Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

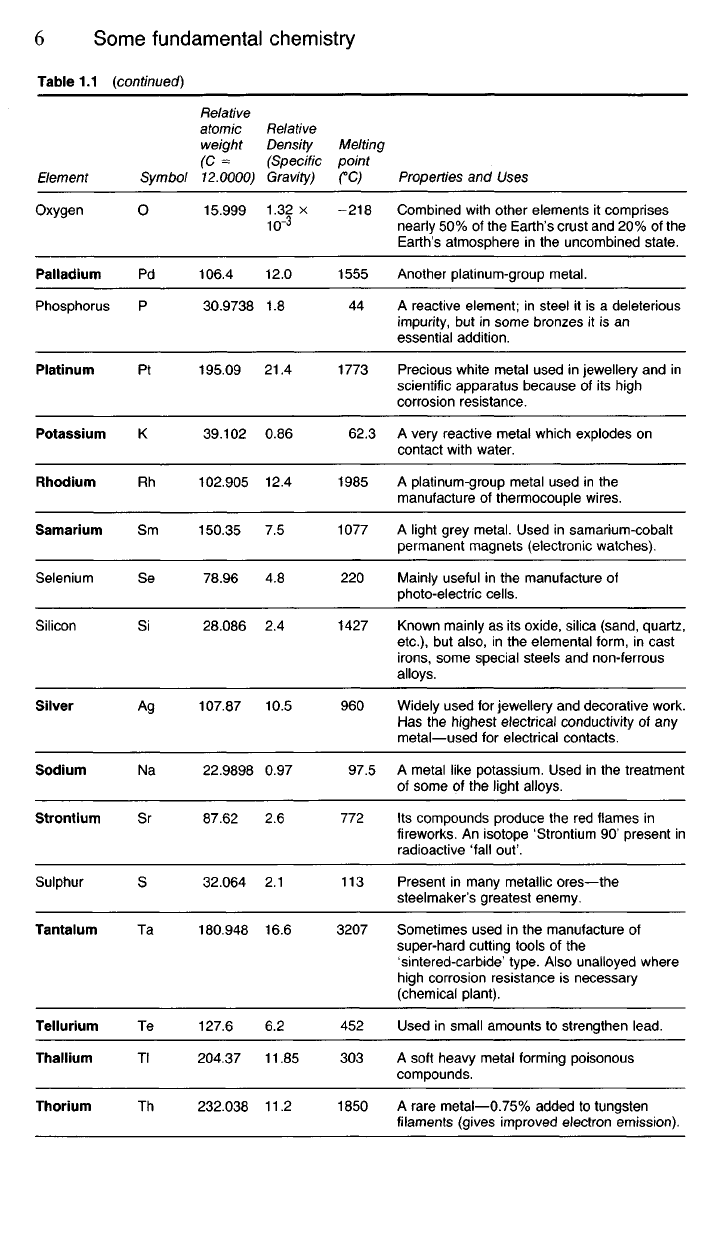
Table 1.1 {continued)
Element
Oxygen
Palladium
Phosphorus
Platinum
Potassium
Rhodium
Samarium
Selenium
Silicon
Silver
Sodium
Strontium
Sulphur
Tantalum
Tellurium
Thallium
Thorium
Symbol
O
Pd
P
Pt
K
Rh
Sm
Se
Si
Ag
Na
Sr
S
Ta
Te
Tl
Th
Relative
atomic
weight
(C =
12.0000)
15.999
106.4
30.9738
195.09
39.102
102.905
150.35
78.96
28.086
107.87
22.9898
87.62
32.064
180.948
127.6
204.37
232.038
Relative
Density
(Specific
Gravity)
1.32 x
10"
3
12.0
1.8
21.4
0.86
12.4
7.5
4.8
2.4
10.5
0.97
2.6
2.1
16.6
6.2
11.85
11.2
Melting
point
CC)
-218
1555
44
1773
62.3
1985
1077
220
1427
960
97.5
772
113
3207
452
303
1850
Properties and Uses
Combined with other elements it comprises
nearly 50% of the Earth's crust and 20% of the
Earth's atmosphere in the uncombined state.
Another platinum-group metal.
A reactive element; in steel it is a deleterious
impurity, but in some bronzes it is an
essential addition.
Precious white metal used in jewellery and in
scientific apparatus because of its high
corrosion resistance.
