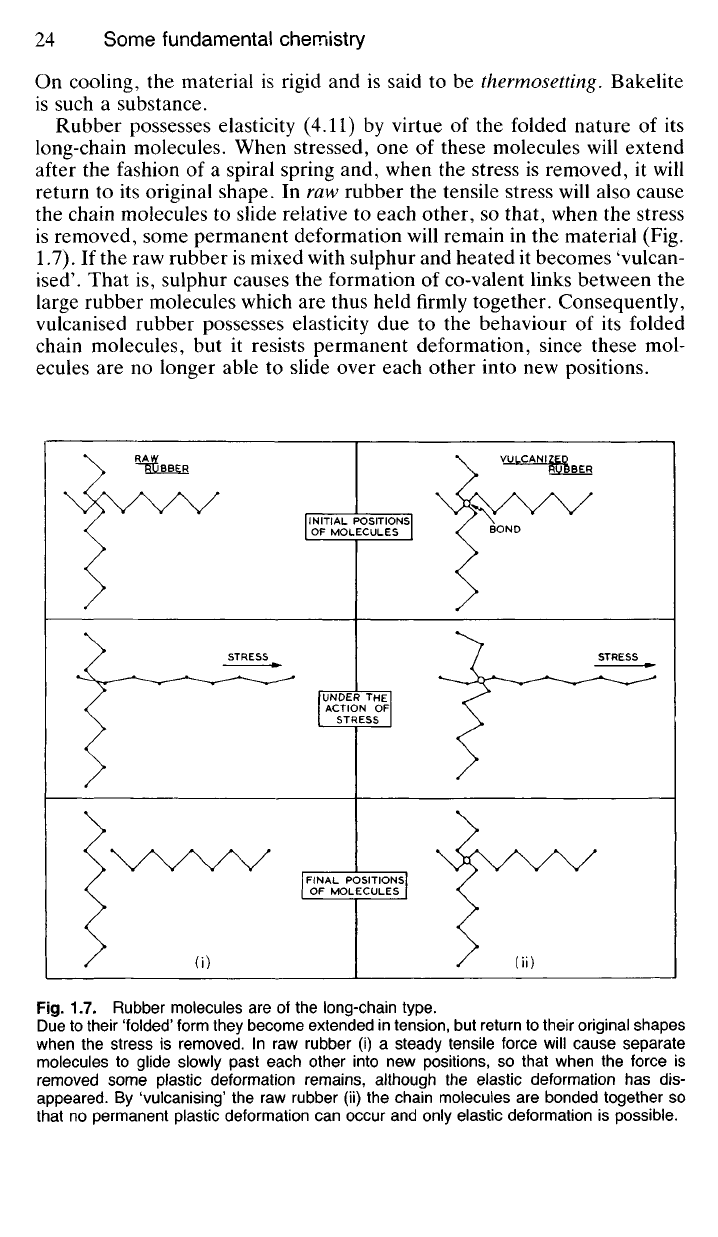
Rubber possesses elasticity (4.11) by virtue of the folded nature of its
long-chain molecules. When stressed, one of these molecules will extend
after the fashion of a spiral spring and, when the stress is removed, it will
return to its original shape. In raw rubber the tensile stress will also cause
the chain molecules to slide relative to each other, so that, when the stress
is removed, some permanent deformation will remain in the material (Fig.
1.7). If the raw rubber is mixed with sulphur and heated it becomes 'vulcan-
ised'. That is, sulphur causes the formation of co-valent links between the
large rubber molecules which are thus held firmly together. Consequently,
vulcanised rubber possesses elasticity due to the behaviour of its folded
chain molecules, but it resists permanent deformation, since these mol-
ecules are no longer able to slide over each other into new positions.
Fig.
1.7. Rubber molecules are of the long-chain type.
Due to their 'folded' form they become extended in tension, but return to their original shapes
when the stress is removed. In raw rubber (i) a steady tensile force will cause separate
molecules to glide slowly past each other into new positions, so that when the force is
removed some plastic deformation remains, although the elastic deformation has dis-
appeared.
By 'vulcanising' the raw rubber (ii) the chain molecules are bonded together so
that no permanent plastic deformation can occur and only elastic deformation is possible.
(i)
(ii)
FINAL POSITIONS
OF MOLECULES
UNDER THE
ACTION OF
STRESS
STRESS
STRESS
INITIAL POSITIONS
OF MOLECULES
BOND
VULCANIZED
RUBBER
RAW
~R"tiBBER
1.76 The Metallic Bond In most pure metals, atoms possess insufficient
valence electrons to be able to form covalent bonds with each other. On
the other hand any metallic ions which may be formed in a pure metal
will carry like positive charges and so tend to repel each other so that
electrovalent bonding is impossible. Yet we know that metals are crystal-
line in the solid state (3.10). How then is this situation achieved?