A very reactive metal which explodes on
contact with
water.
A platinum-group metal used in the
manufacture of thermocouple wires.
A light grey metal. Used in samarium-cobalt
permanent magnets (electronic watches).
Mainly useful in the manufacture of
photo-electric cells.
Known mainly as its oxide, silica (sand, quartz,
etc.),
but also, in the elemental form, in cast
irons,
some special steels and non-ferrous
alloys.
Widely used for jewellery and decorative work.
Has the highest electrical conductivity of any
metal—used for electrical contacts.
A metal like potassium. Used in the treatment
of some of the light alloys.
Its compounds produce the red flames in
fireworks. An isotope 'Strontium 90' present in
radioactive 'fall out'.
Present in many metallic ores—the
steelmaker's greatest enemy.
Sometimes used in the manufacture of
super-hard cutting tools of the
'sintered-carbide' type. Also unalloyed where
high corrosion resistance is necessary
(chemical plant).
Used in small amounts to strengthen
lead.
A soft heavy metal forming poisonous
compounds.
A rare metal—0.75% added to tungsten
filaments (gives improved electron emission).
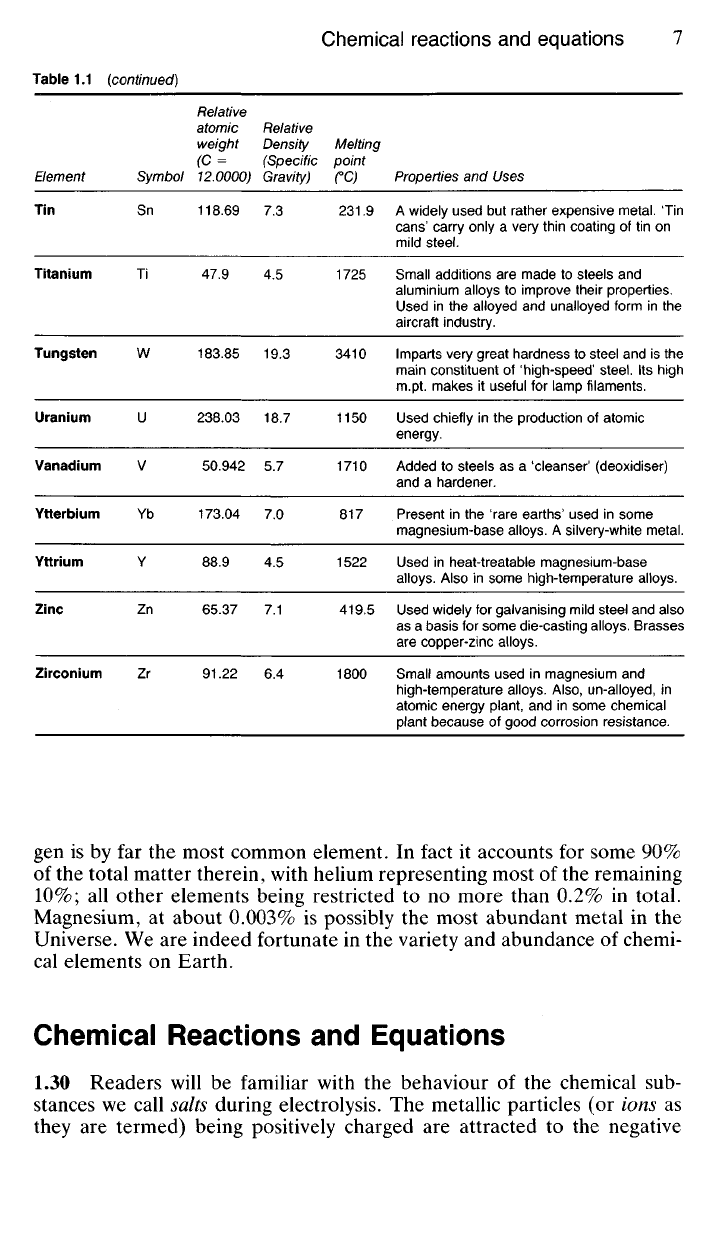
Table 1.1 (continued)
Element
Tin
Titanium
Tungsten
Uranium
Vanadium
Ytterbium
Yttrium
Zinc
Zirconium
Symbol
Sn
Ti
W
U
V
Yb
Y
Zn
Zr
Relative
atomic
weight
(C =
12.0000)
118.69
47.9
183.85
238.03
50.942
173.04
88.9
65.37
91.22
Relative
Density
(Specific
Gravity)
7.3
4.5
19.3
18.7
5.7
7.0
4.5
7.1
6.4
Melting
point
(
0
C)
231.9
1725
3410
1150
1710
817
1522
419.5
1800
Properties and Uses
A widely used but rather expensive metal. Tin
cans'
carry only a very thin coating of tin on
mild steel.
Small additions are made to steels and
aluminium alloys to improve their properties.
Used in the alloyed and unalloyed form in the
aircraft industry.
Imparts very great hardness to steel and is the
main constituent of 'high-speed' steel. Its high
m.pt. makes it useful for lamp filaments.
Used chiefly in the production of atomic
energy.
Added to steels as a 'cleanser' (deoxidiser)
and a hardener.
Present in the 'rare earths' used in some
magnesium-base alloys. A silvery-white metal.
Used in heat-treatable magnesium-base
alloys. Also in some high-temperature alloys.
Used widely for galvanising mild steel and also
as a basis for some die-casting alloys. Brasses
are copper-zinc alloys.
Small amounts used in magnesium and
high-temperature alloys. Also, un-alloyed, in
atomic energy plant, and in some chemical
plant because of good corrosion resistance.
gen is by far the most common element. In fact it accounts for some 90%
of the total matter therein, with helium representing most of the remaining
10%;
all other elements being restricted to no more than 0.2% in total.
Magnesium, at about 0.003% is possibly the most abundant metal in the
Universe. We are indeed fortunate in the variety and abundance of chemi-
cal elements on Earth.
Chemical Reactions and Equations
1.30 Readers will be familiar with the behaviour of the chemical sub-
stances we call salts during electrolysis. The metallic particles (or ions as
they are termed) being positively charged are attracted to the negative

electrode (or cathode), whilst the non-metallic ions being negatively
charged are attracted to the positive electrode (or anode). Because of the
behaviour of their respective ions metals are said to be electropositive and
non-metals electronegative.
In general elements react or combine with each other when they possess
opposite chemical natures. Thus the more electropositive a metal the more
readily will it combine with a non-metal, forming a very stable compound.
If one metal is more strongly electropositive than another the two may
combine forming what is called an intermetallic compound, though usually
they only mix with each other forming what is in effect a 'solid solution'.
This constitutes the basis of most useful alloys.
1.31 Atoms combine with each other in simple fixed proportions. For
example, one atom of the gas chlorine (Cl) will combine with one atom of
the gas hydrogen (H) to form one molecule of the gas hydrogen chloride.
We can write down a formula for hydrogen chloride which expresses at a
glance its molecular constitution, viz. HCl. Since one atom of chlorine will
combine with one atom of hydrogen, its valence is said to be one, the term
valence denoting the number of atoms of hydrogen which will combine
with one atom of the element in question. Again, two atoms of hydrogen
combine with one atom of oxygen to form one molecule of water (H
2
O),
so that the valence of oxygen is two. Similarly, four atoms of hydrogen
will combine with one atom of carbon to form one molecule of methane
(CH
4
).
Hence the valence of carbon in this instance is four. However,
carbon, like several other elements, exhibits a variable valence, since it will
also form the substances ethene (C
2
H
4
), formerly 'ethylene', and ethine
(C
2
H
2
),
formerly acetylene.
1.32 Compounds also react with each other in simple proportions, and
we can express such a reaction in the form of a chemical equation thus:
CaO + 2HCl = CaCl
2
+ H
2
O
Table 1.2 The Approximate Composition of the Earth's Crust (to the Extent of Mining Operations)
Element
Oxygen
Silicon
Aluminium
Iron
Calcium
Sodium
Potassium
Magnesium
Hydrogen
Titanium
Carbon
Chlorine
Phosphorus
Manganese
Sulphur
Barium
Nitrogen
Chromium
% by mass
49.1
26.0
7.4
4.3
3.2
2.4
2.3
2.3
1.0
0.61
0.35
0.20
0.12
0.10
0.10
0.05
0.04
0.03
Element
Nickel
Vanadium
Zinc
Copper
Tin
Boron
Cobalt
Lead
Molybdenum
Tungsten
Cadmium
Beryllium
Uranium
Mercury
Silver
Gold
Platinum
Radium
% by mass
0.02
0.02
0.02
0.01
0.008
0.005
0.002
0.002
0.001
9 x 10"
4
5 x 10"
4
4 x 10"
4
4 x 10"
4
1 x 10"
4
1 x 10~
5
5 x 10"
6
5 x 10"
6
2 x 1O~
10

The above equation tells us that one chemical unit of calcium oxide (CaO
or 'quicklime') will react with two chemical units of hydrogen chloride
(HCl or hydrochloric acid), to produce one chemical unit of calcium chlor-
ide (CaCb) and one chemical unit of water (H2O). Though the total
number of chemical units may change due to the reaction—we began with
three units and ended with two—the total number of atoms remains the
same on either side of the equation. The equation must balance rather like
a financial balance sheet.