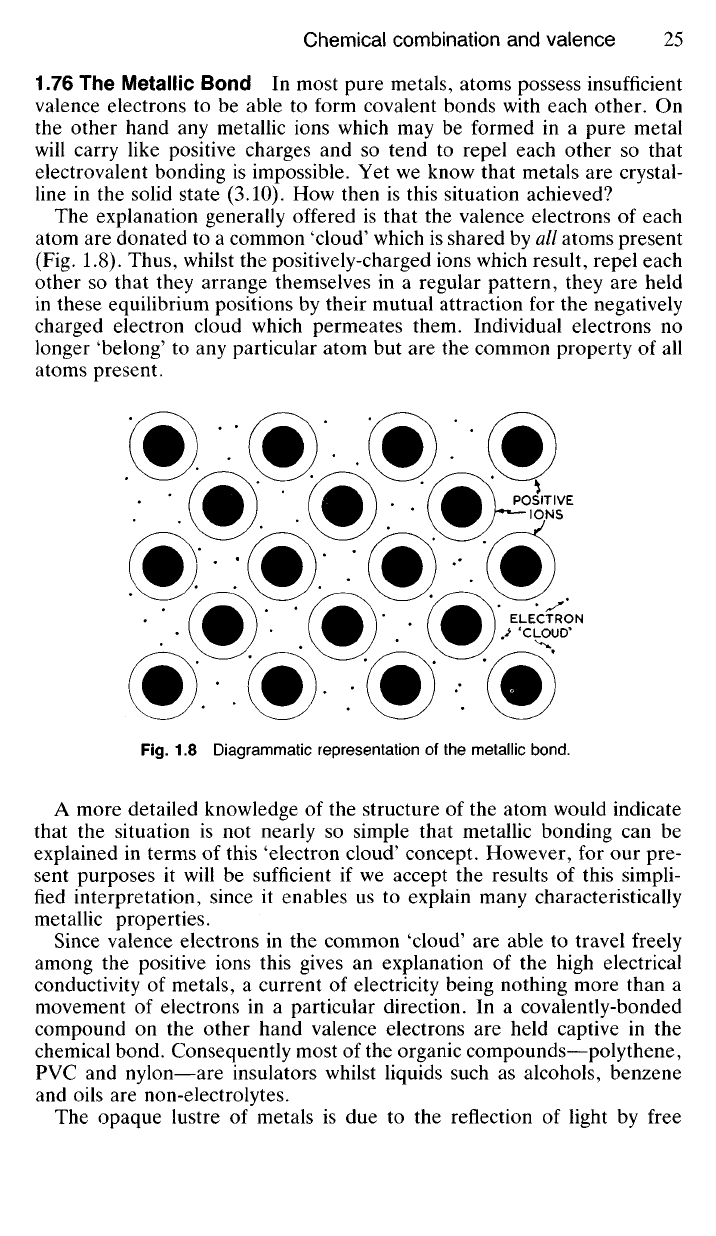
The explanation generally offered is that the valence electrons of each
atom are donated to a common 'cloud' which is shared by all atoms present
(Fig. 1.8). Thus, whilst the positively-charged ions which result, repel each
other so that they arrange themselves in a regular pattern, they are held
in these equilibrium positions by their mutual attraction for the negatively
charged electron cloud which permeates them. Individual electrons no
longer 'belong' to any particular atom but are the common property of all
atoms present.
Fig.
1.8 Diagrammatic representation of the metallic bond.
A more detailed knowledge of the structure of the atom would indicate
that the situation is not nearly so simple that metallic bonding can be
explained in terms of this 'electron cloud' concept. However, for our pre-
sent purposes it will be sufficient if we accept the results of this simpli-
fied interpretation, since it enables us to explain many characteristically
metallic properties.
Since valence electrons in the common 'cloud' are able to travel freely
among the positive ions this gives an explanation of the high electrical
conductivity of metals, a current of electricity being nothing more than a
movement of electrons in a particular direction. In a covalently-bonded
compound on the other hand valence electrons are held captive in the
chemical bond. Consequently most of the organic compounds—polythene,
PVC and nylon—are insulators whilst liquids such as alcohols, benzene
and oils are non-electrolytes.
The opaque lustre of metals is due to the reflection of light by free
ELECTRON
'CLOUD*
POSITIVE
IONS