1.33 By substituting the appropriate atomic masses (approximated
values from Table 1.1) in the above equation we can obtain further useful
information from it.
Thus,
56 parts by weight of quicklime will react with 73 parts by weight of
hydrogen chloride to produce 111 parts by weight of calcium chloride and
18 parts by weight of water. Naturally, instead of 'parts by weight' we can
use grams, kilograms or tonnes as required.
We will now deal with some chemical reactions relevant to a study of
metallurgy.
Oxidation and Reduction
1.40 Oxidation is one of the most common of chemical processes. It
refers,
in its simplest terms, to the combination between oxygen and any
other element—a phenomenon which is taking place all the time around
us.
In our daily lives we make constant use of oxidation. We inhale
atmospheric oxygen and reject carbon dioxide (CO
2
)—the oxygen we
breathe combines with carbon from our animal tissues, releasing energy in
the process. We then reject the waste carbon dioxide. Similarly, heat
energy can be produced by burning carbonaceous materials, such as coal
or petroleum. Just as without breathing oxygen animals cannot live, so
without an adequate air supply fuel cannot burn. In these reactions carbon
and oxygen have combined to form a gas, carbon dioxide (CO
2
), and at
the same time heat energy has been released—the 'energy potential' of
the carbon having fallen in the process.
1.41 Oxidation, however, is also a phenomenon which works to our
disadvantage, particularly in so far as the metallurgist is concerned, since
a large number of otherwise useful metals show a great affinity for oxygen
and combine with it whenever they are able. This is particularly so at high
temperatures so that the protection of metal surfaces by means of fluxes
is often necessary during melting and welding operations. Although cor-
rosion is generally a more complex phenomenon, oxidation is always

involved and expensive processes such as painting, plating or galvanising
must be used to protect the metallic surface.
1.42 It should be noted that, to the chemist, the term oxidation has a
much wider meaning, and in fact refers to a chemical process in which the
electronegative (or non-metallic) constituent of the molecule is increased.
(1.74) For example, iron (II) chloride may be oxidised to iron (III)
chloride—
2FeCl
2
+ Cl
2
= 2FeCl
3
Iron(II) Iron(III)
chloride chloride
The element oxygen is not involved in this reaction, yet we say that iron
(II) chloride has been 'oxidised' by chlorine since the chlorine ion is
electronegative and so the electronegative portion of the iron (III) chlor-
ide molecule is greater than that of the iron (II) chloride molecule.
Since iron exhibits valences of both two and three this is indicated, in
current chemical nomenclature, by writing either iron (II)' or 'iron (III)'
as appropriate. Formerly the terms 'ferrous' or 'ferric' were used to
describe these two series of compounds. In fact any metal exhibiting a
variable valence gave rise to '-ous' and '-/c' series of compounds; -ous
being used to describe that series in which the metal exhibits the lower
valence and -ic that in which the metal exhibits the higher valence.
1.43 Whilst many metals exist in the Earth's crust in combination with
oxygen as oxides, others are combined with sulphur as sulphides. The latter
form the basis of many of the non-ferrous (that is, containing no iron)
metal ores. The separation and removal of the oxygen or sulphur contained
in the ore from the metal itself is often a difficult and expensive process.
Most of the sulphide ores are first heated in air to convert them to oxides,
eg—
2ZnS + 3O
2
= 2ZnO + 2SO
2
Zinc sulphide Zinc oxide Sulphur
('zinc blende') dioxide (gas)
The oxide, whether occurring naturally or produced as indicated in the
above equation, is then generally mixed with carbon in the form of coke
or anthracite and heated in a furnace. In most cases some of the carbon is
burned simultaneously in order to provide the necessary heat which will
cause the reaction to proceed more quickly. Under these conditions carbon
usually proves to have a greater affinity for oxygen than does the metal
and so takes oxygen away from the metal, forming carbon dioxide and
leaving the metal (often impure) behind, eg—
2ZnO-HC = 2Zn+ CO
2
This process of separating the atoms of oxygen from a substance is known
as reduction. Reduction is thus the reverse of oxidation, and again, in the

wider chemical sense, it refers to a reaction in which the proportion of the
electronegative constituent of the molecule is decreased.
1.44 Some elements have greater affinities for oxygen than have others.
Their oxides are therefore more difficult to decompose. Aluminium and
magnesium, strongly electropositive metals, have greater affinities for oxy-
gen than has carbon, so that it is impossible to reduce their oxides in the
normal way using coke—electrolysis, a much more expensive process,
must be used. Metals, such as aluminium, magnesium, zinc, iron and lead,
which form stable, tenacious oxides are usually called base metals, whilst
those metals which have little affinity for oxygen are called noble metals.
Such metals include gold, silver and platinum, metals which will not scale
or tarnish to any appreciable extent due to the action of atmospheric
oxygen.
Acids,
Bases and Salts
1.50 When an oxide of a non-metal combines with water it forms what
we call an
acid.
Thus sulphur trioxide (SO
3
) combines with water to form
the well-known sulphuric acid (H
2
SO
4
), and sulphur trioxide is said to be
the anhydride of sulphuric acid. Though not all acids are as corrosive as
sulphuric, it is fairly well known that in cases of accident involving acids
of this type it is necessary to neutralise the acid with some suitable antidote.
1.51 Substances which have this effect are called bases. These are met-
allic oxides (and hydroxides) which, when they react with an acid, produce
water and a chemical compound which we call a salt. Typical examples of
these acid-base reactions are—
H
2
SO
4
+ CaO = CaSO
4
+ H
2
O
Sulphuric Calcium oxide Calcium Water
acid ('quicklime') sulphate
(a salt)
HCl +NaOH= NaCl +H
2
O
Hydrochloric Sodium Sodium
acid hydroxide chloride
('caustic ('common
soda') salt')
We can generalise in respect of equations like this and say—
Acid + Base = Salt + Water
Similarly, the acid anhydride will often combine with a base, forming a
salt, eg—
SO
3
+ CaO = CaSO
4
This type of reaction occurs quite frequently during the smelting of metallic
ores.

1.52
One of the most common elements in the Earth's crust is silicon,
present in the form of its oxide, silica (SiO
2
). Since silicon is a non-metal,
its oxide is an acid anhydride, and though all the common forms of silica,
such as sand, sandstone and quartz, do not seem to be of a very reactive
nature at normal temperatures, they are sufficiently reactive when heated
to high temperatures to combine with many of the metallic oxides (which
are basic) and produce neutral salts called silicates. Silica occurs entangled
with most metallic ores, and although some of it is rejected by mechanical
means before the ore is charged to the furnace, some remains and could
constitute a difficult problem in that its high melting point of 1780° C would
cause a sort of 'indigestion' in the furnace. To overcome this a sufficient
quantity of a basic flux is added in order to combine with the silica and
produce a slag with a melting point low enough to allow it to run from the
furnace. The cheapest metallic oxide, and the one in general use, is lime.
SiO
2
+2CaO = 2CaO. SiO
2
Acid Base Salt (calcium
anhydride silicate)
The formula for the slag, calcium silicate, is generally written 2CaO.SiO
2
rather than Ca
2
SiO4, since lime and silica will combine in other pro-
portions. When the formula is written in the former manner, the reacting
proportions of silica and lime can be seen at a glance.
1.53 On the other hand acid/basic reactions can constitute a problem
when they involve similar reactions between slags and furnace linings.
Thus,
since we do not wish to liquefy our furnace lining, we must make
sure that it does not react with the charge or the slag covering it. In short,
we must make sure that the furnace lining is of the same chemical nature
as the slag, ie if the slag contains an excess of silica, and is therefore acid,
we must line the furnace with a similar silica-rich refractory, such as silica
brick or ganister; whilst if the slag contains an excess of lime or other basic
material, we must line the furnace with a basic refractory, such as burnt
dolomite (CaO.MgO) or burnt magnesite (MgO). If the chemical nature
of the furnace lining is the same as that of the slag, then, clearly, no
reaction is likely to take place between them. Since silica and ganister, on
the one hand, and dolomite and magnesite, on the other, all have high
softening temperatures, they will also be able to resist the high tempera-
tures encountered in many of the metallurgical smelting processes.
Atomic Structure
1.60 It was the Ancient Greek philosopher Leukippos and his disciple
Demokritos who, during the fifth century BC, suggested that if matter
were progressively subdivided a point would be reached where further
subdivision was impossible. The Greek word for 'indivisible' is 'atomos'.
More than 2000 years later—in 1808—a British chemist, John Dalton
announced his Atomic theory which was based on the original Leukippos/
Demokritos idea. Dalton suggested that chemical reactions could be
explained if it was assumed that each chemical element consisted of
extremely small indivisible particles, which, following the Greek concept,
he called 'atoms'. The Theory was generally accepted but before the end
of the nineteenth century it was discovered that atoms were certainly not
indivisible. Thus the 'atom' is an ill-described particle. But the title became
so firmly established that it is retained to-day, though we usually modify
our description to add that it is 'the smallest stable particle of matter which
can exist'.
1.61 In 1897 the English physicist J. J. Thomson showed that a beam
of 'cathode rays' was in fact a stream of fast-moving negatively charged
particles—they were in fact electrons. Because the electron is negatively
charged it can be deflected from its path by an electrical field and we make
use of this feature in TV tubes where a beam of electrons, deflected by a
system of electro-magnetic fields, builds up a picture by impingement on
a screen which will fluoresce under the impact of electrons. Thomson was
able to make only a rough estimate of the mass of the electron but was
able to show that it was extremely small compared with a hydrogen atom.
Thus Dalton's 'indivisible atom' fell apart.
1.62 Since atoms are electrically neutral—common everyday materials
carry no resultant electrical charge—the discovery of the negatively
charged electron stimulated research to find a positively charged particle.
This was ultimately discovered in the form of the nucleus of the hydrogen
atom, with a mass some 1837 times greater than that of the electron but
with an equal but opposite positive charge. In 1920 the famous New Zea-
land born physicist Ernest Rutherford suggested it be called the proton.
So,
atoms were assumed to be composed of equal numbers of electrons
and protons, the electrons being arranged in 'shells' or 'orbits' around a
bunch of protons which constituted the nucleus of the atom. But there was
a snag: the true atomic weights of the elements, which had been derived
by careful independent experiment over many years, were much greater
than the atomic weights calculated from an assumption of the numbers of
protons and electrons present in the atom of a particular element. Chemists
explained this 'dead weight' in the atomic nucleus by suggesting that there
were extra protons in the nucleus which had been 'neutralised' electrically
by the presence of electrons also lurking there. Thus in the early 1930s
electrons were classed as being either 'planetary' or 'nuclear.'
1.63 At about this time the English physicist Sir James Chadwick dis-
covered the neutron, a particle of roughly the same mass as the proton but
carrying no electrical charge. Its presence in the atomic nucleus made it
easier to explain that part of the atomic mass not attributable to a simple
electron/proton balance. Its electrical neutrality made it a useful particle
in atomic research, since it could be fired into a nucleus without being
repelled by like electrical charges. Chadwick's discovery did in fact alter
the course of history since it made possible the development of the Atomic
Bomb ten years later (18.74).
1.64 Since Chadwick's time the number of elementary particles has
proliferated. These can be classified into three main groups:
1 Baryons (protons and other particles with a mass greater than that of
the proton).
2 Mesons (any of a group of particles with a rest mass between those of
the electron and proton, and with an integral 'spin').
3 Leptons (among which are the electron, positron or 'positive electron'
and the neutrino which possesses neither charge nor mass but only
'spin').
The term 'quark' is used to describe any one of a number of hypothetical
elementary particles with charges of % or -
1
A of the electron charge, and
thought to be fundamental units of all baryons and mesons. It is interesting
to note that the word 'quark' was devised by James Joyce in Finnegan's
Wake. Indeed, to date the existence of more than two hundred different
subatomic particles has been reported. Of these only three—electron, pro-
ton and neutron—appear to have any substantial influence on the distinc-
tive properties of each element. Consequently the rest will receive no
further mention in this book. The essential features of electron, proton
and neutron are summarised in Table 1.3. Since the real mass of these
particles is inconveniently small for calculations their masses relative to
that of the carbon atom (isotope C= 12) are generally used.
Table 1.3
Relative mass
Particle Actual rest mass (kg) (
12
C= 12) Charge (C)
Electron 9.11 x 10~
31
0.000 548 8 -1.602 x 10~
19
\ Equal but opposite
Proton 1.672 x 10"
27
1.007 263 +1.602 x 10"
19 f
Neutron 1.675 x 1O"
27
1.008 665 0 Zero
1.65 In the early days of the twentieth century Rutherford carried out
a series of classical experiments in which he fired a-particles—the nuclei
of helium atoms and therefore positively charged—at very thin gold foil.
Most of these particles passed right through the foil whilst about one in
20 000 rebounded along its incident path. Others were deflected at various
angles to the incident beam. From these experiments Rutherford con-
cluded that the atom consisted of a comparatively small nucleus containing
protons, around which circulated electrons. This concept of the atom was
developed by the Danish physicist Niels Bohr. He proposed that the elec-
trons were placed in a series of fixed orbits which varied in number with
the complexity of the atom. The constitution of an atom was thus regarded
as being similar to that of the solar system and containing about the same
density of actual 'matter'. This model was later modified to the extent that
the definite electron orbit was replaced by a mathematical function which
represents the distribution of 'electrons' in the space occupied by the atom.
This distribution is referred to as the orbital of the electron. In fact many
visualise the electron as being in the nature of a 'cloud' of electricity rather
than as a discrete particle, the orbital indicating the density of that cloud
at any point within the atom. From the point of view of any diagram
representing atomic structure it is more convenient to indicate the electron

as being a definite particle travelling in a simple circular orbit round a
nucleus consisting of protons and neutrons, but diagrams such as Fig. 1.1
should be studied with this statement in mind. Fig. 1.1 in no way represents
what atoms 'look like'.
1.66 The most simple of all atoms is that of ordinary hydrogen. It
consists of one proton with one electron in orbital around it. Since the
positive charge of the proton is balanced by the equal but negative charge
of the electron, the resultant atom will be electrically neutral. The mass of
the electron being very small compared with that of the proton, the mass
of the atom will be roughly that of the proton.
1.67 An atom of ordinary helium comes next in order of both mass
and complexity. Here the nucleus contains two protons which are associ-
ated with two electrons in the same 'shell', ie similar orbitals surrounding
the nucleus. The nucleus also contains two neutrons. However, in Table
1.4, which indicates the proton-electron make-up of some of the simpler
atoms, neutrons have been omitted for reasons which will become apparent
later (1.90). The number of protons in the nucleus, which is equal to the
total number of electrons in successive shells, is called the Atomic number
of the element.
In Table 1.4 it will be noted that with the metal lithium a new electron
shell is formed and that this 'fills up' by the addition of a single electron
with each successive element until, with the 'noble'* gas neon, it contains
a total of eight electrons. With the metal sodium another new shell then
begins and similarly fills so that with the noble gas argon this third shell
also contains eight electrons. The next shell then begins to form with the
metal potassium.
1.68 In the case of the elements dealt with in Table 1.4, this periodicity
in respect of the number of electrons in the outer shell is reflected in the
chemical properties of the elements themselves. Thus the metals lithium,
sodium and potassium each have a single electron in the outer shell and
all are very similar chemically. They will all oxidise very rapidly and react
readily with water, liberating hydrogen and forming soluble hydroxides.
Each of these elements has a valence of one. Physically, also, they are very
similar in that they are all light soft metals, more or less white in colour.
In a similar way the gases fluorine and chlorine, with seven electrons in
the outer shell in each case, have like chemical properties. Both are
coloured gases (at normal temperatures and pressures) with strongly non-
metallic properties.
The noble gases helium, neon and argon occur in small quantities in the
atmosphere. In fact it is only there where they are likely to exist under
natural conditions, since these noble gases are similar in being non-
reactive and, under ordinary circumstances, unable to combine with other
elements. Chemical combination between elements is governed by the
number of electrons present in the outer shell of each atom concerned.
When the outer shell contains eight electrons it becomes, as it were, 'satu-
* In the chemical sense the term 'noble' means that an element is not very reactive—thus, the 'noble'
metals, gold, platinum, etc., are not readily attacked by other reactive substances, such as corrosive acids.
